Abstract
Analgesics, known to be hepatotoxic drugs, are frequently prescribed to patients with liver cirrhosis who are prone to drug-induced liver injury. No guidelines are available regarding the prescription of analgesics in these patients. Therefore, we aimed to evaluate the prescription pattern of most frequently used analgesics in patients with cirrhosis. We assessed the prescription pattern of acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) in patients with liver cirrhosis registered in Health Insurance Review Assessment Service database between January 1, 2012 and December 31, 2012. A total of 125,505 patients with liver cirrhosis were registered from January 1, 2012 to December 31, 2012. Of that group, 50,798 (40.5%) patients claimed reimbursement for at least one prescription for acetaminophen or NSAIDs during the one year follow-up period. Overall, NSAIDs (82.7%) were more prescribed than acetaminophen (64.5%). NSAIDs were more prescribed than acetaminophen even in decompensated cirrhosis compared with compensated cirrhosis (71.5% vs. 68.8%, P value < 0.001). There was a marked difference in prescription preference between acetaminophen and NSAIDs among physicians. Internists more frequently prescribed acetaminophen than NSAIDs compared to other physicians (50.9% vs. 76.2%, P < 0.001). Gastroenterologists more frequently prescribed acetaminophen over NSAIDs compared to other internists (80.9% vs. 51.2%, P < 0.001). Analgesics were prescribed in 40.5% of patients with cirrhosis. NSAIDs were more frequently prescribed although they should be avoided. The prescription pattern of analgesics were different significantly among physicians in patients with liver cirrhosis. The harmful effects of NSAIDs in patients with cirrhosis should be reminded to all physicians prescribing analgesics.
Keywords: Acetaminophen, Non-Steroid Anti-Inflammatory Drugs (NSAIDs), Pattern of Prescription, Liver Cirrhosis
Graphical Abstract
INTRODUCTION
Acute or chronic pain is one of the most common symptoms suffered by patients with cirrhosis secondary to various comorbidities. Pain relief is essential to improve quality of life for these patients. The management of pain in these patients, however, is a clinical challenge to physicians and a source of considerable misconception and concern among physicians. Various types of analgesics can be used in the treatment of pain. The prescription of the proper type of analgesics is important to relieve pain more effectively and avoid unnecessary adverse events.
Analgesics including non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, are some of the most frequently prescribed drugs in patients with cirrhosis. Cirrhotic liver is vulnerable to cellular injury by many drugs. Many drugs increase the risk of hepatic injury and exacerbation of clinical sequelae in patients with cirrhosis.
Although acetaminophen is known as a hepatotoxic drug at higher dose, it can be used safely in maximum daily dose of 2 to 3 grams per day or up to 4 grams per day in the short term in most patients with cirrhosis, including those who regularly consume alcohol (1). NSAIDs can cause gastrointestinal bleeding, hepatic injury, and acute kidney injury more frequently in patients with cirrhosis. NSAIDs can also cause new-onset ascites in patients with compensated cirrhosis or makes it difficult to control existing ascites. As a rule, NSAIDs should be used cautiously in patients with cirrhosis (2).
Although analgesic are commonly used and can affect the natural course of disease in patients cirrhosis, there are few recommendations regarding the proper use of analgesics in this population (2,3,4). To our knowledge, there are only a few reports such as physician opinions or prescription patterns related to analgesics and these reports were based on the survey of a small number of patients (5,6). Therefore, this study assesses the prescription pattern of analgesics in patients with liver cirrhosis and examines the differences in prescription patterns among physician types.
MATERIALS AND METHODS
Data source
This study was a retrospective review of nationwide claims for analgesics prescribed to patients with cirrhosis, which includes adjusted medical and pharmacy claims taken from the HIRA database. Korea has a health care system that is managed and supervised by the government organization. Ninety-seven percent of the population is legally obliged to register in the Korea National Health Insurance Program. All medical institutions must submit data of inpatients and outpatients care to HIRA database to obtain reimbursement of the medical costs. Patient information, including diagnoses (coded according to the International Classification of Disease, Tenth Revision [ICD-10]), procedures, prescription records, demographic information, and direct medical costs, is available from HIRA database.
Patients selection
First, we identified all patients greater than 20 years of age diagnosed with liver cirrhosis using ICD-10 code K746 between January 2012 and December 2012 from HIRA data. Those who had at least one prescription for acetaminophen or NSAIDs with a diagnostic code of cirrhosis (ICD-10 code K746) were included in this study cohort. Patients with concomitant diagnoses of cancer (ICD 10 code C) were excluded. The reimbursement claims for analgesics in patients with cirrhosis were reviewed for one year period following enrollment in this study.
Data collection
Information was collected about the following patient characteristics: age, gender and cause of cirrhosis (e.g., viral hepatitis B and C, as well as other causes). Cause of cirrhosis was defined as cirrhosis with diagnostic code such as viral hepatitis B and C. Compensated cirrhosis was defined as being free from sequelae associated with cirrhosis. Decompensated cirrhosis was defined as cirrhosis with sequelae such as ascites, variceal bleeding, hepatic encephalopathy or hepatorenal syndrome. To identify decompensated cirrhosis, ICD-10 code of each sequelae were selected and patients who have at least one sequelae code during six months prior to study enrolment were defined as decompensated cirrhosis. The duration and number of prescriptions for acetaminophen or NSAIDs were retrieved and collated. Any types of medication with oral acetaminophen or NSAIDs as the chief ingredient were included. NSAIDs included ketoprofen, ibuprofen, dexibuprofen, naproxen, fenoprofen, dexketoprofen, loxoprofen, indomethacin, sulindac, etodolac, ketorolac, diclofenac, aceclofenac, nabumetone, piroxicam, meloxicam, tenoxicam, lornoxicam, mefenamic acid, flufenamic acid and celecoxib. Aspirin was excluded from the analysis because it was rarely prescribed for pain control and there are no studies about the effect of low dose aspirin on disease course in patients with cirrhosis.
We then evaluated the prescription pattern of acetaminophen and NSAIDs. Given the large variety of NSAIDs used, we were unable to analyze all those prescribed. However, the three most commonly prescribed NSAIDs accounted for the majority (70%), and we therefore analyzed the top three drugs. The duration of prescription was based on the sum of the total number of days each drug was prescribed during the one year follow-up period. Long-term prescriptions was defined as drugs prescribed for 15 days or longer. Mean daily dose was calculated by dividing the total dose administered by the total number of patients and days used. Defined daily dose (DDD) is the typical maintenance dose required per day for a drug prescribed for its main indication in adults (7). We evaluated the frequency of drug use by dividing prescriptions into regular use (≥ 0.5 DDD/day) and intermittent use (< 0.5 DDD/day).
Subgroup analysis was performed for compensated cirrhosis and decompensated cirrhosis as well as physician practice type, divided into gastroenterologists, internists, and other physicians. Internists was defined as specialists for internal medicine including gastroenterologists and other physicians was defined as specialists other than internists. When analyzing the prescription pattern between internists and other physicians and between gastroenterologists and other internists, we compared the total number of prescriptions written by each group rather than the total number of prescribing doctors.
Statistical analysis
The distribution of sex, age, medication use and comorbidities was expressed as a frequency (with %). The distribution of sex, age, medication use and comorbidities was expressed as a frequency (with %).
To determine the prescription rate of acetaminophen and NSAIDs, the prescriptions were categorized by chief ingredient code. To determine the day used of analgesics, the prescriptions were categorized by the duration of days in each prescription [divided into two group (< 15 days, ≥ 15 days)]. Mean daily dose was calculated by dividing the total dose administered by the total number of patients and days used. To determine the frequency of drug use, the mean daily dose was categorized by DDD [divided into two group (0 < DDD < 0.5 DDD/day, ≥ 0.5 DDD/day)]. Statistical analysis of categorical variables was performed using χ2 tests. All statistical analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC, USA), and two-sided P value below 0.05 was considered significant.
Ethics statement
This study conformed to the standards of the Declaration of Helsinki and current ethical guidelines and was reviewed and approved by the institutional review board of Pusan National University Yangsan Hospital (PNUYH 05-2015-064). Informed consent was waived by the board.
RESULTS
Overall prescription of analgesics
A total of 125,505 patients with liver cirrhosis were registered from January 1, 2012 to December 31, 2012. The study population consisted of 50,798 (40.5%) patients from that group who claimed reimbursement for at least one prescription for acetaminophen or NSAIDs during the study period, after excluding patients with concomitant diagnoses of cancer (ICD 10 code C, n = 12,621). A majority of the patients were male (61%), with mean age of 56.7 years. Approximately 60% had chronic viral hepatitis as the potential cause of cirrhosis. The characteristics and sequelae of cirrhosis in these patients are summarized in Table 1.
Table 1. Baseline characteristics of study population.
| Characteristics | n = 50,798 |
|---|---|
| Age, yr (mean) | 56.7 |
| Sex, No. (%) | |
| Male | 31,091 (61.2) |
| Female | 19,707 (38.8) |
| Etiology of cirrhosis, No. (%) | |
| Hepatitis B | 25,497 (50.2) |
| Hepatitis C | 5,070 (10.0) |
| Others | 20,231 (39.8) |
| Past cirrhosis decompensation, No. (%) | 1,111 (2.2) |
| Ascites | 247 |
| Variceal bleeding | 513 |
| Hepatic encephalopathy | 352 |
| Hepatorenal syndrome | 25 |
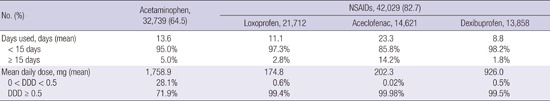
Among 50,798 patients in the study, 32,739 (64.5%) claimed at least one prescription for acetaminophen and 42,029 (82.7%) claimed at least one prescription for NSAIDs. Upper respiratory infections, including acute bronchitis and acute tonsillitis, as well as primary gonarthrosis were the most common diseases precipitating prescription of acetaminophen or NSAIDs. NSAIDs were more frequently prescribed than acetaminophen (82.7% vs. 64.5%). The most frequently prescribed NSAIDs were loxoprofen, aceclofenac and dexibuprofen. NSAIDs and acetaminophens were predominantly used for short-term duration (< 15 days). Acetaminophen was prescribed at a daily dosage of 1,758.9 mg (± 607.1 mg) daily and was prescribed for regular use (at 50% or more of the defined daily dose) in 71.9%. Almost all NSAIDs were prescribed for regular use (Table 2).
Table 2. Overall prescriptions of acetaminophen and NSAIDs.
| No. (%) | Acetaminophen, 32,739 (64.5) | NSAIDs, 42,029 (82.7) | ||
|---|---|---|---|---|
| Loxoprofen, 21,712 | Aceclofenac, 14,621 | Dexibuprofen, 13,858 | ||
| Days used, days (mean) | 13.6 | 11.1 | 23.3 | 8.8 |
| < 15 days | 95.0% | 97.3% | 85.8% | 98.2% |
| ≥ 15 days | 5.0% | 2.8% | 14.2% | 1.8% |
| Mean daily dose, mg (mean) | 1,758.9 | 174.8 | 202.3 | 926.0 |
| 0 < DDD < 0.5 | 28.1% | 0.6% | 0.02% | 0.5% |
| DDD ≥ 0.5 | 71.9% | 99.4% | 99.98% | 99.5% |
NSAIDs, nonsteroidal anti-inflammatory drugs; DDD, defined daily dose.
Prescription patterns for compensated cirrhosis or decompensated cirrhosis
The prescription pattern of acetaminophen was not significantly different for compensated and decompensated cirrhosis patients (64.4% vs. 68.8%). Although the number of prescriptions for NSAIDs were lower for patients with decompensated cirrhosis compared to those with compensated cirrhosis, NSAIDs prescription still exceeded acetaminophen prescription in patients with decompensated cirrhosis (71.5% vs. 68.8%, P < 0.001).
There were no significant differences in duration of prescription, including short-term prescription of less than 15 days, for acetaminophen or NSAIDs between patients with compensated and decompensated cirrhosis. However, NSAIDs tended to be prescribed for a shorter duration in patients with decompensated cirrhosis compared to those with compensated cirrhosis (26.5 days vs. 29.8 days, P = 0.067).
Regular use of acetaminophen (≥ 0.5DDD/day) was decreased in patients with decompensated cirrhosis compared to patients with compensated cirrhosis (54.7% vs. 72.3%). Regular use of NSAIDs (≥ 0.5DDD/day) did not differ between patients with decompensated and compensated cirrhosis. Almost all NSAIDs were prescribed at a level of regular use (≥ 0.5DDD/day) in both patients groups (Table 3).
Table 3. Prescription difference of the acetaminophen and NSAIDs according to hepatic condition.
| Subjects | Compensated cirrhosis (n = 49,687, 97.8%) | Decompensated cirrhosis (n = 1,111, 2.2%) | P |
|---|---|---|---|
| Analgesics, No. (%) | < 0.001 | ||
| Acetaminophen | 31,975 (64.4) | 764 (68.8) | |
| NSAIDs | 41,235 (83.0) | 794 (71.5) | |
| Days used | |||
| Acetaminophen, days (mean) | 13.6 | 14.2 | |
| < 15 days | 95.0% | 93.6% | 0.068 |
| ≥ 15 days | 5.0% | 6.4% | |
| NSAIDs, days (mean) | 29.8 | 26.5 | |
| < 15 days | 86.5% | 85.0% | 0.216 |
| ≥ 15 days | 13.5% | 15.0% | |
| Mean daily dose | |||
| Acetaminophen, mg (mean) | 1,763.8 | 1,551.7 | |
| 0 < DDD < 0.5 | 27.7% | 45.3% | < 0.001 |
| DDD ≥ 0.5 | 72.3% | 54.7% | |
| NSAIDs | |||
| Loxoprofen, mg (mean) | 174.07 | 174.07 | |
| 0 < DDD < 0.5 | 0.6% | 1.7% | 0.024 |
| DDD ≥ 0.5 | 99.4% | 98.3% | |
| Acecloprofen, mg (mean) | 202.35 | 202.3 | |
| 0 < DDD < 0.5 | 0.02% | 0% | 1.000 |
| DDD ≥ 0.5 | 99.98% | 100% | |
| Dexibuprofen, mg (mean) | 926.2 | 911.3 | |
| 0 < DDD < 0.5 | 0.5% | 1.9% | 0.023 |
| DDD ≥ 0.5 | 99.5% | 98.1% |
NSAIDs, nonsteroidal anti-inflammatory drugs; DDD, defined daily dose.
Prescription pattern according to practice types
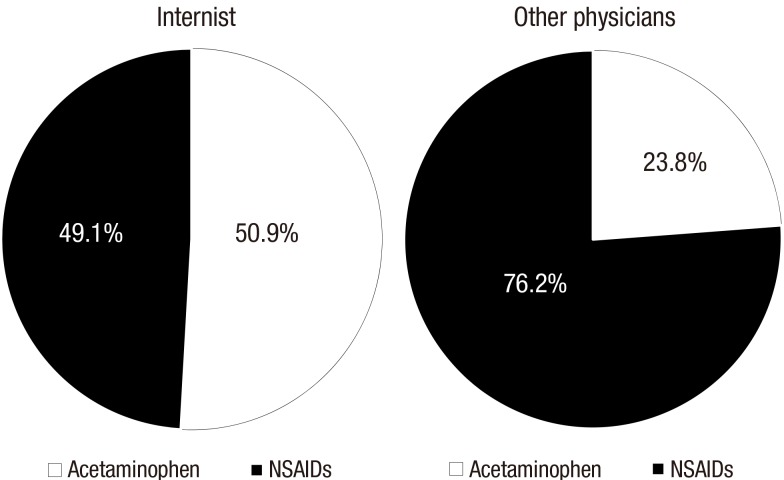
There was a marked difference in prescription preference between internists and other physicians. Internists more frequently prescribed acetaminophen compared to other physicians (50.9% vs. 23.8%, P < 0.001) (Fig. 1). There was no difference in duration of prescription of acetaminophen and NSAIDS between internists and other physician groups. Internists more frequently prescribed acetaminophen for regular use than other physicians (70.3% vs. 66.3%, P < 0.001). Both groups prescribed NSAIDs for regular use in almost all cases (Supplementary Table 1).
Fig. 1.
Prescription difference of the acetaminophen and NSAIDs between internist and other physicians.
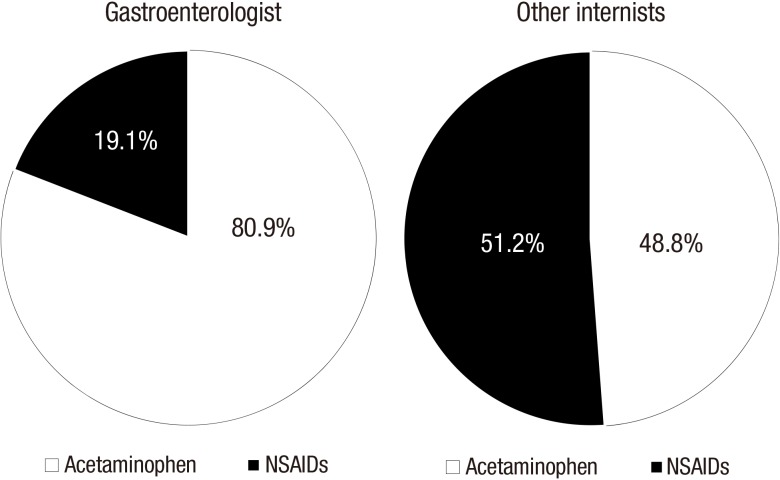
There was also marked difference in prescription preference of acetaminophen and NSAIDs between gastroenterologists and other internists. Gastroenterologists more frequently prescribed acetaminophen than NSAIDs compared to other internists (80.9% vs. 48.8%, P < 0.001) (Fig. 2).
Fig. 2.
Prescription difference of the acetaminophen and NSAIDs between gastroenterologist and other internists.
Gastroenterologists prescribed acetaminophen and NSAIDS for longer use than other internists (7.3 days vs. 4.0 days for acetaminophen and 13.4 days vs. 6.2 days for NSAIDs, P < 0.001 respectively). Gastroenterologists also more frequently prescribed both acetaminophen and NSAIDS for long-term duration (greater than 15 days) compared to other internists (11.5% vs. 2.5% for acetaminophen and 27.7% vs. 8.0% for NSAIDs, P < 0.001 respectively). However, gastroenterologists less frequently prescribed acetaminophen for regular use than other internists (46.7% vs. 73.1%). Both groups prescribed NSAIDs for regular use (50% or more of the defined daily dose) in almost all cases, although, gastroenterologists tended to less prescribe NSAIDs for regular use than other internists (Supplementary Table 2).
DISCUSSION
This study shows that most patients with cirrhosis were exposed to NSAIDs over the course of the study. Considering that NSAIDs are over-the-counter analgesics (OTCAs), we assume that they are used frequently in patients with cirrhosis. Moreover, physicians prescribe the NSAIDs more frequently than acetaminophen in patients with cirrhosis, even when decompensated.
Decompensated cirrhosis is characterized by the development of ascites, variceal bleeding and encephalopathy. It is at this stage of cirrhosis that the patient is at risk of dying from liver disease (8). Acute-on-chronic liver failure (ACLF) which is an increasingly recognized entity can precipitate decompensation in the compensated patient or lead to further deterioration in the decompensated patient (9). ACLF usually results from various precipitating factors, including infections, alcoholic hepatitis, superimposed viral hepatitis, portal thrombosis and ischemic hepatitis. Drug-induced hepatotoxicity is known as one of the most important causes of ACLF. Since acetaminophen and NSAIDs are frequently prescribed, there is concern about their use in patients with cirrhosis secondary to hepatotoxicity.
Acetaminophen is commonly prescribed for acute or chronic pain. It is well known for direct hepatotoxicity potential at high dose, and is one of the most common drugs implicated in acute hepatic failure. Moreover, acetaminophen has been reported to cause hepatic failure and death at therapeutic doses in patients with alcoholism. In one study, although 54% of patients ingested 6 grams or less per day and 30% ingested less than 4 grams per day according to patient report, the overall mortality rate reached 20% (10).
Several studies have evaluated the safety and efficacy of acetaminophen administration in patients with cirrhosis. A study of physician recommendations by Rossi et al. (5) suggested that doses of 2 g or less is safe in patient with cirrhosis. In another survey conducted by Lucena et al. (11), acetaminophen was safely used as a daily dosage of 3 g even in those with alcoholic cirrhosis. It has also been reported that daily doses of 4 g over the course of 2 weeks in healthy adults can lead to marked, but clinically silent, elevations in aspartate transaminase and alanine transaminase (12), although another study showed no significant alanine transaminase elevations with doses up to 4 grams daily when used for shorter periods (1). As a result, most physicians conclude that acetaminophen is safe in patients with cirrhosis at a reduced dose (2-3 grams or less per day) for short treatment duration (5,6).
Most NSAIDs are largely metabolized by hepatic cytochrome P450 enzyme and heavily protein bound (13). Thus serum level of NSAIDs can be increased in patients with cirrhosis due to altered metabolism and bioavailability of NSAIDs (14). NSAIDs can produce deleterious effects on patients with cirrhosis through idiosyncratic acute hepatocellular necrosis or cholestatic damage and can lead to fatal liver injury (15,16). Moreover, NSAIDs can inhibit prostaglandin synthesis in the renal arteries, causing vasoconstriction of the renal arteries, and can lead to a decreased glomerular filtration rate, thereby blunting the natriuretic effect of diuretics and reducing sodium and water excretion in patients with cirrhosis (17,18). Additionally, NSAIDs cause gastroduodenal mucosal injury and lead to bleeding from gastric erosions, peptic ulcers, gastroesophageal varices, or portal hypertensive gastropathy. NSAIDs can likewise increase bleeding risk as a result of thrombocytopenia and coagulopathy associated with advanced liver disease (19). There are several studies that reported renal impairment and gastrointestinal hemorrhage related with NSAIDs (20,21,22,23). It is generally recommended that NSAIDs should be used cautiously in patients with cirrhosis (2).
The findings of this study suggest that physicians generally have lower awareness of the potential harmful effects of NSAIDs, and are more concerned about the hepatotoxicity of acetaminophen, despite occurrences only at higher dose. The difference in prescription patterns was more apparent when compared by physician practice type. Internists prefer acetaminophen to NSAIDs when compared with other physicians and gastroenterologists have a much greater preference for acetaminophen over NSAIDs when compared with other internists. This suggests that physicians with more opportunities to treat patients with liver cirrhosis are generally more aware about the potential harmful effects of NSAIDs, and less concerned about the hepatotoxicity of acetaminophen.
Fortunately, internists and other physicians prescribed both acetaminophen and NSAIDs in short-term duration. However, both groups predominantly prescribed acetaminophen for regular use, at 50% or more of the defined daily dose, and prescribed NSAIDs for regular use in almost all cases. This difference may be related to differences in pain-related disease entities. Gastroenterologists prescribed acetaminophen for longer in general and more frequently for long-term duration than other internists and physicians, but less frequently at 50% or more of the defined daily dose. Gastroenterologists also prescribed NSAIDs for longer in general than other internists and more frequently prescribed NSAIDs for long-term duration compare to other internists. This findings may suggest that even gastroenterologists are not fully attentive to the potential harm of NSAIDs in patients with cirrhosis.
There are only a few reports evaluating physician opinions and prescription patterns for analgesics in patients with cirrhosis (5,6). These reports show that overall prescription pattern of the analgesics favors NSAIDs, which is similar to our findings. In addition, these suggest that the guidelines for the safe use of analgesics in patients with cirrhosis should be further explored because there appears to be hesitancy in recommending analgesics in patients with liver cirrhosis (5). However, these reports do not adequately reflect real community medical practice situations because of the small sample size and also because surveys were performed only in tertiary care hospitals.
These findings of our study should be interpreted cautiously because of a few limitations. Firstly, our study has the possibility of under or overestimation of the number of study population. Although liver cirrhosis is diagnosed as pathologic findings, the diagnosis of the liver cirrhosis is possible using the radiologic and laboratory findings. Because HIRA data do not provide the radiologic and laboratory findings and result in potential limitation of defining liver cirrhosis by ICD code. Secondly, there is the possibility of incomplete coding of sequelae of liver cirrhosis, especially less dramatic events like ascites or jaundice. Therefore, decompensated liver cirrhosis group could not be fully evaluated in this study. Thirdly, we were not able to review prescriptions for analgesics for all disease entities. The small number of disease entities might have led to a bias, which could be related to the prescription frequency and dose. Also, acetaminophen and NSAIDs can be used for fever as well as pain. We should consider that internists who treat more patients with liver cirrhosis can frequently prescribe the acetaminophen as confounding factor. Finally, because we did not identify the actual administration dose and duration of analgesics by reviewing medical records, the findings of our study may be different from the exposure of analgesics to the patients.
In spite of these limitations, this study highlights the physician misconceptions and awareness of potential hepatotoxicity associated with acetaminophen and NSAIDs. Many physicians seem to be more concerned about the hepatotoxicity of acetaminophen and less aware of the adverse effects of NSAIDs. Therefore, the potential harm of NSAIDs in patients with cirrhosis should be emphasized to all physicians who treat patients with cirrhosis.
This large-scale population study will provide valuable information regarding the prescription pattern of acetaminophen and NSAIDs in patients with cirrhosis and will help to establish guidance for the safe use of analgesics in patients with cirrhosis.
ACKNOWLEDGMENT
Statistical analysis is assisted by Research and Statistical Support, Research Institute of Convergence for Biomedical Science and Technology, Pusan National University Yangsan Hospital.
Footnotes
Funding: This study was supported by year 2015 research grant of Pusan National University.
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception & design of the study and drafting: Hong YM, Cho M. Critical revisions related to the important intellectual content of the manuscript: Yoon KT, Heo J, Woo HY, Lim W. Acquisition, analysis and interpretation of the data: An DS, Han JH. Approval of the final manuscript: all authors.
Supplementary Materials
Prescription difference of the acetaminophen and NSAIDs between internist and other physicians
Prescription difference of the acetaminophen and NSAIDs between gastroenterologist and other internists
References
- 1.Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) administration in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther. 2007;26:283–290. doi: 10.1111/j.1365-2036.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- 2.Dwyer JP, Jayasekera C, Nicoll A. Analgesia for the cirrhotic patient: a literature review and recommendations. J Gastroenterol Hepatol. 2014;29:1356–1360. doi: 10.1111/jgh.12560. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis - a practical guide. Aliment Pharmacol Ther. 2013;37:1132–1156. doi: 10.1111/apt.12324. [DOI] [PubMed] [Google Scholar]
- 4.Imani F, Motavaf M, Safari S, Alavian SM. The therapeutic use of analgesics in patients with liver cirrhosis: a literature review and evidence-based recommendations. Hepat Mon. 2014;14:e23539. doi: 10.5812/hepatmon.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi S, Assis DN, Awsare M, Brunner M, Skole K, Rai J, Andrel J, Herrine SK, Reddy RK, Navarro VJ. Use of over-the-counter analgesics in patients with chronic liver disease: physicians’ recommendations. Drug Saf. 2008;31:261–270. doi: 10.2165/00002018-200831030-00007. [DOI] [PubMed] [Google Scholar]
- 6.Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7:994–999. doi: 10.1016/j.cgh.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Collaborating Center for Drug Statistics Methodology. Defined daily dose: definition and general considerations. [accessed on 1 January 2016]. Available at http://www.whocc.no/ddd/definition_and_general_considera/
- 8.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. 1437.e1–1437.e9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman HJ, Maddrey WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology. 1995;22:767–773. [PubMed] [Google Scholar]
- 11.Lucena MI, Andrade RJ, Tognoni G, Hidalgo R, Sanchez de la Cuesta F, Spanish Collaborative Study Group on Therapeutic Management of Liver Diseases Drug use for non-hepatic associated conditions in patients with liver cirrhosis. Eur J Clin Pharmacol. 2003;59:71–76. doi: 10.1007/s00228-003-0586-2. [DOI] [PubMed] [Google Scholar]
- 12.Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Schoene B, Fleischmann RA, Remmer H, von Oldershausen HF. Determination of drug metabolizing enzymes in needle biopsies of human liver. Eur J Clin Pharmacol. 1972;4:65–73. doi: 10.1007/BF00562499. [DOI] [PubMed] [Google Scholar]
- 14.Williams RL, Upton RA, Cello JP, Jones RM, Blitstein M, Kelly J, Nierenburg D. Naproxen disposition in patients with alcoholic cirrhosis. Eur J Clin Pharmacol. 1984;27:291–296. doi: 10.1007/BF00542162. [DOI] [PubMed] [Google Scholar]
- 15.Carson JL, Strom BL, Duff A, Gupta A, Das K. Safety of nonsteroidal anti-inflammatory drugs with respect to acute liver disease. Arch Intern Med. 1993;153:1331–1336. [PubMed] [Google Scholar]
- 16.Fry SW, Seeff LB. Hepatotoxicity of analgesics and anti-inflammatory agents. Gastroenterol Clin North Am. 1995;24:875–905. [PubMed] [Google Scholar]
- 17.Mirouze D, Zipser RD, Reynolds TB. Effect of inhibitors of prostaglandin synthesis on induced diuresis in cirrhosis. Hepatology. 1983;3:50–55. doi: 10.1002/hep.1840030108. [DOI] [PubMed] [Google Scholar]
- 18.Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215–222. [PubMed] [Google Scholar]
- 19.Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35:209–219. doi: 10.1002/j.1552-4604.1995.tb04050.x. [DOI] [PubMed] [Google Scholar]
- 20.Brater DC, Anderson SA, Brown-Cartwright D. Reversible acute decrease in renal function by NSAIDs in cirrhosis. Am J Med Sci. 1987;294:168–174. doi: 10.1097/00000441-198709000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Clària J, Kent JD, López-Parra M, Escolar G, Ruiz-Del-Arbol L, Ginès P, Jiménez W, Vucelic B, Arroyo V. Effects of celecoxib and naproxen on renal function in nonazotemic patients with cirrhosis and ascites. Hepatology. 2005;41:579–587. doi: 10.1002/hep.20595. [DOI] [PubMed] [Google Scholar]
- 22.Lee YC, Chang CH, Lin JW, Chen HC, Lin MS, Lai MS. Non-steroidal anti-inflammatory drugs use and risk of upper gastrointestinal adverse events in cirrhotic patients. Liver Int. 2012;32:859–866. doi: 10.1111/j.1478-3231.2011.02739.x. [DOI] [PubMed] [Google Scholar]
- 23.Luo JC, Leu HB, Hou MC, Huang CC, Lin HC, Lee FY, Chang FY, Chan WL, Lin SJ, Chen JW. Cirrhotic patients at increased risk of peptic ulcer bleeding: a nationwide population-based cohort study. Aliment Pharmacol Ther. 2012;36:542–550. doi: 10.1111/j.1365-2036.2012.05225.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prescription difference of the acetaminophen and NSAIDs between internist and other physicians
Prescription difference of the acetaminophen and NSAIDs between gastroenterologist and other internists