Abstract
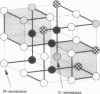
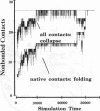
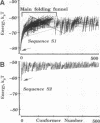
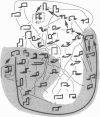
A lattice model of protein folding is developed to distinguish between amino acid sequences that do and do not fold into unique conformations. Although Monte Carlo simulations provide insights into the long-time processes involved in protein folding, these simulations cannot systematically chart the conformational energy surface that enables folding. By assuming that protein folding occurs after chain collapse, a kinetic map of important pathways on this surface is constructed through the use of an analytical theory of probability flow. Convergent kinetic pathways, or "folding funnels," guide folding to a unique, stable, native conformation. Solution of the probability flow equations is facilitated by limiting treatment to diffusion between geometrically similar collapsed conformers. Similarity is measured in terms of a reconfigurational distance. Two specific amino acid sequences are deemed foldable and nonfoldable because one gives rise to a single, large folding funnel leading to a native conformation and the other has multiple pathways leading to several stable conformers. Monte Carlo simulations demonstrate that folding funnel calculations accurately predict the fact of and the pathways involved in folding-specific sequences. The existence of folding funnels for specific sequences suggests that geometrically related families of stable, collapsed conformers fulfill kinetic and thermodynamic requirements of protein folding.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., HABER E., SELA M., WHITE F. H., Jr The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1309–1314. doi: 10.1073/pnas.47.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryngelson J. D., Wolynes P. G. Spin glasses and the statistical mechanics of protein folding. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7524–7528. doi: 10.1073/pnas.84.21.7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covell D. G., Jernigan R. L. Conformations of folded proteins in restricted spaces. Biochemistry. 1990 Apr 3;29(13):3287–3294. doi: 10.1021/bi00465a020. [DOI] [PubMed] [Google Scholar]
- Dill K. A. Theory for the folding and stability of globular proteins. Biochemistry. 1985 Mar 12;24(6):1501–1509. doi: 10.1021/bi00327a032. [DOI] [PubMed] [Google Scholar]
- Harrison S. C., Durbin R. Is there a single pathway for the folding of a polypeptide chain? Proc Natl Acad Sci U S A. 1985 Jun;82(12):4028–4030. doi: 10.1073/pnas.82.12.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalov P. L., Gill S. J. Stability of protein structure and hydrophobic interaction. Adv Protein Chem. 1988;39:191–234. doi: 10.1016/s0065-3233(08)60377-0. [DOI] [PubMed] [Google Scholar]
- Shakhnovich E. I., Gutin A. M. Implications of thermodynamics of protein folding for evolution of primary sequences. Nature. 1990 Aug 23;346(6286):773–775. doi: 10.1038/346773a0. [DOI] [PubMed] [Google Scholar]
- Shakhnovich E, Farztdinov G, Gutin AM, Karplus M. Protein folding bottlenecks: A lattice Monte Carlo simulation. Phys Rev Lett. 1991 Sep 16;67(12):1665–1668. doi: 10.1103/PhysRevLett.67.1665. [DOI] [PubMed] [Google Scholar]
- Skolnick J., Kolinski A. Simulations of the folding of a globular protein. Science. 1990 Nov 23;250(4984):1121–1125. doi: 10.1126/science.250.4984.1121. [DOI] [PubMed] [Google Scholar]
- Zwanzig R., Szabo A., Bagchi B. Levinthal's paradox. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):20–22. doi: 10.1073/pnas.89.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]