Abstract
Altered gap junctional intercellular communication (GJIC) has been associated with chemical carcinogenesis, where both chemical tumor promoters and chemopreventive agents (CPAs) are known to conversely modulate GJIC. The aim of this study was to investigate whether attenuation of chemically inhibited GJIC represents a common outcome induced by different CPAs, which could be effectively evaluated using in vitro methods. Rat liver epithelial cells WB-F344 were pretreated with a CPA for either 30 min or 24 h, and then exposed to GJIC-inhibiting concentration of a selected tumor promoter or environmental toxicant (12-O-tetradecanoylphorbol-13-acetate, lindane, fluoranthene, DDT, perfluorooctanoic acid or pentachlorophenol). Out of nine CPAs tested, quercetin and silibinin elicited the most pronounced effects, preventing the dysregulation of GJIC by all the GJIC-inhibitors, but DDT. Metformin and curcumin attenuated the effects of three GJIC-inhibitors, whereas the other CPAs prevented the effects of two (diallyl sulfide, emodin) or one (indole-3 carbinol, thymoquinone) GJIC-inhibitor. Significant attenuation of chemically induced inhibition of GJIC was observed in 27 (50%) out of 54 possible combinations of nine CPAs and six GJIC inhibitors. Our data demonstrate that in vitro evaluation of GJIC can be used as an effective screening tool for identification of chemicals with potential chemopreventive activity.
Keywords: cancer chemoprevention, gap junctional intercellular communication, metformin, phytochemicals, tumor promotion, phosphatidylcholine-specific phospholipase C
INTRODUCTION
Chemical exposure to environmental and food-borne contaminants has been commonly linked to the etiology of cancers, while consumption of natural or some synthetic compounds has been linked to reduced rates of cancer (1). The underlying cellular properties, known to be critical for cancer development, are DNA alterations by genotoxic mechanisms during the initiation stage of cancer, or alterations of signal transduction pathways and gene expression patterns by non-genotoxic or epigenetic mechanisms, leading primarily to an increase in cell proliferation, inhibition of differentiation, inhibition of apoptosis and inflammatory responses associated with tumor promotion and progression stages of cancer (2, 3).
Conversely, chemical compounds can also counteract these carcinogenic processes, and thus function as cancer chemopreventive agents (CPA), i.e. natural or synthetic chemicals which inhibit, suppress, or reverse the development and progression of cancer (4, 5). Phytochemicals and other natural products are important sources of CPAs, capable of targeting the different stages of the multi-step carcinogenic process (5–7). Dietary intake of phytochemicals, thus, represents an important approach to minimizing cancer risks in healthy individuals, mostly by preventing the tumor-initiating and promoting phases of cancer. The application of phytochemicals in the form of dietary supplements or pharmacological agents can be used for individuals with a high risk of cancer to prevent early phases of cancer, or even for patients in the late stages of cancer to aid chemopreventive, chemoprotective and chemoquiescent effects in chemotherapy during cancer treatment and at the post-cancer stage (5). In the effort to identify novel CPAs and to understand the mechanisms of their chemopreventive activity, plant-derived products and compounds are typically evaluated for their ability to modulate DNA repair, detoxification, free-radical scavenging, carcinogen metabolism, proliferation, angiogenesis, apoptosis, differentiation inflammation and immune responses, i.e. alterations of biochemical and cellular processes connected to acquisition of phenotypic traits characteristic for cancer or transformed cells, so-called hallmarks of cancer (5–7).
In addition to the traditionally recognized hallmarks of cancer, abnormal gap junctional intercellular communication (GJIC) has been documented as another phenotypic hallmark of cancer (8). GJIC facilitates direct exchange of essential signaling molecules and metabolites between adjacent cells, coordinates electrotonic and metabolic events in tissues, and provides the key mechanism of homeostatic regulation (8, 9). Since cancer can be viewed as a disorder of homeostatic regulation, it is consistent with observations that GJIC is dysregulated in vivo or in vitro in response to oncogene activation, exposures to growth factors or chemical tumor promoters (10–14). In different cancer or oncogene-transformed cell lines, CPAs have been reported to increase expression of gap junctional proteins connexin and/or enhanced GJIC, which is typically accompanied by suppression of other phenotypic traits characteristic for cancer cells (9).Various plant-derived products and compounds were also found to prevent inhibition of GJIC, which was induced in normally communicating cells by tumor-promoting chemicals, such as hydrogen peroxide or model tumor promoters like 12-O-tetradecanoylphorbol-13-acetate (TPA) (9). CPAs, like quercetin, resveratrol, green tea or tomato/grape seed extracts, were also reported to prevent inhibition of GJIC caused by several environmental toxicants, such as pentachlorophenol (15), pesticides (16–19), dimethylnitrosamine (20), mercury (21, 22), organic peroxides (23) and perfluorooctanoic acid (PFOA)(24, 25).
Prevention of chemically-induced inhibition of GJIC, thus, might represent an important mechanism contributing to the chemopreventive effects of CPAs. Modulation of GJIC might be relevant especially during the tumor-promotion phase of cancer, where the tumor promoting activity of environmental and food toxicants could be counteracted by co-exposure to CPAs from diet or dietary supplements and reduce the risks of cancer. In vitro evaluation of the ability of chemicals to prevent inhibition of GJIC could then be a very effective approach for rapid identification of agents with biological activities relevant for cancer chemoprevention and suppression. However, there is a lack of studies systematically looking at the effects of different CPAs on GJIC inhibition induced by different environmental toxicants in vitro (9). Therefore, the aim of this study was to investigate whether chemicals with known chemopreventive activity but limited knowledge regarding their effects on GJIC would be able to prevent inhibition of GJIC induced in vitro by tumor promoters and environmental toxicants.
We investigated nine commercially available chemicals previously implicated in cancer chemoprevention (5–7): polyphenols quercetin, silibinin and curcumin, simple phenol indol-3 carbinol, quinones thymoquinone and emodin, unsaturated aromatic carboxylic acid cinnamic acid, the organosulphur compound diallyl sulfide, and an antidiabetic drug, biguanide metformin. These selected CPAs were evaluated for their ability to prevent inhibition of GJIC induced in vitro in rat liver epithelial cells, WB-F344, by six different tumor promoters and/or environmental toxicants with different mechanism of GJIC-inhibition[Sovadinova, 2015 #5885]: TPA, lindane, DDT, fluoranthene, PFOA, and pentachlorophenol. Results of this study suggest that the attenuation of chemically induced inhibition of GJIC represents a typical but signal transduction pathway-specific effect of CPAs, which could be easily evaluated in vitro and utilized as an effective screening tool for identification of chemicals with chemopreventive activity.
MATERIALS AND METHODS
Chemicals
All chemicals, including all GJIC inhibitors (i.e. 12-O-tetradecanoylphorbol-13-acetate (TPA), 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (DDT), fluoranthene, lindane, pentachlorophenol and perfluorooctanoic acid (PFOA)), and CPAs (cinnamic acid, curcumin, diallyl sulfide, emodin, indole-3-carbinol, metformin, quercetin, silibinin and thymoquinone), lucifer yellow, neutral red, dimethylsulfoxide (DMSO), glucose, formaldehyde, inorganic salts for preparation of phosphate-buffered saline (PBS and CaMgPBS) and culture medium preparation were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA). Acetonitrile, acetic acid and ethanol were obtained from Lach-ner (Neratovice, Czech Republic). Stock solutions of GJIC inhibitors were prepared in acetonitrile except TPA, which was dissolved in ethanol. CPAs were dissolved in DMSO. All aqueous solutions were prepared using Milli-Q water produced with a Millipore Synergy water production device (Merck Millipore, Billerica, MA, USA).
Cell culture
WB-F344 rat liver epithelial non-tumorigenic cells were obtained from Drs. Grisham and Tsao, University of North Carolina (26). The cells were grown in a so-called CCD-medium developed by C. C. Chang (27), which was prepared from Eagle’s Minimum Essential Medium (MEM)(Formula M3024, Sigma-Aldrich) supplemented with 1 g/L sodium bicarbonate, 7.635 g/L sodium chloride, 1 mM sodium pyruvate (Gibco, Life Technologies, Carlsbad, CA, USA), 2 mM L-glutamin (Gibco), with concentrations of all vitamins and essential amino acids increased 1.5× by addition of Gibco’s MEM Vitamin solution and MEM Essential amino acids solution, and concentrations of all non-essential amino acids were increased 2× by addition of Gibco’s MEM Non-essential amino acids solution. All components were dissolved in Milli-Q water, and the medium was sterilized by filtration through a polyethersulfone filter with a pore size 0.1 µm (VWR Int., Radnor, PA, USA) and then supplemented with 5% (v/v) fetal bovine serum (Biochrom S0615, Merck Millipore). The cells were routinely cultured in 75 cm2 tissue culture flasks (TPP, Trasadingen, Switzerland) in a humidified 5% CO2 atmosphere at 37 °C, and passaged every other day using trypsin-EDTA (Life Technologies) for cell detachment.
GJIC evaluation
WB-F344 cells were seeded at a density of 20–40 × 103 cell/cm2 on 35 mm-diameter tissue culture Petri dishes (Sterilin, Newport, UK) and cultured for 48 or 72 h to reach complete confluence prior to the addition of the chemical. Confluent cultures were exposed to the selected CPA or DMSO for 30 min or 24 h, followed directly by the addition of a tumor promoter, environmental toxicant or corresponding solvent (acetonitrile or ethanol) for 15 min. In addition to solvent controls, a negative control of cells not treated with any chemical was included in each experiment to account for solvent effects, where solvent concentrations did not exceed 1% (v/v). All chemicals selected as inhibitors of GJIC were previously shown to induce rapid dysregulation of cell-cell communication, and their effects on GJIC occurring within 10–30 min of exposure were shown to be mediated via different signal transduction mechanisms: TPA and lindane inhibited GJIC via MAPK-ERK1/2-dependent mechanism, fluoranthene and DDT through a phopshatidylcholine-specific phospholipase C (PC-PLC) dependent mechanism, PFOA through a mixed, both ERK1/2 and PC-PLC-dependent mechanism, and pentachlorophenol via mechanism independent of ERK1/2 and PC-PLC (25). In order to evaluate the ability of CPAs to target the specific signal transduction pathway implicated in the mechanism of rapid dysregulation of GJIC induced by a given inhibitor, we focused on the attenuation of GJIC after 15 min of exposure to a GJIC inhibitor. The scalpel loading-dye transfer (SL-DT) technique used for the evaluation of GJIC was adapted after the method of (28) and carried out according to the previously published protocol (29). Briefly, the exposed cells were washed three times with CaMgPBS (PBS supplemented with 0.68 mM calcium chloride and 0.49 mM magnesium chloride). Then 1 mg/mL of lucifer yellow dilithium salt diluted in CaMgPBS was added to the cells. By gently pressing the surgical scalpel blade against the dish bottom, the lucifer yellow was introduced into the cell monolayer (three parallel scalpel injections were made per dish). The dye was allowed to diffuse through gap junctions for three minutes, followed by a thorough rinse of cells with CaMgPBS and a fixation step with a 4% (v/v) formaldehyde solution in PBS. A representative microscopic image of the lucifer yellow dye transfer was taken from each cut at 20× magnification using an Olympus IX51 microscope equipped with DP72 CCD camera (Olympus, Hamburg, Germany). The extent of GJIC was evaluated using ImageJ software as an area of the cells stained with lucifer yellow dye. The average fluorescence area in the positive control treated with 70 µM fluoranthene for 15 min was subtracted from each treatment. The adjusted areas of individual treatments were compared with the adjusted area of the vehicle control and expressed as a percentage of GJIC in vehicle control (%control). Each experiment was repeated at least three times independently.
Cell viability assay
WB-F344 cells were seeded onto 96-well microplates (TPP) at the same seeding densities as for GJIC evaluation and cultured for 48–72 h to reach confluence. The selected CPAs or DMSO (max. 1%, v/v) were then added to the cells and incubated for 1 or 24 h. The effects of chemicals on cell viability were evaluated using the neutral red uptake assay. A solution of 150 µg/mL neutral red (NR) dye, i.e. 3-amino-7-(dimethylamino)-2-methylphenazine hydrochloride, in a serum-free culture medium was prepared, equilibrated in a CO2 incubator, and filtered through a 0.22 µm polyethersulfone syringe filter (Merck Millipore). Cells were rinsed twice with PBS, NR solution was added, and the cells were incubated for 1 h in a CO2 incubator to allow dye uptake. After incubation, the cells were rinsed three times with PBS to remove extracellular NR. Intracellular NR was extracted from the cells by the addition of 1% glacial acetic acid in 50% ethanol. After 15 min of incubation on an orbital shaker, the absorbance was measured at 540 nm (reference wavelength = 690 nm) using a TECAN Genios spectrophotometer (TECAN, Männedorf, Switzerland). After subtraction of the blank value, NR dye uptake in an individual treatment was compared with negative control and expressed as percentage of viability. Each experiment was carried out in triplicate and repeated at least three times independently.
RESULTS
Selection of CPA concentration for experiments with GJIC inhibitors
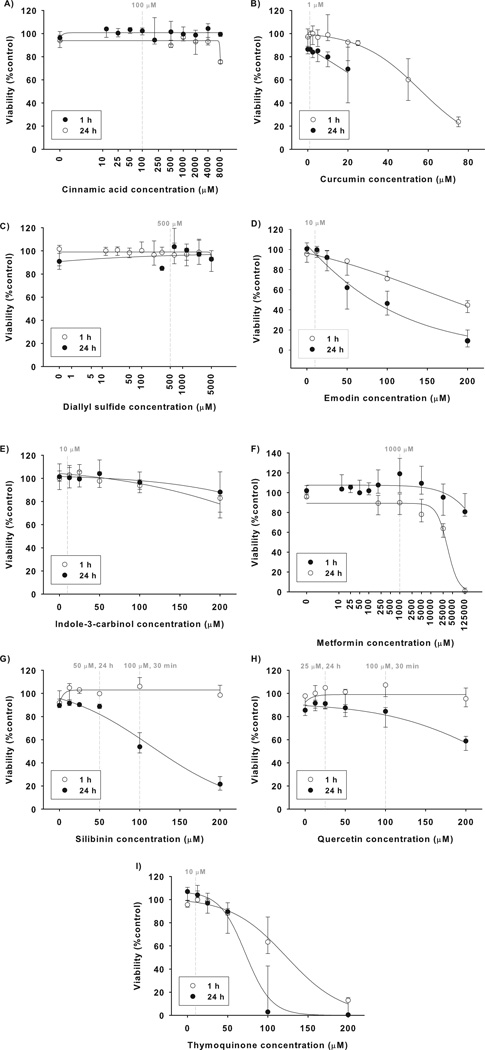
In the first set of experiments, effects of the selected CPAs on viability of WB-F344 cells was evaluated (Fig. 1) along with their effects on GJIC (Fig. S1). This was done to establish a suitable concentration that was neither cytotoxic nor inhibitory of GJIC. Curcumin, emodin and thymoquinone induced the most pronounced cytotoxic effects, when a significant decrease of cell viability was elicited after a one-hour exposure to concentrations of >50 µM, with even more pronounced effects observed after 24 h exposure (Fig. 1B, D, I). Emodin and curcumin also inhibited GJIC (Fig. S1A). Concentrations of 1 µM curcumin and 10 µM of emodin and thymoquinone were found to be both non-toxic (Fig. 1B, D, I) and non-inhibitory of GJIC (Fig. S1B) up to a 24 h exposure and thus selected for experiments with the GJIC inhibitors. Quercetin and silibinin at 100 µM concentration reduced neither cell viability (Fig. 1G, H) nor GJIC (Fig. S1B) after 1 h exposure, and were used for a 30 min pretreatment. However, a 100 µM concentration of silibinin and quercetin was cytotoxic after 24 h exposure, which was manifested by silibinin-induced decrease of the neutral red dye uptake (Fig. 1H) and quercentin-induced increase of cell membrane permeability leading to extensive cell staining with lucifer yellow in SL-DT assays (data not shown). Concentrations of 50 µM silibinin and 25 µM quercetin were found to be non-cytotoxic and used for 24 h cell pretreatment. Similarly to quercetin, indole-3 carbinol did not decrease neutral red dye uptake (Fig. 1E), but at concentrations >25 µM it increased permeability of the cell membrane for lucifer yellow in SL-DT assay (data not shown). Indole-3 carbinol at a concentration of 10 µM was not observed to induce any effect in SL-DT assay (Fig. S1B) and thus selected for further experiments. Cinnamic acid, diallyl sulfide and metformin did not affect cell viability (Fig. 1A, C, F) or GJIC at concentrations up to 5 mM. The final concentrations of these CPAs were selected in preliminary experiments with GJIC inhibitor lindane (data not shown). The final summary of concentrations of CPAs used for experiments with GJIC inhibitors is given in Table 1.
Figure 1. Effects of chemopreventive agents (CPAs) on viability of WB-F344 cells.
WB-F344 cells were treated for 1 or 24 h with CPAs and the cell viability was evaluated using the neutral red uptake assay. The results were expressed as % of the negative control. Data represent medians (circles) with interquartile ranges (error bars) of at least three independent experiments. Sigmoidal regression was used to plot the concentration response curves. Dashed vertical lines indicate concentrations selected for experiments with GJIC inhibitors.
Table 1.
Difference between gap junctional intercellular communication (GJIC) level in WB-F344 cells treated and non-treated with a chemopreventive agent (CPA) prior the addition of a GJIC inhibitor (Δ GJIC).
| ΔGJICa,b = M[GJIC(w/CPA)+(GJIC inhibitor)] − M[GJIC(w/o CPA)+(GJIC inhibitor)] |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemopreventive agent | +GJIC inhibitor (15 min) | |||||||||||||
| Chemical | Concen- tration (µM) |
Pretreat -ment time |
ERK1/2-dependentc | ERK1/2 and PC-PLC- dependent |
ERK1/2 and PC-PLC- independent |
PC-PLC-dependent | ||||||||
| Lindane (70 µM) |
TPA (7.5 nM) |
PFOA (100 µM) |
Pentachlorophenol (70 µM) |
Fluoranthene (50 µM) |
DDT (25 µM) |
|||||||||
| Cinnamic acid | 100 | 30 min | 47 | (***P=0.001)d | 9 | (P=0.244) | 3 | (P=0.116) | 4 | (P=0.725) | 1 | (P=0.66) | −1 | (P=0.608) |
| Curcumin | 1 | 38 | (**P=0.004) | 1 | (P=0.525) | 34 | (**P=0.002) | 15 | (*P=0.05) | −1 | (P=0.367) | 0 | (P=0.788) | |
| Diallyl sulfide | 500 | 47 | (***P=0.001) | −3 | (P=0.169) | 6 | (P=0.19) | 4 | (P=0.34) | 5 | (P=0.159) | 0 | (P=0.606) | |
| Emodin | 10 | 27 | (***P=0.001) | 3 | (P=0.672) | −2 | (P=0.489) | −7 | (P=0.063) | −1 | (P=0.705) | 3 | (P=0.36) | |
| Indol-3-carbinol | 10 | 41 | (***P<0.001) | 10 | (P=0.194) | −1 | (P=0.584) | −3 | (P=0.58) | 0 | (P=1) | 2 | (P=0.909) | |
| Metformin | 1000 | 27 | (**P=0.004) | −2 | (P=0.672) | 6 | (P=0.328) | 14 | (*P=0.031) | 8 | (P=0.075) | 0 | (P=0.492) | |
| Quercetin | 100 | 69 | (***P<0.001) | 27 | (**P=0.008) | 43 | (***P<0.001) | 29 | (**P=0.008) | 19 | (**P=0.009) | 0 | (P=0.762) | |
| Silibinin | 100 | 78 | (***P=0.001) | 25 | (*P=0.011) | 58 | (***P=0.001) | 19 | (*P=0.05) | 26 | (**P=0.002) | 1 | (P=0.34) | |
| Thymoquinone | 10 | 48 | (***P<0.001) | 4 | (P=0.823) | 5 | (P=0.171) | 8 | (P=0.097) | 0 | (P=0.982) | 0 | (P=0.775) | |
| Cinnamic acid | 100 | 24 h | −6 | (P=0.351) | −1 | (P=0.525) | −3 | (P=0.133) | −7 | (P=0.119) | 1 | (P=0.279) | 11 | (*P=0.012) |
| Curcumin | 1 | 4 | (P=0.601) | 1 | (P=0.832) | 1 | (P=0.685) | −3 | (P=0.802) | 7 | (*P=0.013) | 0 | (P=0.689) | |
| Diallyl sulfide | 500 | 1 | (P=0.599) | 2 | (P=1) | −2 | (P=0.353) | −7 | (*P=0.031) | 7 | (*P=0.039) | 30 | (**P=0.005) | |
| Emodin | 10 | 3 | (P=0.501) | 2 | (P=0.112) | 6 | (P=0.121) | 4 | (P=0.88) | 17 | (**P=0.007) | 2 | (P=0.541) | |
| Indol-3-carbinol | 10 | −2 | (P=0.57) | −1 | (P=1) | 5 | (P=0.617) | 6 | (P=0.58) | 4 | (P=0.132) | 9 | (P=0.457) | |
| Metformin | 1000 | 2 | (P=0.699) | 6 | (P=0.138) | 0 | (P=0.867) | 5 | (P=0.898) | 9 | (P=0.066) | 26 | (**P=0.005) | |
| Quercetin | 25 | 42 | (***P=0.001) | 15 | (*P=0.015) | 3 | (P=0.263) | 39 | (**P=0.006) | 38 | (**P=0.002) | 5 | (*P=0.039) | |
| Silibinin | 50 | 26 | (*P=0.028) | 6 | (*P=0.044) | −2 | (P=0.584) | 13 | (**P=0.01) | 24 | (**P=0.002) | 1 | (P=0.391) | |
| Thymoquinone | 10 | −9 | (P=0.285) | −3 | (P=0.459) | 8 | (P=0.121) | 4 | (P=0.802) | 0 | (P=0.33) | 9 | (P=0.423) | |
TPA = 12-O-tetradecanoyl-phorbol-13-acetate, DDT = 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane, PFOA = perfluorooctanoic acid.
ΔGJIC was calculated as a difference between a median (M) value of GJIC (%control) obtained from experiments with a selected chemopreventive agent (CPA) and a given GJIC inhibitor, where the cells were pretreated for 30 min or 24 h with a selected CPA and then treated with GJIC inhibitor, i.e. (M[GJIC(w/ CPA)+(GJIC inhibitor)]), and a median value from experiments with a given GJIC inhibitor, where the cells were not pretreated with CPA, i.e. (M[GJIC(w/o CPA)+(GJIC inhibitor)]).
Shading indicates intensity of GJIC attenuation:  none or weak effect (ΔGJIC ≤10%),
none or weak effect (ΔGJIC ≤10%),  mild effect (ΔGJIC >10 and ≤25%),
mild effect (ΔGJIC >10 and ≤25%),  moderate effect (ΔGJIC >25 and ≤50%),
moderate effect (ΔGJIC >25 and ≤50%),  strong effect (ΔGJIC >50%)
strong effect (ΔGJIC >50%)
Mechanism of GJIC inhibition
Significance of differences between GJIC in the cells treated and non-treated with a chemopreventive agent prior the addition of a GJIC inhibitor was determined by Mann-Whitney test, P values are given in parentheses and labelled with asterisks (*P≤0.05, **P≤0.01, ***P≤0.001)
Selection of GJIC inhibitor concentration
The selected tumor promoters and environmental toxicants rapidly inhibited GJIC within 15 min exposure (Fig. S2). The lowest concentrations of GJIC inhibitors sufficient to repeatedly induce nearly complete inhibition of GJIC (<20%) were used for experiments with CPAs, as indicated in Fig. S2 and summarized in Table 1.
Effects of CPAs on chemically-induced inhibition of GJIC
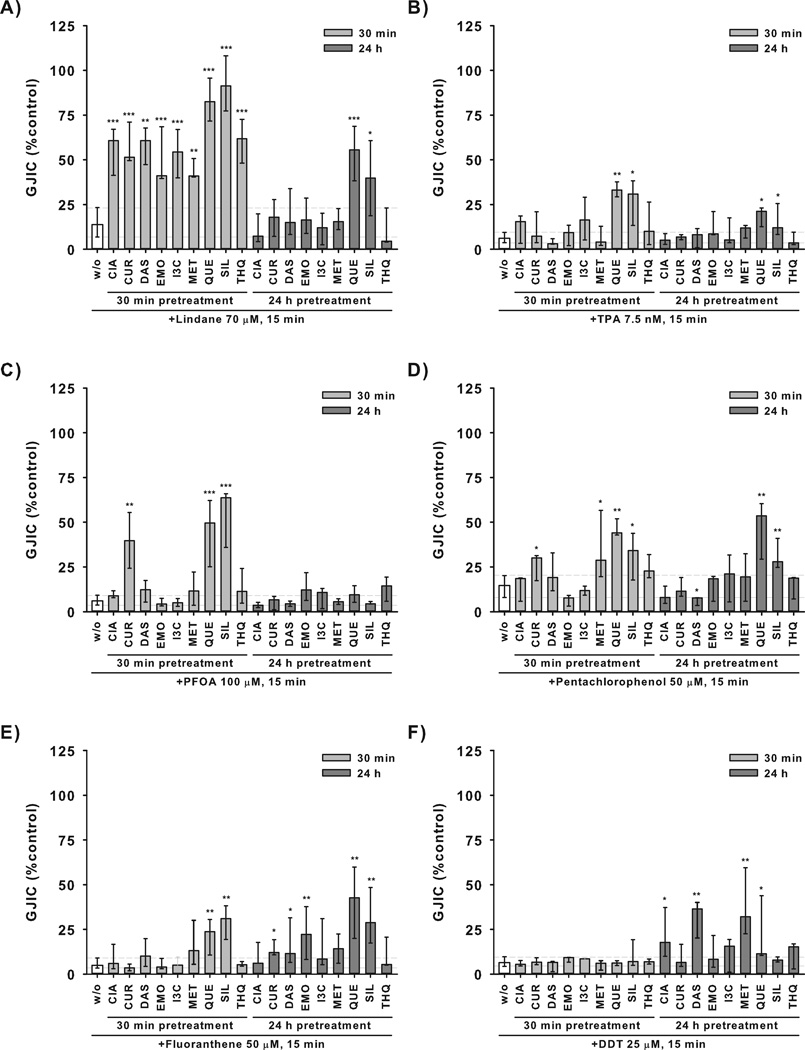
Median GJIC levels in the cells treated with a GJIC inhibitor alone were compared with median GJIC levels in the cells first pretreated with CPAs and then treated with the GJIC inhibitor. The observed differences (ΔGJIC) ranged from slightly negative or positive values (ΔGJIC <10%), indicating none or only a weak effect, up to 78%, which corresponds to almost complete prevention of GJIC inhibition (Table 1). Out of the 54 different combinations of nine CPAs with six GJIC inhibitors, at least mild (ΔGJIC >10%) and statistically significant (P≤0.05) attenuation of GJIC inhibition by CPA pretreatment was observed for 27 unique combinations of a specific CPA and GJIC inhibitor. Such a significant preventive effect was observed for 20 different pairs of CPA-GJIC inhibitor in the case of 30 min pretreatment with a CPA, and for 11 different pairs in the case of 24 h pretreatment (Table 1).
CPAs elicited the most pronounced effects on inhibition of GJIC induced by lindane, when all CPAs significantly attenuated effects of lindane after 30 min pretreatment (Fig. 2A). However, these preventive effects became less frequent and pronounced with prolonged time of CPA pretreatment, where only quercetin and silibinin induced significant effects, but weaker than the 30 min treatment (Fig. 2A). Quercetin and silibinin were also the only CPAs showing significant effects on TPA-induced inhibition of GJIC, again with more pronounced effects observed after a 30 min treatment in comparison with the 24 h incubation with CPA (Fig. 2B). A similar pattern was observed for PFOA, where attenuation of GJIC inhibition by quercetin, silibinin and also curcumin was apparent only in the 30 min pretreatment, but neither CPA elicited significant effects after a 24 h pretreatment (Fig. 2C).
Figure 2. Effects of chemopreventive agents (CPAs) on chemically-induced inhibition of gap junctional intercellular communication (GJIC) in WB-F344 cells.
WB-F344 cells were either not pretreated (w/o) or pretreated with different CPAs for 30 min or 24 h, then treated with an inhibitor of GJIC for 15 min. Concentrations of CPAs were 100 µM for cinnamic acid (CIA), 1 µM for curcumin, 500 µM for diallyl sulfide (DAS), 10 µM for emodin (EMO), indole-3-carbinol (I3C), 1000 µM for metformin (MET), 100 µM (30 min) or 25 µM (24 h) for quercetin (QUE), 100 µM (30 min) or 50 µM (24 h) for silibinin (SIL), 10 µM for thymoquinone (THQ). GJIC was evaluated using scalpel loading-dye transfer assay and expressed as % of the vehicle control. Data represent medians (bars) and interquartile ranges (error bars) of at least three independent experiments. Dashed horizontal lines indicate interquartile range of the treatment with GJIC inhibitor only (w/o). Values significantly different from the treatment with GJIC inhibitor only are labeled by asterisks (Mann-Whitney test, *P≤0.05, **P≤0.01, ***P≤0.001). TPA = 12-O-tetradecanoyl-phorbol-13-acetate, DDT = 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane, PFOA = perfluorooctanoic acid.
Also inhibition of GJIC induced by pentachlorophenol was prevented more efficiently by a 30 min pretreatment with curcumin and metformin, whereas the effects of quercetin and silibinin were comparable for both 30 min and 24 h pretreatment (Fig. 2D).
A different pattern was observed in experiments with fluoranthene and DDT, where attenuation of GJIC inhibition was more frequent when the cells were exposed to CPAs for 24 h (Fig. 2E, F). Effects of fluoranthene on GJIC were prevented by a 24 h treatment with curcumin, diallyl sulfide, emodin, quercetin and silibinin, whereas a 30 min pretreatment modulated fluoranthene-induced inhibition of GJIC only with quercetin and silibinin (Fig. 2E). Similarly, GJIC inhibition by DDT was prevented by a 24 h pretreatment with cinnamic acid, diallyl sulfide, metformin and weakly by quercetin, while DDT effects were not altered by any CPA after a 30 min pretreatment (Fig. 2F).
When comparing effects of individual CPAs, quercetin and silibinin were found to attenuate the effects of all GJIC inhibitors by more than 10% (Table 1), with the exception of DDT, where only quercetin effects were observed (Table 1, Fig. 2F). Curcumin and metformin each prevented GJIC inhibition induced by three different chemicals (Table 1). Pretreatment for 30 min with either curcumin or metformin prevented GJIC inhibition induced by either lindane or pentachlorophenol (Fig. 2A, D). In addition, a 30 min pretreatment with curcumin significantly blocked GJIC inhibition induced by PFOA (Fig. 2C), whereas a 24 h pretreatment with metformin altered DDT-induced inhibition of GJIC (Fig. 2F). Cinnamic acid, diallyl sulfide and emodin prevented effects of two GJIC inhibitors. In addition to the effects of these CPAs in experiments with lindane (30 min pretreatment, Fig. 2A), cinnamic acid and diallyl sulfide also attenuated GJIC inhibition induced by DDT (24 h pretreatment, Fig. 2F), whereas a 24 h pretreatment with emodin prevented GJIC inhibition induced by fluoranthene (Fig. 2E). Indole-3 carbinol and thymoquinone elicited effects only against lindane-induced inhibition of GJIC after a 30 min pretreatment (Table 1, Fig. 2A).
DISCUSSION
Altered connexin expression and function have been found to play a critical role in carcinogenesis, where chronic inhibition of GJIC seem to represent an essential step required for tumor promotion and progression of initiated cells (9–13). Chronic exposure to environmental toxicants inducing the inhibition of GJIC probably represents a very important process involved in the tumor promotion phase of cancer (1, 14), whereas, prevention of GJIC inhibition seems to be crucial for preventing chemically-induced tumor promotion (15, 30) and might be one of the key mechanisms contributing to the potential chemopreventive activity of CPAs. In fact, various plant-derived products and compounds were reported to prevent inhibition of GJIC induced by model tumor promoters (e.g. hydrogen peroxide, TPA, butylated hydroxytoluene or phenobarbital), but the effects of CPAs on the inhibition of GJIC elicited by environmentally relevant toxicants are much less understood (9).
From the set of CPAs evaluated in this study, attenuation of GJIC inhibition induced by hydrogen peroxide or TPA has been reported only for quercetin (16, 31, 32), indole-3-carbinol (33), and curcumin derivates (34), whereas curcumin did not prevent TPA-induced inhibition of GJIC (35). Quercetin was also found to prevent inhibition of GJIC induced by DDT (16), but the ability of these CPAs to attenuate the effects of other GJIC inhibitors represent a novel finding of this study.
The other investigated CPAs, cinnamic acid, diallyl sulfide, emodin, silibinin and thymoquinone represent phytochemicals well-recognized for their antioxidative and chemopreventive activity (5–7), but there is a lack of information on their possible effects on connexins or GJIC. Similarly, a widely used antidiabetic drug, metformin, which is structurally related to French lilac biguanides, has been recently implicated in cancer chemoprevention (36, 37), but its effects on chemically-induced inhibition of GJIC by environmental toxicants have not been specifically addressed so far. Our study thus provides the very first information that prevention of the chemically-induced inhibition of GJIC could be involved in the chemopreventive effects of these CPAs. Importantly, these effects relevant to cancer chemoprevention were elicited in rat liver epithelial cells, which possess characteristics of oval cells, i.e. multipotent progenitors of hepatocytes and biliary duct cells, known to play a crucial role in hepatocarcinogenesis (12, 13, 38). CPAs can thus not only revert or suppress the phenotype of a cell which has already progressed into a neoplastic or malignant stage, they can also directly counteract chemically-induced tumor promoting events such as the inhibition of GJIC in the not-yet-tumorigenic precursors of cancer cells. Such mechanisms relevant for environmental and food toxicant-induced tumor promoting events are especially interesting from the whole chemoprevention perspective, since tumor promotion represents the rate-limiting step of carcinogenesis, which can be probably most effectively targeted via dietary intake of CPAs.
In summary, all CPAs investigated in this study were found to attenuate inhibition of GJIC induced by one or more environmental toxicants. A significant effect on GJIC was observed in 50% of the evaluated combinations of CPA treatment-GJIC inhibitor treatment. These results indicate that in vitro evaluation of phytochemical or natural product effects on chemically-induced inhibition of GJIC can be used as an effective tool suitable for in vitro screening for novel compounds with chemopreventive activity. The effects of CPAs were elicited by concentrations which were lower by factor of 2 or higher than the lowest concentration found to decrease cell viability or induce morphological or membrane-permeability changes in WB-F344 cells. Thus, the observed effects of CPAs were most likely mediated by modulations of specific biochemical and signaling events, rather than by eventual cytotoxic stress reaction to CPAs, which might possibly interfere with the action of GJIC inhibitors. Indeed, our experiments revealed that attenuation of chemically-induced inhibition of GJIC does not seem to be a general activity shared by all different CPAs, since there were substantial qualitative and quantitative differences in the ability of individual CPAs to attenuate the effects of different chemicals inhibiting GJIC through different mechanisms.
Antioxidative activity has been implicated as the principal mechanism responsible for prevention of hydrogen peroxide-dependent inhibition of GJIC by different CPAs (31, 39–41). Such activity could explain also the observed attenuation of lindane-induced inhibition of GJIC in this study, since all investigated CPAs have been associated with antioxidative activity and with reduction of oxidative stress. Moreover, lindane was found to inhibit GJIC via the MAPK-ERK1/2 pathway activated through a redox-dependent mechanism (25, 42, 43). However, despite sharing a similar ERK1/2-dependent mechanism of GJIC inhibition with lindane (25, 44), the effects of TPA on GJIC are most likely not dependent on formation of free radicals (45, 46). Therefore, a different mechanism was probably responsible for the preventive effects of quercetin and silibinin. PFOA inhibits GJIC through MAPK-ERK1/2 and PC-PLC-dependent pathways and antioxidants including resveratrol, N-acetylcysteine and 2-ascorbic acid prevent this inhibition (24, 25).
Such an implication of the redox-dependent mechanism of GJIC-inhibition suggested that the investigated CPAs would prevent PFOA-induced inhibition of GJIC due to their antioxidative activity, as observed in lindane-induced inhibition of GJIC. However, only three CPAs, namely quercetin, silibinin and curcumin, prevented inhibition of GJIC by PFOA. Thus, these effects could be due to modulations of specific mechanisms of redox signaling regulating GJIC rather than due to non-specific antioxidative activities. Since the effects of CPAs on ERK1/2-dependent or co-dependent inhibitors of GJIC, i.e. TPA, lindane and PFOA, were manifested already after 30 min pretreatment with a CPA, this indicates that attenuation of GJIC inhibition was mediated via rapid biochemical and molecular mechanisms, probably involving not only modulations of redox signaling- or oxidative stress, but also interactions of CPAs with cellular receptors or signal transduction enzymes controlling GJIC. Such rapid mechanisms could be responsible also for the effects of metformin and curcumin on GJIC dysregulation caused by pentachlorophenol, which inhibits GJIC via a mechanism independent of ERK1/2 and PC-PLC activity (25). Significant effects observed after 30 min pretreatment with CPAs usually had a transient character and became less pronounced or diminished with prolonged 24 h incubation with a CPA.
However, significant effects of quercetin and silibinin on GJIC inhibition induced by pentachlorophenol or fluoranthene, a PC-PLC-dependent inhibitor of GJIC (25), remained similar or became even more pronounced with an increase of the pretreatment time with a CPA at 24 h. The mechanisms underlying chemopreventive effects, manifested after 24 h incubation with a CPA, included probably more permanent modulations of processes and signal transduction pathways responsible for GJIC dysregulation, possibly involving altered expression of genes involved in gap junction assembly and GJIC control. Moreover, one needs to consider metabolization, transformation or degradation of the tested compounds during the incubation, since transformation products of CPAs might elicit different activities or target different biochemical and molecular events than the parental compounds, and eventually become responsible for the observed differences between 30 min and 24 h treatments with CPAs. Future studies should therefore focus on further characterization of pharmacokinetics and pharmacodynamics of the individual CPAs in relation to their effects on GJIC. Interestingly, 24 h treatment with CPAs induced more pronounced attenuation of GJIC in comparison with 30 min treatment almost exclusively in the case of GJIC inhibition elicited by ERK1/2-independent but PC-PLC-dependent inhibitors, namely DDT and fluoranthene (25). GJIC dysregulation induced by these toxicants was most effectively attenuated by 24 h pretreatment with cinnamic acid, diallyl sulfide, emodin, metformin, quercetin or silibinin. Since the same CPAs were observed to attenuate also ERK1/2-dependent inhibitors, but more effectively after a 30 min pretreatment, it indicates that the same CPA can protect GJIC inhibition induced via multiple mechanisms. These observations support the previous findings of Sovadinova et al. from experiments with resveratrol (25), and strongly suggest that attenuation of chemically-induced inhibition of GJIC is probably a very specific outcome depending on CPA-induced biochemical and molecular events and their specific interactions and cross-talks with signal transduction pathways dysregulating GJIC in response to a GJIC inhibitor. Finally, these studies demonstrate that there will be few, if any, “universal“ chemopreventive agents against all cancers. However, in vitro assessment of CPA effects on chemically-induced inhibition of GJIC can provide mechanistic clues to their chemopreventive effects, and thus represents a possible tool not only for rapid identification of novel chemopreventive compounds, but also for further characterization of mechanisms responsible for their chemopreventive activity.
Supplementary Material
Acknowledgments
This research was supported by the Czech Ministry of Education grant #LH12034, and by NIEHS grant #R01 ES013268-01A2 to Upham. The RECETOX research infrastructure was supported by the projects of the Czech Ministry of Education #LO1214 and #LM2011028.
ABBREVIATIONS
- CPA
Chemopreventive agent
- DDT
1,1,1-Trichloro-2,2-bis(4-chlorophenyl)ethane
- DMSO
Dimethylsulfoxide
- GJIC
Gap junctional intercellular communication
- PFOA
Perfluorooctanoic acid
- SL-DT
Scalpel loading-dye transfer assay
- TPA
12-O-tetradecanoylphorbol-13-acetate
- MEM
Minimum Essential Medium
- NR
Neutral Red
- PBS
Phosphate-buffered saline
- PC-PLC
Phosphatidylcholine-specific phospholipase C
- MAPK
Mitogen activated protein kinases
- ERK1/2
Extracellular receptor kinase 1/2
REFERENCES
- 1.Persano L, Zagoura D, Louisse J, Pistollato F. Role of environmental chemicals, processed food derivatives and nutrients in the induction of carcinogenesis. Stem Cells Dev. 2015 doi: 10.1089/scd.2015.0081. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira PA, Colaco A, Chaves R, Guedes-Pinto H, De-La-Cruz LF, et al. Chemical carcinogenesis. Anais Da Academia Brasileira De Ciencias. 2007;79:593–616. doi: 10.1590/s0001-37652007000400004. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SM, Arnold LL. Chemical Carcinogenesis. Toxicological Sciences. 2011;120:S76–S92. doi: 10.1093/toxsci/kfq365. [DOI] [PubMed] [Google Scholar]
- 4.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of Chemical Carcinogenesis by Vitamin-a and Its Synthetic Analogs (Retinoids) Federation Proceedings. 1976;35:1332–1338. [PubMed] [Google Scholar]
- 5.Mehta RG, Murillo G, Naithani R, Peng XJ. Cancer Chemoprevention by Natural Products: How Far Have We Come? Pharmaceutical Research. 2011;27:950–961. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 6.Shu LM, Cheung KL, Khor TO, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer and Metastasis Reviews. 2010;29:483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Vallinas M, Gonzalez-Castejon M, Rodriguez-Casado A, Ramirez de Molina A. Dietary phytochemicals in cancer prevention and therapy: a complementary approach with promising perspectives. Nutr Rev. 2013;71:585–599. doi: 10.1111/nure.12051. [DOI] [PubMed] [Google Scholar]
- 8.Trosko JE, Chang CC, Upham BL, Tai MH. Ignored hallmarks of carcinogenesis: Stem cells and cell-cell communication. Annals of the New York Academy of Sciences. 2004;1028:192–201. doi: 10.1196/annals.1322.023. [DOI] [PubMed] [Google Scholar]
- 9.Leone A, Longo C, Trosko JE. The chemopreventive role of dietary phytochemicals through gap junctional intercellular communication. Phytochemistry Reviews. 2012 [Google Scholar]
- 10.Trosko JE, Chang CC. Mechanism of up-regulated gap junctional intercellular communication during chemoprevention and chemotherapy of cancer. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis. 2001;480:219–229. doi: 10.1016/s0027-5107(01)00181-6. [DOI] [PubMed] [Google Scholar]
- 11.Trosko JE, Ruch RJ. Gap junctions as targets for cancer chemoprevention and chemotherapy. Current Drug Targets. 2002;3:465–482. doi: 10.2174/1389450023347371. [DOI] [PubMed] [Google Scholar]
- 12.Trosko JE, Chang CC, Upham BL, Tai MH. The role of human adult stem cells and cell-cell communication in cancer chemoprevention and chemotherapy strategies. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis. 2005;591:187–197. doi: 10.1016/j.mrfmmm.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Trosko JE. The role of stem cells and gap junctions as targets for cancer chemoprevention and chemotherapy. Biomedicine & Pharmacotherapy. 2005;59:S326–S331. doi: 10.1016/s0753-3322(05)80065-4. [DOI] [PubMed] [Google Scholar]
- 14.Vinken M, Doktorova T, Decrock E, Leybaert L, Vanhaecke T, et al. Gap junctional intercellular communication as a target for liver toxicity and carcinogenicity. Critical Reviews in Biochemistry and Molecular Biology. 2009;44:201–222. doi: 10.1080/10409230903061215. [DOI] [PubMed] [Google Scholar]
- 15.Sai K, Kanno J, Hasegawa R, Trosko JE, Inoue T. Prevention of the down-regulation of gap junctional intercellular communication by green tea in the liver of mice fed pentachlorophenol. Carcinogenesis. 2000;21:1671–1676. doi: 10.1093/carcin/21.9.1671. [DOI] [PubMed] [Google Scholar]
- 16.Warngard L, Flodstrom S, Ljungquist S, Ahlborg UG. Interaction between quercetin, TPA and DDT in the V79 metabolic cooperation assay. Carcinogenesis. 1987;8:1201–1205. doi: 10.1093/carcin/8.9.1201. [DOI] [PubMed] [Google Scholar]
- 17.Sigler K, Ruch RJ. Enhancement of Gap Junctional Intercellular Communication in Tumor Promoter-Treated Cells by Components of Green Tea. Cancer Letters. 1993;69:15–19. doi: 10.1016/0304-3835(93)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Matesic DF, Blommel ML, Sunman JA, Cutler SJ, Cutler HG. Prevention of organochlorine-induced inhibition of gap junctional communication by chaetoglobosin K in astrocytes. Cell Biology and Toxicology. 2001;17:395–408. doi: 10.1023/a:1013752717500. [DOI] [PubMed] [Google Scholar]
- 19.Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi H, Nomata K, Mori K, Matsuo M, Miyaguchi T, et al. The preventive effect of green tea on the gap junction intercellular communication in renal epithelial cells treated with a renal carcinogen. Anticancer Res. 2004;24:3757–3762. [PubMed] [Google Scholar]
- 21.Zefferino R, Leone A, Piccaluga S, Cincione R, Ambrosi L. Mercury modulates interplay between IL-1beta, TNF-alpha, and gap junctional intercellular communication in keratinocytes: mitigation by lycopene. J Immunotoxicol. 2008;5:353–360. doi: 10.1080/15476910802482854. [DOI] [PubMed] [Google Scholar]
- 22.Leone A, Zefferino R, Longo C, Leo L, Zacheo G. Supercritical CO2-Extracted Tomato Oleoresins Enhance Gap Junction Intercellular Communications and Recover from Mercury Chloride Inhibition in Keratinocytes. Journal of Agricultural and Food Chemistry. 2010;58:4769–4778. doi: 10.1021/jf1001765. [DOI] [PubMed] [Google Scholar]
- 23.Upham BL, Guzvic M, Scott J, Carbone JM, Blaha L, et al. Inhibition of gap junctional intercellular communication and activation of mitogen-activated protein kinase by tumor-promoting organic peroxides and protection by resveratrol. Nutrition and Cancer. 2007;57:38–47. doi: 10.1080/01635580701268188. [DOI] [PubMed] [Google Scholar]
- 24.Upham BL, Park J-S, Babica P, Sovadinova I, Rummel AM, et al. Structure-activity-dependent regulation of cell communication by perfluorinated fatty acids using in vivo and in vitro model systems. Environmental Health Perspectives. 2009;117:545–551. doi: 10.1289/ehp.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sovadinova I, Babica P, Boke H, Kumar E, Wilke A, et al. Phosphatidylcholine specific PLC-induced dysregulation of gap junctions, a robust cellular response to environmental toxicants, and prevention by resveratrol. PLOS One. 2015;10:e0124454. doi: 10.1371/journal.pone.0124454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsao MS, Smith JD, Nelson KG, Grisham JW. A diploid epithelial-cell line from normal adult-rat liver with phenotypic properties of oval cells. Experimental Cell Research. 1984;154:38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- 27.Kao CY, Oakley CS, Welsch CW, Chang CC. Growth requirements and neoplastic transformation of two types of normal human breast epithelial cells derived from reduction mammoplasty. In Vitro Cellular & Developmental Biology-Animal. 1997;33:282–288. doi: 10.1007/s11626-997-0048-8. [DOI] [PubMed] [Google Scholar]
- 28.El Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer - a rapid and simple technique to study gap junctional intercellular communication. Experimental Cell Research. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 29.Upham BL. Role of integrative signaling through gap junctions in toxicology. Current Protocols in Toxicology. 2011;47:1–18. doi: 10.1002/0471140856.tx0218s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choung YH, Choi SJ, Joo JS, Lee JB, Lee HK, et al. Green tea prevents down-regulation of gap junction intercellular communication in human keratinocytes treated with PMA. Eur Arch Otorhinolaryngol. 2011;268:885–892. doi: 10.1007/s00405-010-1411-z. [DOI] [PubMed] [Google Scholar]
- 31.Lee DE, Shin BJ, Hur HJ, Kim JH, Kim J, et al. Quercetin, the active phenolic component in kiwifruit, prevents hydrogen peroxide-induced inhibition of gap-junction intercellular communication. Br J Nutr. 2010;104:164–170. doi: 10.1017/S0007114510000346. [DOI] [PubMed] [Google Scholar]
- 32.Kim YJ, Seo SG, Choi K, Kim JE, Kang H, et al. Recovery effect of onion peel extract against H2 O2 -induced inhibition of gap-junctional intercellular communication is mediated through quercetin. J Food Sci. 2014;79:H1011–H1017. doi: 10.1111/1750-3841.12440. [DOI] [PubMed] [Google Scholar]
- 33.Hwang JW, Jung JW, Lee YS, Kang KS. Indole-3-Carbinol Prevents H2O2-Induced Inhibition of Gap Junctional Intercellular Communication by Inactivation of PKB/Akt. Journal of Veterinary Medical Science. 2008;70:1057–1063. doi: 10.1292/jvms.70.1057. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Fu Z, Chen X, Han R. [Effects of curcumin derivatives on the GJIC of normal and tumor cells] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1996;18:111–115. [PubMed] [Google Scholar]
- 35.Pasti G, Kertai P, Adany R. Curcumin does not alter the phorbol ester effect on cell-cell transfer of lucifer yellow CH. Carcinogenesis. 1995;16:1229–1231. doi: 10.1093/carcin/16.5.1229. [DOI] [PubMed] [Google Scholar]
- 36.Jung JW, Park SB, Lee SJ, Seo MS, Trosko JE, et al. Metformin Represses Self-Renewal of the Human Breast Carcinoma Stem Cells via Inhibition of Estrogen Receptor-Mediated OCT4 Expression. PLOS One. 2011;6 doi: 10.1371/journal.pone.0028068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anisimov VN. Do metformin a real anticarcinogen? A critical reappraisal of experimental data. Ann Transl Med. 2014;2:60. doi: 10.3978/j.issn.2305-5839.2014.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canovas-Jorda D, Louisse J, Pistollato F, Zagoura D, Bremer S. Regenerative toxicology: the role of stem cells in the development of chronic toxicities. Expert Opin Drug Metab Toxicol. 2014;10:39–50. doi: 10.1517/17425255.2013.844228. [DOI] [PubMed] [Google Scholar]
- 39.Lee DE, Kang NJ, Lee KM, Lee BK, Kim JH, et al. Cocoa polyphenols attenuate hydrogen peroxide-induced inhibition of gap-junction intercellular communication by blocking phosphorylation of connexin 43 via the MEK/ERK signaling pathway. Journal of Nutritional Biochemistry. 2010;21:680–686. doi: 10.1016/j.jnutbio.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Kim JH, Lee BK, Lee KW, Lee HJ. Resveratrol counteracts gallic acid-induced down-regulation of gap-junction intercellular communication. Journal of Nutritional Biochemistry. 2009;20:149–154. doi: 10.1016/j.jnutbio.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Choi SH, Kim J, Lee BK, Lee KW, et al. Differential regulation of the hydrogen-peroxide-induced inhibition of gap-junction intercellular communication by resveratrol and butylated hydroxyanisole. Mutat Res. 2009;671:40–44. doi: 10.1016/j.mrfmmm.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Loch-Caruso R, Upham BL, Harris C, Trosko JE. Divergent roles for glutathione in lindane-induced acute and delayed-onset inhibition of rat myometrial gap junctions. Toxicological Sciences. 2005;85:694–702. doi: 10.1093/toxsci/kfi123. [DOI] [PubMed] [Google Scholar]
- 43.Upham BL, Trosko JE. Oxidative-Dependent Integration of Signal Transduction with Intercellular Gap Junctional Communication in the Control of Gene Expression. Antioxidants & Redox Signaling. 2009;11:297–307. doi: 10.1089/ars.2008.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruch RJ, Trosko JE, Madhukar BV. Inhibition of connexin43 gap junctional intercellular communication by TPA requires ERK activation. Journal of Cellular Biochemistry. 2001;83:163–169. doi: 10.1002/jcb.1227. [DOI] [PubMed] [Google Scholar]
- 45.Hasler CM, Frick MA, Bennink MR, Trosko JE. TPA-induced inhibition of gap junctional intercellular communication is not mediated through free radicals. Toxicology and Applied Pharmacology. 1990;103:389–398. doi: 10.1016/0041-008x(90)90312-i. [DOI] [PubMed] [Google Scholar]
- 46.Upham BL, Kang KS, Cho HY, Trosko JE. Hydrogen peroxide inhibits gap junctional intercellular communication in glutathione sufficient but not glutathione deficient cells. Carcinogenesis. 1997;18:37–42. doi: 10.1093/carcin/18.1.37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.