Abstract
The aim of this study was to determine the association between P2X7R rs3751142 and CARD8 rs2043211 polymorphisms and gout susceptibility in male Korean subjects. This study enrolled a total of 242 male patients with gout and 280 healthy controls. The polymorphisms of two individual genes including rs3751142(C>A) in the P2X7R gene and rs2043211(A>T) in the CARD8 gene were assessed using Taq-Man analysis. Statistical analyses were performed using the Chi-square test, Kruskal-Wallis test, and logistic regression analyses. A difference in genotypic frequency of the P2X7R rs3751142 and CARD8 rs2043211 genes was not detected between gout and control patients. Clinical parameters including age, onset age, disease duration, body mass index, and serum uric acid levels were not different among the three genotypes for either P2X7R or CARD8 (P > 0.05 for all). A pair-wise comparison of P2X7R rs3751142 and CARD8 rs2043211 genotype combinations revealed that subjects with the CA P2X7R rs3751142 genotype and the TT CARD8 rs2043211 genotype had a trend toward a higher risk of gout compared to the CC/AA combination (P = 0.056, OR = 2.618, 95% CI 0.975 - 7.031). In conclusion, this study revealed that genetic variability of the P2X7R rs3751142 and CARD8 rs2043211 genes might, in part, be associated with susceptibility for gout.
Keywords: Gout, Polymorphism, P2X7R, CARD8, Inflammasome
Graphical Abstract
INTRODUCTION
The NLRP3 inflammasome, a member of the NLR family, is a key player in the production of uric acid-mediated IL-1β and is an important cytoplasmic protein complex involved in gouty inflammation (1,2). Although the precise pathogenic mechanism of gout has not been clearly determined, several crucial proteins such as the purinergic receptor P2X ligand-gated ion channel 7 (P2X7R) (3,4,5) and caspase activation and recruitment domain 8 (CARD8) (6,7) proteins are known to be responsible for the pathogenesis of gout.
Recent single-nucleotide polymorphism (SNP) studies suggested that genetic alternations of several target molecules such as CARD8 and P2X7R contribute to the process of NLRP3 inflammasome activation. Genetic variants of CARD8 were identified to play a role in the pathogenesis of a variety of inflammatory diseases, such as rheumatoid arthritis (RA) (8) and inflammatory bowel disease (IBD) (9). Chen et al. (6) demonstrated a significantly different genotypic distribution of the CARD8 rs2043211 polymorphism between gout and control patients in the Chinese population. Another candidate gene involved in gouty inflammation might be P2X7R, whose protein product binds with ATP and induces the efflux of K+ ions through the P2X7R channel from cells, finally triggering activation of the NLRP3 inflammasome (3,4). A loss-of-function (1513 A>C) SNP of the P2X7R gene affects ATP-induced cellular functions such as apoptotic cell death (10) and IL-1β release (11). More evidence to support the intimate relationship of P2X7R gene polymorphisms with autoimmune diseases are the demonstrated links between P2X7R polymorphisms and RA and systemic lupus erythematosus (SLE) (12,13). However, there are no available data linking P2X7R SNPs with gout. Here, we investigated the association of P2X7R rs3751142 and CARD8 rs2043211 polymorphisms with the susceptibility and clinical manifestations of gout in the male Korean population.
MATERIALS AND METHODS
Subjects and collection of clinical information
A total of 242 male gout patients fulfilled the preliminary criteria for classification of primary gout proposed by the American College of Rheumatology (14) and a total of 280 healthy male controls were consecutively enrolled in this study. Clinical and laboratory variables, including age at the time of study, disease onset age, body mass index (BMI), disease duration, and serum uric acid level were identified by individual interviews with each patient and medical record review. Medications including corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, allopurinol, and benzbromarone that were used for gout treatment within one month of the study onset were evaluated through medical record reviews. The study protocol was reviewed and approved by the institutional review board/Ethics Committee at each medical center that participated in this study. Informed consent was obtained at the time of study enrollment.
Genotyping
Assay reagents for rs3751142(C>A) in the P2X7R gene and rs2043211(A>T) in the CARD8 gene were designed by Applied Biosystems (Applied Biosystems, Foster City, CA, USA). The reagents consisted of TaqMan MGB probes (FAM and VIC dye-labeled). The reaction in a 10 μL total volume was optimized at 0.125 μL 40X reagents, 5 μL 2X TaqMan Genotyping Master mix (Applied Biosystems) and 2 μL 50 ng genomic DNA. PCR conditions included one cycle at 95˚C for 10 minutes followed by 40 cycles at 95˚C for 15 seconds and 60˚C for 1 minute. The PCR was performed using the ABI plus (Applied Biosystems) system and the samples were read and analyzed using ABI plus (Applied Biosystems) software. The reference sequence was based on the sequence of human chromosome 12, 12q24 for the P2RX7 gene, and human chromosome 19, 19q13.33 for the CARD8 gene. Primer sequences for genotyping are listed in Table 1.
Table 1. Primer sequences used for Taqman probe genotyping for each gene.
| Genes | dbSNP | Primers | Primer sequence (5' → 3') |
|---|---|---|---|
| P2X7R | rs3751142 | Forward | CGGCTGTTGGTGGAATCCA |
| Reverse | GACACGTCCTGCAGTTCCT | ||
| CARD8 | rs2043211 | Forward | AGTTGACACTCAGGAACAGCACGGA |
| Reverse | CAATAATGGCTCTGCCTCTGTCTCA |
SNP, single nucleotide polymorphism; P2X7R, purinergic receptor P2X ligand-gated ion channel 7; CARD8, caspase activation and recruitment domain 8.
Statistical analysis
Clinical parameters are described as the median with interquartile range (IQR) or the number with percent (%). The Hardy-Weinberg equilibrium for gout patients and controls was assessed using a web-based calculator (http://www.had2know.com/academics/hardy-weinberg-equilibrium-calculator-2-alleles.html) (15). The genotypic frequency of each gene was calculated and assessed using the chi-square and Fisher’s exact tests, if appropriate. Both Kolmogorov-Smirnov and Shapiro-Wilk analyses were tested to identify normality of data distribution, which did not show a normal distribution. The Kruskal-Wallis test was used to compare parametric parameters such as age, onset age, disease duration, BMI, and serum uric acid among the three genotypes of each gene. Logistic regression analysis adjusted with age was applied to test whether the combined P2X7R rs3751142 and CARD8 rs2043211 genotype influenced the risk of gout development compared to those in controls. The results are described as the odds ratio (OR) with a 95% confidence interval (CI). A P value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics 19.0 software (IBM Corp., Armonk, NY, USA).
Ethics statement
All patients provided written informed consent, and the protocol of this study was approved by the institutional review board (IRB) of Daegu Catholic University Medical Center (IRB No. CR-12-138-RES-002-R). The authors assert that all procedures contributing to this work comply with the Helsinki Declaration of 1975 and its later amendments.
RESULTS
The baseline characteristics of enrolled patients are outlined in Table 2. The mean age was 55.0 (IQR 45.0–63.0) years for gout patients and 45.0 (IQR 41.0–53.0) for controls, which was significantly different between two groups (P < 0.001).
Table 2. Baseline characteristics of enrolled patients.
| Parameters | Gout (n = 242) | Control (n = 280) | P value |
|---|---|---|---|
| Age, yr | 55.0 (45.0–63.0) | 45.0 (41.0–53.0) | < 0.001 |
| Disease duration, yr | 7.0 (3.0–10.0) | - | - |
| Onset age of disease, yr | 47.0 (38.0–56.0) | - | - |
| Height, cm | 170.0 (168.0–174.0) | - | - |
| Weight, kg | 73.0 (65.3–80.0) | - | - |
| Body mass index, kg/m2 | 25.3 (23.6–26.7) | - | - |
| Uric acid, mg/L | 6.4 (5.1–7.6) | - | - |
| Current medications, No. (%) | - | - | |
| NSAIDs | 123 (50.8) | ||
| Colchicine | 146 (60.3) | ||
| Allopurinol | 154 (63.6) | ||
| Benzobromarone | 61 (25.2) | ||
| Corticosteroid | 85 (35.1) |
Data were expressed as with interquartile range (IQR) for continuous variables and number (%) for categorical variables.
NSAIDs, non-steroidal anti-inflammatory drugs.
No genotypic deviations of rs3751142(C>A) in the P2X7R gene and rs2043211(A>T) in CARD8 based on the Hardy-Weinberg equilibrium were noted for gout or control patients (P > 0.05). Table 3 shows that a frequency difference of alleles and genotypes for rs3751142(C>A) and rs2043211(A>T) was not found between gout or control patients. Under the recessive comparison model (CC vs. CA/AA in P2X7R and AA vs. AT/TT in CARD8), genotypic frequency differences between the two groups were also not detected at each SNP (P > 0.05 of both SNPs).
Table 3. Comparison frequencies of P2X7R and CARD8 SNPs between gout and controls.
| Genes | Gout (n = 242) | Control (n = 280) | P value | |
|---|---|---|---|---|
| P2X7R* | Genotypes | 0.205 | ||
| CC | 180 (74.4) | 198 (70.7) | ||
| CA | 60 (24.8) | 74 (26.4) | ||
| AA | 2 (0.8) | 8 (2.9) | ||
| Alleles | 0.196 | |||
| C | 420 (86.6) | 470 (83.9) | ||
| A | 64 (13.2) | 90 (16.1) | ||
| CARD8* | Genotypes | 0.763 | ||
| AA | 71 (29.3) | 88 (31.4) | ||
| AT | 121 (50.0) | 131 (46.8) | ||
| TT | 50 (20.7) | 61 (21.8) | ||
| Alleles | 0.876 | |||
| A | 263 (54.3) | 307 (54.8) | ||
| T | 221 (45.7) | 253 (45.2) | ||
The statistical analysis was performed using Pearson χ2 test.
*rs3751142(C>A) in P2X7R gene and rs2043211(A>T) in CARD8.
The results of association between clinical variables and genotypes are illustrated in Table 4. There were no differences of clinical variables according to genotypic distribution of P2X7R rs3751142 and CARD8 rs2043211. In a comparison model sub-analysis for onset age (≤ 30 vs. > 30 years) and serum uric acid level (≤ 7.0 vs. > 7.0 mg/L), genotypic differences of each clinical parameter were not observed at the two SNPs (data not shown).
Table 4. Comparison for clinical parameters of P2X7R and CARD8 SNPs in patients with gout.
| Clinical parameters | P2X7R* | P value | CARD8* | P value | ||||
|---|---|---|---|---|---|---|---|---|
| CC | CA | AA | AA | AT | TT | |||
| Age, yr | 55.0 (45.0-63.0) | 56.0 (46.3-62.3) | 63.5 (40.5-54.8) | 0.283 | 56.0 (45.0-61.0) | 55.0 (44.0-64.0) | 56.0 (48.8-63.0) | 0.675 |
| Onset age, yr | 47.0 (37.1-55.0) | 47.5 (38.0-57.0) | 56.5 (47.0-66.0) | 0.574 | 48.0 (38.0-53.0) | 47.0 (36.0-57.0) | 45.5 (39.5-54.6) | 0.911 |
| Disease duration, yr | 7.0 (3.0-11.0) | 6.0 (3.0-10.0) | 7.0 (7.0-7.0) | 0.841 | 7.0 (4.0-11.3) | 7.0 (3.0-10.0) | 5.0 (3.0-10.0) | 0.369 |
| Body mass index, kg/m2 | 25.3 (23.4-26.9) | 24.9 (22.5-27.2) | 21.7 (21.7-21.7) | 0.431 | 25.6 (23.0-27.4) | 25.0 (23.5-27.1) | 24.5 (22.2-26.6) | 0.420 |
| Uric acid, mg/L | 6.4 (5.1-7.6) | 6.3 (5.2-7.7) | 4.8 (3.2-4.0) | 0.411 | 6.5 (5.3-7.9) | 6.4 (5.0-7.5) | 6.2 (5.3-8.0) | 0.690 |
The statistical analysis was performed using Kruskal-Wallis test.
*rs3751142 (C>A) in P2X7R gene and rs2043211(A>T) in CARD8 gene.
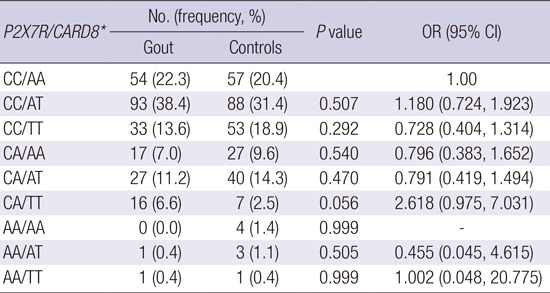
A pair-wise comparison of P2X7R rs3751142 and CARD8 rs2043211 genotype combinations showed that a trend toward higher risk of gout development was identified in subjects who were heterozygous for the CA P2X7R rs3751142 genotype and recessive homozygous for the TT CARD8 rs2043211 genotype compared to the CC/AA combination (P = 0.056, OR = 2.618, 95% CI 0.975-7.031) (Table 5).
Table 5. P2X7R rs3751142 and CARD8 rs2043211 genotype combination between gout and controls.
| P2X7R/CARD8* | No. (frequency, %) | P value | OR (95% CI) | |
|---|---|---|---|---|
| Gout | Controls | |||
| CC/AA | 54 (22.3) | 57 (20.4) | 1 | |
| CC/AT | 93 (38.4) | 88 (31.4) | 0.507 | 1.180 (0.724, 1.923) |
| CC/TT | 33 (13.6) | 53 (18.9) | 0.292 | 0.728 (0.404, 1.314) |
| CA/AA | 17 (7.0) | 27 (9.6) | 0.540 | 0.796 (0.383, 1.652) |
| CA/AT | 27 (11.2) | 40 (14.3) | 0.470 | 0.791 (0.419, 1.494) |
| CA/TT | 16 (6.6) | 7 (2.5) | 0.056 | 2.618 (0.975, 7.031) |
| AA/AA | 0 (0.0) | 4 (1.4) | 0.999 | - |
| AA/AT | 1 (0.4) | 3 (1.1) | 0.505 | 0.455 (0.045, 4.615) |
| AA/TT | 1 (0.4) | 1 (0.4) | 0.999 | 1.002 (0.048, 20.775) |
The statistical analysis was performed using logistic regression analysis after adjustment with age.
OR, odds ratio; CI, confidence interval.
*rs3751142(C>A) in P2X7R gene and rs2043211(A>T) in CARD8.
DISCUSSION
The main objective of this study was to investigate whether genetic variability in the P2X7R rs3751142 and CARD8 rs2043211 genes was involved in the process of NLRP3 inflammasome activation, which might influence susceptibility for the development of gout. This study identified that CARD8 rs2043211 was not associated with increased gout risk, which is in contrast to the known difference of genetic distribution of this SNP in the Chinese and European gout population (6,7). As far as we know, this study is the first to determine genetic association between the P2X7R SNP and gout patients, revealing that P2X7R polymorphism did not increase the risk of gout in our study population.
The NLRP3 inflammasome is a cytoplasmic protein complex that activates caspase-1, inducing the maturation and secretion of IL-1β, which is associated with gout pathogenesis (1,2). The mechanisms by which signaling pathways activate the NLRP3 inflammasome are not clearly determined; however, it is known that cytoplasmic K+ efflux from the cell through the P2X7R channel in response to danger signals allows for NLRP3 inflammasome activation (3,4). Similarly, MSU crystals, ATP, and asbestos are known to induce inflammasome activation by decreasing intracellular K+ levels (16). The P2X7R gene is markedly polymorphic and includes at least 12 non-synonymous SNPs in the coding region (17). P2X7R SNPs that encode Glu496Ala or Ala348Thr substitutions are most commonly found to be associated with IL-1β secretion capacity (11,17). The loss-of-function (1513 A>C) SNP of P2X7R gene induced lower ATP-mediated K+ ion efflux, leading to reduced IL-1β levels in monocytes (11). Tao et al. (18) recently suggested the possibility that P2X7R is a key regulator for the production of IL-1β by MSU crystals during acute gouty arthritis. Human myeloid leukemic KG-1 cells contain several SNPs of the P2X7R gene (19). Among them, KG-1 cells with rs3751142 a revealed neutral effect on P2X7R function. However, there is no evidence as to whether the P2X7R polymorphism has a potent influence on the development of gout. In our data, a minor allele frequency (MAF, A allele) of P2X7R rs3751142 in gout and control patients was 0.132 and 0.161, respectively, which are more compatible to those in the dbSNP rs3751142 (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId05027). This study found that P2X7R rs3751142 was not a genetic risk factor associated with gout susceptibility.
The activation of the NLRP3 inflammasome, which is comprised of multiple cytoplasmic proteins including NLRP3, an ASC protein, and caspase-1, is completed through the binding of the PYD of NLRP3 with the PYD of ASC, which is followed by the recruitment of procaspase-1 by linkages of each CARD within the two proteins. CARD8 was shown to be a negative regulator of nuclear factor-κB (NF-κB) and caspase-1 activation (20,21), which implicates a role in the suppression of NLRP3 inflammasome activation. The rs2043211 SNP in exon 5 of the CARD8 gene is a nonsense mutation resulting in the production of a truncated protein. Although the functional role of polymorphic CARD8 within the NLRP3 inflammasome is complicated, genetic variants of CARD8 rs2043211 contribute to an enhanced inflammatory response in inflammatory diseases such as RA and IBD through increased expression of IL-1β (8,9,22). McKinney et al. (7) demonstrated that the prevalence of CARD8 rs2043211 was different between gout and control patients in the European and Polynesian populations (OR 1.11, P = 0.023 and OR 1.15, P = 0.078, respectively). Another case-control study showed that the MAF (A allele) CARD8 gene was shown to have an increasing trend relative to the T allele in the Chinese male population (OR = 0.084, P = 0.08) (6). However, our study revealed no association of CARD8 rs2043211 with the presence of gout in Korean study population. The frequency of MAF (T allele) is at 0.457 in gout and 0.452 in control patients, which is similar to the frequency in the New Zealand Polynesian (0.499 in gout and 0.439 in control) and Chinese (0.498 in gout and 0.463 in control) patients, whereas a lower frequency of MAF was noted in the European population (7). Based on conflicting data for CARD8 rs2043211 between these three studies, the functional role of the CARD8 gene in gout susceptibility has not been clearly determined. Therefore, the distinct role of the CARD8 gene in the pathogenesis of gout should be considered in a diverse ethnic study population.
The present study has some methodological limitations. First, the lack of completeness for clinical information of control subjects is an important weakness in our study. This study used genomic DNAs provided by the Biobank of Wonkwang University Hospital, Korea as control group samples. We were just able to identify a part of demographic data including gender and age. Limited clinical data of controls could interfere with more detailed analysis of gout susceptibility. Second, this study did not perform functional analysis for genotypes in each target gene. As shown disparity of genetic susceptibility of gout in several ethnic groups (6,7), it is important to identify functional performance of each gene to understand the role of P2X7R and CARD8 SNPs in our study population. Functional analysis is needed in the future investigation of gout.
It is noted that the P2X7R and CARD8 proteins are closely involved in activation of the NLRP3 inflammasome and IL-1β expression, which could explain their role in the pathogenesis of gout (1,2). Our study demonstrated that the P2X7R rs3751142 and CARD8 rs2043211 genetic variants were not implicated in the development of gout in the male Korean population. However, we found that in a pair-wise comparison of the CA/TT P2X7R and CARD8 genotype combination was shown to have an increased trend for the risk of gout (OR = 2.618, P = 0.056). In conclusion, this study provides evidence that the interaction of MSU crystal-mediated P2X7R with CARD8 may be at least in part responsible for the pathogenesis of gout.
ACKNOWLEDGMENT
The genomic DNAs for this study were provided by the Biobank of Wonkwang University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affairs. Language editing was performed by an editing company (eWorldEditing, Inc. Eugene, OR, USA).
Footnotes
Funding: This work was supported by the Dong-A University research fund.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Design of this study: Lee SW, Lee SS, Kim SK. Analysis for polymorphisms: Chae SC, Yun KJ. Interpretation of data: all authors. Collection of data: Lee SW, Lee SS, Oh DH, Park DJ, Kim HS, Choi JR, Chung WT, Choe JY, Kim SK. Drafting the manuscript: Lee SW, Lee SS, Kim SK. Approval of final manuscript: all authors. Full responsibility for the integrity of the study and all parts of the article: all authors.
References
- 1.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 2.Choe JY, Jung HY, Park KY, Kim SK. Enhanced p62 expression through impaired proteasomal degradation is involved in caspase-1 activation in monosodium urate crystal-induced interleukin-1b expression. Rheumatology (Oxford) 2014;53:1043–1053. doi: 10.1093/rheumatology/ket474. [DOI] [PubMed] [Google Scholar]
- 3.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 5.Gong QY, Chen Y. Correlation between P2X7 receptor gene polymorphisms and gout. Rheumatol Int. 2015;35:1307–1310. doi: 10.1007/s00296-015-3258-5. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Ren X, Li C, Xing S, Fu Z, Yuan Y, Wang R, Wang Y, Lv W. CARD8 rs2043211 polymorphism is associated with gout in a Chinese male population. Cell Physiol Biochem. 2015;35:1394–1400. doi: 10.1159/000373960. [DOI] [PubMed] [Google Scholar]
- 7.McKinney C, Stamp LK, Dalbeth N, Topless RK, Day RO, Kannangara DR, Williams KM, Janssen M, Jansen TL, Joosten LA, et al. Multiplicative interaction of functional inflammasome genetic variants in determining the risk of gout. Arthritis Res Ther. 2015;17:288. doi: 10.1186/s13075-015-0802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kastbom A, Johansson M, Verma D, Söderkvist P, Rantapää-Dahlqvist S. CARD8 p.C10X polymorphism is associated with inflammatory activity in early rheumatoid arthritis. Ann Rheum Dis. 2010;69:723–726. doi: 10.1136/ard.2008.106989. [DOI] [PubMed] [Google Scholar]
- 9.Yang SK, Kim H, Hong M, Lim J, Choi E, Ye BD, Park SK, Song K. Association of CARD8 with inflammatory bowel disease in Koreans. J Hum Genet. 2011;56:217–223. doi: 10.1038/jhg.2010.170. [DOI] [PubMed] [Google Scholar]
- 10.Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S, Barden JA, Wiley JS. Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem. 2001;276:11135–11142. doi: 10.1074/jbc.M010353200. [DOI] [PubMed] [Google Scholar]
- 11.Wesselius A, Bours MJ, Arts IC, Theunisz EH, Geusens P, Dagnelie PC. The P2X(7) loss-of-function Glu496Ala polymorphism affects ex vivo cytokine release and protects against the cytotoxic effects of high ATP-levels. BMC Immunol. 2012;13:64. doi: 10.1186/1471-2172-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Shukaili A, Al-Kaabi J, Hassan B, Al-Araimi T, Al-Tobi M, Al-Kindi M, Al-Maniri A, Al-Gheilani A, Al-Ansari A. P2X7 receptor gene polymorphism analysis in rheumatoid arthritis. Int J Immunogenet. 2011;38:389–396. doi: 10.1111/j.1744-313X.2011.01019.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen GM, Feng CC, Ye QL, Tao JH, Li R, Peng H, Zhou M, Leng RX, Li J, Cen H, et al. Association of P2X7R gene polymorphisms with systemic lupus erythematosus in a Chinese population. Mutagenesis. 2013;28:351–355. doi: 10.1093/mutage/get007. [DOI] [PubMed] [Google Scholar]
- 14.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 15.Al-Mohaya MA, Al-Harthi F, Arfin M, Al-Asmari A. TNF-α, TNF-β and IL-10 gene polymorphism and association with oral lichen planus risk in Saudi patients. J Appl Oral Sci. 2015;23:295–301. doi: 10.1590/1678-775720150075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 17.Stokes L, Fuller SJ, Sluyter R, Skarratt KK, Gu BJ, Wiley JS. Two haplotypes of the P2X(7) receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1beta secretion. FASEB J. 2010;24:2916–2927. doi: 10.1096/fj.09-150862. [DOI] [PubMed] [Google Scholar]
- 18.Tao JH, Zhang Y, Li XP. P2X7R: a potential key regulator of acute gouty arthritis. Semin Arthritis Rheum. 2013;43:376–380. doi: 10.1016/j.semarthrit.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Gadeock S, Pupovac A, Sluyter V, Spildrejorde M, Sluyter R. P2X7 receptor activation mediates organic cation uptake into human myeloid leukaemic KG-1 cells. Purinergic Signal. 2012;8:669–676. doi: 10.1007/s11302-012-9320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouchier-Hayes L, Conroy H, Egan H, Adrain C, Creagh EM, MacFarlane M, Martin SJ. CARDINAL, a novel caspase recruitment domain protein, is an inhibitor of multiple NF- kappa B activation pathways. J Biol Chem. 2001;276:44069–44077. doi: 10.1074/jbc.M107373200. [DOI] [PubMed] [Google Scholar]
- 21.Razmara M, Srinivasula SM, Wang L, Poyet JL, Geddes BJ, DiStefano PS, Bertin J, Alnemri ES. CARD-8 protein, a new CARD family member that regulates caspase-1 activation and apoptosis. J Biol Chem. 2002;277:13952–13958. doi: 10.1074/jbc.M107811200. [DOI] [PubMed] [Google Scholar]
- 22.Fontalba A, Martinez-Taboada V, Gutierrez O, Pipaon C, Benito N, Balsa A, Blanco R, Fernandez-Luna JL. Deficiency of the NF-kappaB inhibitor caspase activating and recruitment domain 8 in patients with rheumatoid arthritis is associated with disease severity. J Immunol. 2007;179:4867–4873. doi: 10.4049/jimmunol.179.7.4867. [DOI] [PubMed] [Google Scholar]


