Abstract
Immunoglobulin G subclass deficiency (IgGSCD) is a relatively common primary immunodeficiency disease (PI) in adults. The biological significance of IgGSCD in patients with chronic airway diseases is controversial. We conducted a retrospective study to characterize the clinical features of IgGSCD in this population. This study examined the medical charts from 59 adult patients with IgGSCD who had bronchial asthma or chronic obstructive pulmonary disease (COPD) from January 2007 to December 2012. Subjects were classified according to the 10 warning signs developed by the Jeffrey Modell Foundation (JMF) and divided into two patient groups: group I (n = 17) met ≥ two JMF criteria, whereas group II (n = 42) met none. IgG3 deficiency was the most common subclass deficiency (88.1%), followed by IgG4 (15.3%). The most common infectious complication was pneumonia, followed by recurrent bronchitis, and rhinosinusitis. The numbers of infections, hospitalizations, and exacerbations of asthma or COPD per year were significantly higher in group I than in group II (P < 0.001, P = 0.012, and P < 0.001, respectively). The follow-up mean forced expiratory volume (FEV1) level in group I was significantly lower than it was at baseline despite treatment of asthma or COPD (P = 0.036). In conclusion, IgGSCD is an important PI in the subset of patients with chronic airway diseases who had recurrent upper and lower respiratory infections as they presented with exacerbation-prone phenotypes, decline in lung function, and subsequently poor prognosis.
Keywords: Asthma, Chronic Obstructive Lung Diseases, IgG Subclass Deficiency, Respiratory Tract Infection
Graphical Abstract
INTRODUCTION
Asthma and chronic obstructive pulmonary disease (COPD) are the most common chronic airway diseases worldwide. They differ from each other in their patterns of inflammation, immunological mechanisms, and the extent of reversible airflow limitations (1). However, exacerbations of both asthma and COPD result in significant morbidity and healthcare costs and, in some cases, death. Upper respiratory infections are known as factors that trigger acute exacerbations, with viruses as the primary causative agents (2). In addition, bacterial infections are important factors in the exacerbation of COPD; such infections can act as the primary cause of lower respiratory infections or result from secondary complications of viral infections (3).
Recurrent exacerbations and poorly controlled conditions cause declining lung function and subsequent airway remodeling (4). Previous asthma cohort studies have described the presence of several asthma phenotypes in asthmatics, with one subset showing an intrinsic phenotype of exacerbation-prone asthma (2). This subset had a history of multiple exacerbations requiring three or more bursts of oral corticosteroids per year and characteristic features including chronic rhinosinusitis, irreversible airflow limitation, and bronchiectatic changes on radiologic studies. Previous studies using COPD cohorts suggested that patients suffering from frequent exacerbations belonged to a distinct phenotype associated with an accelerated decline in lung function, reduced physical activity, poorer quality of life (QOL), and increased risk of mortality (5). In patients with moderate-to-severe COPD, the prevalence of bronchiectasis was higher and it was associated with COPD exacerbation and hospitalization (6). These exacerbations were likely caused by the complex interactions between the host, respiratory viruses, airway bacteria, environmental pollution, increased susceptibility to viruses and bacteria, and both innate and adaptive immune dysfunction.
Adult-onset primary immunodeficiency diseases (PIs) are mostly humoral immune deficiencies, such as common variable immunodeficiencies, hypogammaglobulinemia, immunoglobulin G subclass deficiency (IgGSCD), and selective IgA deficiency (7,8). Previous studies have shown that IgGSCD may be associated with increased susceptibility to sinopulmonary infections and airway obstruction in patients with chronic bronchitis or COPD (9,10,11). In severe asthmatics, immunoglobulin replacement provided corticosteroid sparing effect and reduced hospital admissions (12). However, asymptomatic individuals who have decreased levels of one or more IgG subclasses can have normal responses to polysaccharide antigens and present no infectious complications. Presentation due to recurrent infections has long been the hallmark of immunodeficiency, yet, more recently it is becoming recognized that some patients may present with other alterations in immunity, such as autoimmunity (13). Diagnosis of PI is challenging and relies on clinical suspicion and laboratory confirmation. The 10 Warning Signs of PI, developed for the purpose of early diagnosis and awareness of PI, are based on expert consensus developed at the Jeffrey Modell Foundation (JMF). This system is currently used as a screening tool for the diagnosis of PI (14).
We conducted this study to assess IgGSCD and its correlation with infectious complications in patients with chronic airway diseases such as asthma or COPD using the 10 warning signs of PI. Furthermore, we aimed to evaluate the clinical value of serum IgG subclass levels in this population.
MATERIALS AND METHODS
Study subjects
We enrolled 59 patients with IgGSCD who were diagnosed with bronchial asthma, asthma COPD overlap syndrome (ACOS) or COPD between January 2007 and December 2012. Asthma was defined as episodic respiratory symptoms and reversible airflow obstruction with bronchodilator response (BDR) or positivity to the methacholine bronchoprovocation test. Patients with COPD had incompletely reversible airflow obstruction with a post-bronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) < 70%, a post-bronchodilator FEV1 < 80% of predicted, and no airway hyper-responsiveness (AHR) or BDR. Patients with ACOS had respiratory symptoms and positive bronchodilator responses as well as incompletely reversible airflow obstruction (post-bronchodilator FEV1/FVC < 70%). Each patient was followed in our clinic for the treatment of asthma or COPD for at least 2 consecutive years, based on the Global Initiative for Asthma (GINA) (15) or the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (16). The diagnosis of IgGSCD was made according to the published guidelines (17). Patients had an IgG subclass level that was > 2 standard deviations below the mean on at least two separate occasions. Patients with secondary immunodeficiency diseases, such as human immunodeficiency virus (HIV) infection, and chronic systemic corticosteroid or other immunosuppressant users were excluded. Quantification of IgG subclasses was done by using turbidimetric assay and normal ranges for IgG subclasses were presented in Table 1.
Table 1. Immunoglobulin G subclass deficiencies in the study subjects.
| IgG subclasses | No. (%) of patients | P value | ||
|---|---|---|---|---|
| All (n = 59) | Group I (n =17) | Group II (n = 42) | ||
| IgG1 (382.4-928.6 mg/dL) | 1 (1.7) | 0 (0.0) | 1 (2.4) | 1.000 |
| IgG2 (241.8-700.3 mg/dL) | 1 (1.7) | 0 (0.0) | 1 (2.4) | 1.000 |
| IgG3 (21.8-176.1 mg/dL) | 52 (88.1) | 14 (82.4) | 38 (90.5) | 0.399 |
| IgG4 (3.9-86.4 mg/dL) | 9 (15.3) | 6 (35.2) | 3 (7.1) | 0.013 |
Baseline data and exacerbation variables
The medical records of each patient included age, sex, smoking history, previous medications, comorbidity, type of infection, exacerbation, sputum eosinophil count, total IgE, and atopic status. Asthma or COPD exacerbations were defined as worsening of respiratory symptoms that required treatment with antibiotics or systemic glucocorticoids alone or in combination for > 3 days in an outpatient clinic or hospitalization. Sputum specimens for bacteria culture were obtained during the exacerbations. Pneumonia was defined as a new chest radiographic infiltration plus respiratory symptoms such as cough, dyspnea, fever, discolored sputum or pleuritic chest pain. Patients without the evidence of pneumonic infiltration on chest X-ray were diagnosed as having bronchitis and recurrent bronchitis was defined as three or more episodes of acute bronchitis per year. Atopy was defined as 1) at least one positive specific IgE, or 2) one positive skin-prick test to aeroallergens (cats, dogs, house dust mites, trees, grasses, weeds and fungi). The skin-prick test was considered positive with a wheal diameter ≥ 3 mm and erythema ≥ 10 mm compared with the negative control.
Pulmonary function tests
Spirometry was conducted using handheld spirometers (Vmax 2130; Sensor Medics, Yorba Linda, CA, USA) and was performed and interpreted according to the American Thoracic Society recommendations (18). The instruments were calibrated according to the manufacturers' instructions before each testing day. All spirometry tests were performed by three trained technicians. The spirometry results were reevaluated and finally interpreted by respiratory specialists. Forced expiratory maneuvers were repeated until three reproducible acceptable readings were obtained, and the best FEV1, FVC, and FEV1/FVC ratios were analyzed. Reversibility testing to exclude individuals with asthma was performed 15 minutes after inhalation of 400 μg Ventolin® via metered-dose inhaler (salbutamol; GlaxoSmithKline plc, London, UK) in all patients. Airway hyperresponsiveness to methacholine was determined by a method described previously (19). An aerosol of 0.9% NaCl, followed by serial doubling concentrations of methacholine (0.625-25.0 mg/mL), was inhaled. FEV1 was measured 5 minutes after each inhalation until the FEV1 had fallen by 20% from the post saline value. Airway hyperresponsiveness was considered present if a patient demonstrated more than a 20% decrease in FEV1 after inhalation of any concentration (0.625-25 mg/mL).
Statistical analysis
Continuous variables are presented as means ± standard deviations of the mean and analyzed using the Mann-Whitney U-test for two groups (group I and II) and Kruskal-Wallis test for three subgroups (asthma, ACOS, COPD). Categorical variables are presented as frequencies and percentages and analyzed using Pearson's χ2 tests or Fisher's exact tests. Wilcoxon signed rank test was used to evaluate whether the decline of FEV1 during the study period. All tests were two-sided, and P < 0.05 was considered statistically significant. All analyses were performed using the SPSS computer package, version 18.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
This study was approved by the institutional review board of the Hallym Sacred Heart Hospital for the evaluation and publication of information from patient records (IRB No. 2014-I004). The requirement for informed consent was waived because of the retrospective nature of the study.
RESULTS
Patient characteristics
This study enrolled a total of 59 patients (30 females and 29 males) with a mean age of 60.5 ± 15.9 years. Most patients were diagnosed as having IgGSCD 5.4 years after initial treatment of asthma or COPD. Thirty-three percent of patients were diagnosed as having bronchial asthma and half of the subjects had never smoked. The major underlying diseases were bronchiectasis (37.9%), history of pulmonary tuberculosis (31.0%), cardiovascular disease (15.5%), and diabetes (10.3%). According to the 10 JMF warning signs, patients were classified into two groups: group I (n = 17) met ≥ 2 criteria, and group II (n = 42) met none of the criteria as they had no recurrent infections even though they had IgGSCD (Supplementary Table 1). The numbers of infections, hospitalizations, and exacerbations of asthma or COPD in a year were significantly higher in group I than in group II (P < 0.001, P = 0.012, and P < 0.001, respectively). Other baseline characteristics such as atopy, smoking status, underlying airway diseases, and comorbidities did not differ between the two groups (Table 2). When we compared the number of infection, hospitalization and exacerbation in patients with asthma, ACOS, and COPD of each group, there were no significant differences (Supplementary Table 2).
Table 2. Baseline characteristics of the study subjects.
| Parameters | No. (%) of patients | P value | ||
|---|---|---|---|---|
| All (n = 59) | Group I (n = 17) | Group II (n = 42) | ||
| Male gender | 29 (49.2) | 7 (41.2) | 22 (52.4) | 0.436 |
| Age at diagnosis of IgGSCD, yr | 60.5 ± 15.9 | 59.0 ± 21.6 | 61.1 ± 13.3 | 0.947 |
| Age at asthma or COPD, yr | 55.1 ± 16.2 | 54.8 ± 22.4 | 55.2 ± 13.3 | 0.709 |
| Atopy | 14 (23.7) | 4 (23.5) | 41 (23.8) | 0.995 |
| Never smoker | 32 (54.2) | 12 (70.6) | 20 (47.6) | 0.276 |
| Follow up duration, yr | 5.9 ± 4.1 | 3.3 ± 2.7 | 7.0 ± 4.1 | < 0.001 |
| No. of infection, /yr | 1.9 ± 1.6 | 1.9 ± 0.5 | 0.6 ± 0.1 | < 0.001 |
| No. of hospitalization, /yr | 0.6 ± 0.7 | 1.0 ± 0.2 | 0.4 ± 0.1 | 0.012 |
| No. of exacerbation, /yr | 1.1 ± 1.3 | 1.8 ± 0.4 | 0.5 ± 0.1 | < 0.001 |
| Chronic airway diseases | 0.564 | |||
| Asthma | 20 (33.9) | 4 (23.5) | 16 (38.1) | |
| ACOS | 9 (15.3) | 3 (17.6) | 6 (14.3) | |
| COPD | 30 (50.8) | 10 (58.8) | 20 (47.6) | |
| Comorbidities | 0.651 | |||
| Bronchiectasis | 22 (37.9) | 5 (33.3) | 17 (39.5) | |
| Tuberculosis | 18 (31.0) | 4 (26.7) | 14 (32.6) | |
| Cardiovascular disease | 9 (15.5) | 3 (20.0) | 6 (14.0) | |
| Diabetes mellitus | 6 (10.3) | 2 (13.3) | 4 (9.3) | |
| Chronic liver disease | 3 (5.2) | 1 (6.7) | 2 (4.7) | |
IgGSCD, immunoglobulin G subclass deficiency; COPD, chronic obstructive pulmonary disease; ACOS, asthma COPD overlap syndrome.
Immunoglobulin levels and IgG subclass deficiencies
Among the types of IgGSCD, IgG3 subclass deficiency was the most common (n = 52, 88.1%), followed by IgG4 subclass deficiency (n = 9, 15.3%). Four patients showed at least two or more IgG subclass deficiencies. Three patients had both IgG3 and IgG4 deficiency and one patient showed both IgG1 and IgG3 deficiency. All patients with both IgG3 and IgG4 deficiency belonged to group I. When we compared the types of IgG subclass deficiencies between the two groups, the prevalence of IgG4 was significantly higher in group I than group II (P = 0.013, Table 1). There was no significant difference in the IgG subclass deficiency among the patients with asthma, ACOS, and COPD (Supplementary Table 3).
Pulmonary function tests at base line and follow-up
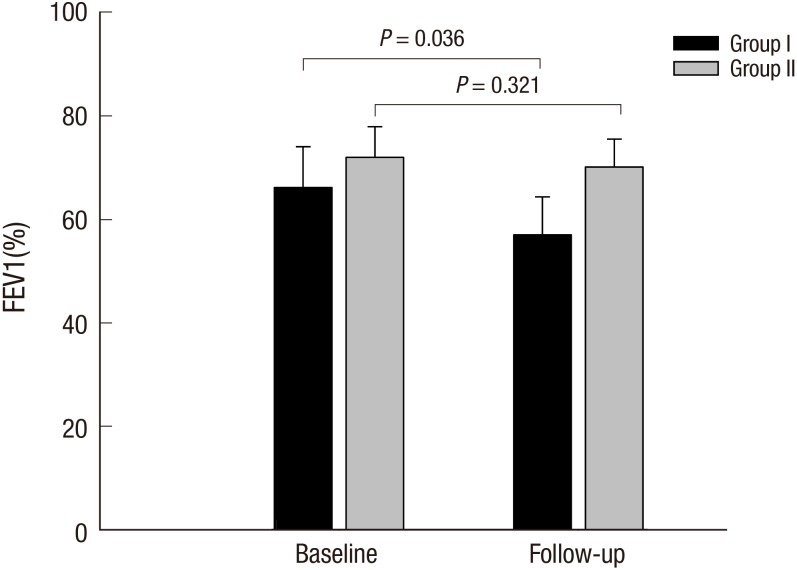
The mean post- albuterol FEV1 value was 70.5% ± 32.0% and 66.9% ± 29.6% predicted at baseline and follow-up visits in all subjects. There were no significant differences between the two groups regarding FEV1, FVC, the ratio of FEV1/FVC, and BDR at baseline and follow-up visits (Table 3). However, the mean values of FEV1, FVC, and the ratio tended to be lower in group I than in group II at both baseline and follow-up visits. The follow up mean FEV1 was significantly decreased in patients in group I compared with their baseline values despite regular treatment of asthma or COPD (P = 0.036). No significant changes were noted between the baseline and follow-up FEV1 values in group II (P = 0.321) (Fig. 1). When we compared the FEV1 values between the initial and follow up visits among the three subgroups (asthma, ACOS, and COPD) of group I and group II, there were significant differences, with ACOS poorer than asthma, and COPD worst of all groups. Longitudinal changes of the FEV1 values in the three subgroups of each group did not differ between baseline and follow up visits (Supplementary Fig. 1).
Table 3. Spirometric results in the study subjects.
| Spirometric parameters | All (n = 59) | Group I (n = 17) | Group II (n = 42) | P value |
|---|---|---|---|---|
| 1st visit | ||||
| FEV1 (% of predicted) | 70.5 ± 32.0 | 66.2 ± 26.1 | 72.0 ± 34.2 | 0.616 |
| FVC (% of predicted) | 82.7 ± 20.3 | 82.2 ± 14.6 | 83.0 ± 22.0 | 0.714 |
| FEV1/FVC (%) | 58.0 ± 17.4 | 56.5 ± 18.8 | 58.5 ± 17.2 | 0.587 |
| Follow-up visit | ||||
| FEV1 (% of predicted) | 66.9 ± 29.6 | 57.0 ± 24.4 | 70.2 ± 30.8 | 0.188 |
| FVC (% of predicted) | 79.3 ± 22.2 | 71.7 ± 16.2 | 81.8 ± 23.6 | 0.193 |
| FEV1/FVC (%) | 59.1 ± 16.9 | 56.3 ± 19.0 | 60.7 ± 16.2 | 0.551 |
FEV1, Forced expiratory volume in one second; FVC, Forced vital capacity.
Fig. 1.
FEV1% predicted at baseline and follow-up visit in patients of group I and II.
Frequencies of infectious complications and identified organisms
The most common infectious complication was pneumonia, followed by recurrent bronchitis, rhinosinusitis, and extrapulmonary site infections including herpes zoster, urinary tract infection, and gasteroenteritis (Table 4). The types of infectious complications were similar between the groups. In patients with pneumonia, 17 organisms were isolated in cultured sputum specimens during exacerbations. Pseudomonas aeruginosa was the most commonly detected organism (n = 11), followed by Klebsiella pneumonia (n = 4), Streptococcal pneumonia (n = 1), and Moraxella catarrhalis (n = 1). The number and type of isolated organisms were similar between group I and group II. The prevalence of P. aeruginosa tended to be higher in patients with COPD compared to those with asthma or ACOS without a statistical significance.
Table 4. The frequencies of infectious complications.
| Infections | No. (%) of patients | ||
|---|---|---|---|
| All (n = 88) | Group I (n = 28) | Group II (n = 60) | |
| Pneumonia | 53 (60.2) | 14 (50.0) | 39 (65.0) |
| Recurrent bronchitis | 14 (15.9) | 5 (17.9) | 9 (15.0) |
| Rhinosinusitis | 11 (12.5) | 4 (14.3) | 7 (11.7) |
| Others | 4 (4.5) | 1 (3.6) | 3 (5.0) |
| Herpes zoster | 3 (3.4) | 1 (3.6) | 2 (3.3) |
| Urinary tract infection | 2 (2.3) | 2 (7.1) | 0 (0.0) |
| Gastroenteritis | 1 (1.1) | 1 (3.6) | 0 (0.0) |
DISCUSSION
In this study, we found that one-third of patients with chronic airway diseases who had been diagnosed with IgGSCD showed recurrent respiratory infections. In addition, these patients showed an increased number of exacerbations, hospitalizations and a subsequent decline in lung function despite regular anti asthmatic or COPD treatment.
IgGSCD is defined as an abnormally low level of one or more IgG subclasses in patients with normal levels of total IgG level. The IgG subclasses are composed of four different isotypes, and each subclass has different structural and biological properties (20). IgG3 comprises only 4%–8% of total serum IgG and has a shorter half-life in comparison with other IgG subclasses. IgG3 plays an important role in complement system activation. Moreover, it binds with high affinity to Fc receptors on macrophages and may be important in antibody-mediated phagocytosis (21). Selective IgG3 deficiencies are a commonly detected type of IgGSCD in adults and recurrent respiratory tract infections and allergy are major clinical associations with IgGSCD (21,22). While the role of selective IgG4 deficiency in infection susceptibility is still unknown, IgG4 deficiency usually occurs in association with other isotype deficiencies and increased risk of sinopulmonary infection (8,11). In this study, we found isolated IgG3 was the most common type of IgGSD, followed by IgG4, and IgG3 combined with other types.
Exacerbations, caused by respiratory viruses, airway bacteria, allergens, and environmental pollution, can lead to increased airway inflammation. Such inflammatory processes may be amplified by host factors such as immune deficiencies and impaired lung functions. In our study, one-third of patients with reduced IgG3 or G4 levels experienced frequent exacerbations due to upper or lower respiratory infections and subsequent declining lung functions. A recent study demonstrated that a subset of patients with selective IgG3 deficiency had combined T and B cell defects, suggesting the propensity for infections may not be solely attributable to IgG3 deficiency but may involve more complicated immune dysfunctions (21). Nevertheless, a clinical diagnosis of IgGSCD is controversial and some patients with IgGSCD also showed specific antibody deficiency (SAD) as well (17). In this study, we confirmed IgGSCD based on laboratory findings; however, we did not measure antibody responses after vaccination to exclude SAD, which is a major limitation of our study.
Exacerbations appear to accelerate the decline in lung function, resulting in reduced physical activity, poor QOL, and high healthcare costs. Thus, the prevention of exacerbations is a key component of the management strategies for both asthma and COPD. In a comparison of lung functions between the two groups, we found no significant change between baseline and follow-up visits. However, a longitudinal analysis of lung function over 3 years showed a significant difference between groups I and II. The mean FEV1 level in group I, which had IgGSCD and a history of recurrent infections, was significantly reduced from baseline despite treatment of asthma or COPD; in contrast, we found no significant change in the follow-up mean FEV1 level in group II. These results were consistent with prior studies showing that repeated infectious exacerbations had negative effects on lung functions (2).
One important phenotype is the frequent exacerbator in COPD or exacerbation-prone asthma, which is now recognized as a distinct clinical subgroup in COPD or asthma cohort studies (23,24,25). In this study, group I had more than three times as many exacerbations per person per year as group II (1.8 vs. 0.5 exacerbation/person/year). Such symptoms are compatible with the typical characteristics of exacerbation-prone phenotypes, implying that these patients require basic immunological evaluations, in particular, if they have histories of recurrent exacerbations triggered by virus or bacterial infections.
The principle of IgGSCD management includes infection control and use of gammaglobulin in selected patients (26). Although few reports have suggested the efficacy of immunoglobulin replacement therapy in patients with IgGSCD (21,27), these reports demonstrate that immunoglobulin replacement therapy significantly improved the QOL, reduced the number of infections, and decreased the need for antibiotics and hospitalization (22,27). In addition, a recent retrospective study showed patients with selective IgG3 deficiency responded clinically to immunoglobulin replacement (21). Several open trials suggested that immunoglobulin replacement had corticosteroid-sparing effects in severe asthmatics because of the potent anti-inflammatory properties of immunoglobulin (28,29). However, randomized controlled studies failed to demonstrate the effect of immunoglobulin in asthmatics, although significant corticosteroid-sparing effect was noted in a subgroup that required high daily doses of oral corticosteroids (12,29). To evaluate the effect of immunoglobulin replacement on the exacerbations and the lung functions of patients with chronic airway diseases, future research should include a prospective study among carefully defined patients.
In this study, we investigated the clinical features of patients with IgGSCD and chronic airway diseases. IgGSCD exists as the subset of exacerbation prone phenotype of asthma or COPD patients. Detailed historical data regarding infectious complications in both upper and lower airway is the only predictable factor and the measurements of immunoglobulin levels are supportive to detect symptomatic patients with IgGSCD in chronic airway disease. Immunoglobulin replacement has beneficial effects when patients with IgGSCD demonstrate recurrent infections. However, further studies are required to determine whether immunoglobulin replacement therapy could be a therapeutic option in this population. Furthermore, a detailed study evaluating B cells, T cells, and other components of the innate immune system of a large cohort of these patients is needed.
Footnotes
Funding: This study was supported by Hallym University Research Fund 2014 (HURF-2014-58).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study concept and design: Kim JH, Park S, Hwang YI, Jang SH, Jung KS, Kim DG. Study implementation, analysis and interpretation of data: Kim JH, Sim YS, Kim CH, Kim DG. Drafting of article or critical revision: Kim JH, Jung KS, Kim CH, Kim DG. Final approval of the version to be published: all authors.
Supplementary Material
Proportion of patients who met the 10 warning signs for adults by Jeffrey Modell Foundation
Frequency of infection, hospitalization and exacerbation according to the obstructive airway diseases (asthma, ACOS, and COPD)
Immunoglobulin G subclass deficiency of the patients with asthma, ACOS, and COPD
FEV1% predicted in patients with asthma, ACOS, and COPD at baseline and follow-up visit in group I (A) and group II (B).
References
- 1.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saturni S, Contoli M, Spanevello A, Papi A. Models of respiratory infections: virus-induced asthma exacerbations and beyond. Allergy Asthma Immunol Res. 2015;7:525–533. doi: 10.4168/aair.2015.7.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holgate ST. Mechanisms of asthma and implications for its prevention and treatment: a personal journey. Allergy Asthma Immunol Res. 2013;5:343–347. doi: 10.4168/aair.2013.5.6.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-García MA, de la Rosa Carrillo D, Soler-Cataluña JJ, Donat-Sanz Y, Serra PC, Lerma MA, Ballestín J, Sánchez IV, Selma Ferrer MJ, Dalfo AR, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:823–831. doi: 10.1164/rccm.201208-1518OC. [DOI] [PubMed] [Google Scholar]
- 7.Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, Kobrynski LJ, Levinson AI, Mazer B, Nelson RP, Jr, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94:S1–63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 8.Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, Keller M, Kobrynski LJ, Komarow HD, Mazer B, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136:1186–1205.e1-78. doi: 10.1016/j.jaci.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 9.Popa V. Airway obstruction in adults with recurrent respiratory infections and IgG deficiency. Chest. 1994;105:1066–1072. doi: 10.1378/chest.105.4.1066. [DOI] [PubMed] [Google Scholar]
- 10.O'Keeffe S, Gzel A, Drury R, Cullina M, Greally J, Finnegan P. Immunoglobulin G subclasses and spirometry in patients with chronic obstructive pulmonary disease. Eur Respir J. 1991;4:932–936. [PubMed] [Google Scholar]
- 11.Schwitzguébel AJ, Jandus P, Lacroix JS, Seebach JD, Harr T. Immunoglobulin deficiency in patients with chronic rhinosinusitis: Systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136:1523–1531. doi: 10.1016/j.jaci.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Salmun LM, Barlan I, Wolf HM, Eibl M, Twarog FJ, Geha RS, Schneider LC. Effect of intravenous immunoglobulin on steroid consumption in patients with severe asthma: a double-blind, placebo-controlled, randomized trial. J Allergy Clin Immunol. 1999;103:810–815. doi: 10.1016/s0091-6749(99)70424-0. [DOI] [PubMed] [Google Scholar]
- 13.Lehman H, Hernandez-Trujillo V, Ballow M. Diagnosing primary immunodeficiency: a practical approach for the non-immunologist. Curr Med Res Opin. 2015;31:697–706. doi: 10.1185/03007995.2014.1001063. [DOI] [PubMed] [Google Scholar]
- 14.Costa-Carvalho BT, Grumach AS, Franco JL, Espinosa-Rosales FJ, Leiva LE, King A, Porras O, Bezrodnik L, Oleastro M, Sorensen RU, et al. Attending to warning signs of primary immunodeficiency diseases across the range of clinical practice. J Clin Immunol. 2014;34:10–22. doi: 10.1007/s10875-013-9954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 16.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 17.Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, Cunningham-Rundles C, Etzioni A, Franco JL, Gaspar HB, Holland SM, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2014;5:162. doi: 10.3389/fimmu.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 19.Popa V. ATS guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2001;163:292–293. doi: 10.1164/ajrccm.163.1.16310b. [DOI] [PubMed] [Google Scholar]
- 20.Buckley RH. Immunoglobulin G subclass deficiency: fact or fancy? Curr Allergy Asthma Rep. 2002;2:356–360. doi: 10.1007/s11882-002-0067-1. [DOI] [PubMed] [Google Scholar]
- 21.Abrahamian F, Agrawal S, Gupta S. Immunological and clinical profile of adult patients with selective immunoglobulin subclass deficiency: response to intravenous immunoglobulin therapy. Clin Exp Immunol. 2010;159:344–350. doi: 10.1111/j.1365-2249.2009.04062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olinder-Nielsen AM, Granert C, Forsberg P, Friman V, Vietorisz A, Björkander J. Immunoglobulin prophylaxis in 350 adults with IgG subclass deficiency and recurrent respiratory tract infections: a long-term follow-up. Scand J Infect Dis. 2007;39:44–50. doi: 10.1080/00365540600951192. [DOI] [PubMed] [Google Scholar]
- 23.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weatherall M, Travers J, Shirtcliffe PM, Marsh SE, Williams MV, Nowitz MR, Aldington S, Beasley R. Distinct clinical phenotypes of airways disease defined by cluster analysis. Eur Respir J. 2009;34:812–818. doi: 10.1183/09031936.00174408. [DOI] [PubMed] [Google Scholar]
- 26.Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, Buckley R, Chinen J, El-Gamal Y, Mazer BD, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117:S525–53. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Abdou NI, Greenwell CA, Mehta R, Narra M, Hester JD, Halsey JF. Efficacy of intravenous gammaglobulin for immunoglobulin G subclass and/or antibody deficiency in adults. Int Arch Allergy Immunol. 2009;149:267–274. doi: 10.1159/000199723. [DOI] [PubMed] [Google Scholar]
- 28.Landwehr LP, Jeppson JD, Katlan MG, Esterl B, McCormick D, Hamilos DL, Gelfand EW. Benefits of high-dose i.v. immunoglobulin in patients with severe steroid-dependent asthma. Chest. 1998;114:1349–1356. doi: 10.1378/chest.114.5.1349. [DOI] [PubMed] [Google Scholar]
- 29.Haque S, Boyce N, Thien FC, O'Hehir RE, Douglass J. Role of intravenous immunoglobulin in severe steroid-dependent asthma. Intern Med J. 2003;33:341–344. doi: 10.1046/j.1445-5994.2003.t01-1-00419.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proportion of patients who met the 10 warning signs for adults by Jeffrey Modell Foundation
Frequency of infection, hospitalization and exacerbation according to the obstructive airway diseases (asthma, ACOS, and COPD)
Immunoglobulin G subclass deficiency of the patients with asthma, ACOS, and COPD
FEV1% predicted in patients with asthma, ACOS, and COPD at baseline and follow-up visit in group I (A) and group II (B).