Abstract
Macrophages represent a widely distributed and functionally diverse population of innate myeloid cells involved in inflammatory response to pathogens, tissue homeostasis and tissue repair (Murray and Wynn, 2011). Macrophages can be broadly grouped into two subpopulations with opposing activites: M1 or pro-inflammatory macrophages that promote T-helper type 1 (Th1) cell immunity and tissue damage, and M2 or anti-inflammatory/alternatively activated macrophages implicated in Th2 response and resolution of inflammation. Here we describe a rapid assay we used previously to monitor changes in pro-inflammatory and anti-inflammatory cytokine production by lipopolysaccharide (LPS)-activated macrophages in response to therapeutic paracrine factors produced by adult stem cells (Bartosh et al., 2010; Ylostalo et al., 2012; Bartosh et al., 2013). The assay can be adapted appropriately to test macrophage response to other agents as well that will be referred to herein as ‘test reagents’ or ‘test compounds’.
In this protocol, the mouse macrophage cell line J774A.1 is expanded as an adherent monolayer on petri dishes allowing for the cells to be harvested easily without enzymes or cell scrapers that can damage the cells. The macropahges are then stimulated in suspension with LPS and seeded into 12-well cell culture plates containing the test reagents. After 16–18 h, the medium conditioned by the macrophages is harvested and the cytokine profile in the medium determined with enzyme-linked immunosorbent assays (ELISA). We routinely measure levels of the pro-inflammtory cytokine TNF-alpha and the anti-inflammatory cytokine interleukin-10 (IL-10).
Materials and Reagents
J774A.1 mouse macrophages (ATCC, catalog number: TIB-67)
0.1 mg/ml lipopolysaccharide (LPS) (Sigma-Aldrich, catalog number: L4130) solution in PBS
Mouse TNF-alpha Quantikine ELISA kit (R&D Systems, catalog number: MTA00B)
Mouse Interleukin 10 (IL-10) Quantikine ELISA kit (R&D Systems, catalog number: M1000B)
High glucose Dulbecco’s modified Eagle medium (DMEM) containing Glutamax (Life Technologies, catalog number: 10569)
Fetal bovine serum (Atlanta Biologicals, catalog number: S11550)
Penicillin-streptomycin (Life Technologies, catalog number: 15140)
Macrophage medium (see Recipes)
Equipment
150 × 15 mm petri dish (BD Biosciences, Falcon®, catalog number: 351058)
Stericup-GP 0.22 µm vacuum filtration device (EMD Millipore, catalog number: SCGPU05RE)
12-well tissue culture treated plates (Corning, catalog number: 3512)
10 ml capacity serological pipette (VWR International, catalog number: 89130)
1.5 ml microcentrifuge tubes
50 ml sterile conical tube (BD Biosciences, Falcon®, catalog number: 352070)
Water bath set to 37 °C
Pipette-aid
Centrifuge with swinging-bucket rotor and adaptors for 50-ml conical tubes
Humidified cell culture incubator set to 37 °C and 5% CO2
Upright microscope with 10× objective
Microplate reader (capable of measuring absorbance at 450 nm, with a background correction wavelength of 540 nm or 570 nm)
Procedure
-
Preparation of macrophage cultures
-
1.
Expand the J774A.1 mouse macrophages in macrophage medium on a 15 cm petri dish.
Notes:
A detailed method for culture of mouse macrophages can be found in references below.
Macrophage medium should be changed every 2–3 days.
The use of petri dishes allows the macrophages to be harvested for the assay without dissociating enzymes and without the requirement of a cell scraper that can be damaging to the cells.
-
2.
Upon reaching 70–80% confluence, aspirate the culture medium from the dish and add 10 ml of fresh macrophage medium.
-
3.
Harvest the macrophages by washing them from the petri dish with the macrophage medium using a pipette-aid and 10 ml serological pipette.
Note: Spray the medium over the macrophages multiple times to dislodge the cells from the dish.
-
4.
Transfer the macrophage suspension from the plate to a 50 ml conical tube. Add an additional 10 ml macrophage medium to the plate and repeat wash step.
-
5.
Pellet the cells by centrifugation at 200–250 × g for 5–7 min.
-
6.
Suspend the macrophages in macrophage medium and count the cells.
Notes:
A 15 cm petri dish containing macrophages at 70% confluence should yield approximately 15 million macrophages.
The macrophages will consistently have high viability when cultured and harvested as described. Therefore, the use of a viability dye such as trypan blue for distinguishing live versus dead cells is not mandatory.
-
7.
Prepare a suspension of the macrophages in macrophage medium at a density of 200,000 cells per ml.
-
1.
-
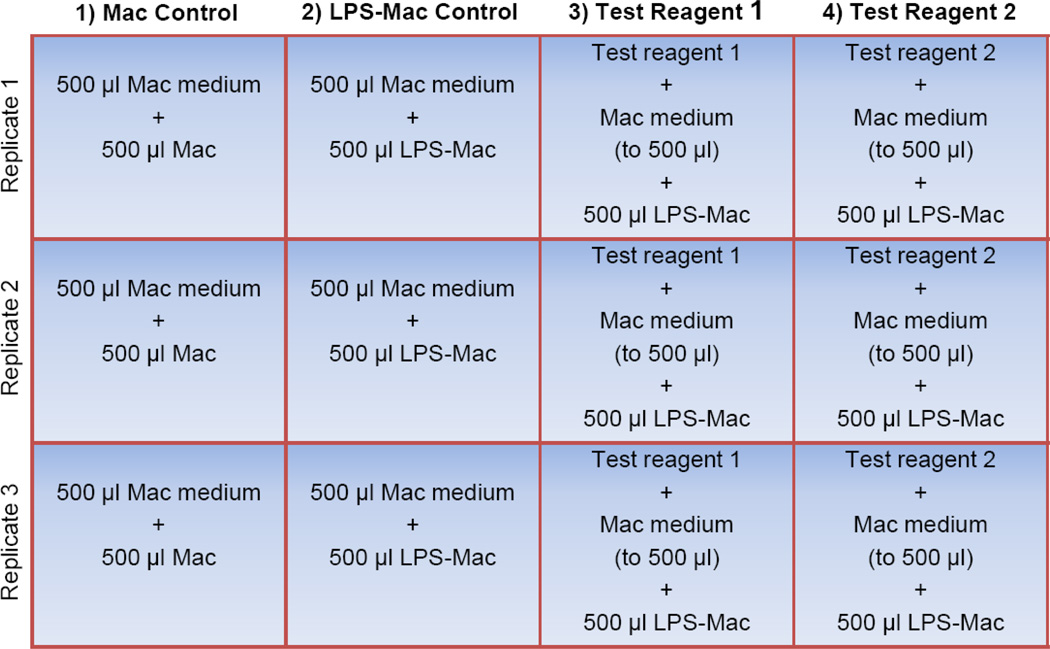
Preparation of assay plates (Figure 1)
-
8.
Determine the number of 12-well cell culture plates necessary to assay samples of interest in triplicate.
-
9.
Transfer the test compound(s) of interest in triplicate to the appropriate wells of a 12-well plate.
Note: We have routinely tested the effects of mesenchymal stem cell conditioned medium, prostaglandins such as PGE2, various pharmacological compounds, neutralizing antibodies and recombinant proteins.
-
10.
Add macrophage medium to all wells containing a test reagent to a final volume of 500 µl.
Note: If the volume of the test compound employed is minute, it is advantageous to first add the macrophage medium to the wells and then transfer the test compound of interest (from step B9) to the appropriate wells. The final volume in each well should remain 500 µl.
-
11.
Add 500 µl macrophage medium to 6 additional wells not containing a test reagent.
Note: These wells will provide controls for the assay.
-
12.
Transfer 500 µl of the macrophage cell suspension (100,000 cells) to each of 3 wells containing 500 µl of macrophage medium only (without a test reagent).
Note: These wells will serve as unstimulated macrophage controls (Mac controls, Figure 1).
-
8.
-
Macrophage stimulation with LPS
-
13.
Stimulate the macrophages by adding a 1:500 dilution of the 0.1 mg/ml solution of LPS to the remainder of the macrophage suspension. To distribute the LPS, immediately mix the cell suspension by pipetting up and down 10–15 times.
-
14.
Place the cap on the 50 ml tube containing the LPS-stimulated macrophages and incubate the cells in the 50 ml tube for 5 min at room temperature.
-
15.
Mix the cells again then transfer 500 µl (100,000 cells) to the appropriate wells containing the test samples. Also, add 500 µl of the macrophage suspension to the three remaining wells containing 500 µl macrophage medium only (LPS-stimulated macrophage controls) (Figure 1).
-
16.
Rock the plate 3 times to distribute the macrophages evenly across the well and incubate at 37 °C for up to 24 h. Each well for the assay finally contains 100,000 macrophages in 1.0 ml macrophage medium and 100 ng/ml LPS (except the three unstimulated control wells).
Note: We routinely incubate the macrophages with LPS for approximately 16–18 h. However, an abundance of macrophage-derived cytokines can be detected in the conditioned medium as early as 4–6 h after LPS stimulation.
-
13.
-
Collection of conditioned medium for assays of cytokine production
-
17.
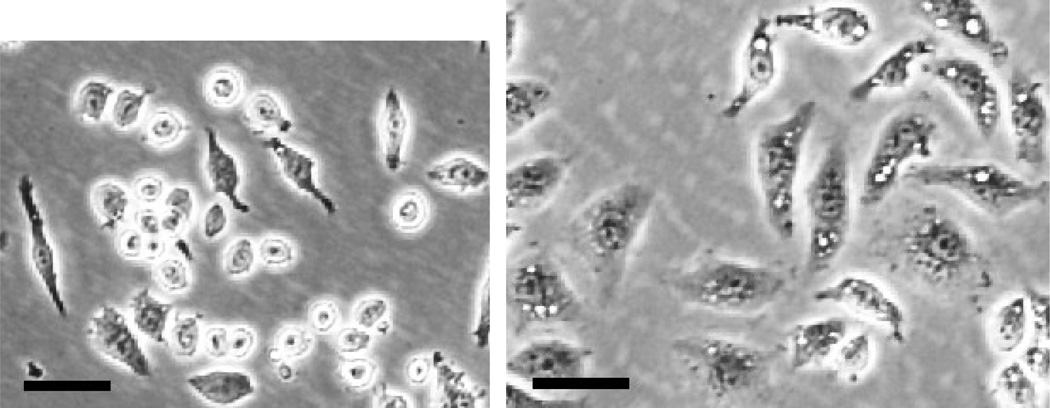
Prior to collecting the medium conditioned by the macrophages, observe the cultures to verify the cells were properly activated (Figure 2).
Note: J774A.1 macrophages stimulated with LPS appear larger, flatter, and more granular than un-stimulated macrophages.
-
18.
Collect the medium conditioned by the macrophages and place it into 1.5 ml microcentrifuge tubes.
-
19.
Centrifuge the samples for 5 min at 500 × g, room temperature.
-
20.
Transfer the supernatant to new 1.5 ml tubes.
Notes:
Although a cell pellet should not be visible, avoid touching the bottom of the tube when removing the supernatant.
The samples can be used immediately for ELISA assay or aliquoted and stored at −80 °C.
-
21.
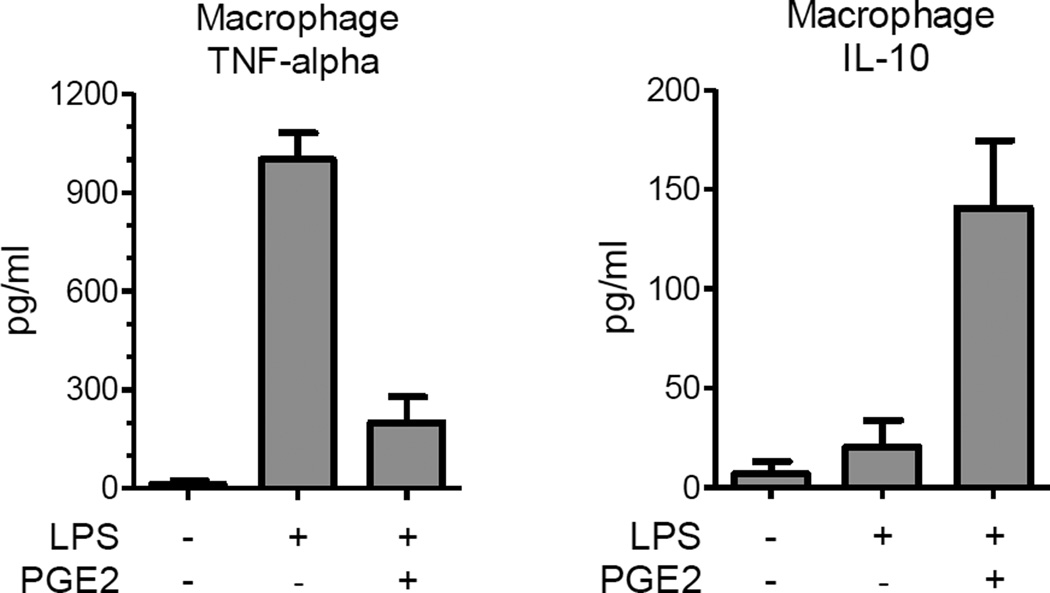
Vortex the medium samples prior to their use in TNF-alpha and IL-10 ELISA assays. Follow instructions provided by the manufacturer of the ELISA kits. The absorbance of the wells can be read on a microplate reader with a wavelength of 450 nm and background wavelength correction set at 540 nm or 570 nm. Representative amounts of TNF-alpha and IL-10 produced by macrophages in response to LPS and a test compound (PGE2) are shown in the graphs below (Figure 3).
-
17.
Figure 1. Sample plate layout for the macrophage assay.
To prepare the 12-well assay plate, the test reagents of interest are first added to the appropriate wells in triplicate (shown here in columns 3 and 4). Macrophage (Mac) medium is then added to every well to a final volume of 500 µl followed by addition of 500 µl of the Mac cell suspension to each well in the first column (unstimulated Mac control). 500 µl of the LPS-stimulated macrophages (LPS-Mac) are transferred to each of the remaining 9 wells (columns 2, 3, and 4). Note that three of these wells do not contain a test reagent (shown here in column 2) and therefore serve as the LPS-stimulated macrophage control (LPS-Mac control). The final volume per well is 1.0 ml. For simplicity, wells containing a vehicle control for the test reagents have been omitted.
Figure 2. Changes in macrophage morphology in response to LPS.
Brightfield images of unsimlulated macrophages (left) and macrophages stimulated with LPS for 18 h (right). Scale bar = 50 µm.
Figure 3. Effects of LPS and PGE2 on TNF-alpha and IL-10 production by macrophages.
Macrophages were stimulated for 18 h with 100 ng/ml LPS alone or LPS in combination with 1 ng/ml of the immune modulatory factor prostaglandin E2 (PGE2). LPS increases levels of the pro-inflammatory cytokine TNF-alpha produced by the macrophages. PGE2 attenuates macrophage production of TNF-alpha elicited by LPS stimulation but enhances levels of the anti-inflammatory cytokine IL-10. Values are mean +/− SD, n=3 per group.
Notes
We have routinely used mouse TNF-alpha and IL-10 ELISA kits to monitor the specific activity of mouse macrophages in response to paracrine factors, such as PGE2, produced by mesenchymal stem cells. However, production of other macrophage-derived cytokines is also increased by LPS stimulation including CXCL2/MIP2, MCP-1, IL-6, IL-1 beta, and IL12-p40 (Ylostalo et al., 2012). These cytokines can also be measured by ELISA.
When the assay is performed as described above, levels of TNF-alpha in the conditioned medium increases from 10–30 pg/ml in unstimulated macrophage cultures to 800–1,200 pg/ml in macrophages stimulated for 16–18 h with LPS. Levels of IL-10 increase only slightly with LPS stimulation. However, IL-10 levels can be markedly enhanced in LPS-stimulated macrophage cultures by factors that promote an anti-inflammatory macrophage phenotype such as PGE2 (Figure 3).
Different preparations of LPS can elicit variations in macrophage cytokine production. The concentration of LPS used in the assay can be adjusted to achieve the desired results. We have routinely used LPS concentrations ranging from 10 ng/ml to 1 µg/ml.
Recipes
-
Macrophage medium (500 ml)
High glucose DMEM containing Glutamax, 445 ml
Fetal bovine serum, 50 ml
Penicillin-streptomycin, 5 ml
Filters sterilize (0.22 µm) and store up to one month at 4 °C
Prewarm to 37 °C in water bath immediately prior to use
Acknowledgments
This protocol was adapted from our original work (Bartosh et al., 2013; Ylostalo et al., 2012) that was supported by grants from the NIH (P40RR17447) and Cancer Prevention & Research Institute of Texas (RP110620) to Darwin J. Prockop, Institute for Regenerative Medicine, Texas A&M University Health Science Center.
References
- 1.Bartosh TJ, Ylostalo JH, Bazhanov N, Kuhlman J, Prockop DJ. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1) Stem Cells. 2013;31(11):2443–2456. doi: 10.1002/stem.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartosh TJ, Ylostalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107(31):13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ylostalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30(10):2283–2296. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]