Abstract
Background
Genome-wide association studies have identified variants within the FTO (fat mass and obesity associated) locus as the strongest predictors of obesity amongst all obesity-associated gene loci. Recent evidence suggests that variants in FTO directly affect human adipocyte function through targeting IRX3 and IRX5 and thermogenesis regulation.
Aim
We addressed the relevance of this proposed FTO-IRX pathway in adipose tissue (AT) of children.
Results
Expression of IRX3 was higher in adipocytes compared to SVF. We found increased adipocyte-specific expression of IRX3 and IRX5 with the presence of the FTO risk haplotype in lean children, whereas it was unaffected by risk variants in obese peers. We further show that IRX3 expression was elevated in isolated adipocytes and AT of lean compared to obese children, particularly in UCP1-negative adipocytes, and inversely correlated with BMI SDS. Independent of BMI, IRX3 expression in adipocytes was significantly related to adipocyte hypertrophy, and subsequent associations with AT inflammation and HOMA-IR in the children.
Conclusion
One interpretation of our observation of FTO risk variants linked to IRX3 expression and adipocyte size restricted to lean children, along with the decreased IRX3 expression in obese compared to lean peers, may reflect a defense mechanism for protecting body-weight, which is pertinent for lean children.
Introduction
Genetic variants in the FTO (fat mass and obesity associated) gene have been discovered and repeatedly confirmed to pose the strongest (poly)genetic risk for human obesity [1–3]. Multiple studies have focused on deciphering potential mechanisms, by which variants within a region of high linkage disequilibrium in introns 1 and 2 of FTO confer the obesity risk.
Initial observations that fasting is associated with decreased hypothalamic Fto expression and that manipulations in hypothalamic Fto expression affect food intake in mice [4] suggested a central mode of action for FTO. In humans, presence of the risk genotype only subtly modulated the amount and/or the preferences of ingested food [5, 6]. Studies in Fto knockdown mice showing reduced adipose tissue (AT) mass despite hyperphagia or unaltered food intake but increased energy expenditure [7, 8] questioned whether the susceptibility alleles and FTO convey their effects entirely in the brain and point to the adipose tissue itself as a potential target. Very recently, Claussnitzer et al. delineated a new mechanism whereby variants in the FTO gene affect human adipocyte function through targeting IRX3 and IRX5 [9]. The potential functional link between FTO and IRX3 is supported by evidence from murine, human, and in vitro studies showing that the IRX3 promoter strongly interacts with the obesity-associated interval within FTO, and that obesity-linked SNPs are associated with IRX3 but not with FTO expression [10, 11]. Knockout of Irx3 in mice led to a significant reduction in body weight, increased activation of brown AT and increased energy expenditure [11]. In the human study, the causal rs1421085 variant resulted in the activation of IRX3 and IRX5 expression during early adipocyte differentiation and a developmental shift from energy-dissipating beige adipocytes to energy-storing white adipocytes [9] and thereby may contribute to the predisposition for obesity, even though the study was restricted to lean healthy adults.
Within this study, we aimed to assess whether the link between the FTO risk genotype and IRX3 and IRX5 expression in AT is related to the development of obesity and to alterations of AT biology in children.
Materials and Methods
Subjects and samples (Leipzig Childhood Adipose Tissue cohort)
Subcutaneous AT samples from 45 lean and 47 overweight and obese children included in the previously described Leipzig Childhood Adipose Tissue cohort [12] were obtained during elective surgery. Children underwent detailed anthropometric, clinical and metabolic assessments [12]. The study was approved by the ethics committee of the Medical Faculty, University of Leipzig (Reg.No: 265-08-ff; NCT02208141) and written informed consent was obtained from all parents.
Preparation of and analyses of AT samples was performed according to previously published protocols [12]. Briefly, adipocytes and stromal vascular fraction (SVF) were isolated by collagenase digestion and adipocyte diameter was determined after osmium fixation using a Coulter counter (Multisizer III; Beckmann Coulter). Macrophage infiltration was analysed by immunohistochemical staining of AT sections with CD68 antibody (M0718, DAKO).
Prior to surgery, fasting blood samples were obtained and stored at -80°C. Analyses of serum parameters (adiponectin, leptin, high sensitivity C-reactive protein (hsCRP), glucose and insulin) were performed by a certified laboratory (Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University of Leipzig).
DNA-Isolation and genotyping
DNA from EDTA blood samples was isolated using the QIAmp DNA Blood MiniKit (Qiagen). Samples were genotyped as part of the LIFE Child study (43 plates comprising 4,128 samples) using the Affymetrix Axiom Genome-Wide CEU 1 Array. Sample quality control included dish-qc> = 0.82, call rate> = 97%, sex-mismatches, implausible relatedness and outliers of principal components analysis. Duplicates and controls were removed resulting in a total of 3,797 successfully genotyped samples. SNP quality control was passed with cluster plot criteria of Affymetrix, call rate ≥97%, p-value of Hardy-Weinberg disequilibrium test >10−6 and plate-association p-value >10−7 in 541.835 autosomal SNPs. Data were imputed on the 1000Genomes reference panel (phase 1, release V3 of CEU, HG19, dbSNP-build 135) using SHAPEIT Version v2.r778 with standard settings for European populations and IMPUTE2, version 2.3.0.
According to Claussnitzer et al., participants were grouped into risk-, heterozygous, or nonrisk-allele carries depending on their genotype for the FTO obesity variants rs9930506, rs1421085 (directly genotyped) and rs1558902 (imputed) [9].
RNA isolation and mRNA expression analyses
RNA isolation and cDNA synthesis from whole AT samples, isolated adipocytes or SVF cells was performed as previously described [12]. IRX3 and IRX5 expression levels were determined by quantitative real-time RT-PCR using SYBR green. UCP1 expression was determined as described [13]. Expression levels were normalized to the reference genes TBP, ACTB and HPRT [14]. Primer and probe sequences are listed in S1 Table.
Protein isolation and immunoblot analyses
Protein isolation was performed using TRIzol Reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. Equivalent amounts of proteins were resolved by 10% SDS-PAGE and immunoblotting using antibodies against IRX3 (abcam, ab174307) and beta-ACTIN (abcam, ab8227).
Statistical analyses
All statistical analyses were performed using the Statistica 10.0 software package (StatSoft). Before analyses, normal distribution of the data was assessed by Shapiro-Wilks W test and quantile quantile plots. Non-normally distributed data were log-transformed before analyses. Quantitative traits were analysed using parametric tests (Pearson correlation analysis, Student's t test, one-way ANOVA with Dunnett’s post-hoc test). Categorical variables were analysed by chi square test. For statistical analyses, overweight and obese children were combined. For multiple regression analyses, the stepwise forward model was employed.
Results
General characteristics of patients and samples of the Leipzig Childhood AT cohort included in this study are summarized in Table 1. As previously described we detected characteristic obesity-related alterations in AT and serum parameters, such as hypertrophy and inflammation [12].
Table 1. Characteristics of the Childhood Adipose Tissue Cohort (n = 92).
| Lean | Obese | ||||||
|---|---|---|---|---|---|---|---|
| n | Mean±SEM | Range | n | Mean±SEM | Range | p | |
| Anthropometric parameters | |||||||
| Male/Female (% male) | 20/25 (44.4) | 18/29 (38.3) | 0.549 | ||||
| Age [years] | 45 | 10.0±0.8 | 1.1–18.3 | 47 | 13.3±0.5 | 4.8–18.4 | <0.001 |
| PH | 40 | 2.5±0.3 | 1–6 | 45 | 3.4±0.2 | 1–6 | 0.011 |
| BMI SDS | 45 | -0.1±0.1 | -2.4–1.1 | 47 | 2.5±0.1 | 1.4–4.3 | <0.001 |
| Adipose tissue parameters | |||||||
| Adipocyte diameter [μm] | 25 | 111.9±2.4 | 90.9–131.2 | 33 | 125.8±2.4 | 98.0–146.2 | <0.001 |
| Macrophage infiltration | |||||||
| Macrophages per 100 adipocytes | 37 | 11.1±1.4 | 0–29 | 39 | 20.5±3.4 | 0–115 | 0.031a |
| CD68 mRNA in SVF | 36 | 0.6±0.1 | 0.1–1.7 | 32 | 1.3±0.2 | 0.0–3.8 | <0.001a |
| Serum parameters | |||||||
| Adiponectin [mg/l] | 34 | 10.9±1.3 | 3.7–43.8 | 38 | 5.7±0.4 | 1.7–11.7 | <0.001a |
| Leptin [ng/ml] | 30 | 5.1±0.8 | 0.4–17.5 | 38 | 31.0±3.3 | 1.3–83.6 | <0.001a |
| hsCRP [mg/l] | 34 | 0.6±0.1 | 0.3–3.2 | 38 | 1.9±0.3 | 0.3–9.9 | <0.001a |
| HOMA-IR | 34 | 1.4±0.2 | 0.1–5.6 | 37 | 4.0±0.3 | 0.6–8.2 | <0.001a |
| Genetic parameters | |||||||
| FTO haplotype(nonrisk/heterozygous/risk) | 14/14/7 | 7/16/11 | 0.188 | ||||
Data are given as mean ± SEM. For gender and FTO haplotype, statistical significance was analysed by chi square test. Statistical significance for differences between groups was determined by Students t-test. Significant p-values are indicated in bold. PH, pubertal stage; BMI, body-mass index; SDS, standard deviation score; SVF, stroma-vascular fraction; hsCRP, high sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance.
aStatistical analyses were performed for log-transformed parameters.
We first evaluated potential differences in IRX3 and IRX5 expression in lean and obese children. Considering that obese children are slightly older compared to lean children and, hence, show a more advanced pubertal stage, we analyzed the effect of pubertal stage on IRX3 and IRX5 expression in SVF cells, adipocytes and whole AT of lean children. We did not detect significant puberty-related alterations in IRX3 and IRX5 expression (S1 Fig). If FTO variants confer obesity risk by driving the expression of IRX3 [9], one may expect increased expression of IRX genes in obese subjects. However, IRX3 expression was unchanged in SVF of obese children and appeared to be even lower in whole adipose tissue samples and isolated adipocytes of obese compared to lean children (Fig 1A). IRX5 expression was not different between lean and obese children. Concordantly, adipocyte IRX3 expression negatively correlated with BMI SDS (R = -0.265, P = 0.016), which was not found for IRX5.
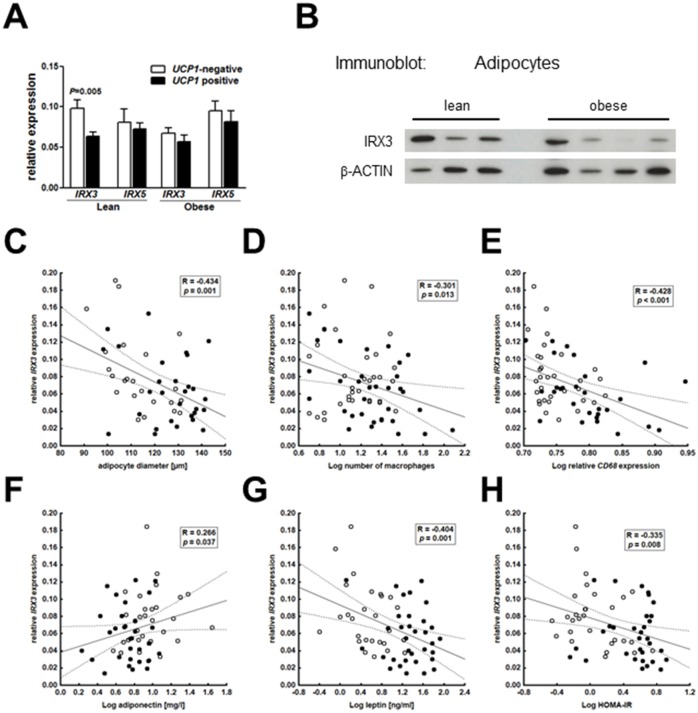
Fig 1. Expression of IRX3 and IRX5 in adipose tissue of children and association with FTO risk haplotype and with UCP1 expression.
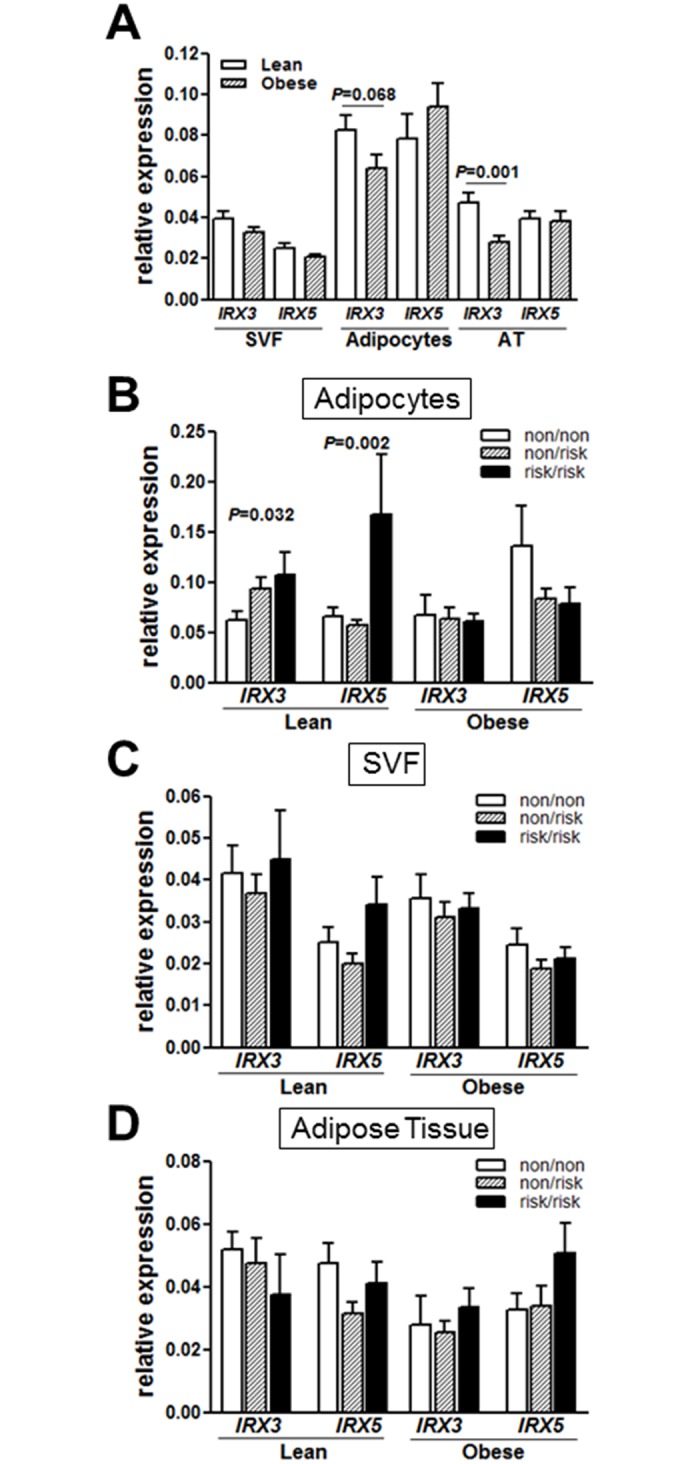
Adipocyte and whole AT expression of IRX3 was reduced in obese compared to lean children (A). Adipocyte-specific expression of IRX3 and IRX5 was significantly increased in lean children carrying the FTO risk haplotype compared to lean children carrying the nonrisk haplotype (B). There were no alterations in IRX3 and IRX5 expression between FTO haplotype groups in SVF (C) or whole AT (D) of lean children. Obese children did not show differences in IRX3 and IRX5 expression levels between FTO haplotype groups in any of the analyzed tissues (B-D). Significant differences between FTO nonrisk versus risk haplotype groups were assessed by Student’s t-test and significant p-values are indicated in the barplots.
As this observation may point to potentially distinct regulation in AT subfractions, we compared IRX3 and IRX5 expression in whole adipose tissue and freshly isolated SVF cells and adipocytes. We detected highest expression of both IRX3 and IRX5 in adipocytes (Fig 1A).
To evaluate whether the IRX expression might be genetically driven by FTO risk variants, we next assessed the expression of IRX3 and IRX5 according to FTO risk haplotype. Only in the lean subcohort, adipocyte-specific expression of both IRX3 and IRX5 was increased in risk-allele carriers compared to nonrisk-allele carriers, which was not observed in obese children (Fig 1B) similar to what was found in lean adults [9]. In line with Claussnitzer et al. we did not detect differences in IRX3 and IRX5 expression between genotype groups in whole AT or SVF (Fig 1C and 1D).
Considering that IRX3 and IRX5 expression were directly linked to mitochondrial thermogenesis and negatively associated with the expression of thermogenic genes [9], we were interested in the existence of a similar link in adipocytes of children. When we stratified our samples into UCP1-negative and UCP1-positive (depending on detectable UCP1 expression), we found increased expression of IRX3 in UCP1-negative compared to UCP1-positive adipocytes of lean children, while there was no difference in IRX3-expression in obese children (Fig 2A). Again, we did not find any alterations in IRX5 expression according to UCP1-expression in lean nor in obese children (Fig 2A). Quantitatively, neither IRX3 nor IRX5 expression correlated with UCP1 expression in UCP1-positive adipocytes.
Fig 2. Adipocyte IRX3 expression is associated with obesity-related alterations in AT and related clinical traits.
Expression of IRX3 but not IRX5 was significantly lower in UCP1-positive (18 lean, 19 obese) compared UCP1-negative adipocytes (21 lean, 23 obese) derived from lean, but not of obese children (A). Significant differences between UCP1-positive versus UCP1-negative adipocyte samples were assessed by Student’s t-test and p-values are indicated in the barplots. Protein levels of IRX3 are reduced in adipocyte of obese (n = 4) compared to lean (n = 3) children (B). Expression of IRX3 in adipocytes of children was associated with adipocyte diameter (C), macrophage infiltration as indicated by the number of adipocytes in AT (D) and CD68 expression in the stroma-vascular fraction (E), adiponectin (F) and leptin (G) serum levels, as well as HOMA-IR as a measure insulin resistance (H). Pearson correlation coefficient R and p-value are given in each scatter plot. Significant p-values (p<0.05) are indicated in bold.
Finding IRX3 mRNA (Fig 1A) and protein (Fig 2B) expression decreased in adipocytes of obese children, we further evaluated whether IRX3 expression may also be related to obesity related AT alterations. Indeed, IRX3 expression in adipocytes negatively correlated with obesity-related parameters of AT dysfunction, i.e. adipocyte diameter (Fig 2C) and AT inflammation as indicated by the number of infiltrating macrophages (Fig 2D) and CD68 mRNA expression in the SVF (Fig 2E). These correlations withstood adjustment for BMI SDS and age of children in partial correlation analysis (adipocyte diameter: Radj = -0.358, p = 0.006; macrophage number: Radj = -0.254, p = 0.041; CD68 expression in SVF: Radj = -0.371, p = 0.008). Moreover, we detected significant associations of adipocyte IRX3 expression with adiponectin (Fig 2F) and leptin (Fig 2G) serum levels and with HOMA-IR (Fig 2H) as a measure of insulin resistance, which were, however, lost after adjustment for BMI SDS and age. All of these correlations were independent of pubertal development, which has a major impact on insulin resistance (S2 Table).
Finally, we performed multiple regression analyses to determine the main predictors for adipocyte IRX3 and IRX5 expression in AT of children. Adipocyte diameter and the FTO risk haplotype independently determined adipocyte IRX3 expression accounting for 12 and 6% of variability. For IRX5 expression, the FTO risk haplotype was not predictive (Table 2).
Table 2. Multiple regression analyses for predictors of adipocyte IRX3/5 expression.
| Step | Parameter | Delta R2 | Beta ± SEM | p |
|---|---|---|---|---|
| independent variables for all models: | ||||
| age, gender, PH, BMI SDS, adipocyte diameter, Log macrophage number, FTO haplotype | ||||
| dependent variable: IRX3 expression in adipocytes (R2 = 0.30, p = 0.002, n = 44) | ||||
| 1 | adipocyte diameter | 0.12 | -0.42±0.14 | 0.005 |
| 2 | FTO haplotype | 0.06 | -0.29±0.14 | 0.038 |
| 3 | Log macrophage number | 0.12 | -0.23±0.14 | 0.110 |
| dependent variable: IRX5 expression in adipocytes (R2 = 0.26, p = 0.019, n = 44) | ||||
| 1 | adipocyte diameter | 0.39 | -0.48±0.18 | 0.010 |
| 2 | BMI SDS | 0.33 | 0.37±0.17 | 0.035 |
| 3 | age | 0.20 | 0.28±0.15 | 0.073 |
| 4 | Log macrophage number | 0.11 | -0.16±0.15 | 0.273 |
PH, pubertal stage; BMI, body-mass index; SDS, standard deviation score. Significant p-values (p<0.05) are indicated in bold.
Discussion
In this study, we have addressed the relevance of the proposed FTO-IRX pathway in AT samples of children. Our findings of increased adipocyte-specific expression of IRX3 and IRX5 with the presence of the FTO risk haplotype in lean individuals not only complement those of the previous study, which was restricted to lean adults. Moreover, we show higher expression levels of IRX3 and IRX5 in adipocytes compared to SVF and we further show that IRX3 expression is elevated in AT and isolated adipocytes of lean compared to obese children and negatively correlates with BMI SDS. Independent of BMI, IRX3 expression in adipocytes was significantly related to adipocyte hypertrophy, which may explain subsequent associations with AT inflammation and parameters of insulin resistance.
Claussnitzer et al. performed studies in adipocytes of lean adult humans and delineated the activation of two genes near the FTO locus, IRX3 and IRX5, during early adipocyte differentiation as a new mechanism conferring genetic obesity risk whereby variants in FTO directly affect adipocyte function [9]. We provide evidence that this link between FTO risk variants and IRX3/5 expression is already active in children. Nevertheless, this proposed FTO-IRX association may be restricted to lean subjects as indicated by increased IRX3 and IRX5 expression in adipocytes of lean children (and adults) carrying the FTO risk haplotype, whereas it was unaffected by risk variants in obese children. In contrast to our study, previous studies did not analyze IRX expression or a potential association of FTO risk variants with IRX expression in AT of obese patients. It would, hence, be interesting whether the proposed FTO-IRX association is restricted to lean adults similar to what we have seen in children.
According to Claussnitzer et al., enhanced expression of IRX3 and IRX5 results in a shift from white adipocyte browning to lipid storage [9]. In line with these data we observed increased IRX3 expression in UCP1-negative adipocytes compared to UCP1-positive adipocytes, but we did not observe an association of IRX5 and UCP1 expression in adipocytes of children indicating that IRX3 might be the main mediator of obesity risk in FTO risk variant carriers in children.
Concluding from the observed association of IRX3 expression with FTO obesity risk variants and UCP1 expression in adipocytes of lean children, one may expect increased IRX3 expression in obese subjects. However, IRX3 expression was even lower in isolated adipocytes of obese compared to lean children and correlated with obesity-related measures of adipocyte hypertrophy and inflammation. Interestingly, adipocyte diameter was the strongest independent predictor of adipocyte IRX3 expression. An interpretation for this and Claussnitzer´s finding of an IRX-dependent shift from energy consumption to energy storage in adipocytes may be the protection of body weight under circumstances of limited energy supply. Similar hypotheses have been discussed for downregulation of leptin levels to signal energy insufficiency [15]. Interestingly, obesity-associated FTO risk alleles have been discussed as candidate thrifty alleles, which have been driven to high frequency by positive selection [16]. According to the thrifty gene hypothesis, populations whose ancestral environments were characterized by alternating periods of food abundance and food shortage experienced positive selection for alleles that promote storage of fat in order to provide a survival advantage [17]. Such defense mechanisms for preserving body weight would be pertinent for lean subjects, particularly children, and may be attenuated in the obese state.
One strength of our study is that we provide data on whole AT as well as freshly isolated SVF cells and adipocytes of humans, which might be closer to physiological conditions compared to analyses performed in cell cultures of primary cells. However, we are limited by the often small sample volumes in children, which precluded more mechanistic analyses.
In conclusion, our results indicate a relationship between FTO variants and IRX3 expression and adipocyte phenotype in lean children, which is attenuated in the obese state.
Supporting Information
There were no significant differences in IRX3 and IRX5 expression in SVF cells, adipocytes or AT between pre-pubertal, pubertal and post-pubertal children. Differences between puberty stages were assessed by one-way ANOVA and Dunnett’s post-hoc test. A P-value of less than 0.05 was considered significant. SVF, stroma-vascular fraction. AT, adipose tissue.
(TIF)
(DOC)
(DOC)
Acknowledgments
We thank Antje Berthold and Roy Tauscher for technical assistance. This work was supported by the German Research Council (DFG) for the Collaborative Research Center "Obesity Mechanisms" CRC1052, the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1501 (IFB AdiposityDiseases), and the European Community’s 7th Framework Programme project Beta-JUDO n° 279153. Genotyping was facilitated the LIFE Child study (Leipzig Research Center for Civilization Diseases, Universität Leipzig), funded by the European Union, by the European Regional Development Fund (ERFD) by means of the Free State of Saxony within the framework of the excellence initiative. We acknowledge support from the German Research Foundation (DFG) and University of Leipzig within the program of Open Access Publishing.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the German Research Council (DFG) for the Collaborative Research Center "Obesity Mechanisms" CRC1052, the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1501 (IFB AdiposityDiseases), and the European Community’s 7th Framework Programme project Beta-JUDO n° 279153. Genotyping was facilitated the LIFE Child study (Leipzig Research Center for Civilization Diseases, Universität Leipzig), funded by the European Union, by the European Regional Development Fund (ERFD) by means of the Free State of Saxony within the framework of the excellence initiative.
References
- 1.Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nature genetics. 2007;39(6):724–6. 10.1038/ng2048 . [DOI] [PubMed] [Google Scholar]
- 2.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94. 10.1126/science.1141634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loos RJ, Yeo GS. The bigger picture of FTO: the first GWAS-identified obesity gene. Nature reviews Endocrinology. 2014;10(1):51–61. 10.1038/nrendo.2013.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tung YC, Ayuso E, Shan X, Bosch F, O'Rahilly S, Coll AP, et al. Hypothalamic-specific manipulation of Fto, the ortholog of the human obesity gene FTO, affects food intake in rats. PloS one. 2010;5(1):e8771 10.1371/journal.pone.0008771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi Q, Downer MK, Kilpelainen TO, Taal HR, Barton SJ, Ntalla I, et al. Dietary Intake, FTO Genetic Variants, and Adiposity: A Combined Analysis of Over 16,000 Children and Adolescents. Diabetes. 2015;64(7):2467–76. 10.2337/db14-1629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. The New England journal of medicine. 2008;359(24):2558–66. 10.1056/NEJMoa0803839 . [DOI] [PubMed] [Google Scholar]
- 7.Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nature genetics. 2010;42(12):1086–92. 10.1038/ng.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC, et al. Inactivation of the Fto gene protects from obesity. Nature. 2009;458(7240):894–8. 10.1038/nature07848 . [DOI] [PubMed] [Google Scholar]
- 9.Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. The New England journal of medicine. 2015;373(10):895–907. 10.1056/NEJMoa1502214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragvin A, Moro E, Fredman D, Navratilova P, Drivenes O, Engstrom PG, et al. Long-range gene regulation links genomic type 2 diabetes and obesity risk regions to HHEX, SOX4, and IRX3. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(2):775–80. 10.1073/pnas.0911591107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gomez-Marin C, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507(7492):371–5. 10.1038/nature13138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landgraf K, Rockstroh D, Wagner IV, Weise S, Tauscher R, Schwartze JT, et al. Evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes. 2015;64(4):1249–61. 10.2337/db14-0744 . [DOI] [PubMed] [Google Scholar]
- 13.Rockstroh D, Landgraf K, Wagner IV, Gesing J, Tauscher R, Lakowa N, et al. Direct evidence of brown adipocytes in different fat depots in children. PloS one. 2015;10(2):e0117841 10.1371/journal.pone.0117841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernhard F, Landgraf K, Kloting N, Berthold A, Buttner P, Friebe D, et al. Functional relevance of genes implicated by obesity genome-wide association study signals for human adipocyte biology. Diabetologia. 2013;56(2):311–22. 10.1007/s00125-012-2773-0 . [DOI] [PubMed] [Google Scholar]
- 15.Flier JS. Clinical review 94: What's in a name? In search of leptin's physiologic role. The Journal of clinical endocrinology and metabolism. 1998;83(5):1407–13. 10.1210/jcem.83.5.4779 . [DOI] [PubMed] [Google Scholar]
- 16.Stratigopoulos G, Martin Carli JF, O'Day DR, Wang L, Leduc CA, Lanzano P, et al. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell metabolism. 2014;19(5):767–79. 10.1016/j.cmet.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman JM. A war on obesity, not the obese. Science. 2003;299(5608):856–8. 10.1126/science.1079856 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
There were no significant differences in IRX3 and IRX5 expression in SVF cells, adipocytes or AT between pre-pubertal, pubertal and post-pubertal children. Differences between puberty stages were assessed by one-way ANOVA and Dunnett’s post-hoc test. A P-value of less than 0.05 was considered significant. SVF, stroma-vascular fraction. AT, adipose tissue.
(TIF)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.