Abstract
Discovering prognostic factors that simultaneously describe tumor characteristics and improve risk stratification is a priority in cutaneous T-cell lymphoma (CTCL). More than a third of advanced stage CTCL patients in this cohort had detectable cell free plasma Epstein–Barr virus (EBV)-DNA (pEBVd) using quantitative real-time polymerase chain reaction. An increased level of pEBVd was highly concordant with EBV (ie, Epstein–Barr virus RNAs) in tumor tissue and was associated with inferior survival.
Introduction
Outcomes in advanced stage (AS) cutaneous T-cell lymphomas (CTCL) are poor but with great variability. Epstein–Barr virus (EBV) is associated with a subset of non-Hodgkin lymphomas. Frequency of plasma EBV-DNA (pEBVd) detection, concordance with EBV RNA (EBER) in tumor tissue, codetection of plasma cytomegalovirus DNA (pCMVd), and prognostic effect in AS CTCL are unknown.
Patients and Methods
Patients (n = 46; 2006–2013) with AS CTCL (≥IIB) were retrospectively studied. pEBVd and pCMVd were longitudinally measured using quantitative real-time polymerase chain reaction. EBER in situ hybridization (ISH) was performed on tumor samples. Survival from time of diagnosis (ToD) and time of progression to AS was assessed.
Results
Plasma EBV-DNA and pCMVd were detected in 37% (17 of 46) and 17% (8 of 46) of AS CTCL patients, respectively. pCMVd detection was significantly more frequent in pEBVd-positive (pEBVd+) than pEBVd− patients (35% vs. 7%; P = .038). Tumor tissue for EBER-ISH was available in 14 of 17 pEBVd+ and 22 of 29 pEBVd− patients; 12 of 14 (85.7%) pEBVd+ patients were EBER+ versus 0 of 22 pEBVd− patients. Frequency of large cell transformation (LCT) tended to be greater in pEBVd+ patients, but was not significant (10 of 14 pEBVd+ vs. 10 of 23 pEBVd−; P = .17). No notable differences in rates of increased levels of serum lactate dehydrogenase (LDH) were observed (17 of 17 pEBVd+ vs. 27 of 29 pEBVd−). pEBVd detection was associated with significantly worse survival from ToD (P = .021) and time of progression to AS (P = .0098).
Conclusion
Detection of cell-free plasma EBV-DNA was highly concordant with the presence of EBERs in tumor tissue, predicted survival independent of LDH and LCT, and should be further studied as a biomarker in AS CTCL.
Clinical Practice Points: Biomarker, CTCL, EBV, Mycosis fungoides, MF, biomarker
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a heterogeneous group of extranodal non-Hodgkin lymphomas of mature T cells. The most common types of CTCL (approximately 70%) are mycosis fungoides (MF) and Sezary syndrome (SS). MF is characterized by an indolent course, generally with a stepwise progression toward greater tumor burden in the skin, followed in some cases by extracutaneous dissemination and rarely evolution to SS.1 SS is characterized by erythroderma, lymphadenopathy, and circulating clonal atypical T cells. Evolution from MF to SS, and vice versa, is infrequent but well documented and this clinical plasticity is reflected by a substantial molecular overlap.2
Survival in MF/SS is strongly influenced by stage (Supplemental Tables 1 and 2 in the online version).3 Patients with “early stage” (stages IA–IIA) have a low tumor burden in the skin, with superficial patches or plaques, and an expected survival of >10 years.4–7 Patients with “advanced stage” (AS; stages ≥ IIB, including SS) have skin tumors, erythroderma, and/or extracutaneous involvement, and a poor, albeit variable, median survival of <5 years.4–7 Factors responsible for variability within AS CTCL are not well known.8 Although survival outcomes are generally assessed on the basis of stage at diagnosis, the chronic and recurring natural history of MF/SS implies that stage, insofar as it is used as a reflection of tumor burden, is also a time-dependent covariate, and the same might be true for other prognostic factors. The identification of new variables that predict outcome independent of or better than stage alone, is an important priority.9
Epstein–Barr virus (EBV) and cytomegalovirus (CMV) are ubiquitous human herpesviruses that infect leukocytes and are characterized by a biological cycle of primary infection, latency, and lytic reactivation.10,11 EBV is associated with a broad variety of lymphoproliferative disorders.12 EBV-associated cancers are canonically identified on the basis of the detection of EBV-encoded RNA (EBER) in tumor tissue using in situ hybridization (ISH).12 In some EBV-associated lymphomas, detectable cell-free plasma EBV-DNA (pEBVd) is prognostic and a biomarker of disease status.13–15 A number of groups have looked for the presence of EBV in the skin and blood of CTCL patients, mostly in small series, using different methodologies, with conflicting results.16–38 In the largest study,39 conducted in a cohort of patients of all stages, Novelli et al observed that detection of EBV-DNA using quantitative real-time (qRT)-polymerase chain reaction (PCR) in lesional skin and/or peripheral blood mononuclear cells (PBMCs) predicted a worse survival compared with EBV-negative patients.38 No follow-up study to confirm and expand these observations has been published.
In light of the conflicting literature about the presence and clinical effect of EBV in CTCL and need for better risk stratification in AS CTCL, we conducted a retrospective study of longitudinal pEBVd monitoring in AS CTCL patients at The Ohio State University (OSU). We also explored plasma CMV-DNA (pCMVd) as a surrogate marker of impaired immune surveillance, especially treatment-related. The primary objectives of this study were: (1) to estimate the frequency of pEBVd detection in AS CTCL patients; (2) to assess concordance between pEBVd and EBER in tumor tissue; and (3) to estimate the possible prognostic effect of detectable pEBVd.
Patients and Methods
Patient Selection and End Points
Numerous instances of detectable pCMVd and/or pEBVd in patients with T-cell lymphoma prompted us to integrate the measurement of pEBVd and pCMVd in parallel via qRT-PCR in the OSU Clinical Laboratory Improvement Amendments (CLIA)-approved molecular microbiology laboratory as a standard of care for patients with T-cell lymphoma. pEBVd and pCMVd are measured at each follow up, before initiation of new systemic therapies, and also when clinically indicated. Detection of pEBVd or pCMVd prompts a clinical assessment of the patient, but in a non-transplant setting antiviral therapy is initiated only in the presence of symptomatic infection, without predefined cut off values for therapy. Data from the electronic medical record (EMR) were reviewed, and patients with AS CTCL (≥IIB) who were followed at OSU James Cancer Hospital between November 1, 2006 and October 24, 2013 were identified. Patients who had at least 1 paired pEBVd and pCMVd measurement at any time after having reached stage ≥ IIB and a pathologically confirmed diagnosis of CTCL according to World Health Organization-European Organization of Research and Treatment of Cancer (EORTC) criteria, were included in the analysis.40,41 Pathology was reviewed in all cases by 1 of the authors (A.A.G.). Stage was defined according to the tumor-node-metastasis-blood (TNMB) classification, as modified by the International Society for Cutaneous Lymphomas (ISCL)/EORTC.3 Time of diagnosis (ToD) was defined as the date of the first skin biopsy showing a diagnosis of CTCL. Time of progression to AS (ToAS) was defined as the date when the patient was first documented to have stage ≥ IIB. Disease progression post-ToAS was defined as any disease-specific event, such as the development of new cutaneous, nodal, or visceral disease, or an increase in blood stage (ISCL B0–2) that necessitated a change in systemic therapy. Dates of death were confirmed using our institutional EMR as well as the Social Security Death Index. The OSU institutional review board approved the analysis of patient clinical course, outcomes and laboratory studies (2013C0125). Supplemental methods describes plasma EBV and CMV DNA quantification by qRT-PCR and EBER in situ hybridization testing on tissue specimens.
Statistical Analysis
Epstein–Barr virus RNA, pEBVd, and pCMVd status were calculated as dichotomous outcomes of positive versus negative. Clinical characteristics and outcomes as well as the markers of interest were all descriptively summarized over the entire cohort as well as within subgroups of interest. Differences in rates between groups (eg, pEBVd-positive [pEBVd+] vs. pEBVd−) were calculated using Fisher exact test. Differential distributions in continuous measures between groups were calculated using Wilcoxon rank sum tests. Concordance between EBER and pEBVd status was assessed using the McNemar test for paired data. The primary clinical outcome of interest was overall survival (OS), where this was defined in 2 ways: from the ToD and from the ToAS. Because 1 of our goals was to further stratify AS patients and better clarify prognosis for this patient population, our primary focus was on OS from ToAS. For all OS analyses, an event was defined as death from any cause; data for patients alive at last follow-up were censored at that time point. Kaplan–Meier analyses and Log-rank statistics were used to evaluate and compare survival differences between groups of interest. Because of the limited number of deaths, multivariable Cox regression analyses were restricted to two-covariate models and were seen as exploratory. Statistical significance was defined as P < .05. SAS version 9.3 for Windows (SAS Institute Inc) was used for these analyses.
Results
Patient Characteristics and pEBVd and pCMVd Measurements
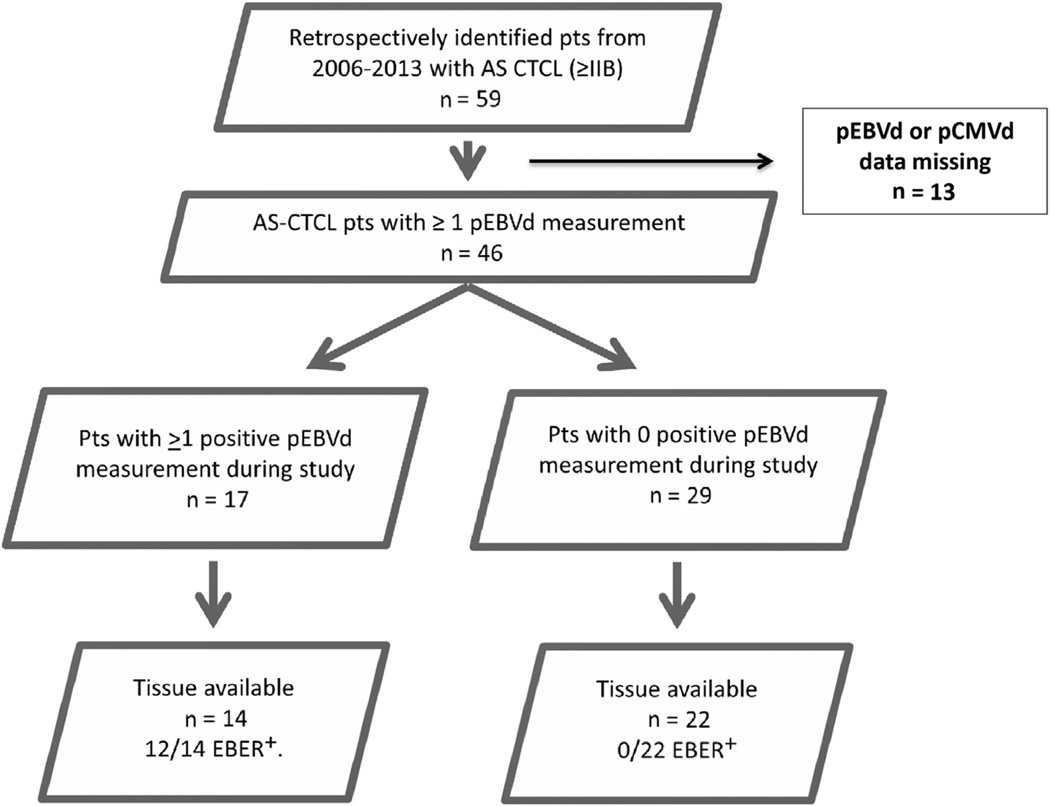
Of a total of 59 AS CTCL patients (stage ≥ IIB) entered in the OSU database between 2006 and 2013, 46 patients had at least 1 set of pEBVd and pCMVd measurements obtained using qRT-PCR during the longitudinal observation period of this study (Figure 1). Thus, 46 patients were assessable for the key primary objective (ie, to determine the number of instances of detection of increased levels of pEBVd in the study population at any time during the course of their disease). Any episode of pEBVd detection was scored as pEBVd+ even if detection occurred only once during the entire longitudinal observation time.
Figure 1.
Patient Selection and Study Overview
Abbreviations: AS = advanced stage; CTCL = cutaneous T-cell lymphoma; EBER = Epstein–Barr virus RNA; pCMVd = plasma cytomegalovirus DNA; pEBVd = plasma Epstein–Barr virus DNA; pts = patients.
Demographic and clinical features of the entire cohort and of the pEBVd+ and pEBVd− subsets are listed in Table 1. Most patients (42 of 46) had MF or SS, whereas 4 were diagnosed with other types of CTCL but had a disease course clinically indistinguishable from MF. One was diagnosed with primary cutaneous CD4+ medium/small-sized T-cell lymphoma, and 3 were diagnosed with peripheral T-cell lymphoma but had only skin and superficial lymph node involvement. No patient had features of extranodal natural killer (NK)-/T-cell lymphoma. Of the study cohort, 21 patients (47%) had AS disease at the time of initial diagnosis (Table 1). The remaining 24 patients (53%) initially presented with early stage disease (<IIB), but subsequently progressed to AS. Median age of the patients at ToD was 63 (range 29–89) years and 27 (59%) were male. Median time from ToD to ToAS was 32 (range 0–171) months, but was skewed by the 21 (47%) diagnosed with AS. Serological status for CMV was available in 59% of the patients (27 of 46) and 21 (78%) were seropositive. Patients were heavily pretreated with a variety of skin-directed and systemic therapies (Table 1 and Supplemental Table 3 in the online version). The median number of systemic regimens was 3 (range, 0–9). Median follow-up for the entire cohort was 55 months (range, 6–302 months) and at the time of these analyses 14 patients had died.
Table 1.
Patient Characteristics According to pEBVd Status
| Characteristic | All Patients (n = 46) | pEBVd+ (n = 17) | pEBVd− (n = 29) | Fisher Exact/Rank Sum P |
|---|---|---|---|---|
| Stage Breakdowna | ||||
| IIB | 15 (33) | 4 (24) | 11 (38) | .35 |
| III/IV | 31 (67) | 13 (76) | 18 (62) | |
| Sex | ||||
| Male | 27 (59) | 11 (65) | 16 (55) | .55 |
| Female | 19 (41) | 6 (35) | 13 (45) | |
|
Median Age at ToD (Range), Years |
63 (29–89) | 71 (41–84) | 62 (29–89) | .14 |
| Initial Diagnosis | ||||
| MF | 33 (72) | 10 (59) | 23 (79) | .15 |
| SS | 9 (20) | 6 (35) | 3 (10) | |
| Other | 4 (9) | 1 (6) | 3 (10) | |
| Stage at ToDb | ||||
| <IIB | 24 (53) | 11 (65) | 13 (46) | .36 |
| ≥IIB | 21 (47) | 6 (35) | 15 (54) | |
|
Median Time From ToD to ToAS, Months (Range) |
31.5 (0–171) | 32.7 (11–171) | 18 (0–97) | .14 |
| Median OS in Months (Range) | ||||
| From ToD | 302.5 (68–302) | 58 (40–176) | 302.5 (NR-NR) | .021 |
| From ToAS | 302.5 (42–303) | 42.3 (10-NR) | 302.5 (NR-NR) | .0098 |
| Status at Last Follow-Up | ||||
| Death/hospice/advancing disease |
23 (50) | 13 (76) | 10 (34) | .013 |
| Stable/no evidence of disease | 23 (50) | 4 (24) | 19 (66) | |
| Elevated LDH Level | ||||
| Yes | 44 (96) | 17 (100) | 27 (93) | .52 |
| No | 2 (4) | 0 | 2 (7) | |
| Large Cell Transformation | ||||
| Yes | 20 (54) | 10 (71) | 10 (43) | |
| No | 17 (46) | 4 (29) | 13 (57) | .17 |
| Not available | 9 | 3 | 6 | |
| EBER-ISH+ | ||||
| Yes | 12 (33) | 12 (86) | 0 | |
| No | 24 (67) | 2 (14) | 22 (100) | .48 |
| Not available | 10 | 3 | 7 | |
|
Number of pEBVd Measurements |
||||
| Median (range) | 13 (1–65) | 17 (7–35) | 11 (1–65) | .043 |
| CMV IgG Serology | ||||
| Positive | 21 (78) | 10 (71) | 11 (85) | .65 |
| Negative | 6 (22) | 4 (29) | 2 (15) | |
| Not tested | 19 | 3 | 16 | |
| pCMVd Detection | ||||
| Yes | 8 (17) | 6 (35) | 2 (7) | .038 |
| No | 38 (83) | 11 (65) | 27 (93) | |
| Alemtuzumab Exposure | ||||
| Yes | 15 (33) | 8 (47) | 7 (24) | .19 |
| No | 31 (67) | 9 (53) | 22 (76) | |
| HDACi Exposure | ||||
| Yes | 21 (46) | 10 (59) | 11 (38) | .23 |
| No | 25 (54) | 7 (41) | 18 (62) | |
|
Median Number of Systemic Treatments (Range) |
3 (0–9) | 3 (1–9) | 3 (0–9) | .41 |
Data are presented as n (%) except where otherwise stated. Significant p-values are italicized.
Abbreviations: CMV = cytomegalovirus; EBER-ISH+ = Epstein–Barr virus RNA detected using in situ hybridization; HDACi = histone deacetylase inhibitor; LDH = lactate dehydrogenase; MF = mycosis fungoides; OS = overall survival; pCMVd = plasma cytomegalovirus DNA; pEBVd = plasma Epstein–Barr virus DNA; SS = Sezary syndrome; ToAS = Time/date of progression to advanced stage; ToD = Time/date of initial diagnosis.
Stage breakdown according to tumor-node-metastasis-blood (TNMB) at study entry.
One patient had an unclear stage at the time of diagnosis.
As stated, there was no formal schedule for pEBVd testing. The frequency and longitudinal duration of pEBVd testing for each patient varied, ranging from weekly (during alemtuzumab therapy) to every 2 to 3 months (during surveillance). As expected, there were more measurements in the pEBVd+ cohort (median, 17; range, 7–35) compared with the pEBVd− cohort (median, 11; range, 1–65); however, even patients who repeatedly tested negative for pEBVd continued to have longitudinal measurements for the duration of the observation time. Overall, with a median of 13 pEBVd measurements per patient (range, 1–65), 17 of 46 patients (37%) had at least one instance of detectable pEBVd whereas 29/46 (63%) never had detectable pEBVd. In the pEBVd+ cohort, the median number of pEBVd+ measurements was 2 (range, 1–8) and the median pEBVd viral load was 4773 copies/mL (range, 1500–111,035 copies/mL). There were no significant differences between the pEBVd+ and pEBVd− cohorts with regard to age, sex, subtype of CTCL, stage, number of previous treatments, frequency of increased levels of lactate dehydrogenase (LDH), and large cell transformation (LCT; Table 1).
Exposure to chemotherapy, T–cell-depleting monoclonal antibodies, and epigenetic agents are known to affect viral latency and induce lytic reactivation of EBV and CMV.42,43 Therefore, we looked at previous therapy in relation to time of first measurable pEBVd to estimate if detection of pEBVd might be treatment-related. Supplemental Table 3 (in the online version) shows systemic therapies received in the pEBVd+ cohort before the first measurable pEBVd compared with the EBV− cohort. In the entire cohort, 15 patients received alemtuzumab and 21 received histone deacetylase inhibitor (HDACi), of which 8 of 15 and 10 of 21, respectively, were pEBVd+ (Table 1). However, pEBVd detection predated exposure to alemtuzumab in 2 of 8 and to HDACi in 4 of 10 (Supplemental Figure 1 in the online version).
Because pCMVd was measured in parallel with pEBVd we explored CMV viremia as a surrogate marker of immune competence, in particular in relation to alemtuzumab therapy, which often leads to CMV reactivation. Of the entire AS CTCL cohort, only 8 of 46 (17%) had detectable pCMVd, although a significantly higher percentage of pEBVd+ than pEBVd− patients had detectable pCMVd (N = 6; 35% vs. N = 2; 7%; P = .038; Table 1). The pCMVd viral load ranged from 284 to 27,872 copies/mL (median, 1024 copies/mL; upper limit of normal [ULN] <250 copies/mL). Of the pEBVd+ patients who were pCMVd+, 4 of 6 were treated with alemtuzumab and had synchronous pEBVd and pCMVd detection. In summary (Supplemental Figure 2 in the online version), although more than 50% (9 of 17) of the pEBVd+ patients were alemtuzumab-naive, only 22% (2 of 8) of the pCMVd+ patients were alemtuzumab-naive. Although the numbers are small and the data set is incomplete, these data suggest a degree of discordance between detection of pEBVd versus pCMVd, at least in relation to alemtuzumab exposure, which might reflect different mechanisms of release of pEBVd and pCMVd and needs to be further explored.
Concordance Between pEBVd and EBER
To assess the concordance between detectable pEBVd and EBER in tumor tissue, EBER-ISH was performed on all patients who had tumor samples available for analysis at any time (n = 14 of 17 pEBVd+; n = 22 of 29 pEBVd−). Overall, 50 tumor samples from the 36 patients were available for EBER-ISH analysis (46 skin, 3 lymph node, and 1 lung). In the pEBVd+ cohort, there were a total of 28 EBER-assessable samples from 14 patients, acquired at different times during the observation period. We observed that 16 of 28 samples (57%) from the pEBVd+ group were EBER+ (12 skin, 3 lymph node, and 1 lung). In the pEBVd− cohort, there were a total of 22 samples from 29 patients, acquired at different times during the observation period, and none were EBER+. Thus, in summary, 12 of 14 pEBVd+ patients (85.7%) were EBER+ versus 0 of 22 pEBVd− patients. With regard to the degree of positivity for EBER-ISH, 10 samples were 1+ (9 skin and 1 lymph node), 2 were 2+ (1 skin and 1 lung), and 4 were 3+ (2 skin and 2 lymph node). There was a trend for higher pEBVd levels in patients with ≥2+ EBER-ISH (for definition of EBER 1+, 2+, and 3+, see Supplemental Figure 3 in the online version).
These results show a high level of concordance between pEBVd and EBER status, with 12 (86%) of the 14 EBER-assessable, pEBVd+ patients had at least 1 EBER+ sample. Specificity for this cohort was 100%, in which none of the patients classified as pEBVd− were EBER+. There are some caveats with this assessment in that the number of samples analyzed per patient was more limited in the pEBVd− cohort and the tissue analyzed for EBER was not always obtained at the same time as the pEBVd measurements. Regardless, there was still a high level of concordance between these 2 markers (McNemar test P = .48), with only 2 patients who had discordant results (pEBVd+ but EBER−). Despite the variability in timing, our data indicate that pEBVd and EBER positivity are highly concordant markers in AS CTCL.
Effect of pEBVd on Outcome
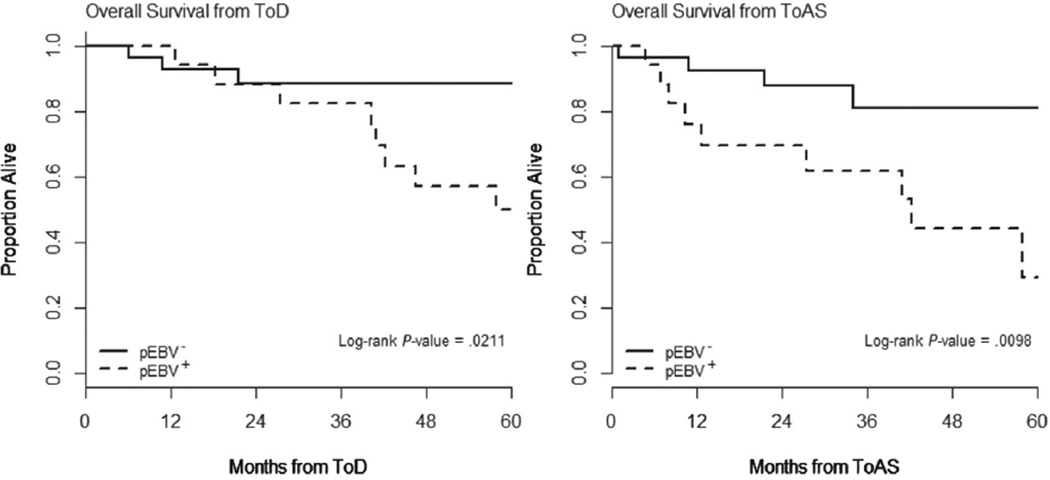
Since discovering prognostic factors with more discriminating power than stage alone is a priority in CTCL, one of the goals in this study was to ask whether pEBVd was prognostic for survival invariant of stage, and specifically if it provided further prognostic insight within those classified as. Therefore, we focused our multivariable analyses only on patients with AS CTCL (≥IIB), and evaluated the potential utility of pEBVd as a prognostic marker in relation to other putative prognostic factors such as LCT and increased levels of serum LDH after controlling for stage. In the 46 AS CTCL patients, pEBVd positivity was significantly associated with worse OS from ToD (P = .021) and from ToAS (P = .0098; Figure 2). With a median follow-up of 35.2 months from ToAS and 14 deaths reported to date, the estimated 2-year, 3-year, and 4-year OS rates from ToAS for pEBVd+ patients were 69.7%, 61.9%, and 44.2%, respectively, versus 87.7%, 81%, and 81%, respectively, for the pEBVd− patients.
Figure 2.
Overall Survival From Time of Diagnosis (ToD) and From Time of Progression to Advanced Stage (ToAS) Cutaneous T-Cell Lymphoma (ie, ≥IIIB) in Patients With Detectable Plasma Epstein–Barr Virus DNA (pEBV+) Compared With Patients With No Detectable Plasma Epstein–Barr Virus DNA (pEBVd−)
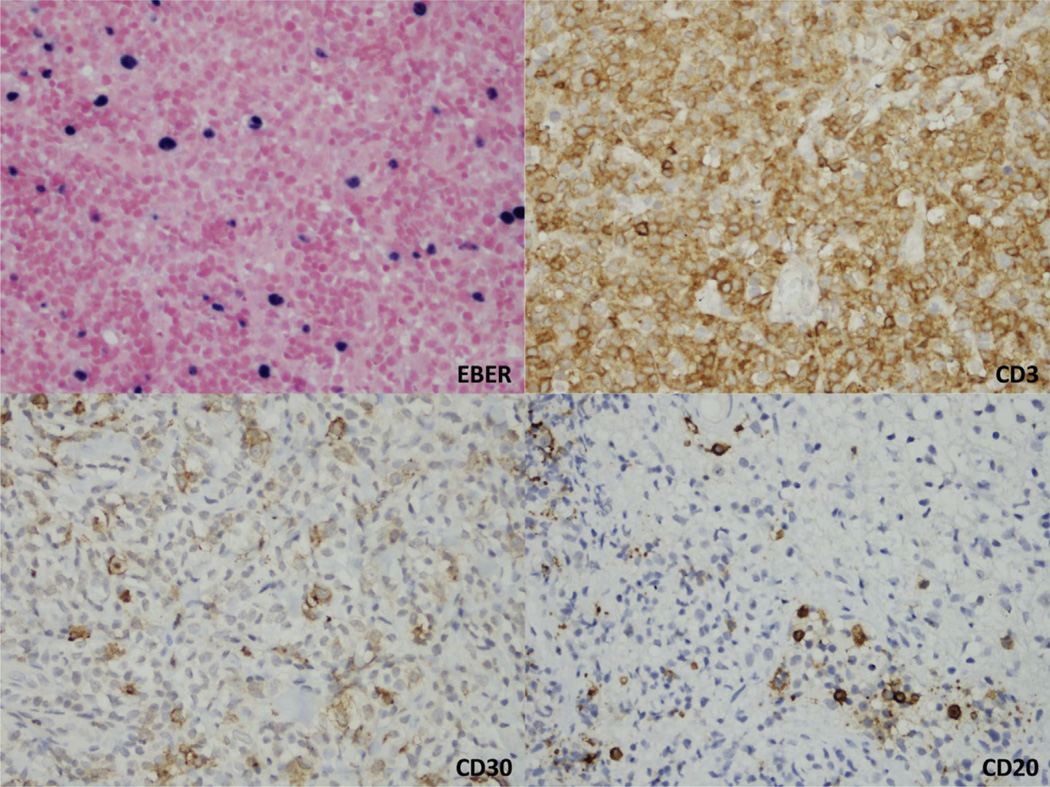
No notable differences in rates of increased levels of serum LDH were observed (17 of 17 pEBVd+ vs. 27 of 29 pEBVd−). Having observed that detectable pEBVd was highly concordant with EBER in AS CTCL and that pEBVd detection identified a poor-risk subset, we asked if pEBVd might be associated with LCT. Sixty-two tumor samples from 37 patients (14 pEBVd+ and 23 pEBVd−) were available and were examined for the presence of LCT. Ten of 14 (71%) pEBVd+ patients had evidence of LCT compared with 10 of 23 (43%) pEBVd− patients (P = .17). We did not see significant differences in OS in the subset of AS CTCL patients (N = 20, 54%) with LCT (P = .20). Because CD30 is often associated with LCT and is upregulated in EBV-infected cells, we evaluated CD30 expression in 40 available samples from 32 patients (12 pEBVd+, 20 pEBVd−). Overall, approximately half of the samples analyzed had CD30+ cells, with no notable difference between pEBVd+ and pEBVd− cases. In the pEBVd+ subset, CD30 was detected only in EBER+ samples with concomitant LCT, and was expressed by large atypical T-cells in 4 cases (representative case shown in Figure 3).
Figure 3.
Skin Biopsy From a Patient With Detectable Plasma Epstein–Barr Virus DNA. Epstein–Barr Virus RNA (EBER) In Situ Hybridization Is Positive in Medium and Large Cells, Which Are Strong and Diffusely Positive for CD3. Immunohistochemical Stains Also Show a Subset of Cells Positive for CD30. CD20 Is Negative in Most of the Lymphocytes, Including the EBER-Positive Cells
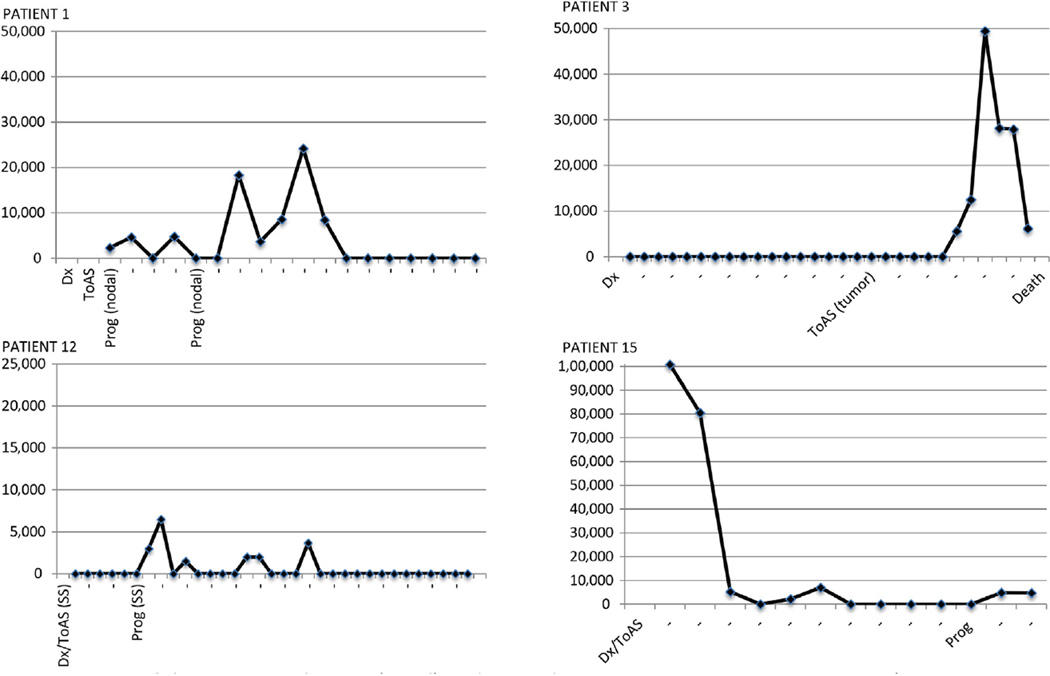
Finally, we explored the possible role of pEBVd as an indicator of disease activity. The display of longitudinal pEBVd measurements in a subset of patients (Figure 4 and Supplemental Figure 1 in the online version) shows that pEBVd corresponded to periods of disease activity, with detection around the time of progression and lack of detection during response, with a trend for higher pEBVd levels in the setting of blood and nodal involvement. This supports prospective study of pEBVd as a prognostic and predictive biomarker.
Figure 4.
Patterns of Plasma Epstein–Barr Virus DNA (pEBVd) Detection in Relation to Disease Activity In 4 Representative Patients. The X-Axis Is the Time From Diagnosis to Last Follow-Up. The Y-Axis Is pEBVd Copies/mL Measured Using quantitative real-time (qRT)-Polymerase Chain Reaction (Note the Different Y-Axis Ranges). In 14 of 17 Patients With Detectable pEBVd (pEBVd+) We Observed Similar Patterns of pEBVd Detection in These Examples. These 14 of 17 Patients All Had pEBVd >2000 Copies/mL. The Remaining 3 of 17 pEBVd+ Patients Had pEBVd Ranges of 1500 to 2000 Copies/mL and the Time of pEBVd Detection Did Not Track Disease Activity
Abbreviations: Dx = diagnosis; prog = time of progressive disease defined as measurable progression requiring change in therapy (type of progression in parentheses [ie, nodal, tumor stage, or to SS (Sezary syndrome)]); ToAS = time of progression to advanced stage.
Discussion
In the search for new prognostic markers in CTCL, we studied the frequency of plasma cell-free EBV-DNA (pEBVd) detection and association with outcomes in a cohort of AS CTCL patients. Our emphasis on pEBVd, as opposed to EBER, derives from the fact that peripheral blood is a more practical and adaptable biosample for longitudinal biomarker studies. An easily attainable prognostic marker provides opportunity to identify patients in transition from a period of indolent disease to an aggressive AS disease. The selection of plasma versus PBMC to measure EBV-DNA levels is justified by studies showing that the latter does not correlate well with EBER-ISH, disease burden, or prognosis in other EBV-associated neoplasms.14,44–46
In our retrospective study, we show that more than a third (N = 17, 37%) of the 46 patients in this AS CTCL cohort had increased levels of pEBVd and often at multiple time points (median, 2; range, 1–8). The detection of pEBVd was associated with worse OS from ToD (P = .021) and ToAS (P = .0098), differentiating prognosis independent of LDH and better than LCT. The difference in outcome for the 2 groups is remarkable: the 4-year OS from ToAS for pEBVd− patients was nearly double that of pEBVd+ patients (81% vs. 44.2%). We showed concordance between EBER and pEBVd, suggesting that detection of pEBVd reflects the presence of EBV in tumor tissue; in light of the availability of EBV-targeting therapies, pEBVd monitoring could have a significant clinical effect. To our knowledge, this is the largest study to analyze the association between EBV and AS CTCL and the first to longitudinally assess pEBVd. Despite the limitations of the 2-covariate model (LDH and LCT), our data suggest that pEBVd is a candidate as an independent prognostic marker in AS CTCL. Interestingly, the highest levels of pEBVd, and stronger EBER positivity, were observed in the setting of blood and nodal involvement and appeared to coincide with disease progression. pEBVd might, therefore be a marker of extracutaneous and visceral progression in CTCL and identify patients transitioning to an “accelerated” phase.
Our results advance the observations made by Novelli et al39 and offer some perspective on the conflicting reports about EBV and CTCL in the literature. The longitudinal analysis shows that pEBVd is a time-dependent covariate in CTCL and the level might be increased or undetectable at different time points in the same patient during the course of AS disease. A similar phenomenon was also observed by Kanakry et al in pEBVd+ Hodgkin’s lymphoma patients, where a small subset was negative at diagnosis but positive at 6 months.14 Thus, the acquisition time of blood and tissue samples from CTCL patients, in relation to stage, disease progression and therapy is important in determining whether EBV (EBER or pEBVd) will be detected. To our knowledge, this is also the first study to longitudinally measure pEBVd and pCMVd in AS CTCL in parallel. The main goal of the CMV analysis was to provide context to the pEBVd data by offering a surrogate view of immune surveillance against a related human herpesvirus in the same patient. Despite similarly high CMV seroprevalences in the pEBVd+ (71%) and pEBVd− (85%) subsets, most of the pEBVd+ patients did not have detectable pCMVd and only 8 (17%) patient in the entire cohort had increased levels of pCMVd, suggesting a more frequent association with EBV.
A number of weaknesses limit the value of this study to hypothesis generation. The lack of a standardized sampling schedule for pEBVd measurements prevents conclusive inferences about the relationship with EBER status and clinical end points. The multivariable Cox regression modeling of pEBVd as an independent prognostic factor was restricted to evaluation in 2-covariate models (adjusting for LDH and LCT). The significance of defining pEBVd status as a binary value (pEBVd+ vs. pEBVd−) on the basis of the detection of pEBVd above a CLIA-approved threshold at a single time point over a prolonged observation time can be questioned, although in most cases there was more than 1 occurrence. Lastly, all patients in this study were heavily pretreated, therefore we were not able to rigorously asses the effect of therapy on pEBVd and ascertain whether differences might exist in the frequency of pEBVd detection between AS CTCL patients and appropriate control subjects, including those with early stage CTCL. It should be noted, however, that even if pEBVd was to be detected in control groups, it would not disprove our current hypothesis, which is that pEBVd distinguishes subsets with different outcome within AS CTCL, and not that pEBVd is a specific marker for AS CTCL.
Although detection of increased pEBVd levels in AS CTCL might have prognostic value regardless of the specific mechanisms leading to its increase, we did not address the issue of latency and lineage of the EBV-infected cells, which are critical to understand if the virus is a true tumor marker or simply reflects lytic reactivation secondary to immune suppression. These questions cannot be addressed by the quantitative measurement of pEBVd alone. It is well known that in some EBV-associated cancers, detectable pEBVd (colloquially known as “viral load”) reflects a degree of lytic EBV reactivation and release of intact infectious particles, and in others it is predominantly because of shedding of cell-free, tumor-associated EBV-DNA fragments from latently infected, neoplastic cells. The former is typically seen with florid immune deficiency, as in post-transplant and HIV-associated lymphomas. The latter is seen in nasopharyngeal carcinoma and EBV-associated Hodgkin lymphoma. In many EBV-associated neoplasms, including CTCL and other T-/NK-cell lymphomas, the latency type, the cellular sources, and the process by which EBV-DNA is released into the plasma are unknown. Unfortunately, because of limitations of available biospecimens we were unable to investigate this further in our AS CTCL cohort.
In conclusion, pEBVd was detected in more than a third of AS CTCL patients and was associated with significantly worse survival, independent of LDH and LCT. Even if the prevalence and clinical effect of pEBVd detection are confirmed, a number of challenges remain. Although the association between lymphoma and EBV is relatively well understood in B-cell lymphomas, the role of EBV in hematologic neoplasms derived from cell types that are not the virus’ natural reservoir remains unclear.47 The rate of infection, the transcriptional programs, and the effect of EBV on the tumor microenvironment in T-/NK-cell lymphomas are unknown. In that regard, methodologies to address questions about latency and disease burden in the same biospecimen would represent an important innovation. Finally, it is possible that most, if not all, of the EBV-infected cells detected in T-/NK-cell lymphomas are B cells rather than T cells.48 Some have suggested that tumor-infiltrating B cells might have a role in sustaining the growth and survival of neoplastic T cells in CTCL; B cell depletion with anti-CD20 monoclonal antibodies was shown to have clinical benefit in a small number of patients.49 Although pEBVd and EBER status were not assessed in those patients, these observations highlight the value of characterizing the interplay between B cells, neoplastic T-cells, and EBV in the tumor microenvironment of T-/NK-cell lymphomas.
Conclusions
Improved risk stratification is needed in AS CTCL. Easily attainable biomarkers that define risk groups independent of stage and at the same time identify patients who might benefit from specific therapies would be ideal. The EBV causes B-cell lymphoma in immune-suppressed as well as in immune-competent patients, is the target of increasingly effective treatment strategies, and is found in a variable subset of patients with T-/NK-cell lymphomas. We observed that the level of pEBVd was measurably increased in approximately a third of patients with AS CTCL, was highly concordant with EBV (EBER) in tumor tissue (skin and lymph nodes), and was increased in active disease. Furthermore, survival was worse in the patients identified as being pEBVd+ independent of LDH and better than LCT. These data support further study of pEBVd as a potential biomarker for risk stratification, disease monitoring, and targeted therapy in AS CTCL.
Supplementary Material
Clinical Practice Points.
Although the prognosis of patients with AS CTCL is poor, compared with those with early stage disease, there is significant variability in survival. Easily attainable biomarkers that define risk groups independent of stage are needed.
A number of groups have looked for the presence of EBV in the skin and blood of patients with CTCL, mostly in small series, using different methodologies, and with conflicting results. Previous studies also suggest a worse survival in EBV+ patients and that detection of EBV-DNA is more common in patients with AS disease.
We observed that the level of pEBVd was increased in approximately a third of patients with AS-CTCL (ie, ≥IIB), and was highly concordant with EBV (EBER) in tumor tissue (skin and lymph nodes), and was increased during active disease.
Survival was twice as short in AS CTCL patients with increased levels of cell free plasma EBV-DNA and was independently prognostic compared with LDH and LCT.
These data support further study of pEBVd as a potential biomarker for risk stratification, disease monitoring, and targeted therapy in AS CTCL.
Acknowledgments
The study was funded through an NCI R21 (CA164911, Porcu/Wong) and received additional support through an intramural T-cell Lymphoma Research grant (Pelotonia Idea Grant) and philanthropic funding. Dr Haverkos was supported by an NIH/NCI T32 (T32CA165998) at the time the study was conducted.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Supplemental Data
Supplemental figures, tables, and Patients and Methods accompanying this article can be found in the online version at http://dx.doi.org/10.1016/j.clml.2016.02.014.
References
- 1.Wong HK, Mishra A, Hake T, Porcu P. Evolving insights in the pathogenesis and therapy of cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome) Br J Haematol. 2011;155:150–166. doi: 10.1111/j.1365-2141.2011.08852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CS, Ungewickell A, Bhaduri A, et al. Transcriptome sequencing in Sezary syndrome identifies Sezary cell and mycosis fungoides-associated lncRNAs and novel transcripts. Blood. 2012;120:3288–3297. doi: 10.1182/blood-2012-04-423061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 4.Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730–4739. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- 5.Talpur R, Singh L, Daulat S, et al. Long-term outcomes of 1,263 patients with mycosis fungoides and Sezary syndrome from 1982 to 2009. Clin Cancer Res. 2012;18:5051–5060. doi: 10.1158/1078-0432.CCR-12-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quaglino P, Pimpinelli N, Berti E, et al. Time course, clinical pathways, and long-term hazards risk trends of disease progression in patients with classic mycosis fungoides: a multicenter, retrospective follow-up study from the Italian Group of Cutaneous Lymphomas. Cancer. 2012;118:5830–5839. doi: 10.1002/cncr.27627. [DOI] [PubMed] [Google Scholar]
- 7.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 8.Boonk SE, Putter H, Koolhof L, Willemze R, Vermeer MH. Quantitation of tumour development correlates with prognosis in tumour stage (stage IIB) mycosis fungoides. Br J Dermatol. 2014;170:1080–1086. doi: 10.1111/bjd.12763. [DOI] [PubMed] [Google Scholar]
- 9.Scarisbrick JJ, Kim YH, Whittaker SJ, et al. Prognostic factors, prognostic indices and staging in mycosis fungoides and Sezary syndrome: where are we now? Br J Dermatol. 2014;170:1226–1236. doi: 10.1111/bjd.12909. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman PM. Virology. Epstein–Barr virus turns 50. Science. 2014;343:1323–1325. doi: 10.1126/science.1252786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezk SA, Weiss LM. Epstein–Barr virus-associated lymphoproliferative disorders. Hum Pathol. 2007;38:1293–1304. doi: 10.1016/j.humpath.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Kimura H, Ito Y, Suzuki R, Nishiyama Y. Measuring Epstein–Barr virus (EBV) load: the significance and application for each EBV-associated disease. Rev Med Virol. 2008;18:305–319. doi: 10.1002/rmv.582. [DOI] [PubMed] [Google Scholar]
- 14.Kanakry JA, Li H, Gellert LL, et al. Plasma Epstein–Barr virus DNA predicts outcome in advanced Hodgkin lymphoma: correlative analysis from a large North American cooperative group trial. Blood. 2013;121:3547–3553. doi: 10.1182/blood-2012-09-454694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung SF, Zee B, Ma BB, et al. Plasma Epstein–Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006;24:5414–5418. doi: 10.1200/JCO.2006.07.7982. [DOI] [PubMed] [Google Scholar]
- 16.Anagnostopoulos I, Hummel M, Kaudewitz P, Korbjuhn P, Leoncini L, Stein H. Low incidence of Epstein–Barr virus presence in primary cutaneous T-cell lymphoproliferations. Br J Dermatol. 1996;134:276–281. [PubMed] [Google Scholar]
- 17.Angel CA, Slater DN, Royds JA, Nelson SN, Bleehen SS. Absence of Epstein–Barr viral encoded RNA (EBER) in primary cutaneous t-cell lymphoma. J Pathol. 1996;178:173–175. doi: 10.1002/(SICI)1096-9896(199602)178:2<173::AID-PATH428>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Bonin S, Tothova SM, Barbazza R, Brunetti D, Stanta G, Trevisan G. Evidence of multiple infectious agents in mycosis fungoides lesions. Exp Mol Pathol. 2010;89:46–50. doi: 10.1016/j.yexmp.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Brice SL, Jester JD, Friednash M, et al. Examination of cutaneous T-cell lymphoma for human herpesviruses by using the polymerase chain reaction. J Cutan Pathol. 1993;20:304–307. doi: 10.1111/j.1600-0560.1993.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 20.de Bruin PC, Jiwa M, Oudejans JJ, et al. Presence of Epstein–Barr virus in extranodal T-cell lymphomas: differences in relation to site. Blood. 1994;83:1612–1618. [PubMed] [Google Scholar]
- 21.Dreno B, Celerier P, Fleischmann M, Bureau B, Litoux P. Presence of Epstein–Barr virus in cutaneous lesions of mycosis fungoides and Sezary syndrome. Acta Derm Venereol. 1994;74:355–357. doi: 10.2340/0001555574355357. [DOI] [PubMed] [Google Scholar]
- 22.Foulc P, N’Guyen JM, Dreno B. Prognostic factors in Sezary syndrome: a study of 28 patients. Br J Dermatol. 2003;149:1152–1158. doi: 10.1111/j.1365-2133.2003.05677.x. [DOI] [PubMed] [Google Scholar]
- 23.Hellier I, Dereure O, Segondy M, Guillot B, Baldet P, Guilhou JJ. Unlikely role of Epstein–Barr virus in the pathogenesis of primary cutaneous CD30+ anaplastic large cell lymphoma. Eur J Dermatol. 2001;11:203–208. [PubMed] [Google Scholar]
- 24.Kanavaros P, Ioannidou D, Tzardi M, et al. Mycosis fungoides: expression of C-myc p62 p53, bcl-2 and PCNA proteins and absence of association with Epstein–Barr virus. Pathol Res Pract. 1994;190:767–774. doi: 10.1016/S0344-0338(11)80423-1. [DOI] [PubMed] [Google Scholar]
- 25.Kim YC, Yang WI, Lee MG, et al. Epstein–Barr virus in CD30 anaplastic large cell lymphoma involving the skin and lymphomatoid papulosis in South Korea. Int J Dermatol. 2006;45:1312–1316. doi: 10.1111/j.1365-4632.2006.02951.x. [DOI] [PubMed] [Google Scholar]
- 26.Nagore E, Ledesma E, Collado C, Oliver V, Perez-Perez A, Aliaga A. Detection of Epstein–Barr virus and human herpesvirus 7 and 8 genomes in primary cutaneous T- and B-cell lymphomas. Br J Dermatol. 2000;143:320–323. doi: 10.1046/j.1365-2133.2000.03657.x. [DOI] [PubMed] [Google Scholar]
- 27.Noorali S, Yaqoob N, Nasir MI, Moatter T, Pervez S. Prevalence of mycosis fungoides and its association with EBV and HTLV-1 in Pakistanian patients. Pathol Oncol Res. 2002;8:194–199. doi: 10.1007/BF03032394. [DOI] [PubMed] [Google Scholar]
- 28.Peris K, Niedermeyer H, Cerroni L, Radaskiewicz T, Chimenti S, Hofler H. Detection of Epstein–Barr virus genome in primary cutaneous T and B cell lymphomas and pseudolymphomas. Arch Dermatol Res. 1994;286:364–368. doi: 10.1007/BF00371794. [DOI] [PubMed] [Google Scholar]
- 29.Peris K, Niedermeyer H, Chimenti S, Radaskiewicz T, Kerl H, Hoefler H. Detection of Epstein–Barr virus in cutaneous and lymph nodal anaplastic large cell lymphomas (Ki-1+) Br J Dermatol. 1995;133:542–546. doi: 10.1111/j.1365-2133.1995.tb02701.x. [DOI] [PubMed] [Google Scholar]
- 30.Su IJ, Tsai TF, Cheng AL, Chen CC. Cutaneous manifestations of Epstein–Barr virus-associated T-cell lymphoma. J Am Acad Dermatol. 1993;29:685–692. doi: 10.1016/0190-9622(93)70231-h. [DOI] [PubMed] [Google Scholar]
- 31.Suzushima H, Asou N, Fujimoto T, et al. Lack of the expression of EBNA-2 and LMP-1 in T-cell neoplasms possessing Epstein–Barr virus. Blood. 1995;85:480–486. [PubMed] [Google Scholar]
- 32.Shimakage M, Sasagawa T, Kawahara K, Yutsudo M, Kusuoka H, Kozuka T. Expression of Epstein–Barr virus in cutaneous T-cell lymphoma including mycosis fungoides. Int J Cancer. 2001;92:226–231. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1172>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Lee DY, Kim WS, Ko YH. Primary cutaneous Epstein–Barr virus-associated T-cell lymphoproliferative disorder-2 cases with unusual, prolonged clinical course. Am J Dermatopathol. 2010;32:832–836. doi: 10.1097/DAD.0b013e3181d68381. [DOI] [PubMed] [Google Scholar]
- 34.Borisch B, Boni J, Burki K, Laissue JA. Recurrent cutaneous anaplastic large cell (CD30+) lymphoma associated with Epstein–Barr virus. A case report with 9-year follow-up. Am J Surg Pathol. 1992;16:796–801. doi: 10.1097/00000478-199208000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Mouly F, Baccard M, Rybojad M, Lebbe C, Morinet F, Morel P. Aggressive cutaneous T-cell lymphoma associated with the presence of Epstein–Barr virus. 2 cases [in French] Ann Dermatol Venereol. 1996;123:574–576. [PubMed] [Google Scholar]
- 36.Cho KH, Kim CW, Lee DY, Sohn SJ, Kim DW, Chung JH. An Epstein–Barr virus-associated lymphoproliferative lesion of the skin presenting as recurrent necrotic papulovesicles of the face. Br J Dermatol. 1996;134:791–796. [PubMed] [Google Scholar]
- 37.Tournadre A, D’Incan M, Dubost JJ, et al. Cutaneous lymphoma associated with Epstein–Barr virus infection in 2 patients treated with methotrexate. Mayo Clin Proc. 2001;76:845–848. doi: 10.1016/S0025-6196(11)63231-X. [DOI] [PubMed] [Google Scholar]
- 38.Erkek E, Sahin S, Atakan N, Kocagoz T, Olut A, Gokoz A. Examination of mycosis fungoides for the presence of Epstein–Barr virus and human herpesvirus-6 by polymerase chain reaction. J Eur Acad Dermatol Venereol. 2001;15:422–426. doi: 10.1046/j.1468-3083.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- 39.Novelli M, Merlino C, Ponti R, et al. Epstein–Barr virus in cutaneous T-cell lymphomas: evaluation of the viral presence and significance in skin and peripheral blood. J Invest Dermatol. 2009;129(6):1556–1561. doi: 10.1038/jid.2008.396. [DOI] [PubMed] [Google Scholar]
- 40.Weaver J, Mahindra AK, Pohlman B, Jin T, Hsi ED. Non-mycosis fungoides cutaneous T-cell lymphoma: reclassification according to the WHO-EORTC classification. J Cutan Pathol. 2010;37:516–524. doi: 10.1111/j.1600-0560.2010.01526.x. [DOI] [PubMed] [Google Scholar]
- 41.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 42.Ritchie D, Piekarz RL, Blombery P, et al. Reactivation of DNA viruses in association with histone deacetylase inhibitor therapy: a case series report. Haematologica. 2009;94:1618–1622. doi: 10.3324/haematol.2009.008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skoetz N, Bauer K, Elter T, et al. Alemtuzumab for patients with chronic lymphocytic leukaemia. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD008078.pub2. CD008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu CL, Chan SC, Chang KP, et al. Clinical scenario of EBV DNA follow-up in patients of treated localized nasopharyngeal carcinoma. Oral Oncol. 2013;49:620–625. doi: 10.1016/j.oraloncology.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Wang H, Wang JH, et al. Post-treatment plasma EBV-DNA positivity predicts early relapse and poor prognosis for patients with extranodal NK/T cell lymphoma in the era of asparaginase. Oncotarget. 2015;6:30317–30326. doi: 10.18632/oncotarget.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang ZY, Liu QF, Wang H, et al. Clinical implications of plasma Epstein–Barr virus DNA in early-stage extranodal nasal-type NK/T-cell lymphoma patients receiving primary radiotherapy. Blood. 2012;120:2003–2010. doi: 10.1182/blood-2012-06-435024. [DOI] [PubMed] [Google Scholar]
- 47.George LC, Rowe M, Fox CP. Epstein-barr virus and the pathogenesis of T and NK lymphoma: a mystery unsolved. Curr Hematol Malig Rep. 2012;7:276–284. doi: 10.1007/s11899-012-0136-z. [DOI] [PubMed] [Google Scholar]
- 48.Dupuis J, Emile JF, Mounier N, et al. Prognostic significance of Epstein–Barr virus in nodal peripheral T-cell lymphoma, unspecified: A Groupe d’Etude des Lymphomes de l’Adulte (GELA) study. Blood. 2006;108:4163–4169. doi: 10.1182/blood-2006-04-017632. [DOI] [PubMed] [Google Scholar]
- 49.Theurich S, Schlaak M, Steguweit H, et al. Targeting tumor-infiltrating B cells in cutaneous T-cell lymphoma. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.50.9471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.