Abstract
Dysmorphic nuclei are commonly seen in cancers and provide strong motivation for studying the main structural proteins of nuclei, the lamins, in cancer. Past studies have also demonstrated the significance of microenvironment mechanics to cancer progression, which is extremely interesting because the lamina was recently shown to be mechanosensitive. Here, we review current knowledge relating cancer progression to lamina biophysics. Lamin levels can constrain cancer cell migration in 3D and thereby impede tumor growth, and lamins can also protect a cancer cell's genome. In addition, lamins can influence transcriptional regulators (RAR, SRF, YAP/TAZ) and chromosome conformation in lamina associated domains. Further investigation of the roles for lamins in cancer and even DNA damage may lead to new therapies or at least to a clearer understanding of lamins as bio-markers in cancer progression.
Keywords: Nuclear lamina, cancer, mechanotransduction, homeostasis, SRF, YAP/TAZ, LADs
Introduction
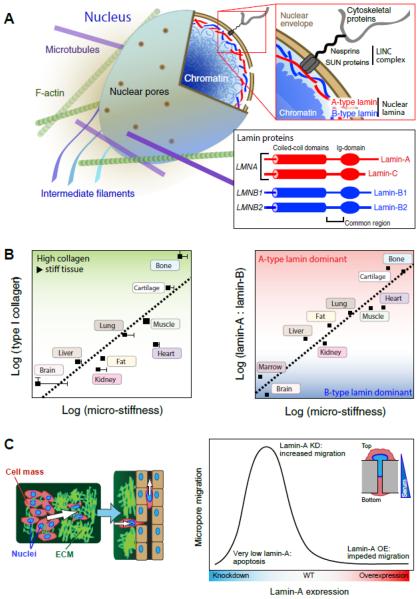
Evolution has likely driven our tissues and organs to fulfill their roles with sustained viability. Mature tissues in particular need to be resistant to the mechanical demands of an active life. Our bones, cartilage, skeletal muscle and heart tissues are stiff, making them robust to routine physical exertion such as walking or running, during which they are subjected to high-frequency shocks, stresses and strains. Tissue-level deformations might even be amplified within cells and their nuclei35. A close correlation between the amount of fibrous collagen extra-cellular matrix (ECM) components and tissue micro-elasticity was recently discovered for mouse tissues79. Surprisingly, we also discovered a systematic positive scaling between tissue elasticity and lamin levels in the nucleoskeleton, which implies a novel role for the lamina as a “mechanostat” that mirrors tissue stiffness—that is, nuclei in stiffer tissues will be stiffer due to higher lamin content. Previous studies have also shown a correlation between matrix stiffness, lamin levels and various transcription factors' activation, suggesting a role of lamins in signaling pathways79. Continuity between the ECM, the cytoskeleton network (with which lamins interact via the LINC complex) and the chromatin might even provide a direct mechanotransduction bridge for the extracellular environment to alter chromosomal organization59.
The lamina is a meshwork of intermediate filament (IF) proteins called lamins that lies inside the nuclear envelope and that interacts with both the chromatin and the cytoskeleton (Fig. 1A). In somatic cells in humans and mice as well as most vertebrates, the major forms of lamin protein are expressed from three genes: lamins A and C are alternative splicing products of the LMNA gene (collectively `A-type' lamins) and lamins B1 and B2 are encoded by LMNB1 and LMNB2 genes (`B-type' lamins). The lamins show some commonality in amino acid sequence and share structural features, but they differ in their post-translational modifications. B-type lamins are permanently modified by a membrane-inserting farnesyl group that is cleaved from mature lamin-A27, 38. Like other IFs, such as keratin and vimentin, the lamins form coiled-coil parallel dimers that assemble into higher-order filamentous structures which fulfill important structural roles36.
Fig. 1. Nuclear lamin levels scale with matrix stiffness and dictate cancer cell migration potential.
(A) A-type and B-type lamins form juxtaposed networks on the inside of the nuclear envelope; they are effectively located at an interface between chromatin and the cytoskeleton, to which the lamina is attached through the `LINC' (linker of nucleo- and cytoskeleton) complex. `A-type lamins', lamins A and C are alternative spliceoform products of the LMNA gene; `B-type lamins', lamins B1 and B2 are protein products of LMNB1 and LMNB2 respectively (adapted from ©Buxboim et al. 2010, originally published in The Journal of Cell Science). (B - left) The quantity of collagen-1 present in tissues scales with tissue micro-elasticity80. As collagen is one of the most prevalent proteins in the body, it is perhaps expected that it defines mechanical properties. (B - right) The composition of the nuclear lamina scales with tissue microelasticity. A-type lamins dominate the lamina in stiff tissue, whereas B-type lamins are prevalent in soft tissue80. (C - left) As the largest and stiffest organelle in the cell, the nucleus can act as an `anchor', preventing cell movement through the matrix or into surrounding vasculature. (C - right) As a model of migration through matrix, cells are induced to pass through 3 μm-pores, a diameter sufficiently small to require deformation of the nucleus (inset). Lamin-A overexpression inhibits migration, whereas knockdown increases migration, up to a point at which significant apoptosis is observed. Thus extremely low or high lamin-A,C levels are unfavorable for cell migration, an observation with potential impact on understanding of processes such as cell migration during development and cancer metastasis.
Dysmorphic nuclei are common markers of cancer. Various studies have also demonstrated the influence of a cancer cell's microenvironment in tumor progression, including the effects of niche stiffness that affect cytoskeleton and cell shape. Nuclear shape changes have long been known to correlate with cell shape changes, but relationships to the lamina are just emerging.
The cancer lamina and what little is known of its function
Evidence from several cancer types as well as from development and aging suggests that nuclear architecture serves as a master integrator for multifactorial microenvironmental signals. Indeed, the microenvironment specifies not just cytoskeletal structure, but also nuclear structure43, 45, 76, 94 and perhaps some aspects of chromatin organization35, 40, 49. Physical signals can thus propagate from the ECM, through adhesions and the cytoskeleton through the LINC complex, and then into the lamina, the nucleus and chromatin, as postulated decades ago11, 13. In flattened 2D tissues and 2D cultures, the nucleus flattens and orients with cell shape, but the interplay of ECM, adhesions, cytoskeleton, and nucleus in 3D tissues remains understudied. It is now clear that the stiffness of non-condensed, well-hydrated nuclei is controlled by the nuclear lamina, and it is clear that lamin-A,C normally adjusts to the stiffness of 3D tissues, with ECM being a major determinant of tissue stiffness (Fig. 1B). In 2D culture models of soft or stiff matrix, cytoskeleton forces and linkages convey mechanical information from outside-to-inside: both lung cancer and primary mesenchymal stem cells exhibit higher lamin-A,C levels when grown on stiff matrix. Similar results have been found for some human cancer cell lines in vitro and in xenografts in vivo16, 20, 79. Three-dimensional xenografts of a human glioblastoma line in stiff mouse subcutaneous tissue lead to significantly higher levels of lamin-A,C than xenografts in mouse brain which is soft16, 79. Further study into mechanisms is needed with 3D systems in vitro as well as in vivo, which is more relevant to cancers, but trends for 2D and 3D appear consistent thus far. Patterned substrates might be useful for example, but it is clear that matrix elasticity is upstream of cell shape since a cell will not spread to fill a large pattern of matrix on a soft substrate even if it fills a small pattern on a stiff substrate. Fibrillar matrices might also be useful6, but crosslinking between fibers requires careful attention because shear rigidity (and Young's modulus) vanishes for any one-dimensional system.
The nuclear envelope mechanically couples the nucleus to the cytoskeleton and to ECM such that the nucleus deforms with the cell14, 79. Indeed, trans-envelope protein interactions such as SUN-KASH directly link the lamin network to cytoskeletal proteins via nuclear envelope spectrin related proteins (nesprins, Fig. 1A)52, 82. When the actin cytoskeleton is cut with laser scissors, the nucleus is observed to move both laterally and away from the culture substratum, demonstrating physical tethering between the cytoskeleton and the nuclear envelope51, 58. These linkages not only help move the nucleus, but are also a major regulator of cytoplasmic stiffness52, 79. Embryos are soft as they lack much ECM, and embryonic cells in vivo and embryonic stem cells in culture have low lamin-A,C and high levels of the nuclear envelope membrane protein lamin-B receptor (LBR)71. However, during normal differentiation to mechanically stiff cell types (e.g. muscle, bone), lamin-A becomes predominant, accompanied by changes in LBR and SUN-KASH proteins22, 60, 79. While understanding of the variable expression of these factors—as perhaps co-regulated by mechanical cues14, 79—is just emerging for normal tissue cells, similar descriptions are emerging more slowly for cancer. Several studies have shown that lamin levels change in cancers of many organ types when compared to normal tissue (Table 1). One very recent immunohistochemical study of human breast tumors and a few breast cancer cell lines reports global loss of about 80–90% of lamin-A,C, SUN1, SUN2, and nesprin-250. However, it is not yet known whether or why such changes in lamin level are associated with changes in LBR and/or other SUN-KASH proteins. Such coordinated changes could suggest reversion of cancer cells to an embryonic state and/or changes in the coupling of nucleus to cytoskeleton, adhesions, and ECM.
Table 1.
Lamins in cancer.
| Type of Cancer | Lamin-A,C | Lamin-B |
|---|---|---|
| Lung cancer15 | ↓ | |
| Breast cancer56, 88 | ↓ | ↓ |
| Colon cancer8, 56 | ↓ | ↓ |
| Colorectal cancer3, 90 | ↑ | ↑ |
| Colonic and grastic adenocarcinomas56 | ↓ | |
| Primary gastric carcinoma93 | ↓ | |
| Basal cell skin carcinoma85 | ↓ | |
| Skin cancer83 | ↑ | |
| Leukemia1 | ↓ | |
| Ovarian serous cancer87 | ↑ | |
| Ovarian cancer9, 17 | ↓ | ↑ |
| Prostate cancer23, 70 | ↓ | ↑ |
| Liver cancer78 | ↑ | |
| Pancreatic cancer46 | ↑ |
Lamin levels, both lamin-A,C and lamin-B, change in cancers of various organs, suggesting that lamins either play a role in cancer progression or alter in response to it. adapted from28. Arrows indicate decrease (down) or increase (up) of either gene or protein expression.
Aging is amongst the highest risk factors for cancer in humans, and so it is intriguing that the only accelerated aging syndromes that affect most human organs and that are currently known in humans involve either DNA repair factors (e.g. Werner Syndrome) or lamin-A,C (e.g. Progeria). Children with such mutations have the striking appearance of octogenarians. Degradation of metabolic pathways is closely associated with aging progression7, but mutations in metabolic factors (and other factors implicated in aging) seem less significant to broad phenotype, accelerated aging conditions in humans than the aforementioned DNA repair proteins and lamin-A,C. As expected of mutations in DNA repair, Werner Syndrome increases the risk for multiple types of cancer. On the other hand, it remains unclear whether lamin-A,C contributes to chromatin stability like a DNA repair factor41, 54, 92. Different adult cell types normally express very different amounts of both lamin-A,C and LBR, whereas B-type lamin levels (especially lamin-B2) vary minimally across adult tissue lineages79, although such trends have yet to be quantified in aging and Progeria. At the molecular scale, lamin-A,C is more mobile and dynamic than B-type lamins66, and Progeria mutants of lamin-A,C, with the farnesylated end intact, behave like B-type lamins24. In numerous cancers, both A- and B-type lamins change28, but there are no reports that the Progerin form of lamin-A is altered or important despite aging as a risk factor in cancer.
Since lamins are the main structural proteins of nuclei, efforts to document changes in lamin levels in cancers have long been motivated by the dysmorphic nuclei that are a hallmark of cancer—often called “nuclear atypia.” In breast cancer, for example, evidence of higher mean levels of lamin-A have been associated with better clinical outcomes17, 26, 88. Culture studies suggest that cells with stiffer nuclei might have greater nuclear integrity14, 33, 79, 81, but it is also clear that a stiffer nucleus prevents invasive migration through small micro-pores (3 μm diameter) even though modest changes in lamin-A levels have no effect on migration in 2D nor in migration through large pores64, 67. In lung cancer, lamin-A levels also tend to be low15. Mechanistic studies of one human lung cancer line have shown that such cells in the periphery of 3D dermal xenografts (with similar stiffness as normal lung) exhibit more distended nuclei compared to the tumor core and express lower levels of lamin-A relative to lamin-B34. Partial transient knockdown of lamin-A,C also led to 3-fold more rapid growth of tumors initially, and made nuclei softer by about 4-fold while allowing cells to migrate about 4-fold more efficiently through small micro-pores (Fig. 1C). Deep knockdown of lamin-A,C in the same studies increased apoptosis only after migration through the small micro-pores, suggesting that DNA damage within a relatively unprotected nucleus during invasion through rigid and constraining microenvironments could be sufficiently high to initiate cell death. Inhibition of at least one factor required for DNA repair was indeed found to decrease the number of cells that successfully migrated through micro-pores34. Beyond the reported changes in lamin-A,C, elevated levels of lamin-B1 have also been reported at least in human liver cancer patients77. Causes and consequences of all such lamin changes in cancer remain in need of much deeper study.
As for animal models, transgenic mice have thus far provided very limited mechanistic insight. For example, in humans, reduced expression of lamin-A,C in late-stage colon cancer patients has been associated with disease recurrence8, but in mice, tissue-specific ablation of lamin-A,C in the gastrointestinal (GI) epithelium shows no effect on overall growth, longevity, or morphology86. Crossing these mice into another strain of transgenic mice susceptible to cancer-associated GI polyps also produces only a small increase in polyp size. Such lineage-specific engineering is motivated by the fact that tissue-wide knockout of the mouse lamin-A,C gene as well as expression of the accelerated aging Progeria-causing mutation result in early lethality, usually within weeks of birth, with stunted growth of the musculoskeletal system and evident fibrosis in the cardiovascular system, all of which are stiff tissues that normally exhibit high levels of lamin-A,C79. Lamin-B knockouts die at birth with small brains (a soft tissue) and defective innervation42. Early death of such mice undermines almost any analysis of the role of lamins in mouse carcinogenesis (perhaps also in humans), but a complete, informative, and cancer-probing rescue was achieved with a mosaic mouse engineered to possess 50% of cells with one form of Progeria and 50% normal cells, achieving a 1:1 ratio that was surprisingly maintained throughout the normal lifespan of the mouse25. The mosaic mice exhibited normal susceptibility to carcinogenic agents applied to lung and skin but reduced invasiveness of tumors caused by a mutagenic agent in the upper GI tract. Perhaps the DNA damage from the latter agent enhances invasion-associated death of the Progeria population of cells that should have a higher level of pre-existing DNA damage57; this could be consistent with speculations from the above studies of lung cancer cells migrating through rigid 3D pores34. Although altered nuclear compliance of Progeria nuclei23 needs to be considered, the stiff tissues in mosaic mice exhibit less fibrotic ECM than the same tissues in 100% Progeria mice, leading to the conclusion that accelerated aging of 100% Progeria mice is due to such cell-extrinsic factors. Since mice do not fibrose as dramatically as humans and typically display lessened forms of otherwise highly fibrotic diseases in humans (e.g. muscular dystrophies29), insights into human diseases from mouse models might be generally limited when ECM is a major contributor. Given the currently limited findings on the role and regulation of the nuclear lamina in cancer, it is evident that further studies are required.
Cancer gene expression regulation: transport into the nucleus and chromatin modulation
The changing structure of the cytoskeleton and nucleus in response to microenvironmental physical stimuli can in turn regulate accumulation of nuclear actin, transcriptional co-activators YAP and TAZ of the Hippo pathway, and retinoic acid receptor (RAR) transcription factors61, 73, 79. These three pathways have all been implicated in cancer and are likely representative of many other physically regulated pathways.
Nuclear actin modulates a number of cellular functions68, including a switch between quiescence and gene transcription in epithelial cells72. Lamin-A contains a nuclear actin binding region and interacts with many actin-binding proteins that affect nuclear actin levels37, which suggests that lamin-A contributes more globally to regulation of cell activity. Nuclear actin indeed controls nuclear import of megakaryoblastic leukemia 1 (MKL1), which is a co-activator of the transcription factor serum response factor (SRF)37 and is also a gene associated with acute megakaryocytic leukemia (AML) via translocation to a dysfunctional fusion protein. SRF normally regulates its own expression together with many other actin-interacting genes, including vinculin, smooth muscle actin, and nonmuscle myosin-IIA (MYH9), such that knockdown of lamin-A decreases expression of SRF pathway genes more so than any other single pathway16, 37, 79, 84. These mechanisms thus regulate a feedback loop between the acto-myosin cytoskeleton and the nuclear lamina. Human breast and melanoma cancer cell lines depleted of MKL1, SRF, or even just myosin-IIA failed to colonize mouse lung from the bloodstream as they were unable to persist after arrival53. Increased cancer rates are not reported in humans with heterozygous, weakly dominant negative mutations of MYH9-related diseases that otherwise do cause diseased platelet, kidney and cataract phenotypes74. On the other hand, myosin-IIA has also been reported to be a tumor suppressor in a mouse screen for genes involved in skin cancer65. Myosin-IIA levels may only become critical when they are very low, facilitating strong feedback effects on SRF and driving cancer. Overall, these findings suggest that low levels of lamin-A might also be accompanied not only by low SUN2, etc. per above, but also by low levels of myosin-IIA, affecting cell tension and contributing to nuclear atypia in cancer.
Hippo pathway signaling involving YAP/TAZ is well-known to regulate growth in response to key contributions from cell junctions, polarity, and cytoskeleton12; loss of such spatial control is a hallmark of cancer. Increased YAP/TAZ activity has been implicated in the progression of some cancers: for example, YAP is limited to progenitor compartments of normal colon, lung, and ovary tissues, but in tumor tissues there is strong and diffuse nuclear and cytoplasmic YAP expression75. In mammary cells, nuclear YAP/TAZ also affects lineage specification19, 69 and mediates contact inhibition via the actin cytoskeleton5, with up-regulation in breast cancer31. Notably, YAP/TAZ activity responds to mechanical stresses involving the ECM-cytoskeleton-lamin interconnectivity. In epithelial sheets in culture, YAP/TAZ activity is regulated by F-actin-capping/severing proteins (Cofilin, CapZ, and Gelsolin), and it is confined to cells exposed to mechanical stress, such as those on the sheet edge or along curved contours5. In mesenchymal stem cells (MSCs) in 2D culture on stiff mechanical environments, YAP accumulates to affect differentiation fates even after cells have been transferred to a different mechanical environment95. Interestingly, lamin-A,C overexpression actually decreases nuclear YAP in similar culture systems, which is consistent with decreased levels of YAP in a rigid normal tissue such as bone compared to muscle (Fig. 2)79; the same set of studies also suggests that some fraction of nuclear YAP localizes specifically near the nuclear lamina, with only one envelope protein (ELYS) identified by mass spectrometry analyses of proteins co-immunoprecipitated with YAP. As for myosin, 3D spheroids of an immortalized retinal pigment epithelial cell line, together with data from a fish model, have recently indicated that YAP contributes to 3D tissue shape and fibronectin assembly, at least partly via ARHGAP18-related proteins that activate Rho-GTPase in a manner that cannot be simply rescued by activating myosin-II contractility62. Given the frequent parallels between development and cancer, it seems important to now address similar regulatory mechanisms of YAP/TAZ activity or dysfunction in 2D as well as 3D cancer models.
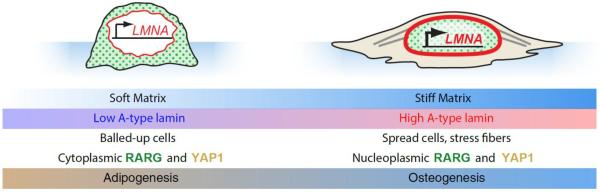
Fig. 2. Decisions of cell fate downstream of lamin-A,C regulation.
MSCs cultured on soft and stiff substrates take on differing phenotypes and favor alternate cell fates32. On soft substrate, MSCs exhibit small nuclear and cellular spread areas, and the nuclear lamina is thinned by a stress-sensitive phosphorylation feedback mechanism80. The transcription factors RARG and YAP131 remain in the cytoplasm, and adipogenic cell fate is preferred. Conversely, on stiff substrate, cells spread extensively with nuclei that are pinned down by well-developed stress fibers. Lamin-A,C is less phosphorylated under strain, thus strengthening the lamina; RARG also translocates to the nucleus, increasing LMNA transcription. Activity of the transcription factor SRF (downstream of lamin-A,C) increases expression of cytoskeletal components37. Under these conditions, YAP1 translocates to the nucleus and cells favor osteogenesis. On both soft and stiff substrates, the effects of matrix elasticity and lamin level cooperate to enhance differentiation: lamin-A,C knockdown on soft matrix leads to more adipogenesis; lamin-A overexpression on stiff matrix leads to more osteogenesis.
Retinoic acid receptor (RAR) transcription factors have long been known to be mutated in leukemia89, but nuclear levels of RARs also regulate diverse developmental pathways, including mammary gland and hematopoiesis18, 21. Lamin-A,C was one predictable target of RARs, but it was surprising to find that RARG, as one of the three RAR isoforms, forms a feedback loop with lamin-A,C79. In at least one line of human lung cancer cells as well as in primary mesenchymal stem cells, rigid matrix was shown to cause cytoskeletal tension and nuclear stress that stabilizes high lamin-A,C (Fig. 2), which helps retain SUN2 at the nuclear envelope; this shift of SUN2 from the endoplasmic reticulum into the nucleus also facilitates RARG entry via SUN2-RARG interaction. Such interplay between the nuclear envelope and various microenvironmental factors allows a cell to coordinate signals from physical cues with signals from purely soluble growth factors and cytokines, resulting in altered expression of gene programs by broadly acting transcription factors. Parallels between development and cancer once again motivate a more careful focus on RAR regulatory mechanisms or dysfunction, especially in 3D cancer models.
Beyond the regulated trafficking of transcription-related factors, interactions of chromatin with the nuclear envelope lead to lamina associated domains (LADs) that have a tendency to display histone marks typical of heterochromatin55. LADs can influence gene expression10, 44 with up to 1000-fold lower expression than genes located elsewhere in the nucleus of murine ES cells2. Cancer stem cells—assuming they exist—are sometimes said to exhibit characteristics of ES cells91, and so repression of differentiation state by sequestration into LADs could be an attractive hypothesis for the cancer field to pursue. Indeed, during differentiation of stem cells, genes specific to stem cell function, such as pluripotency genes, move into LADs, while tissue-specific genes tend to move out47, 63. Likewise, the physical location of the gene locus for the mammary specific milk proteins, whey acidic protein and beta casein, correlate with their activity: when milk proteins are transcribed, the gene is often found at the central edge of its chromosome territory, whereas in hepatocytes, these genes are found in the nuclear periphery96.
Despite what might be presumed from the name “lamina associated domain,” whether and how lamins influence so-called LADs remains an open question. Indeed, as chromatin tagging methods have improved and as DNA sequencing has become more affordable and extensive, the most recent and complete studies of murine ES cells in culture show that nuclear lamins have surprisingly zero role in mediating genome-wide LAD organization in these cells4. These latest studies do imply a role for non-lamin nuclear envelope components in genome organization via LADs (at least in ES cells). Artificial tethering of chromatin to the NE can still suppress gene expression30, 97. Meanwhile, loss of envelope-mediated anchorage of a chromosome in murine cells might increase transcription of genes on that chromosome48 even if it is confirmed across multiple cell types, including cancer cells in 2D and 3D, that lamins are not major determinants of LADs.
Conclusions
We have sought to describe how studies in lamin characterization shed light on cancer progression. Lamins play a direct role in constraining cancer cell migration potential and, according to limited mouse models, modulating tumor growth, with lower lamin levels conferring a growth advantage. In addition, the lamina might indirectly affect cancer progression by altering gene expression via either transcription factors or changes in chromosomal conformation through LADs. These lamin-cancer connections raise numerous possibilities: perhaps the lamina plays a role in epi-genetic modification, leading to oncogene activation, or maybe it acts as a tumor suppressor of sorts by inhibiting migration or by protecting the chromatin from damage that could lead to genomic mutation. Our 2015 report of DNA damage in constricted migration of human cancer-derived cells raises the question of whether `invasion-mutation' mechanisms contribute to the mutation rates and genomic heterogeneity that are highest in the stiffest tissues39. Further work is required to elaborate the mechanistic link between lamins and cancer, which may lead to new treatments or at least a clearer understanding of lamins as bio-markers in cancer progression.
Acknowledgement
The authors in this review were supported by the National Cancer Institute of the National Institutes of Health under Award Number U54. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Conflict of interest Jerome Irianto, Charlotte R. Pfeifer, Irena L. Ivanovska, Joe Swift, and Dennis E. Discher declare that they have no conflicts of interest
Ethical standards No human studies were carried out by authors for this article.
No animal studies were carried out by authors for this article.
References
- 1.Agrelo R, Setien F, Espada J, Artiga MJ, Rodriguez M, Perez-Rosado A, Sanchez-Aguilera A, Fraga MF, Piris MA, Esteller M. Inactivation of the lamin A/C gene by CpG island promoter hypermethylation in hematologic malignancies, and its association with poor survival in nodal diffuse large B-cell lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(17):3940–7. doi: 10.1200/JCO.2005.11.650. [DOI] [PubMed] [Google Scholar]
- 2.Akhtar W, de Jong J, Pindyurin AV, Pagie L, Meuleman W, de Ridder J, Berns A, Wessels LFA, van Lohuizen M, van Steensel B. Chromatin Position Effects Assayed by Thousands of Reporters Integrated in Parallel. Cell. 2013;154(4):914–927. doi: 10.1016/j.cell.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso P, Canamero M, Fernandez-Carbonie F, Nunez A, Casal JI. Proteome analysis of membrane fractions in colorectal carcinomas by using 2D-DIGE saturation labeling. Journal of proteome research. 2008;7(10):4247–55. doi: 10.1021/pr800152u. [DOI] [PubMed] [Google Scholar]
- 4.Amendola M, van Steensel B. Nuclear lamins are not required for lamina-associated domain organization in mouse embryonic stem cells. EMBO reports. 2015;16(5):610–7. doi: 10.15252/embr.201439789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047–59. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 6.Baker BM, Trappmann B, Wang WY, Sakar MS, Kim IL, Shenoy VB, Burdick JA, Chen CS. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nature materials. 2015;14(12):1262–8. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–22. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belt EJ, Fijneman RJ, van den Berg EG, Bril H, Delis-van Diemen PM, Tijssen M, van Essen HF, de Lange-de Klerk ES, Belien JA, Stockmann HB, Meijer S, Meijer GA. Loss of lamin A/C expression in stage II and III colon cancer is associated with disease recurrence. European journal of cancer. 2011;47(12):1837–45. doi: 10.1016/j.ejca.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Bengtsson S, Krogh M, Szigyarto CA, Uhlen M, Schedvins K, Silfversward C, Linder S, Auer G, Alaiya A, James P. Large-scale proteomics analysis of human ovarian cancer for biomarkers. Journal of proteome research. 2007;6(4):1440–50. doi: 10.1021/pr060593y. [DOI] [PubMed] [Google Scholar]
- 10.Berman BP, Weisenberger DJ, Aman JF, Hinoue T, Ramjan Z, Liu Y, Noushmehr H, Lange CP, van Dijk CM, Tollenaar RA, Van Den Berg D, Laird PW. Regions of focal DNA hypermethylation and long-range hypomethylation in colorectal cancer coincide with nuclear lamina-associated domains. Nature genetics. 2012;44(1):40–6. doi: 10.1038/ng.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? Journal of Theoretical Biology. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 12.Boggiano JC, Fehon RG. Growth control by committee: intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Dev Cell. 2012;22(4)):695–702. doi: 10.1016/j.devcel.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boudreau N, Myers C, Bissell MJ. From laminin to lamin: regulation of tissue-specific gene expression by the ECM. Trends in cell biology. 1995;5(1):1–4. doi: 10.1016/s0962-8924(00)88924-2. [DOI] [PubMed] [Google Scholar]
- 14.Broers JL, Peeters EA, Kuijpers HJ, Endert J, Bouten CV, Oomens CW, Baaijens FP, Ramaekers FC. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Human molecular genetics. 2004;13(21):2567–80. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- 15.Broers JL, Raymond Y, Rot MK, Kuijpers H, Wagenaar SS, Ramaekers FC. Nuclear A-type lamins are differentially expressed in human lung cancer subtypes. The American journal of pathology. 1993;143(1):211–20. [PMC free article] [PubMed] [Google Scholar]
- 16.Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PC, Athirasala A, Kao YR, Cho S, Harada T, Shin JW, Discher DE. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Current biology: CB. 2014;24(16):1909–17. doi: 10.1016/j.cub.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capo-chichi CD, Cai KQ, Simpkins F, Ganjei-Azar P, Godwin AK, Xu XX. Nuclear envelope structural defects cause chromosomal numerical instability and aneuploidy in ovarian cancer. BMC medicine. 2011;9:28. doi: 10.1186/1741-7015-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chanda B, Ditadi A, Iscove NN, Keller G. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell. 2013;155(1):215–27. doi: 10.1016/j.cell.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes & development. 2014;28(5):432–437. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chin L, Xia Y, Discher DE, Janmey PA. Mechanotransduction in cancer. Current Opinion in Chemical Engineering. 2016;11:77–84. doi: 10.1016/j.coche.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho KW, Kwon HJ, Shin JO, Lee JM, Cho SW, Tickle C, Jung HS. Retinoic acid signaling and the initiation of mammary gland development. Developmental biology. 2012;365(1):259–66. doi: 10.1016/j.ydbio.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C Expression Is a Marker of Mouse and Human Embryonic Stem Cell Differentiation. STEM CELLS. 2006;24(1):177–185. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- 23.Coradeghini R, Barboro P, Rubagotti A, Boccardo F, Parodi S, Carmignani G, D'Arrigo C, Patrone E, Balbi C. Differential expression of nuclear lamins in normal and cancerous prostate tissues. Oncology reports. 2006;15(3):609–13. [PubMed] [Google Scholar]
- 24.Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(27):10271–6. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de la Rosa J, Freije JM, Cabanillas R, Osorio FG, Fraga MF, Fernandez-Garcia MS, Rad R, Fanjul V, Ugalde AP, Liang Q, Prosser HM, Bradley A, Cadinanos J, Lopez-Otin C. Prelamin A causes progeria through cell-extrinsic mechanisms and prevents cancer invasion. Nature communications. 2013;4:2268. doi: 10.1038/ncomms3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Las Heras JI, Batrakou DG, Schirmer EC. Cancer biology and the nuclear envelope: a convoluted relationship. Seminars in cancer biology. 2013;23(2):125–37. doi: 10.1016/j.semcancer.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD. Nuclear lamins. Cold Spring Harbor perspectives in biology. 2010;2(20826548):a000547. doi: 10.1101/cshperspect.a000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denais C, Lammerding J. In: Nuclear Mechanics in Cancer, in Cancer Biology and the Nuclear Envelope, Advances in Experimental Medicine and Biology. Schirmer EC, Heras J.I.d.l., editors. Springer Science+Business Media; New York: 2014. pp. 435–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dingal PC, Bradshaw AM, Cho S, Raab M, Buxboim A, Swift J, Discher DE. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nature materials. 2015 doi: 10.1038/nmat4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du J-Y, Chen M-C, Hsu T-C, Wang J-H, Brackenbury L, Lin T-H, Wu Y-Y, Yang Z, Streuli CH, Lee Y-J. The RhoA-Rok-myosin II pathway is involved in extracellular matrix-mediated regulation of prolactin signaling in mammary epithelial cells. J Cell Physiol. 2012;227(4):1553–1560. doi: 10.1002/jcp.22886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 32.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 33.Hammerick KE, Huang Z, Sun N, Lam MT, Prinz FB, Wu JC, Commons GW, Longaker MT. Elastic properties of induced pluripotent stem cells. Tissue engineering. Part A. 2011;17(3–4):495–502. doi: 10.1089/ten.tea.2010.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harada T, Swift J, Irianto J, Shin J-W, Spinler KR, Athirasala A, Diegmiller R, Dingal PCDP, Ivanovska IL, Discher DE. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. The Journal of cell biology. 2014;204(5):669–682. doi: 10.1083/jcb.201308029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson, Jonathan T, Shannon G, Veress Alexander I., Neu Corey P. Direct Measurement of Intranuclear Strain Distributions and RNA Synthesis in Single Cells Embedded within Native Tissue. Biophysical Journal. 2013;105(10):2252–2261. doi: 10.1016/j.bpj.2013.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrmann H, Strelkov SV, Burkhard P, Aebi U. Intermediate filaments: primary determinants of cell architecture and plasticity. J. Clin. Invest. 2009;119(7):1772–1783. doi: 10.1172/JCI38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497(7450):507–11. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho CY, Lammerding J. Lamins at a glance. Journal of Cell Science. 2012;125(9):2087–2093. doi: 10.1242/jcs.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irianto J, Pfeifer CR, Xia Y, Athirasala A, Ivanovska IL, Greenberg RE, Discher DE. bioRxiv. 2015. Constricted cell migration causes nuclear lamina damage, DNA breaks, and squeeze-out of repair factors. [Google Scholar]
- 40.Iyer KV, Pulford S, Mogilner A, Shivashankar GV. Mechanical Activation of Cells Induces Chromatin Remodeling Preceding MKL Nuclear Transport. Biophysical Journal. 2012;103(7):1416–1428. doi: 10.1016/j.bpj.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kashiyama K, Nakazawa Y, Pilz DT, Guo C, Shimada M, Sasaki K, Fawcett H, Wing JF, Lewin SO, Carr L, Li TS, Yoshiura K, Utani A, Hirano A, Yamashita S, Greenblatt D, Nardo T, Stefanini M, McGibbon D, Sarkany R, Fassihi H, Takahashi Y, Nagayama Y, Mitsutake N, Lehmann AR, Ogi T. Malfunction of nuclease ERCC1-XPF results in diverse clinical manifestations and causes Cockayne syndrome, xeroderma pigmentosum, and Fanconi anemia. American journal of human genetics. 2013;92(5):807–19. doi: 10.1016/j.ajhg.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, Gaiano N, Ko MS, Zheng Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334(6063):1706–10. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kress C, Ballester M, Devinoy E, Rijnkels M. Epigenetic Modifications in 3D: Nuclear Organization of the Differentiating Mammary Epithelial Cell. J Mammary Gland Biol Neoplasia. 2010;15(1):73–83. doi: 10.1007/s10911-010-9169-x. [DOI] [PubMed] [Google Scholar]
- 44.Kubben N, Adriaens M, Meuleman W, Voncken JW, van Steensel B, Misteli T. Mapping of lamin A- and progerin-interacting genome regions. Chromosoma. 2012;121(5):447–64. doi: 10.1007/s00412-012-0376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Beyec J, Xu R, Lee S-Y, Nelson CM, Rizki A, Alcaraz J, Bissell MJ. Cell shape regulates global histone acetylation in human mammary epithelial cells. Experimental Cell Research. 2007;313(14):3066–3075. doi: 10.1016/j.yexcr.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, Du Y, Kong X, Li Z, Jia Z, Cui J, Gao J, Wang G, Xie K. Lamin B1 is a novel therapeutic target of betulinic acid in pancreatic cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(17):4651–61. doi: 10.1158/1078-0432.CCR-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lund E, Oldenburg AR, Delbarre E, Freberg CT, Duband-Goulet I, Eskeland R, Buendia B, Collas P. Lamin A/C-promoter interactions specify chromatin state–dependent transcription outcomes. Genome Research. 2013;23(10):1580–1589. doi: 10.1101/gr.159400.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malhas A, Lee CF, Sanders R, Saunders NJ, Vaux DJ. Defects in lamin B1 expression or processing affect interphase chromosome position and gene expression. The Journal of cell biology. 2007;176(5):593–603. doi: 10.1083/jcb.200607054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences. 1997;94(3):849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto A, Hieda M, Yokoyama Y, Nishioka Y, Yoshidome K, Tsujimoto M, Matsuura N. Global loss of a nuclear lamina component, lamin A/C, and LINC complex components SUN1, SUN2, and nesprin-2 in breast cancer. Cancer medicine. 2015;4(10):1547–57. doi: 10.1002/cam4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazumder A, Shivashankar GV. Emergence of a prestressed eukaryotic nucleus during cellular differentiation and development. Journal of The Royal Society Interface. 2010;7(Suppl 3):S321–S330. doi: 10.1098/rsif.2010.0039.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKee, Clayton T, Raghunathan Vijay K., Nealey Paul F., Russell P, Murphy Christopher J. Topographic Modulation of the Orientation and Shape of Cell Nuclei and Their Influence on the Measured Elastic Modulus of Epithelial Cells. Biophysical Journal. 2011;101(9):2139–2146. doi: 10.1016/j.bpj.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medjkane S, Perez-Sanchez C, Gaggioli C, Sahai E, Treisman R. Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nature cell biology. 2009;11(3)):257–68. doi: 10.1038/ncb1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohaghegh P, Karow JK, Brosh RM, Jr, Bohr VA, Hickson ID. The Bloom's and Werner's syndrome proteins are DNA structure-specific helicases. Nucleic Acids Research. 2001;29(13):2843–2849. doi: 10.1093/nar/29.13.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moro L, Dolce L, Cabodi S, Bergatto E, Erba EB, Smeriglio M, Turco E, Retta SF, Giuffrida MG, Venturino M, Godovac-Zimmermann J, Conti A, Schaefer E, Beguinot L, Tacchetti C, Gaggini P, Silengo L, Tarone G, Defilippi P. Integrin-induced Epidermal Growth Factor (EGF) Receptor Activation Requires c-Src and p130Cas and Leads to Phosphorylation of Specific EGF Receptor Tyrosines. Journal of Biological Chemistry. 2002;277(11):9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- 56.Moss SF, Krivosheyev V, de Souza A, Chin K, Gaetz HP, Chaudhary N, Worman HJ, Holt PR. Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms. Gut. 1999;45(5):723–9. doi: 10.1136/gut.45.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Musich PR, Zou Y. Genomic instability and DNA damage responses in progeria arising fromdefective maturation of prelamin A. Aging. 2009;1(1):28–37. doi: 10.18632/aging.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagayama K, Yahiro Y, Matsumoto T. Stress fibers stabilize the position of intranuclear DNA through mechanical connection with the nucleus in vascular smooth muscle cells. FEBS letters. 2011;585(24):3992–7. doi: 10.1016/j.febslet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Osmanagic-Myers S, Dechat T, Foisner R. Lamins at the crossroads of mechanosignaling. Genes & development. 2015;29(3):225–37. doi: 10.1101/gad.255968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proceedings of the National Academy of Sciences. 2007;104(40):15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pelissier FA, Garbe JC, Ananthanarayanan B, Miyano M, Lin C, Jokela T, Kumar S, Stampfer MR, Lorens JB, LaBarge MA. Age-related dysfunction in mechanotransduction impairs differentiation of human mammary epithelial progenitors. Cell Rep. 2014;7(6):1926–39. doi: 10.1016/j.celrep.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porazinski S, Wang H, Asaoka Y, Behrndt M, Miyamoto T, Morita H, Hata S, Sasaki T, Krens SF, Osada Y, Asaka S, Momoi A, Linton S, Miesfeld JB, Link BA, Senga T, Castillo-Morales A, Urrutia AO, Shimizu N, Nagase H, Matsuura S, Bagby S, Kondoh H, Nishina H, Heisenberg CP, Furutani-Seiki M. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature. 2015;521(7551):217–21. doi: 10.1038/nature14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, Aiden EL. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–80. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowat AC, Jaalouk DE, Zwerger M, Ung WL, Eydelnant IA, Olins DE, Olins AL, Herrmann H, Weitz DA, Lammerding J. Nuclear Envelope Composition Determines the Ability of Neutrophil-type Cells to Passage through Micron-scale Constrictions. Journal of Biological Chemistry. 2013;288(12):8610–8618. doi: 10.1074/jbc.M112.441535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schramek D, Sendoel A, Segal JP, Beronja S, Heller E, Oristian D, Reva B, Fuchs E. Direct in vivo RNAi screen unveils myosin IIa as a tumor suppressor of squamous cell carcinomas. Science. 2014;343(6168):309–13. doi: 10.1126/science.1248627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, Goldman RD. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes & development. 2008;22(24):3409–21. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin JW, Spinler KR, Swift J, Chasis JA, Mohandas N, Discher DE. Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(47):18892–7. doi: 10.1073/pnas.1304996110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simon DN, Zastrow MS, Wilson KL. Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin A tail. Nucleus. 2010;1(3):264–72. doi: 10.4161/nucl.1.3.11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skibinski A, Breindel Jerrica L., Prat A, Galván P, Smith E, Rolfs A, Gupta Piyush B., LaBaer J, Kuperwasser C. Cell Reports. 2014. The Hippo Transducer TAZ Interacts with the SWI/SNF Complex to Regulate Breast Epithelial Lineage Commitment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skvortsov S, Schafer G, Stasyk T, Fuchsberger C, Bonn GK, Bartsch G, Klocker H, Huber LA. Proteomics profiling of microdissected low- and high-grade prostate tumors identifies Lamin A as a discriminatory biomarker. Journal of proteome research. 2011;10(1):259–68. doi: 10.1021/pr100921j. [DOI] [PubMed] [Google Scholar]
- 71.Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, Herrmann H, Blum H, Engelkamp D, Stewart CL, Leonhardt H, Joffe B. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 2013;152(3):584–98. doi: 10.1016/j.cell.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 72.Spencer VA, Costes S, Inman JL, Xu R, Chen J, Hendzel MJ, Bissell MJ. Depletion of nuclear actin is a key mediator of quiescence in epithelial cells. J Cell Sci. 2011;124(Pt 1):123–32. doi: 10.1242/jcs.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spencer VA, Costes S, Inman JL, Xu R, Chen J, Hendzel MJ, Bissell MJ. Depletion of nuclear actin is a key mediator of quiescence in epithelial cells. Journal of Cell Science. 2011;124(1):123–132. doi: 10.1242/jcs.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spinler KR, Shin JW, Lambert MP, Discher DE. Myosin-II repression favors pre/proplatelets but shear activation generates platelets and fails in macrothrombocytopenia. Blood. 2015;125(3):525–33. doi: 10.1182/blood-2014-05-576462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA. Expression of Yes-associated protein in common solid tumors. Human pathology. 2008;39(11):1582–9. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Storch K, Eke I, Borgmann K, Krause M, Richter C, Becker K, Schröck E, Cordes N. Three-Dimensional Cell Growth Confers Radioresistance by Chromatin Density Modification. Cancer Res. 2010;70(10):3925–3934. doi: 10.1158/0008-5472.CAN-09-3848. [DOI] [PubMed] [Google Scholar]
- 77.Sun A, Jiang Y, Wang X, Liu Q, Zhong F, He Q, Guan W, Li H, Sun Y, Shi L, Yu H, Yang D, Xu Y, Song Y, Tong W, Li D, Lin C, Hao Y, Geng C, Yun D, Zhang X, Yuan X, Chen P, Zhu Y, Li Y, Liang S, Zhao X, Liu S, He F. Liverbase: a comprehensive view of human liver biology. Journal of proteome research. 2010;9(1):50–8. doi: 10.1021/pr900191p. [DOI] [PubMed] [Google Scholar]
- 78.Sun S, Xu MZ, Poon RT, Day PJ, Luk JM. Circulating Lamin B1 (LMNB1) biomarker detects early stages of liver cancer in patients. Journal of proteome research. 2010;9(1):70–8. doi: 10.1021/pr9002118. [DOI] [PubMed] [Google Scholar]
- 79.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149):1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, Pajerowski JD, Spinler KR, Shin J-W, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149):1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tamiello C, Kamps MA, van den Wijngaard A, Verstraeten VL, Baaijens FP, Broers JL, Bouten CC. Soft substrates normalize nuclear morphology and prevent nuclear rupture in fibroblasts from a laminopathy patient with compound heterozygous LMNA mutations. Nucleus. 2013;4(1):61–73. doi: 10.4161/nucl.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tapley EC, Starr DA. Connecting the nucleus to the cytoskeleton by SUN-KASH bridges across the nuclear envelope. Current opinion in cell biology. 2013;25(1):57–62. doi: 10.1016/j.ceb.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tilli CM, Ramaekers FC, Broers JL, Hutchison CJ, Neumann HA. Lamin expression in normal human skin, actinic keratosis, squamous cell carcinoma and basal cell carcinoma. The British journal of dermatology. 2003;148(1):102–9. doi: 10.1046/j.1365-2133.2003.05026.x. [DOI] [PubMed] [Google Scholar]
- 84.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear Actin Regulates Dynamic Subcellular Localization and Activity of the SRF Cofactor MAL. Science. 2007;316(5832):1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 85.Venables RS, McLean S, Luny D, Moteleb E, Morley S, Quinlan RA, Lane EB, Hutchison CJ. Expression of individual lamins in basal cell carcinomas of the skin. British journal of cancer. 2001;84(4):512–9. doi: 10.1054/bjoc.2000.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang AS, Kozlov SV, Stewart CL, Horn HF. Tissue specific loss of A-type lamins in the gastrointestinal epithelium can enhance polyp size. Differentiation; research in biological diversity. 2015;89(1–2):11–21. doi: 10.1016/j.diff.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Wu R, Cho KR, Thomas DG, Gossner G, Liu JR, Giordano TJ, Shedden KA, Misek DE, Lubman DM. Differential protein mapping of ovarian serous adenocarcinomas: identification of potential markers for distinct tumor stage. Journal of proteome research. 2009;8(3):1452–63. doi: 10.1021/pr800820z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wazir U, Ahmed MH, Bridger JM, Harvey A, Jiang WG, Sharma AK, Mokbel K. The clinicopathological significance of lamin A/C, lamin B1 and lamin B receptor mRNA expression in human breast cancer. Cell Mol Biol Lett. 2013;18(4)):595–611. doi: 10.2478/s11658-013-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, Wartman LD, Lamprecht TL, Liu F, Xia J, Kandoth C, Fulton RS, McLellan MD, Dooling DJ, Wallis JW, Chen K, Harris CC, Schmidt HK, Kalicki-Veizer JM, Lu C, Zhang Q, Lin L, O'Laughlin MD, McMichael JF, Delehaunty KD, Fulton LA, Magrini VJ, McGrath SD, Demeter RT, Vickery TL, Hundal J, Cook LL, Swift GW, Reed JP, Alldredge PA, Wylie TN, Walker JR, Watson MA, Heath SE, Shannon WD, Varghese N, Nagarajan R, Payton JE, Baty JD, Kulkarni S, Klco JM, Tomasson MH, Westervelt P, Walter MJ, Graubert TA, DiPersio JF, Ding L, Mardis ER, Wilson RK. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–78. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Willis ND, Cox TR, Rahman-Casans SF, Smits K, Przyborski SA, van den Brandt P, van Engeland M, Weijenberg M, Wilson RG, de Bruine A, Hutchison CJ. Lamin A/C is a risk biomarker in colorectal cancer. PloS one. 2008;3(8):e2988. doi: 10.1371/journal.pone.0002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong DJ, Segal E, Chang HY. Stemness, cancer and cancer stem cells. Cell cycle. 2008;7(23):3622–4. doi: 10.4161/cc.7.23.7104. [DOI] [PubMed] [Google Scholar]
- 92.Worman HJ, Bonne G. “Laminopathies”: A wide spectrum of human diseases. Experimental Cell Research. 2007;313(10):2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu Z, Wu L, Weng D, Xu D, Geng J, Zhao F. Reduced expression of lamin A/C correlates with poor histological differentiation and prognosis in primary gastric carcinoma. Journal of experimental & clinical cancer research: CR. 2009;28:8. doi: 10.1186/1756-9966-28-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu R, Nelson CM, Muschler JL, Veiseh M, Vonderhaar BK, Bissell MJ. Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. The Journal of cell biology. 2009;184(1):57–66. doi: 10.1083/jcb.200807021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nature materials. 2014 doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou G-L, Xin L, Song W, Di L-J, Liu G, Wu X-S, Liu D-P, Liang C-C. Active Chromatin Hub of the Mouse α-Globin Locus Forms in a Transcription Factory of Clustered Housekeeping Genes. Molecular and Cellular Biology. 2006;26(13):5096–5105. doi: 10.1128/MCB.02454-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zullo, Joseph M, Demarco Ignacio A., Piqué-Regi R, Gaffney Daniel J., Epstein Charles B., Spooner Chauncey J., Luperchio Teresa R., Bernstein Bradley E., Pritchard Jonathan K., Reddy Karen L., Singh H. DNA Sequence-Dependent Compartmentalization and Silencing of Chromatin at the Nuclear Lamina. Cell. 2012;149(7):1474–1487. doi: 10.1016/j.cell.2012.04.035. [DOI] [PubMed] [Google Scholar]