Abstract
Objective:
To determine the extent to which deficits associated with autism spectrum disorder (ASD) in toddlers with tuberous sclerosis complex (TSC) overlap with those in toddlers with nonsyndromic ASD (nsASD) and to examine cognitive function and epilepsy severity in toddlers with TSC and comorbid ASD. This is the endpoint analysis from a longitudinal investigation of ASD risk factors in children with TSC.
Methods:
Measures included the Autism Diagnostic Observation Schedule (ADOS), the Mullen Scales of Early Learning, and clinical epilepsy variables. A repeated-measures analysis of variance was performed with between-subjects factor of group (typically developing, TSC/no ASD, TSC/ASD, nsASD) and within-subjects factors of individual ADOS item scores in the social communication and repetitive behavior/restricted interest domains. Within the TSC group, comparisons of epilepsy characteristics and cognitive domains were performed using independent-samples t tests.
Results:
Children with TSC/ASD demonstrated a profile of social communication impairment that had complete convergence with nsASD. Measured social communication impairments included gestures, pointing, eye contact, responsive social smile, and shared enjoyment. This convergence was observed despite the high comorbidity between ASD and cognitive impairment in TSC.
Conclusions:
This study supports the clinical diagnosis of ASD in young children with TSC and demonstrates remarkable convergence of autism symptoms between TSC/ASD and nsASD. Our results strongly suggest the need for early intervention in toddlers with TSC, with treatment strategies targeting social communication function as well as broader developmental domains, before the onset of autism symptoms.
Tuberous sclerosis complex (TSC) is an autosomal dominant genetic disorder resulting from a TSC1/TSC2 mutation, leading to widespread growth of hamartomas in multiple organ systems, including the brain.1 Children with TSC are at high risk for neuropsychiatric syndromes that include developmental delay, intellectual disability (ID), mood disorders, and autism spectrum disorder (ASD), collectively referred to as TSC-associated neurocognitive deficits.2,3 In fact, TSC is one of the most commonly occurring single-gene disorders associated with ASD.4–6 Given the high rate of ASD in TSC and the fact that TSC is often diagnosed in utero or in early infancy,7 TSC has been considered a model system for understanding mechanisms underlying ASD.8–11 However, the specific phenotypic profile of ASD in TSC and the extent to which it converges with nonsyndromic ASD (nsASD) has not been well-established.12,13
The current study, which represents the endpoint analysis from a longitudinal investigation of autism risk factors in children with TSC,14 had 2 primary aims: first, to determine the extent to which deficits associated with ASD in TSC overlap with those in toddlers with nsASD by examining item-level symptom profiles from a play-based diagnostic measure; and second, to examine cognitive profiles and epilepsy characteristics of toddlers with TSC and ASD. Given our findings of nonverbal cognitive slowing predicting ASD in children with TSC, we hypothesized that a distinctive autism symptom profile would emerge in TSC that would differentiate these toddlers behaviorally from those with nsASD. Moreover, we hypothesized that children with TSC and ASD would demonstrate greater epilepsy severity and greater cognitive impairment than those without ASD.
METHODS
Procedures.
The data from the TSC and typically developing (TD) children are gathered from a longitudinal study of behavioral and electrophysiologic characteristics in children with and without TSC, aged 3–36 months. Recruitment and testing were performed at the University of California, Los Angeles (UCLA) Center for Autism Research and Treatment and Boston Children's Hospital (BCH) Laboratories of Cognitive Neuroscience. Data from the ASD cohort were collected from a study of toddlers with nsASD through the UCLA Autism Center of Excellence (PI Kasari). Participants were recruited from an early intervention (EI) program prior to the start of intervention. Parents were provided with a consent form if their child was younger than 36 months, had a clinical diagnosis of ASD confirmed by independent testers with the Autism Diagnostic Interview–Revised15 and the Autism Diagnostic Observation Schedule (ADOS),16 had no significant physical disabilities, and parent and child were available for follow-up assessments. All assessments were administered prior to the onset of intervention.
Standard protocol approvals, registrations, and patient consents.
For the TSC and TD samples, institutional review board (IRB) approval was obtained from the UCLA and BCH sites (UCLA IRB no. 11-002349; BCH IRB no. P00001144). All families gave informed consent before participation. The nsASD sample signed consents for their data to be used for future research (UCLA IRB no. 11-000032).
Participants.
TSC diagnosis was based on clinical presentation. Genetics reports were available for 31/44 (70%) children. TD exclusion criteria included prematurity, birth trauma, developmental concerns, or immediate family history of neurodevelopmental disorders. The mean age of the nsASD cohort (n = 82 months, mean age 31.4 months, range 19–36 months) did not differ from the mean age of the TSC cohort (n = 44 months, mean age 32.1 months, range 23–39 months, p = 0.44), while the TD cohort (n = 18 months, mean age 28.7 months, range 24–37 months) was significantly younger (p = 0.02 and p < 0.01, respectively). There were significant differences in sex, with the ASD cohort being 83% male, the TSC cohort being 62% male, and the TD cohort being 39% male (χ2 = 16.0, p < 0.01).
Epilepsy data.
Data regarding epilepsy were gathered from interim medical questionnaires from parents and review of medical records when available. Given the variability in clinical epilepsy data available across studies, we drew from a standardized measure of epilepsy severity, The Early Childhood Epilepsy Severity Scale, which has been used to quantify epilepsy severity in children with TSC.17 Variables included age at seizure onset, number and identity of antiepileptic drugs, frequency of seizures (daily, weekly, monthly), treatment response (fully controlled, partially controlled, refractory), and presence of infantile spasms. Epilepsy data were gathered at each assessment time point.
Behavioral testing.
TSC and TD.
The Mullen Scales of Early Learning (MSEL) is a standardized cognitive measure for children 0–69 months of age, testing gross motor, fine motor (FM), visual reception (VR), receptive language (RL), and expressive language (EL) function. Raw scores were converted to age-standardized T scores, which facilitates the distinction between normative and non-normative development. A developmental quotient (DQ) was also calculated based on an average of the FM, VR, RL and EL scores.18
The ADOS is a semi-structured, play-based assessment with standardized probes and scoring for social interaction, communication, repetitive behaviors, and play. The ADOS has been demonstrated to have excellent interrater reliability among formally trained examiners.19 ASD diagnoses were determined on the basis of children meeting criteria for ASD on the ADOS using the revised algorithm along with clinical judgment.20
Statistical methods and results.
For the first set of analyses, 4 groups were identified: TSC/ASD (n = 18), TSC/no ASD (n = 18), nsASD (n = 82), and TD (n = 16).
Cognitive measures.
Repeated-measures analysis of variance (ANOVA) was performed, with the between-subjects factor of group (TSC/no ASD, TSC/ASD, nsASD, TD) and within-subjects factor of cognitive domain (FM, VR, RL, EL).
ADOS profile analysis.
We performed a repeated-measures ANOVA with the between-subjects factor of group and within-subjects factors of each ADOS item in the social communication and repetitive behavior/restricted interest domains. Post hoc analyses were performed by comparing mean ADOS scores and item × group interaction between all pairs. The items included in the ADOS included the Reciprocal Social Interaction items and the Restricted and Repetitive Behaviors items.
TSC/ASD clinical characteristics.
For the TSC-specific analyses, only 2 groups were compared: TSC/ASD vs TSC/no ASD. Independent-samples t tests were performed to compare groups on MSEL domain T scores (FM, VR, EL, RL) and overall standard score (DQ). Independent-samples t tests were used to compare groups on continuous variables including age at onset of seizures and number of antiepileptics; χ2 tests were used to compare groups based on categorical variables: history of active spasms, frequency of seizures (daily, weekly, monthly), and treatment response (controlled/no seizures, partially controlled/reduced seizure frequency, refractory/no change in seizure frequency).
RESULTS
Cognitive function.
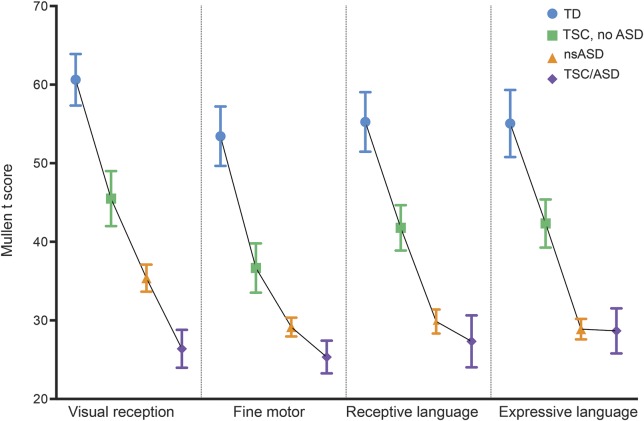
Data from 16 TD, 82 ASD, and 36 TSC were collected. Of the 36 toddlers with TSC, 18 (50%) met criteria for ASD. The mean age of each group was as follows: TD: 29.2 months (SD 6.1); nsASD: 31.4 months (SD 3.2); TSC/no ASD: 34.6 months (SD 3.9); and TSC/ASD: 32.0 months (SD 5.8). In a profile analysis of the 4 groups, there were main effects of cognitive domain (F3,390 = 8.3, p < 0.01) and group (F3,130 = 26.8, p < 01) but no group × domain interaction (F9,390 = 1.6, p = 0.11), suggesting that while the overall level of cognition differed among the 4 groups, there was no unique profile of cognitive impairment associated with each group. Post hoc analyses of the group effect showed that while the estimated marginal mean of the cognition scores of the TD group (EMM 56.1) was significantly higher than any of the other groups (TSC/no ASD: EMM: 41.6, p < 0.01; nsASD: EMM: 30.8, p < 0.01; and TSC/ASD: EMM: 26.9, p < 0.01), and TSC/no ASD was significantly higher than nsASD (p < 0.01) and TSC/ASD (p < 0.01), there was no significant difference in cognition between the nsASD and the TSC/ASD cohort (p = 0.19) (figure 1).
Figure 1. Cognitive domains across 4 groups: Typically developing (TD), tuberous sclerosis complex (TSC)/no autism spectrum disorder (ASD), nonsyndromic ASD (nsASD), and TSC/ASD.
Mullen T scores by ASD groupings in TSC, TD children, and nonsyndromic ASD.
Autism features.
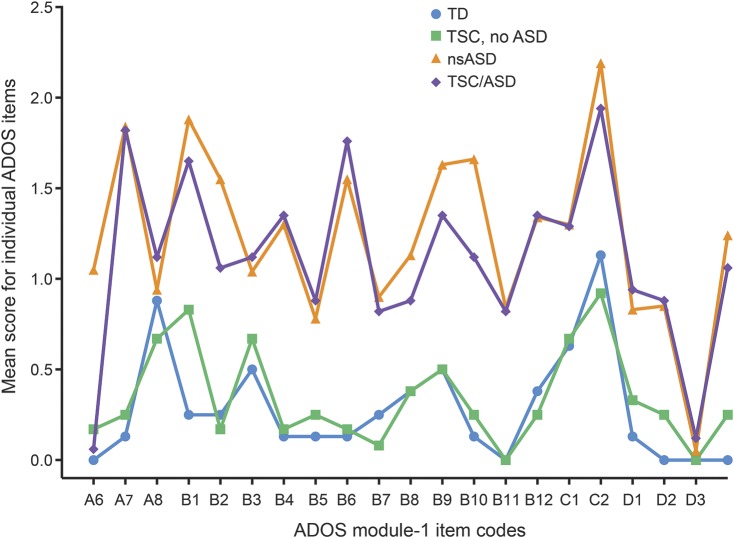
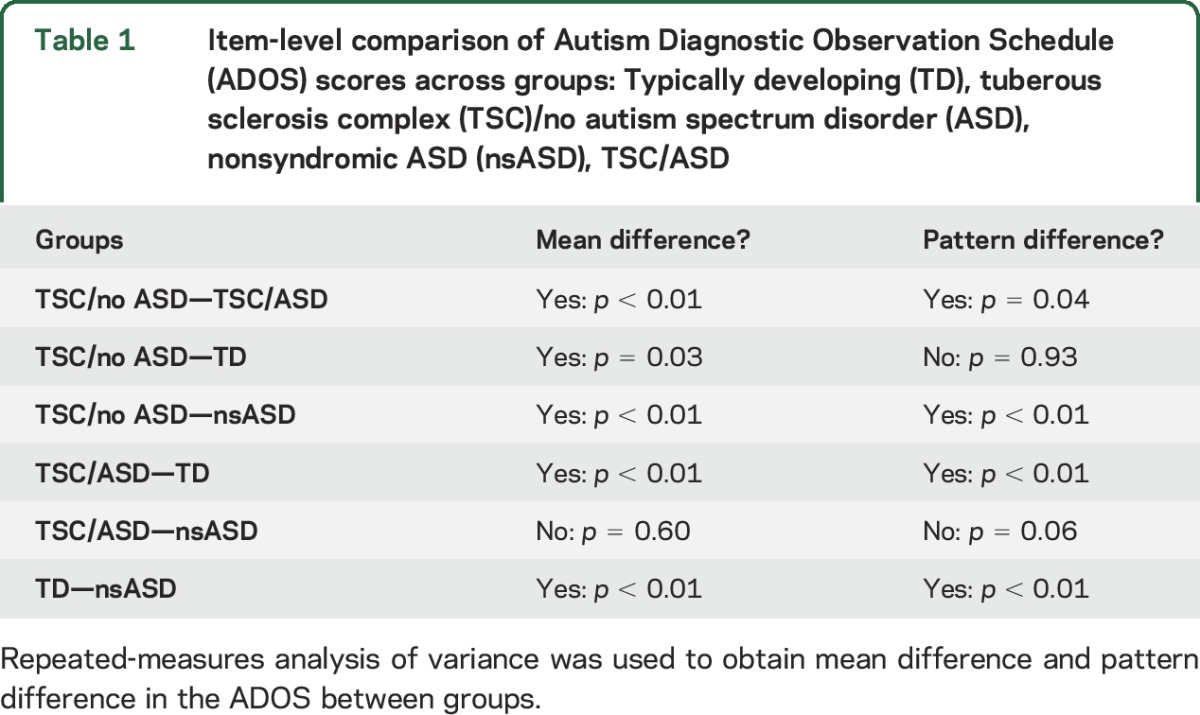
The ANOVA was performed controlling for DQ. Only participants with both a DQ score and an ADOS module 1 were included, yielding a sample of 8 TD, 81 nsASD, 12 TSC/no ASD, and 17 TSC/ASD. There were significant differences in DQ among these 4 subsets of participants (F3,114 = 8.8, p < 0.01; TD: mean 99.6, SD 21.6; nsASD: mean 68.6, SD 20.6; TSC/no ASD: mean 73.8, SD 15.0; and TSC/ASD: mean 57.4, SD 14.23). There was a significant main effect of group (F3,121 = 34.06, p < 0.0001) and a factor × group interaction (F3,121 = 33.6, p < 0.001) (figure 2). Post hoc analyses (table 1) revealed that these group and group × item effects were driven by a difference between TSC/ASD and TSC/no ASD, TSC/ASD and TD, and nsASD and TD. There were no significant differences between nsASD and ASD/TSC in the mean or pattern of ADOS scores and no significant differences between TD and TSC/no ASD (figure 2 and table 1). See figure e-1 on the Neurology® Web site at Neurology.org for the range in item scores by group.
Figure 2. Item-level comparison of Autism Diagnostic Observation Schedule (ADOS) scores across 4 groups: Typically developing (TD), tuberous sclerosis complex (TSC)/no autism spectrum disorder (ASD), nonsyndromic ASD (nsASD), and TSC/ASD.
Item-level ADOS profiles for each group: TD, TSC/no ASD, nsASD, TSC/ASD.
Table 1.
Item-level comparison of Autism Diagnostic Observation Schedule (ADOS) scores across groups: Typically developing (TD), tuberous sclerosis complex (TSC)/no autism spectrum disorder (ASD), nonsyndromic ASD (nsASD), TSC/ASD
TSC/ASD characterization.
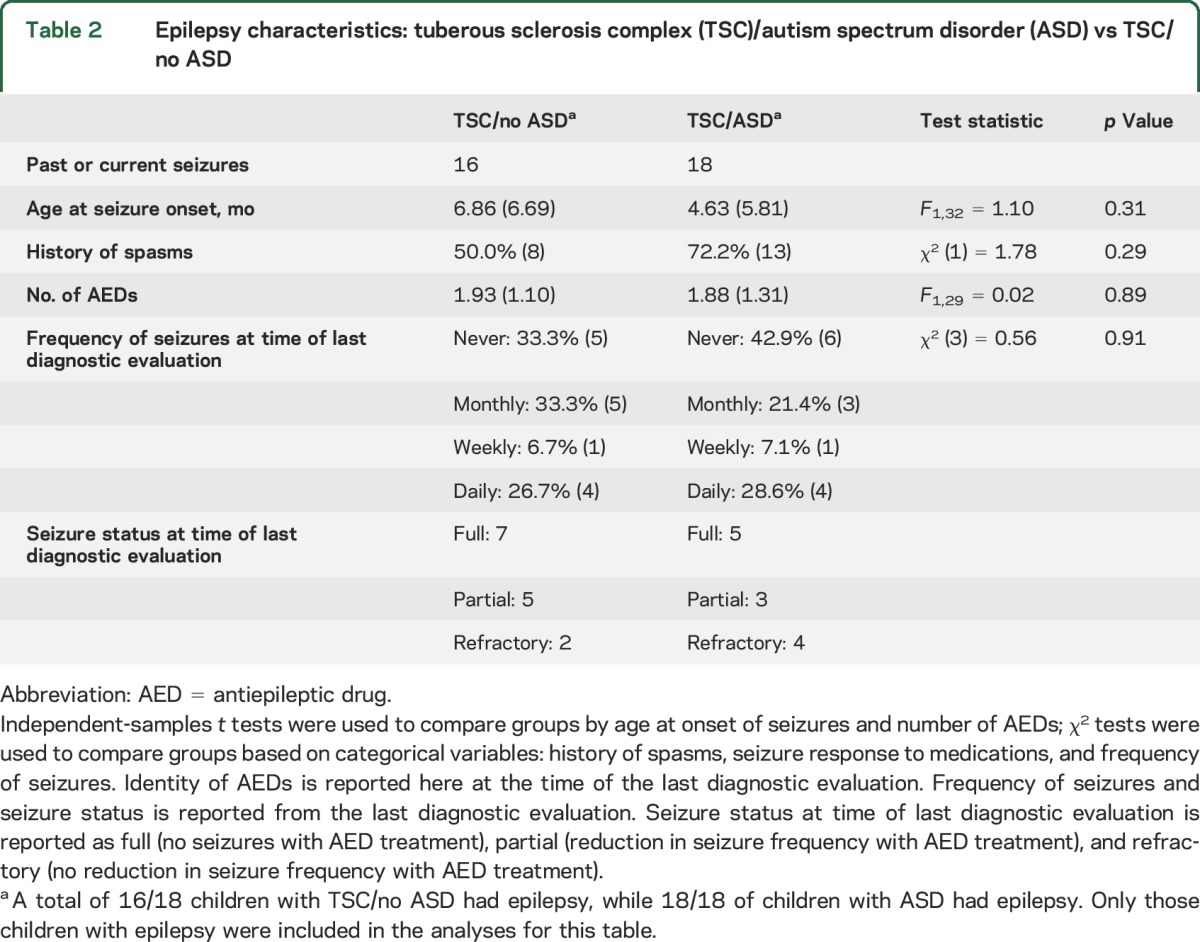
Of the children with TSC with ASD, 68.2% (n = 15) had genetic testing; of those, 73.3% (n = 11) had mutations in the TSC2 gene, and 26.7% (n = 4) had mutations in the TSC1 gene. Of the TSC/no ASD children, 68.2% (n = 16) had genetic testing; of those, 93.7% (n = 15) had mutations in the TSC2 gene, and 6.3% (n = 1) had mutations in the TSC1 gene. There were significant differences in all MSEL domain scores between ASD and no ASD in TSC, with the ASD group showing significantly more impairment across domains (see figure e-2). There were no significant differences in epilepsy characteristics between groups (see table 2 and table e-1).
Table 2.
Epilepsy characteristics: tuberous sclerosis complex (TSC)/autism spectrum disorder (ASD) vs TSC/no ASD
DISCUSSION
We examined symptom profiles of ASD in toddlers with TSC in order to determine if (1) there were distinctive social communication profiles that characterized this genetic syndrome, (2) global developmental delay would confound the identification of autism symptoms, and (3) epilepsy severity differed based on ASD diagnosis. TSC has long been considered an ideal model to study the development of ASD, not only because of the high prevalence of ASD in TSC but also because TSC is often diagnosed prior to the onset of atypical development. ASD is a behavioral, not etiologic, diagnosis based on core deficits in social communication function and the presence of repetitive behaviors and restricted interests. With advances in molecular diagnostic methods, an increasing number of genetic etiologies have been identified that lead to an ASD diagnosis, with up to 20% of children with ASD diagnosed with a causative genetic variant.21 However, few studies have examined whether nosologically distinct syndromes demonstrate convergence in autism symptoms, particularly in comparison to children with nsASD. Were we to identify behaviors that may distinguish specific syndromes, we would be able to develop more targeted intervention strategies that are, in fact, rooted in etiologic subgroups and not simply based on diagnostic classifications.
In our prospective study of children with TSC, we found that developmental slowing in nonverbal cognition predicted ASD, highlighting the strong comorbidity of developmental delay and ASD in TSC,14 and raising the question of whether ASD in TSC actually reflects a more global cognitive impairment rather than specific deficits in social communication. To examine social communication profiles, we used the ADOS, a standardized play-based assessment, with a calibrated severity score metric.20 The ADOS is a play-based measure of social communication skills that takes age and language level into account in the quantification of discrete skills, such as pointing, gestures, eye contact, and shared enjoyment. While not required for diagnostic purposes in the clinical setting, the ADOS is widely used by researchers and clinicians because of its reliability and ability to detect impairments that may be missed in a diagnostic interview or a brief clinical evaluation. ASD diagnosis on the ADOS results from a threshold score, but this threshold can be reached through a wide range of item-level scores. Therefore, the ADOS has the potential to detect subtle differences in behaviors across the spectrum of autism.
Fifty percent of our TSC cohort met criteria for ASD, consistent with prior studies in TSC.5,14 Results revealed that children with TSC and ASD demonstrate a profile of social communication impairment that, at the behavioral level, was virtually identical to that of children with nsASD; indeed, there were no individual markers that distinguished the 2 groups. This convergence was observed in the context of high comorbidity between ASD and cognitive impairment in both the TSC/ASD and nsASD groups. The comorbidity of ASD in TSC has been well-established in retrospective,22–24 prospective,5,14,25 and meta-analytic6 studies. To date, most studies in TSC have focused on older age groups, treating ASD as a categorical outcome for classification, and they have relied on parent report for diagnoses, which can be limited in a child with multiple medical challenges that may obscure the parent's attention to subtle developmental manifestations. Our results reinforce the utility of the ADOS as a diagnostic measure for ASD in TSC, as it does differentiate groups based on ASD diagnosis. The striking convergence of autism symptoms in TSC/ASD and nsASD despite the variability in pathways to those deficits suggests either that the mechanisms underlying social communication delays may not lead to the expression of distinct behavioral phenotypes (in other words, these behaviors are truly “final common pathways”) or that behavioral measures alone may not capture subtle, yet meaningful, distinctions in abilities that may further guide intervention strategies and treatment targets. It is also possible that at this early developmental stage, when diagnoses are just being made, the range of potential social and communicative behaviors is narrow, and so symptoms do not differentiate etiologic subtypes. The differentiation may occur later in development, as comorbidities emerge and the effect of ID on later development becomes more pronounced.
However, contrary to that hypothesis, there exist both neural and behavioral data in older children that point toward ongoing convergence between TSC/ASD and nsASD, potentially suggestive of a final common pathway to social communication impairments. Using EEG, common alterations in network topology have been found in TSC/ASD and nsASD, marked by reduced long- over short-range coherence in individuals across a wide age range (infancy through to young adulthood).26 At the behavioral level, there is a higher degree of convergence between parent-reported ASD symptomatology in children (at approximately 10 years of age) with TSC and nsASD than other high-risk groups (such as Down syndrome and Klinefelter syndrome).27 Our findings extend this line of research to show item-level convergence based on direct observation in social communication deficits between TSC/ASD and nsASD within the first 3 years of life.
We also directly examined epilepsy and cognitive function in toddlers with TSC who did and did not meet criteria for ASD in order to highlight potential pathways contributing to the manifestation of ASD in TSC. There exists a rich literature supporting the comorbidity of epilepsy and neurodevelopmental disorders in TSC.2,4,28 In particular, early seizure onset and the presence of infantile spasms have been strongly associated with neurodevelopmental and cognitive impairment, leading to the question of the temporal relationship of epilepsy and neurodevelopmental disorders in TSC.13 While there was a trend towards greater epilepsy burden in the TSC/ASD group, based on higher rates of infantile spasms and earlier age at seizure onset, the results were not statistically significant. In part this lack of significance may reflect small sample size. The absence of a significant difference also may be rooted in the fact that most children had an epilepsy diagnosis, therefore limiting the variability of the epilepsy characteristics. Most likely, these data support the contention that more refined measures of epilepsy and underlying neurophysiologic status are required to understand the relationship between epilepsy and ASD. They also highlight the fact that other neurobiological factors (such as tuber burden, connectivity, or genetic background) likely contribute to the neurodevelopmental sequelae of TSC.
Finally, the TSC/no ASD group requires further consideration. Despite demonstrating similar epilepsy burden, these toddlers maintained higher cognitive skills than either of the 2 ASD groups and demonstrated typical social communication symptom profiles. The nature of the longitudinal study design and early recruitment may select for higher functioning children who may otherwise go unmonitored by their clinicians. This cohort affords a unique and valuable opportunity to identify the behavioral or neurobiological factors (such as genetic background), and resulting compensatory mechanisms, that may distinguish these children early in infancy and may explain their fairly preserved neurodevelopmental function. Such insight could inform strategies to improve outcomes in those children without these protective or compensatory factors.
Our results reinforce the need for developmental screening of all children with TSC, with particular attention to features of ASD within the first 3 years of life. Moreover, these findings necessitate a study of EI in toddlers with TSC, with treatment strategies targeting social communication function, with a direct comparison of outcomes from various autism interventions and, perhaps, across subgroups within the autism spectrum. Given the high rate of ASD and the emergence of cognitive delays in the first 2 years of life, intervention studies may need to take a tiered approach, with an initial goal of supporting global developmental domains followed by more targeted, developmentally informed approaches that focus on social communication skills, prior to the onset of autism symptoms.
Supplementary Material
GLOSSARY
- ADOS
Autism Diagnostic Observation Schedule
- ANOVA
analysis of variance
- ASD
autism spectrum disorder
- BCH
Boston Children's Hospital
- DQ
developmental quotient
- EI
early intervention
- EL
expressive language
- EMM
estimated marginal mean
- FM
fine motor
- ID
intellectual disability
- IRB
institutional review board
- MSEL
Mullen Scales of Early Learning
- nsASD
nonsyndromic autism spectrum disorder
- RL
receptive language
- TD
typically developing
- TSC
tuberous sclerosis complex
- UCLA
University of California, Los Angeles
- VR
visual reception
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Jeste was primarily responsible for study design, supervision of data acquisition, behavioral and clinical data analysis, and manuscript preparation. Dr. Varcin was actively involved in processing and analysis of clinical data and manuscript preparation and revisions. Dr. Hellemann was primarily responsible for statistical analysis of data and contributed to manuscript preparation. Dr. Gulsrud was actively involved in data collection, processing and analysis of clinical data, and manuscript preparation and revisions. Dr. Bhatt was actively involved with data analysis and manuscript preparation and revisions. Dr. Wu was involved with study design, analysis of the clinical data, and manuscript preparation and revisions. Dr. Sahin was involved with study design and manuscript preparation. Dr. Nelson was primary responsible for study design, study implementation, data analysis, and manuscript preparation and revisions.
STUDY FUNDING
Supported by Department of Defense (DOD CDMRP TSCRP: 2011–2014) and UCLA CTRC UL1TR000124.
DISCLOSURE
S. Jeste serves as a consultant for Roche Pharmaceuticals and on the professional advisory board for the Tuberous Sclerosis Alliance. K. Varcin, G. Hellemann, A. Gulsrud, R. Bhatt, and C. Kasari report no disclosures relevant to the manuscript. J. Wu serves on the professional advisory board for the Tuberous Sclerosis Alliance; and has received honoraria from and serves on the scientific advisory board and the speakers' bureau for Novartis Pharmaceuticals Inc. and Lundbeck. M. Sahin receives research support from Novartis and Shire and is on the Scientific Advisory Board of Sage Therapeutics. Dr. Nelson reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Crino PB. Evolving neurobiology of tuberous sclerosis complex. Acta Neuropathol 2013;125:317–332. [DOI] [PubMed] [Google Scholar]
- 2.Curatolo P, Moavero R, de Vries PJ. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol 2015;14:733–745. [DOI] [PubMed] [Google Scholar]
- 3.de Vries PJ, Whittemore VH, Leclezio L, et al. . Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND checklist. Pediatr Neurol 2015;52:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton PF, Park RJ, Higgins JN, Griffiths PD, Pickles A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain 2002;125:1247–1255. [DOI] [PubMed] [Google Scholar]
- 5.Jeste SS, Sahin M, Bolton P, Ploubidis GB, Humphrey A. Characterization of autism in young children with tuberous sclerosis complex. J Child Neurol 2008;23:520–525. [DOI] [PubMed] [Google Scholar]
- 6.Richards C, Jones C, Groves L, Moss J, Oliver C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry 2015;2:909–916. [DOI] [PubMed] [Google Scholar]
- 7.Datta AN, Hahn CD, Sahin M. Clinical presentation and diagnosis of tuberous sclerosis complex in infancy. J Child Neurol 2008;23:268–273. [DOI] [PubMed] [Google Scholar]
- 8.Bateup HS, Johnson CA, Denefrio CL, Saulnier JL, Kornacker K, Sabatini BL. Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron 2013;78:510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tye C, Bolton P. Neural connectivity abnormalities in autism: insights from the Tuberous Sclerosis model. BMC Med 2013;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai PT, Hull C, Chu Y, et al. . Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 2012;488:647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis PE, Peters JM, Krueger DA, Sahin M. Tuberous sclerosis: a new frontier in targeted treatment of autism. Neurotherapeutics 2015;12:572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res 2009;53:852–873. [DOI] [PubMed] [Google Scholar]
- 13.van Eeghen AM, Pulsifer MB, Merker VL, et al. . Understanding relationships between autism, intelligence, and epilepsy: a cross-disorder approach. Dev Medicine Child Neurol 2013;55:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeste SS, Wu JY, Senturk D, Varcin K, et al. . Early developmental trajectories associated with ASD in infants with tuberous sclerosis complex. Neurology 2014;83:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lord C, Storoschuk S, Rutter M, Pickles A. Using the ADI-R to diagnose autism in preschoolers. Infant Ment Health J 1993;14:234–252. [Google Scholar]
- 16.Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Services; 2001. [Google Scholar]
- 17.Humphrey A, Ploubidis GB, Yates JR, Steinberg T, Bolton PF. The Early Childhood Epilepsy Severity Scale (E-Chess). Epilepsy Res 2008;79:139–145. [DOI] [PubMed] [Google Scholar]
- 18.Mullen EM. Mullen Scales of Early Learning: AGS Edition. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 19.Lord C, Risi S, Lambrecht L, et al. . The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205–223. [PubMed] [Google Scholar]
- 20.Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord 2007;37:613–627. [DOI] [PubMed] [Google Scholar]
- 21.Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol 2014;10:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prather P, de Vries P. Behavioral and cognitive aspects of tuberous sclerosis complex. J Child Neurol 2004;19:666–674. [DOI] [PubMed] [Google Scholar]
- 23.de Vries PJ, Hunt A, Bolton PF. The psychopathologies of children and adolescents with tuberous sclerosis complex (TSC): a postal survey of UK families. Eur J Child Adolesc Psychiatry 2007;16:16–24. [DOI] [PubMed] [Google Scholar]
- 24.Curatolo P, Napolioni V, Moavero R. Autism spectrum disorders in tuberous sclerosis: pathogenetic pathways and implications for treatment. J Child Neurology 2010;25:873–880. [DOI] [PubMed] [Google Scholar]
- 25.Humphrey A, Williams J, Pinto E, Bolton PF. A prospective longitudinal study of early cognitive development in tuberous sclerosis: a clinic based study. Eur J Child Adolesc Psychiatry 2004;13:159–165. [DOI] [PubMed] [Google Scholar]
- 26.Peters JM, Taquet M, Vega C, et al. . Brain functional networks in syndromic and non-syndromic autism: a graph theoretical study of EEG connectivity. BMC Med 2013;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruining H, Eijkemans MJ, Kas MJ, Curran SR, Vorstman JA, Bolton PF. Behavioral signatures related to genetic disorders in autism. Mol Autism 2014;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joinson C, O'Callaghan FJ, Osborne JP, Martyn C, Harris T, Bolton PF. Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis complex. Psychol Med 2003;33:335–344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.