Abstract
Background
Talaromyces marneffei is an opportunistic dimorphic fungus prevalent in Southeast Asia. We previously demonstrated that Mp1p is an immunogenic surface and secretory mannoprotein of T. marneffei. Since Mp1p is a surface protein that can generate protective immunity, we hypothesized that Mp1p and/or its homologs are virulence factors.
Methodology/Principal Findings
We examined the pathogenic roles of Mp1p and its homologs in a mouse model. All mice died 21 and 30 days after challenge with wild-type T. marneffei PM1 and MP1 complemented mutant respectively. None of the mice died 60 days after challenge with MP1 knockout mutant (P<0.0001). Seventy percent of mice died 60 days after challenge with MP1 knockdown mutant (P<0.0001). All mice died after challenge with MPLP1 to MPLP13 knockdown mutants, suggesting that only Mp1p plays a significant role in virulence. The mean fungal loads of PM1 and MP1 complemented mutant in the liver, lung, kidney and spleen were significantly higher than those of the MP1 knockout mutant. Similarly, the mean load of PM1 in the liver, lung and spleen were significantly higher than that of the MP1 knockdown mutant. Histopathological studies showed an abundance of yeast in the kidney, spleen, liver and lung with more marked hepatic and splenic necrosis in mice challenged with PM1 compared to MP1 knockout and MP1 knockdown mutants. Likewise, a higher abundance of yeast was observed in the liver and spleen of mice challenged with MP1 complemented mutant compared to MP1 knockout mutant. PM1 and MP1 complemented mutant survived significantly better than MP1 knockout mutant in macrophages at 48 hours (P<0.01) post-infection. The mean fungal counts of Pichia pastoris GS115-MP1 in the liver (P<0.001) and spleen (P<0.05) of mice were significantly higher than those of GS115 at 24 hours post-challenge.
Conclusions/Significance
Mp1p is a key virulence factor of T. marneffei. Mp1p mediates virulence by improving the survival of T. marneffei in macrophages.
Author Summary
Talaromyces (Penicillium) marneffei is an opportunistic thermal dimorphic fungus most prevalent in Southeast Asia. Our team has previously shown that Mp1p, a protein encoded by the MP1 gene, is an immunogenic surface and secretory protein of T. marneffei. In this study, we showed that mice challenged with T. marneffei with the MP1 gene died but those challenged with T. marneffei without the MP1 gene did not die. There was also significantly higher fungal load and more necrosis in organs of mice challenged with T. marneffei with the MP1 gene than T. marneffei without the MP1 gene. Furthermore, T. marneffei with the MP1 gene survived better in macrophages than T. marneffei without the MP1 gene and Pichia pastoris with the MP1 gene survived in mice better than P. pastoris without the MP1 gene. Our data support that Mp1p is a key virulence factor of T. marneffei and Mp1p mediates virulence by improving the survival of T. marneffei in macrophages.
Introduction
Talaromyces (Penicillium) marneffei is an opportunistic thermal dimorphic fungus most prevalent in Southeast Asia [1–6]. Bamboo rats and the soil of their burrows are known to be important sources of T. marneffei. Since the 1980s, a marked increase in the number of infections caused by T. marneffei has been observed, primarily as a result of the HIV pandemic. In addition to tuberculosis and cryptococcosis, T. marneffei infection is one the most important indicator of AIDS in our locality. However, in recent years, there has been a surge in the number of T. marneffei infections in HIV-negative patients owing to the use of a variety of immunosuppressive therapies and also due to the increased recognition of underlying immunodeficiency syndromes [3, 7–11].
The first line defense of the human body against T. marneffei infection is achieved mainly through tissue macrophages; however, the mechanisms by which T. marneffei evades host defense is not well understood [12, 13]. In 1998, we described the cloning of a cell surface and abundantly secreted immunogenic mannoprotein, Mp1p, in T. marneffei [14]. Mp1p is a 462-amino acid protein with two homologous domains, which are named as lipid binding domain 1 (Mp1p-LBD1) and lipid binding domain 2 (Mp1p-LBD2). We demonstrated that Mp1p based enzyme-linked immunosorbent assays (ELISAs) can be used for antigen and antibody detection in patients with T. marneffei infections and showed that Mp1p has the ability to generate protective immunity in mice [15–17]. Through analysis of the genome sequence of T. marneffei, we observed the presence of 13 Mp1p homologs in its genome [18]. Moreover, the amino acid sequence of Mp1p in different strains of T. marneffei was found to be highly variable, especially in Mp1p-LBD1; and by using Mp1p and four additional Mp1p homologs, we constructed a multilocus sequence typing scheme for T. marneffei [19]. Recently, we have solved the X-ray crystallographic structure of Mp1p-LBD2, the relatively more conserved LBD of Mp1p, and have shown that it is able to bind palmitic acid [20].
Since Mp1p is a surface protein that can generate protective immunity, we hypothesize that Mp1p and/or its homologs are virulence factors of T. marneffei. To test this hypothesis, we systematically knocked down MP1 and its 13 homologs in T. marneffei and examined their roles in virulence in a mouse model. We demonstrated that Mp1p, but not its homologs, is a key virulence factor of T. marneffei and its virulence is achieved by improving the survival of T. marneffei in macrophages.
Methods
Ethics statement
The experimental protocols were approved by the Committee on the Use of Live Animals in Teaching and Research, The University of Hong Kong, in accordance with the Guidelines laid down by the NIH in the USA regarding the care and use of animals for experimental procedures.
Strains
All fungal strains are summarized in Table 1. T. marneffei PM1 and the genetically- modified derivatives of PM1 were grown on Sabouraud dextrose agar (SDA) (Oxoid), while Pichia pastoris GS115 and its derivatives were grown on yeast extract peptone dextrose agar (Sigma).
Table 1. Fungal strains used in this study.
| Strains | Abbreviation | Characteristics | Relative gene expression level (%) | Source or reference |
|---|---|---|---|---|
| Talaromyces marneffei | ||||
| PM1 | Human strain isolated from an HIV-negative patient | [18] | ||
| MP1 knockdown mutant | shRNA MP1 | PM1 derivative with MP1 knockdown | 45.3±11.4 | This study |
| MPLP1 knockdown mutant | shRNA MPLP1 | PM1 derivative with MPLP1 knockdown | 4.7±3.6 | This study |
| MPLP2 knockdown mutant | shRNA MPLP2 | PM1 derivative with MPLP2 knockdown | 21.6±5.6 | This study |
| MPLP3 knockdown mutant | shRNA MPLP3 | PM1 derivative with MPLP3 knockdown | 32.7±6.9 | This study |
| MPLP4 knockdown mutant | shRNA MPLP4 | PM1 derivative with MPLP4 knockdown | 57.6±2.8 | This study |
| MPLP5 knockdown mutant | shRNA MPLP5 | PM1 derivative with MPLP5 knockdown | 8.9±5.5 | This study |
| MPLP6 knockdown mutant | shRNA MPLP6 | PM1 derivative with MPLP6 knockdown | 4.7±0.9 | This study |
| MPLP7 knockdown mutant | shRNA MPLP7 | PM1 derivative with MPLP7 knockdown | 23.3±9.5 | This study |
| MPLP8 knockdown mutant | shRNA MPLP8 | PM1 derivative with MPLP8 knockdown | 48.4±1.7 | This study |
| MPLP9 knockdown mutant | shRNA MPLP9 | PM1 derivative with MPLP9 knockdown | 19.3±5.2 | This study |
| MPLP10 knockdown mutant | shRNA MPLP10 | PM1 derivative with MPLP10 knockdown | 22.6±7.9 | This study |
| MPLP11 knockdown mutant | shRNA MPLP11 | PM1 derivative with MPLP11 knockdown | 34.1±9.0 | This study |
| MPLP12 knockdown mutant | shRNA MPLP12 | PM1 derivative with MPLP12 knockdown | 23.9±12.7 | This study |
| MPLP13 knockdown mutant | shRNA MPLP13 | PM1 derivative with MPLP13 knockdown | 57±8.3 | This study |
| MP1 knockout mutant | △MP1 | PM1 derivative with MP1 knockout | 45.3±11.4 | This study |
| MP1 complemented mutant | △MP1(pAN8-1 MP1) | MP1 knockout mutant derivative with MP1 complemented | This study | |
| Pichia pastoris | ||||
| GS115 | Pichia pastoris strain for protein expression | Purchased from Invitrogen | ||
| GS115-MP1 | GS115 derivative with Mp1p expression | This study | ||
Identification of Mp1p homologs in T. marneffei
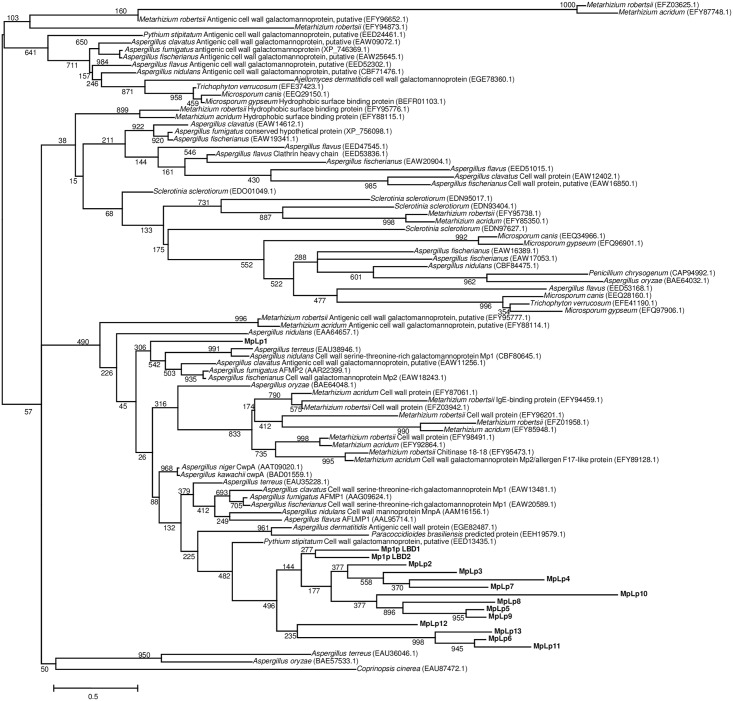
Mp1p homologs in the T. marneffei genome were identified using TBLASTN searches with Mp1p as query [21]. Phylogenetic relationships of Mp1p homologs [MpLp1 (Mp1p-Like protein 1) to MpLp13] and Mp1p were determined using maximum likelihood method with Mega 5 [22].
Knockdown of Mp1p homologs in T. marneffei
DNA extraction and plasmid construction were performed as previously described [23–25]. Expression vector pSilent-1, which can express the short hairpin RNAs (shRNA) against target gene, was used to construct pKD-MP1 and pKD-MPLP1 to 13 for MP1 homolog knockdown. Firstly, the internal gene fragments (sense) were amplified using primers LPW9895, LPW9896, LPW11195, LPW11196, LPW11199, LPW11200, LPW11203, LPW11204, LPW11207, LPW11208, LPW11211, LPW11212, LPW11215, LPW11216, LPW11219, LPW11220, LPW11223, LPW11224, LPW11227, LPW11228, LPW11231, LPW11232, LPW11235, LPW11236, LPW11239, LPW11240, LPW11243 and LPW11244 (S1 Table) (Invitrogen). The PCR mixture (25 μl) contained T. marneffei DNA, PCR buffer (10 mM Tris-HCl pH 8.3, 50 mM KCl, 2 mM MgCl2 and 0.01% gelatin), 200 μM of each dNTPs and 1.0 U Taq polymerase (Applied Biosystem). The mixtures were amplified in 32 cycles of 95°C for 30 seconds, 56°C for 30 seconds and 72°C for 40 seconds, and a final extension at 72°C for 10 minutes (Applied Biosystem). The PCR products were purified using the QIAquick Gel Extraction kit (Qiagen), digested with XhoI and HindIII, and cloned into the XhoI-HindIII site of the pSilent-1 plasmid, resulting in pKD-MP1-1 and pKD-MPLP1-1 to pKD-MPLP13-1. Second, the internal gene fragments (antisense) were amplified with primers LPW 9897, LPW10358, LPW11197, LPW11198, LPW11201, LPW11202, LPW11205, LPW11206, LPW11209, LPW11210, LPW11213, LPW11214, LPW11217, LPW11218, LPW11221, LPW11222, LPW11225, LPW11226, LPW11229, LPW11230, LPW11233, LPW11234, LPW11237, LPW11238, LPW11241, LPW11242, LPW11245 and LPW11246 (S1 Table), using the PCR conditions described above. Amplified fragments were purified as described above, digested with BglII and KpnI, and cloned into the BglII-KpnI sites of pKD-MP1-1 and pKD-MPLP1-1 to pKD-MPLP13-1 respectively, resulting in pKD-MP1 and pKD-MPLP1 to 13.
pKD-MP1 and pKD-MPLP1 to 13 were linearized using EcoICRI and transformed into PM1 respectively. Transformation of T. marneffei was achieved by heat shock using the yeast form of T. marneffei. T. marneffei yeast cells obtained from cultures grown on SDA at 37°C for 10 days were used to inoculate 50 ml yeast extract peptone dextrose (YPD) broth in a 250 ml conical flask with shaking in a gyratory shaker and were further incubated at 37°C with shaking at 200 rpm for 24 hours. T. marneffei yeast cells were harvested by centrifugation at 2,500 rpm for 5 minutes at 4°C and then washed with TE buffer (10mM Tris-HCl pH7.5, 1mM EDTA) and Li-TE buffer (0.1 M lithium acetate in TE pH7.5). T. marneffei yeast cells were resuspended in 200 μl Li-TE buffer and 50 μl of yeast cells were used in each reaction. Three hundred microliters of 40 weight/volume percent (w/v %) freshly prepared polyethylene glycol (PEG) 4000 (Sigma), 5 μl of 10 mg/ml single-stranded sheared salmon sperm DNA (Invitrogen), and 1–2 μg linearized plasmid were sequentially added and mixed with T. marneffei yeast cells and the reactions were subsequently incubated at 30°C for 30 minutes and then at 42°C for 40 minutes. After the heat shock process, yeast cells were collected by three short spins at room temperature and the yeast pellets were resuspended in 10 ml of YPD broth and incubated at 37°C with shaking at 200 rpm for 24 hours. T. marneffei transformants were plated onto SDA containing 150 μg/ml hygromycin B (Invitrogen) and incubated at 37°C for 10–14 days for selecting the knockdown strains MP1 knockdown mutant and MPLP1-13 knockdown mutant. The RNA of the respective transformants was extracted, reverse transcribed, and checked by real-time quantitative RT-PCR (qRT-PCR). The relative gene expression levels of each knockdown mutant compared to PM1 were calculated using 2-ΔΔCT method [26].
Generation of MP1 knockout mutant
For deletion of MP1, pKO-MP1 was generated using a homologous recombination method as previously described [27]. Two DNA fragments, comprising the 1313-bp upstream and the 1406-bp downstream flanking sequences of MP1, were generated by PCR using LPW2558/2559 and LPW2560/2561 respectively (S1 Table). The PCR products of upstream/downstream flanking fragments were ligated into BglII/HindIII sites of vector pAN7-1 that harbored the hygromycin B resistance gene to generate plasmid pKO-MP1, which was then linearized with AspEI and used for transformation. Hygromycin-resistant colonies were screened for homologous recombination by amplification of two fragments which harbored partial genomic sequence, MP1 upstream/downstream fragment and vector sequence using primers LPW2815/LPW2575 and LPW392/LPW2816, whereas one set of gene-specific primers (LPW2562/LPW2772) was used to confirm successful target gene knockout (S1 Table).
Mp1p expression by western blot analysis
Western blot analysis was performed as previously described [15]. Twenty micrograms of protein from the cell lysates of T. marneffei was loaded onto a sodium dodecyl sulfate–10% polyacrylamide gel and the proteins were subsequently onto a nitrocellulose membrane (Bio-Rad). The blot was incubated with 1:1000 dilution of guinea pig anti-Mp1p antibodies, followed by 1:4000 dilution of goat anti-guinea pig IgG (H+L) secondary antibody conjugated with horse radish peroxidase (HRP). Antigen-antibody interaction was then detected with an enhanced chemiluminescence fluorescence system (GE healthcare).
Mp1p expression by ELISA
ELISA was performed as previously described [28]. Briefly, microwell plates (Corning) were coated with 100 μl/well of Mp1p monoclonal antibodies by incubation overnight at 4°C followed by incubation with a blocking reagent containing 2.5 g casein sodium salt, 1.21 g Tris-base, 2 g gelatin, 20 g sucrose, 0.2 g merthiolate, and 5 ml Tween 20 in 1000 ml dH2O (Sigma). The blocking solution was then removed and 100 μl of culture filtrates of wild type or mutant T. marneffei was serially diluted in 1:10 in 0.1% bovine serum albumin and incubated at 37°C for 1 hour. After the plates were washed, biotinylated monoclonal antibody (100 μl/well) was added and the plates were incubated for 30 minutes at 26°C. Following incubation with streptavidin-HRP (Sigma), 3,3′,5,5′-tetramethylbenzidine substrate was added. The reaction was stopped after 10 minutes by addition of 0.3 N sulfuric acid, and the plates were examined in an ELISA plate reader (Bio-Tek) at 450 nm.
Southern blot analysis of homologous recombination
Southern blot analysis was performed as previously described [29]. For MP1-knockout mutant, homologous recombination at the desired locus was confirmed by Southern blot analysis of SpeI-digested genomic DNA probed with a 625-bp PCR product, generated by primers LPW5140/5141 (S1 Table), located at the 5’ upstream flanking region of MP1. Deletion of MP1 was further confirmed by Southern blot analysis with a 680-bp PCR product, generated by primers LPW5142/2772 (S1 Table), which targeted nucleotides 191–650 of the MP1 gene.
Complementation of Mp1p in MP1 knockout mutant
To examine whether the virulence properties of Mp1p can be restored in MP1 knockout mutant, the MP1 gene was complemented in the MP1 knockout mutant. Plasmid pAN8-1 was used to construct pAN8-1MP1 for MP1 complementation. The promoter region of A. nidulans gpd gene and the terminator region of the A. nidulans trpC gene were ligated to the 5’ and 3’ ends of MP1 gene respectively. The MP1 fragment containing promoter and terminator was cloned into NarI and NdeI sites of vector pAN8-1 that harbored the Streptococcus hindustanus phleomycin resistance gene using primers LPW19020/ LPW18915 to give plasmid pAN8-1MP1 (S1 Table). The pAN8-1-MP1 was linearized with PciI and used for transformation.
T. marneffei strain MP1 knockout mutant was transformed with linearized pAN8-1MP1, using 100 μg/ml phleomycin (Invivogen) for selection, generating MP1 complemented mutant. Successful complementation of MP1 gene and Mp1p production were confirmed by PCR, Western blot and ELISA respectively.
Construction of P. pastoris expressing Mp1p
P. pastoris GS115 expressing Mp1p was generated using the Multi-Copy Pichia Expression Kit (Invitrogen). The coding region of MP1 was amplified using primers in S1 Table, digested with EcoRI and XhoI and cloned into the EcoRI-XhoI sites of pPIC9K (Invitrogen) to generate pPIC9K-MP1. The plasmid was first transformed and propagated in Escherichia coli BL21(DE3), followed by transformation into GS115 to generate GS115-MP1. Mp1p expression was induced with buffered methanol complex medium at 30°C with shaking at 300 rpm for 24 hours and expression was confirmed by western blot.
Relative gene expression by real-time quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using RiboPure-Yeast (Ambion). Extracted RNA was eluted in 70 μl of RNase-free water and then used as the template for real-time qRT-PCR. Reverse transcription was performed using the SuperScript III kit (Invitrogen). Real-time qRT-PCR was performed as described previously [30], with primers as listed in S1 Table and using actin primers LPW20631/LPW20160 for normalization. cDNA was amplified in a LightCycler 2.0 (Roche) with 20 μl reaction mixtures containing FastStart DNA Master SYBR Green I Mix reagent kit (Roche), 2 μl cDNA, 2 mM MgCl2 and 0.5 mM primers at 95°C for 10 minutes followed by 50 cycles of 95°C for 10 seconds, 57°C (55°C for actin gene) for 5 seconds and 72°C for 23 seconds (36 seconds for actin gene). All experiments were performed in triplicates.
Animal experiments
Balb/c (H-2d) mice (6-8-week-old, 18–22 g) were housed under standard conditions as described previously [23, 31]. Ten mice each were challenged intravenously with 8×106 spores of PM1, MP1 knockout mutant, MP1 complemented mutant, MP1 knockdown mutant and the MPLP1-13 knockdown mutants; and 1×107 spores of GS115 and GS115-MP1 according to viable counts. Mice survival was recorded daily for 60 days. All experiments were performed in triplicates.
Five mice from the four groups challenged with PM1, MP1 knockout mutant, MP1 complemented mutant and MP1 knockdown mutant were sacrificed on day 12 post-challenge. Five mice from the two groups challenged with GS115 and GS115-MP1 were sacrificed at 24 hours post-challenge. One half of each organ was homogenized in 1× PBS for colony counts, and the other half fixed in 10% neutral buffered formalin and embedded in paraffin. Paraffin-embedded sections were stained with hematoxylin & eosin (H&E), Grocott’s methenamine silver (GMS) or Periodic acid-Schiff (PAS).
Intracellular survival of T. marneffei
Murine macrophage-like cell line J774 (ATCC no. TIB-67) was maintained in Dulbecco's Modified Eagle's Medium (DMEM) (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (Gibco) in 5% CO2 at 37°C in 75 cm2 tissue culture flask (Cellstar). J774 macrophages were differentiated by treatment with 0.32 μM phorbol myristate acetate (PMA) for 72 hours prior to the antifungal assay [23]. PMA-differentiated J774 cells were seeded in duplicates in a 24-well plate at 1×105 cells/well in complete medium. Spores of T. marneffei strains PM1, MP1 knockout mutant, MP1 complemented mutant and MP1 knockdown mutant were harvested and inoculated into J774 cells at 2×105 spores/well (multiplicity of infection of 2) and incubated at 37°C in 5% CO2 incubator for 2 hours for phagocytosis. After phagocytosis, cell monolayers were washed sequentially with 240 U/ml nystatin (Sigma) and warm PBS to remove non-phagocytized Spores and maintained in DMEM supplemented with 1 μg/ml of lipopolysaccharides from E. coli serotype O111:B4 (Sigma) and 400 U/ml of recombinant mouse interferon-γ (R&D System) and further incubated for 48 hours. J774 cells were then harvested and lysed with 1% (w/v) Triton X-100 (Sigma) for colony forming unit (CFU) count at 2 hours, 8 hours, 16 hours, 24 hours and 48 hours post-inoculation. Macrophages lysed with 1% Triton X100 which consisted of the phagocytized yeasts were plated in serial dilutions in duplicate in SDA and incubated for 5 days at 37°C. The results were expressed as mean CFU ± standard deviations from three different experiments.
Statistical analyses
Means between groups were compared with Student’s t-test. Survival of mice was tested by Kaplan-Meier method and Log-rank test.
Results
T. marneffei possesses 13 Mp1p homologs
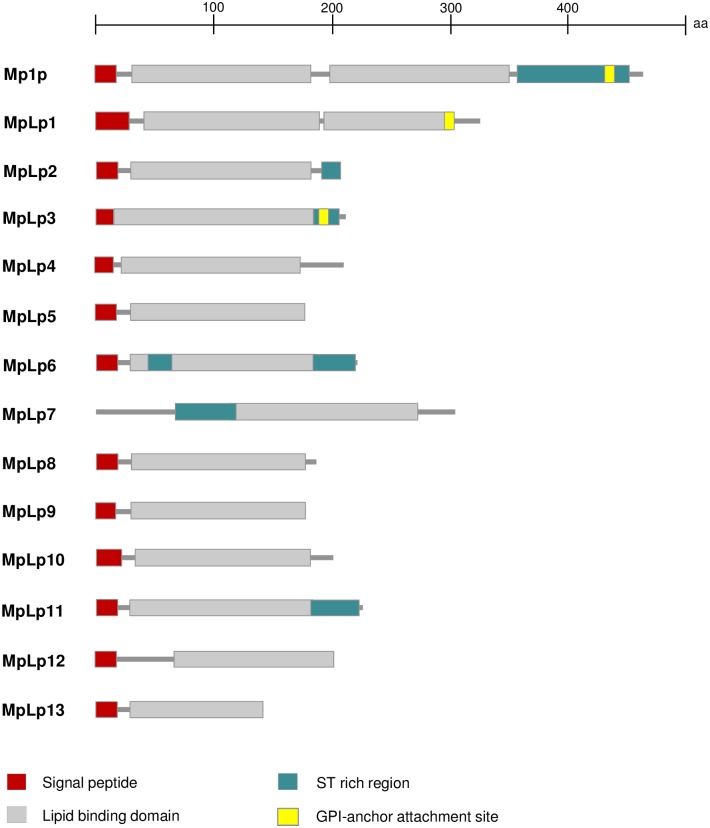
Using TBLASTN searches and the amino acid sequence of Mp1p as query, we observed 13 additional open reading frames in the T. marneffei (strain PM1) draft genome (AGCC00000000) [18] that encodes for putative homologs of Mp1p [MpLp1 to MpLp13 (Table 2 and Fig 1)]. Unlike Mp1p which possesses two LBDs (Mp1p-LBD1 and Mp1p-LBD2), MpLp1 to MpLp13 have only one LBD each. Phylogenetically, Mp1p-LBD1 and Mp1p-LBD2 were clustered with high bootstrap support (Fig 2), suggesting that they are results of duplication of the Mp1p-LBD ancestor during its evolution in T. marneffei. Similar to Mp1p, most of these Mp1p homologs also contain putative signal peptides, variable numbers of putative N-glycosylation and O-glycosylation sites and glycosylphosphatidylinositol (GPI) anchors, and they are all expressed in both the yeast and mold phases of T. marneffei (S1 Fig).
Table 2. Characteristics of Mp1p homologs in T. marneffei.
| Mp1p homolog | pI | Size (aa) | Molecular mass (kDa) | Intron | Subcellular localization | Lipid binding domain (aa) | N-glycosylation site | ST rich region | Signal peptide | GPI-anchor |
|---|---|---|---|---|---|---|---|---|---|---|
| Mp1p | 5.38 | 462 | 47.8 | 0 | Extracellular | 151 | 1 | 354–447 | Yes | Yes |
| MpLp1 | 4.73 | 324 | 31.3 | 1 | Extracellular | 148 | 1 | 193–299 | Yes | Yes |
| MpLp2 | 4.79 | 206 | 22.5 | 0 | Extracellular | 152 | 0 | 188–203 | Yes | No |
| MpLp3 | 5.16 | 210 | 23.4 | 0 | Extracellular | 168 | 2 | 187–208 | Yes | Yes |
| MpLp4 | 5.33 | 205 | 19.6 | 0 | Cytoplasmic | 151 | 0 | No | No | No |
| MpLp5 | 5.94 | 176 | 19.4 | 0 | Extracellular | 147 | 0 | No | Yes | No |
| MpLp6 | 6.51 | 220 | 24.1 | 0 | Extracellular | 157 | 1 | 44–63; 183–218 | Yes | No |
| MpLp7 | 8.46 | 303 | 33.9 | 1 | Nuclear | 159 | 1 | 68–118 | No | No |
| MpLp8 | 8.71 | 186 | 20.7 | 0 | Extracellular | 147 | 1 | No | Yes | No |
| MpLp9 | 8.89 | 176 | 19.6 | 0 | Extracellular | 147 | 0 | No | Yes | No |
| MpLp10 | 8.93 | 200 | 22.3 | 1 | Extracellular | 148 | 1 | No | Yes | No |
| MpLp11 | 8.98 | 224 | 24.1 | 0 | Extracellular | 155 | 1 | 182–222 | Yes | No |
| MpLp12 | 9.32 | 201 | 22.2 | 0 | Extracellular | 128 | 0 | No | Yes | No |
| MpLp13 | 5.57 | 218 | 23.3 | 0 | Extracellular | 153 | 0 | No | Yes | No |
Fig 1. Predicted domains of Mp1p homologs in T. marneffei.
Fig 2. Phylogenetic analysis of the putative LBD of Mp1p homologs in T. marneffei and orthologs in other fungi.
Homologs of Mp1p in T. marneffei are shown in bold. The tree was constructed by maximum likelihood method with bootstrap values calculated from 1,000 trees and rooted on midpoint. The scale bar indicates the branch lengths that correspond to 0.5 substitutions per site as indicated. Names and accession numbers are given as cited in the GenBank database.
Mp1p is a virulence factor of T. marneffei
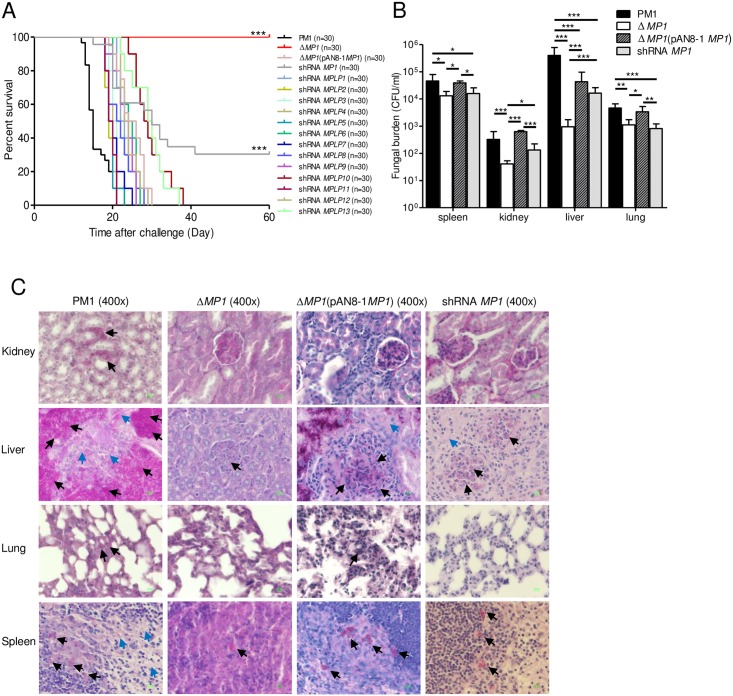
We challenged Balb/c mice intravenously with spores of wild-type T. marneffei strain PM1, MP1 knockout mutant, MP1 complemented mutant, MP1 knockdown mutant, and knockdown mutants of each of the 13 Mp1p homologs (MPLP1 to MPLP13 knockdown mutant), respectively. Site-specific knockout of MP1 was confirmed by PCR, Southern blot, western blot and ELISA (S1 Table and S2–S5 Figs). Complementation of MP1 was confirmed by PCR, western blot and ELISA (S1 Table and S3–S5 Figs). Knockdown of MP1 and its homologs MPLP1 to MPLP13 were confirmed by the corresponding real-time qRT-PCR. All mice died 21 and 30 days after being challenged with PM1 and MP1 complemented mutant respectively (Fig 3A). None of the mice died 60 days after challenge with MP1 knockout mutant (P<0.0001). Seventy percent of mice died 60 days after challenge with MP1 knockdown mutant (P<0.0001), showing a dose-response effect. All mice died after challenge with MPLP1 to MPLP13 knockdown mutant, suggesting that only Mp1p played a significant role in virulence.
Fig 3. Mp1p is a virulence factor of T. marneffei.
(A) Survival curves of Balb/c mice challenged with T. marneffei PM1, MP1 knockout mutant, MP1 complemented mutant and knockdown mutants (***P<0.0001). (B) Mean fungal burden in spleen, kidney, liver and lung of mice challenged with T. marneffei PM1, MP1 knockout mutant, MP1 complemented mutant and MP1 knockdown mutant at day 12 post-challenge (***P<0.001, **P<0.01, *P<0.05). Error bars represent standard deviations. (C) Histopathological examination of PAS stained internal organs of mice at day 12 post-challenge. T. marneffei yeast cells are shown in black arrows and tissue necrosis in blue arrows.
At day 12 post-challenge, five mice from each of the PM1, MP1 knockout mutant, MP1 complemented mutant and MP1 knockdown mutant groups were sacrificed for fungal counts and histopathological studies. The mean fungal loads of PM1 and MP1 complemented mutant in the liver, lung, kidney and spleen were significantly higher than those of MP1 knockout mutant and those of PM1 in the liver, lung and spleen were significantly higher than those of MP1 knockdown mutant (Fig 3B). In the liver, the mean fungal loads of PM1 were >10-fold higher than those of MP1 knockdown mutant and >100-fold higher than those of MP1 knockout mutant. Histopathological studies showed a higher abundance of yeast in the kidney, spleen, liver and lung with more marked hepatic and splenic necrosis in mice challenge with PM1 compared to MP1 knockout mutant and MP1 knockdown mutant (Fig 3C). It also showed an abundance of yeast in the liver and spleen of mice challenged with MP1 complemented mutant compared to MP1 knockout mutant (Fig 3C).
Mp1p enhances survival of T. marneffei in macrophages
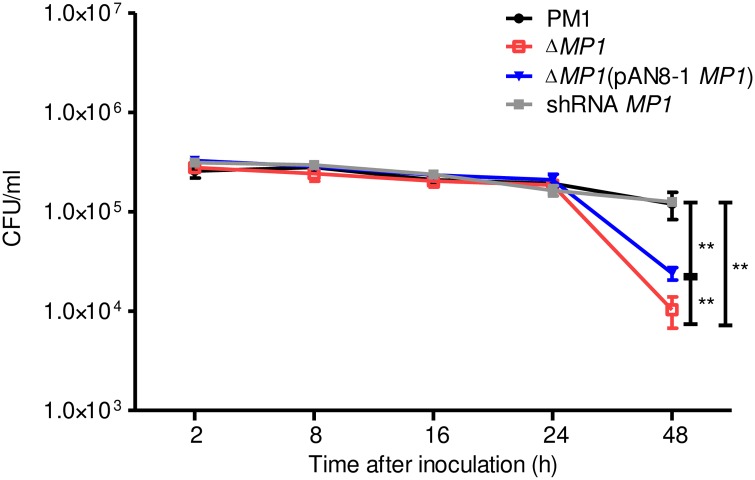
To examine whether Mp1p can improve the intracellular survival of T. marneffei in murine macrophages, we measured the survival of PM1, MP1 knockout mutant, MP1 complemented mutant and MP1 knockdown mutant in murine macrophages. PM1 and MP1 complemented mutant survived significantly better than MP1 knockout mutant at 48 hours (P<0.01) post-infection (Fig 4), suggesting that Mp1p mediates virulence by improving the survival of T. marneffei in macrophages, the primary defensive mechanism against the fungus.
Fig 4. Mp1p enhances T. marneffei survival in macrophages.
Intracellular survival of T. marneffei PM1, MP1 knockout mutant, MP1 complemented mutant and MP1 knockdown mutant in murine macrophages at 2, 8, 16, 24 and 48 hours post-inoculation (**P<0.01). Error bars represent standard deviations.
Mp1p improves survival of P. pastoris in mice
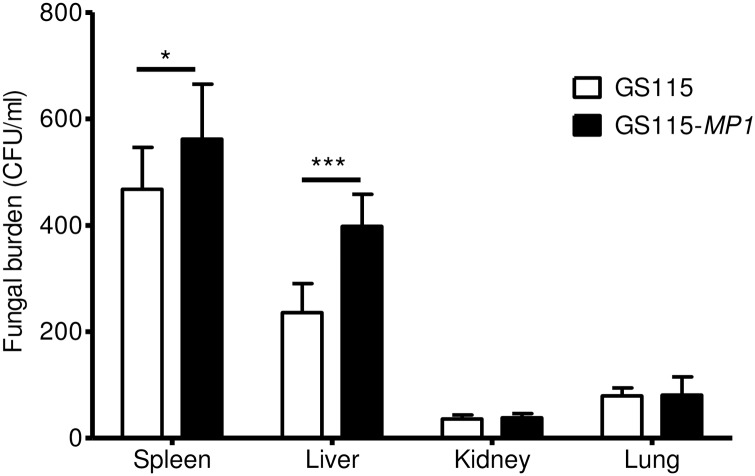
To determine if Mp1p can improve the survival of P. pastoris in mice, we cloned MP1 into expression plasmid pPIC9K and transformed into P. pastoris GS115 (GS115-MP1) and challenged Balb/c mice with GS115 and GS115-MP1 respectively (Table 1). The mean fungal counts of GS115-MP1 in the liver (P<0.001) and spleen (P<0.05) of mice were significantly higher than those of GS115 at 24 hours post-challenge, indicating a gain-of-function (Fig 5).
Fig 5. Mp1p improves survival of Pichia pastoris in mice.
Fungal burden in spleens, livers, kidneys, and lungs of mice challenged with P. pastoris GS115 and GS115-MP1 at 24 hours post-challenge (*P<0.05, ***P<0.001). Error bars represent standard deviations.
Discussion
In this study, we documented that Mp1p is a novel and key virulence factor of T. marneffei. In the literature, six genes (sodA, cpeA, hsp70, alb1, pks11 and pks12) have been suggested to encode potential virulence factors of T. marneffei (superoxide dismutase, catalase-peroxidase, heat shock protein 70 and polyketide synthases for their biosynthetic pathways) [23, 31–34]. Among these six genes, only those encoding the polyketide synthases for the biosynthesis of melanin, mitorubrinic acid and mitorubrinol, which we characterized recently, were shown to have virulence properties in an animal model. However, knocking down of the alb1 (for melanin biosynthesis), pks11 (for mitorubrinic acid biosynthesis) or pks12 (for mitorubrinol biosynthesis) gene could only rescue 10–20% of the mice, suggesting that these are not major virulence factors of T. marneffei [23, 31]. As for sodA, cpeA, and hsp70, studies have demonstrated that the expression of their transcripts in T. marneffei was higher during macrophage infection, oxidative stress or mycelium to yeast phase transition, although these proteins have also been implicated as virulence factors in other fungi [32–34]. In the present study, a T. marneffei strain isolated from an HIV-negative patient with the typical clinical features and with genome sequence available was used [18]. Results showed that after knocking out of MP1 in T. marneffei, all mice survived (Fig 3A). With partial knocking down of MP1, a significant proportion of mice survived (Fig 3A). Moreover, the virulence properties of T. marneffei were restored by complementation of the MP1 gene in its knockout strain. The deaths of the mice were a result of invasion of T. marneffei, as demonstrated by higher fungal counts with massive necrosis in the internal organs of mice challenged with wild-type T. marneffei as compared to both the MP1 knockout mutant and knockdown mutants (Fig 3B and 3C). Further direct evidence to show that Mp1p is a bona fide virulence factor was shown by the cloning of MP1 into P. pastoris enhanced survival of the fungus in mice was observed, indicating a gain-of-function (Fig 5).
The molecular mechanism of virulence for Mp1p remains to be determined. At the cellular level, Mp1p improved the survival of T. marneffei in macrophages (Fig 4), the key defensive cells against the fungus. Although we have shown previously that Mp1p is able to bind palmitic acid [20], this does not seem to provide a direct clue to the molecular mechanism of virulence, as in vitro binding of a protein to other proteins, lipids or other molecules is not uncommon and may not have physiological roles. Since palmitic acid is a fatty acid, further experiments to examine the capability of Mp1p to bind other fatty acids as well as site-directed mutagenesis experiments to look for mutants that affect both the binding activities and virulence properties of T. marneffei will help shed light on the mechanism of virulence of Mp1p. It is noteworthy that the T. marneffei genome contains 13 Mp1p homologs in addition to Mp1p. Similar to Mp1p, these 13 Mp1p homologs are also expressed in significant amounts in both the mold and yeast phases of the fungus (S1 Fig). Overall, their LBDs possessed 21–40% and 25–43% amino acid identities to those of Mp1p-LBD1 and Mp1p-LBD2 respectively and most of their LBDs are comparable in size to Mp1p (Table 2). Moreover, some of these homologs possess N-glycosylation sites, ST rich regions and GPI anchor, which are regions that are also found in Mp1p (Table 2). Interestingly, in contrast to Mp1p which is a strong virulence factor of T. marneffei, the other 13 Mp1p homologs present in the T. marneffei genome do not contribute significantly to virulence as demonstrated by the mice challenge experiments using the corresponding knockdown mutants (Fig 3). Further studies are required to determine the reason for the differential virulence properties of Mp1p and its homologs.
The virulence property of Mp1p may also be present in Mp1p homologs found in other fungi. Phylogenetic analysis of the mitochondrial genomes of T. marneffei and other fungi showed that T. marneffei is closely related to the Aspergillus species [25], which are highly virulent molds that cause high fatalities in patients with hematological malignancies, transplant recipients, HIV positive patients and patients on corticosteroid therapy [35, 36]. We previously showed that A. fumigatus and A. flavus both possess Mp1p homologs (Afmp1p and Afmp2p in A. fumigatus and Aflmp1p in A. flavus) and these homologous proteins in A. fumigatus and A. flavus are also immunogenic proteins which can be used for serological diagnosis in the corresponding fungi [37–41]. Since the LBDs of these proteins are homologous to Mp1p (Fig 2), we speculate that they may also help the corresponding Aspergillus species to evade host immunity. Further experiments will reveal the virulence spectrum of Mp1p homologs in different fungal pathogens.
Supporting Information
The constitutively expressed actin was used as control.
(TIF)
The genomic DNA was digested with SpeI and probed with 1-kb MP1 upstream region probe and MP1 probe. Homologous recombination of the deletion construct at the MP1 locus resulted in integration of the hygromycin resistance gene that increased the size of the hybridizing band. (a) Wild-type T. marneffei strain PM1 and MP1 knockout mutant probed with 680-bp MP1 probe. (b) Wild-type T. marneffei strain PM1 and MP1 knockout mutant probed with 625-bp 1-kb MP1 upstream region probe.
(TIF)
Lane 1, Wild-type T. marneffei strain PM1. Lane 2, MP1 knockout mutant. Lane 3, MP1 complemented mutant.
(TIF)
Lane 1, Wild-type T. marneffei strain PM1. Lane 2, MP1 knockout mutant. Lane 3, MP1 complemented mutant. Lane 4, MP1 knockdown mutant.
(TIF)
Diluted culture supernatants of wild-type T. marneffei strain PM1, MP1 knockout mutant and MP1 complemented mutant were used for Mp1p detection in ELISA. Culture medium was used as control.
(TIF)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was partly supported by Mona Shaw and the Shaw Foundation; the Health and Medical Research Fund (No. HKM-15-M07 [commissioned project]), Food and Health Bureau, Government of the Hong Kong Special Administrative Region, Hong Kong; the Strategic Research Theme Fund of The University of Hong Kong; Research Grant Council Grant; Croucher Senior Medical Research Fellowship; HKU Award for CAE Membership and Dr Hector T.G. Ma; and a grant from the Canadian Institutes of Health Research (MOP-125879, to NER). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yuen KY, Wong SS, Tsang DN, Chau PY. Serodiagnosis of Penicillium marneffei infection. Lancet. 1994;344(8920):444–5. [DOI] [PubMed] [Google Scholar]
- 2.Wong SS, Siau H, Yuen KY. Penicilliosis marneffei—West meets East. J Med Microbiol. 1999;48(11):973–5. [DOI] [PubMed] [Google Scholar]
- 3.Woo PC, Lau SK, Lau CC, Chong KT, Hui WT, Wong SS, et al. Penicillium marneffei fungaemia in an allogeneic bone marrow transplant recipient. Bone Marrow Transplant. 2005;35(8):831–3. [DOI] [PubMed] [Google Scholar]
- 4.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in southeast Asia. Lancet. 1994;344(8915):110–3. [DOI] [PubMed] [Google Scholar]
- 5.Vanittanakom N, Cooper CR Jr., Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA, Peterson SW, et al. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol. 2011;70(1):159–83. 10.3114/sim.2011.70.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang BS, Chan JF, Chen M, Tsang OT, Mok MY, Lai RW, et al. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin Vaccine Immunol. 2010;17(7):1132–8. 10.1128/CVI.00053-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo CY, Chan DT, Yuen KY, Li FK, Cheng KP. Penicillium marneffei infection in a patient with SLE. Lupus. 1995;4(3):229–31. [DOI] [PubMed] [Google Scholar]
- 9.Wang JL, Hung CC, Chang SC, Chueh SC, La MK. Disseminated Penicillium marneffei infection in a renal-transplant recipient successfully treated with liposomal amphotericin B. Transplantation. 2003;76(7):1136–7. [DOI] [PubMed] [Google Scholar]
- 10.Wong SS, Woo PC, Yuen KY. Candida tropicalis and Penicillium marneffei mixed fungaemia in a patient with Waldenstrom's macroglobulinaemia. Eur J Clin Microbiol Infect Dis. 2001;20(2):132–5. [DOI] [PubMed] [Google Scholar]
- 11.Chan JF, Chan TS, Gill H, Lam FY, Trendell-Smith NJ, Sridhar S, et al. Disseminated Infections with Talaromyces marneffei in Non-AIDS Patients Given Monoclonal Antibodies against CD20 and Kinase Inhibitors. Emerg Infect Dis. 2015;21(7):1101–6. 10.3201/eid2107.150138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taramelli D, Tognazioli C, Ravagnani F, Leopardi O, Giannulis G, Boelaert JR. Inhibition of intramacrophage growth of Penicillium marneffei by 4-aminoquinolines. Antimicrob Agents Chemother. 2001;45(5):1450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper CR, Vanittanakom N. Insights into the pathogenicity of Penicillium marneffei. Future Microbiol. 2008;3(1):43–55. 10.2217/17460913.3.1.43 [DOI] [PubMed] [Google Scholar]
- 14.Cao L, Chan CM, Lee C, Wong SS, Yuen KY. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect Immun. 1998;66(3):966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao L, Chan KM, Chen D, Vanittanakom N, Lee C, Chan CM, et al. Detection of cell wall mannoprotein Mp1p in culture supernatants of Penicillium marneffei and in sera of penicilliosis patients. J Clin Microbiol. 1999;37(4):981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao L, Chen DL, Lee C, Chan CM, Chan KM, Vanittanakom N, et al. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. J Clin Microbiol. 1998;36(10):3028–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong LP, Woo PC, Wu AY, Yuen KY. DNA immunization using a secreted cell wall antigen Mp1p is protective against Penicillium marneffei infection. Vaccine. 2002;20(23–24):2878–86. [DOI] [PubMed] [Google Scholar]
- 18.Woo PC, Lau SK, Liu B, Cai JJ, Chong KT, Tse H, et al. Draft genome sequence of Penicillium marneffei strain PM1. Eukaryot Cell. 2011;10(12):1740–1. 10.1128/EC.05255-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo PC, Lau CC, Chong KT, Tse H, Tsang DN, Lee RA, et al. MP1 homologue-based multilocus sequence system for typing the pathogenic fungus Penicillium marneffei: a novel approach using lineage-specific genes. J Clin Microbiol. 2007;45(11):3647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao S, Tung ET, Zheng W, Chong K, Xu Y, Dai P, et al. Crystal structure of the Mp1p ligand binding domain 2 reveals its function as a fatty acid-binding protein. J Biol Chem. 2010;285(12):9211–20. 10.1074/jbc.M109.057760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo PC, Chong KT, Tse H, Cai JJ, Lau CC, Zhou AC, et al. Genomic and experimental evidence for a potential sexual cycle in the pathogenic thermal dimorphic fungus Penicillium marneffei. FEBS Lett. 2006;580(14):3409–16. [DOI] [PubMed] [Google Scholar]
- 22.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–9. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo PC, Lam CW, Tam EW, Leung CK, Wong SS, Lau SK, et al. First discovery of two polyketide synthase genes for mitorubrinic acid and mitorubrinol yellow pigment biosynthesis and implications in virulence of Penicillium marneffei. PLoS Negl Trop Dis. 2012;6(10):e1871 10.1371/journal.pntd.0001871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo PC, Lam CW, Tam EW, Lee KC, Yung KK, Leung CK, et al. The biosynthetic pathway for a thousand-year-old natural food colorant and citrinin in Penicillium marneffei. Sci Rep. 2014;4:6728 10.1038/srep06728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo PC, Zhen H, Cai JJ, Yu J, Lau SK, Wang J, et al. The mitochondrial genome of the thermal dimorphic fungus Penicillium marneffei is more closely related to those of molds than yeasts. FEBS Lett. 2003;555(3):469–77. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 27.Sanglard D, Ischer F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40(10):2300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YF, Cai JP, Wang YD, Dong H, Hao W, Jiang LX, et al. Immunoassays based on Penicillium marneffei Mp1p derived from Pichia pastoris expression system for diagnosis of penicilliosis. PLoS One. 2011;6(12):e28796 10.1371/journal.pone.0028796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo PC, Lau SK, Teng JL, Que TL, Yung RW, Luk WK, et al. Association of Laribacter hongkongensis in community-acquired gastroenteritis with travel and eating fish: a multicentre case-control study. Lancet. 2004;363(9425):1941–7. [DOI] [PubMed] [Google Scholar]
- 30.Lau SK, Tse H, Chan JS, Zhou AC, Curreem SO, Lau CC, et al. Proteome profiling of the dimorphic fungus Penicillium marneffei extracellular proteins and identification of glyceraldehyde-3-phosphate dehydrogenase as an important adhesion factor for conidial attachment. FEBS J. 2013;280(24):6613–26. 10.1111/febs.12566 [DOI] [PubMed] [Google Scholar]
- 31.Woo PC, Tam EW, Chong KT, Cai JJ, Tung ET, Ngan AH, et al. High diversity of polyketide synthase genes and the melanin biosynthesis gene cluster in Penicillium marneffei. FEBS J. 2010;277(18):3750–8. 10.1111/j.1742-4658.2010.07776.x [DOI] [PubMed] [Google Scholar]
- 32.Thirach S, Cooper CR Jr., Vanittanakom P, Vanittanakom N. The copper, zinc superoxide dismutase gene of Penicillium marneffei: cloning, characterization, and differential expression during phase transition and macrophage infection. Med Mycol. 2007;45(5):409–17. [DOI] [PubMed] [Google Scholar]
- 33.Pongpom M, Sawatdeechaikul P, Kummasook A, Khanthawong S, Vanittanakom N. Antioxidative and immunogenic properties of catalase-peroxidase protein in Penicillium marneffei. Med Mycol. 2013;51(8):835–42. 10.3109/13693786.2013.807445 [DOI] [PubMed] [Google Scholar]
- 34.Kummasook A, Pongpom P, Vanittanakom N. Cloning, characterization and differential expression of an hsp70 gene from the pathogenic dimorphic fungus, Penicillium marneffei. DNA Seq. 2007;18(5):385–94. [DOI] [PubMed] [Google Scholar]
- 35.Yuen KY, Woo PC, Ip MS, Liang RH, Chiu EK, Siau H, et al. Stage-specific manifestation of mold infections in bone marrow transplant recipients: risk factors and clinical significance of positive concentrated smears. Clin Infect Dis. 1997;25(1):37–42. [DOI] [PubMed] [Google Scholar]
- 36.Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12(2):310–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chong KT, Woo PC, Lau SK, Huang Y, Yuen KY. AFMP2 encodes a novel immunogenic protein of the antigenic mannoprotein superfamily in Aspergillus fumigatus. J Clin Microbiol. 2004;42(5):2287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo PC, Chong KT, Leung AS, Wong SS, Lau SK, Yuen KY. AFLMP1 encodes an antigenic cel wall protein in Aspergillus flavus. J Clin Microbiol. 2003;41(2):845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo PC, Chan CM, Leung AS, Lau SK, Che XY, Wong SS, et al. Detection of cell wall galactomannoprotein Afmp1p in culture supernatants of Aspergillus fumigatus and in sera of aspergillosis patients. J Clin Microbiol. 2002;40(11):4382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan CM, Woo PC, Leung AS, Lau SK, Che XY, Cao L, et al. Detection of antibodies specific to an antigenic cell wall galactomannoprotein for serodiagnosis of Aspergillus fumigatus aspergillosis. J Clin Microbiol. 2002;40(6):2041–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuen KY, Chan CM, Chan KM, Woo PC, Che XY, Leung AS, et al. Characterization of AFMP1: a novel target for serodiagnosis of aspergillosis. J Clin Microbiol. 2001;39(11):3830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The constitutively expressed actin was used as control.
(TIF)
The genomic DNA was digested with SpeI and probed with 1-kb MP1 upstream region probe and MP1 probe. Homologous recombination of the deletion construct at the MP1 locus resulted in integration of the hygromycin resistance gene that increased the size of the hybridizing band. (a) Wild-type T. marneffei strain PM1 and MP1 knockout mutant probed with 680-bp MP1 probe. (b) Wild-type T. marneffei strain PM1 and MP1 knockout mutant probed with 625-bp 1-kb MP1 upstream region probe.
(TIF)
Lane 1, Wild-type T. marneffei strain PM1. Lane 2, MP1 knockout mutant. Lane 3, MP1 complemented mutant.
(TIF)
Lane 1, Wild-type T. marneffei strain PM1. Lane 2, MP1 knockout mutant. Lane 3, MP1 complemented mutant. Lane 4, MP1 knockdown mutant.
(TIF)
Diluted culture supernatants of wild-type T. marneffei strain PM1, MP1 knockout mutant and MP1 complemented mutant were used for Mp1p detection in ELISA. Culture medium was used as control.
(TIF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.