Abstract
Polyethylene glycols (PEGs) are believed to be chemically inert agents, but larger PEG polymers could have immunogenicity. A 39-year-old man was referred to emergency room for loss of consciousness and dyspnea after taking of PEG-3350 (Colyte®). In laboratory findings, the initial serum tryptase level was increased to 91.9 mg/L (normal range: 0.00-11.40 mg/L) without any other laboratory abnormalities. The intradermal test with 10 mg/mL Colyte® showed a 5 × 5 mm wheal, but basophil activation and histamine releasability tests were negative. PEG-3350 is widely used as an osmotic laxative due to its lack of absorption from the gastrointestinal tract. However, the loss of mucosal integrity at gastrointestinal membrane such as diverticulitis may be a predisposing factor for anaphylaxis to Colyte®. We report a case of anaphylaxis induced by the ingestion of PEG-3350 in a patient with diverticulitis which might be a risk factor of anaphylaxis.
Keywords: Polyethylene Glycols (PEG), Anaphylaxis, Diverticulitis
Graphical Abstract
INTRODUCTION
Polyethylene glycols (PEGs) are nonionic hydrophilic polymers of ethylene oxide that have been widely used to produce cosmetics and medications in the cosmetic and pharmaceutical industry. PEG products are of liquid or low-melting solid depending on their molecular weights. In general, PEGs are believed to be chemically inert, but larger PEG polymers could have immunogenic properties because they are potentially large enough to illicit an immune response without haptenization (1).
CASE DESCRIPTION
A 39-year-old man was referred to the Emergency Department for loss of consciousness and shortness of breath which had developed one and a half hours after intake of an evacuant solution (PEG-3350, Colyte®, Taejoon pharma Co., Ltd., Seoul, Korea) during preparation of colonoscopy in 2013. He was diagnosed with mild atopic dermatitis, but he did not have any other allergic diseases to any drug or food. Ten years ago, he had colonoscopy safely with Colyte®. One month ago, he was admitted for diverticulitis and received cefixime (suprax®, Dong-A ST Co., Ltd., Seoul, Korea) for discharge medication.
When he arrived at the Emergency Department, his blood pressure was 80/66 mmHg, and he had severe respiratory distress with wheezing sounds in whole lung fields. Intramuscular administration of epinephrine (epinephrine injection solution, Daihan Pharm Co., Ltd., Seoul, Korea) 0.3 mg rapidly restored blood pressure and alleviated respiratory symptoms. Fortunately, his clinical conditions fully improved without sequelae. In laboratory test, serum total IgE level was not increased (69 KU/L) and skin prick test revealed strong positive only to house dust mites. However, the initial serum tryptase level was significantly increased to 91.9 mg/L (0.00-11.40 mg/L).
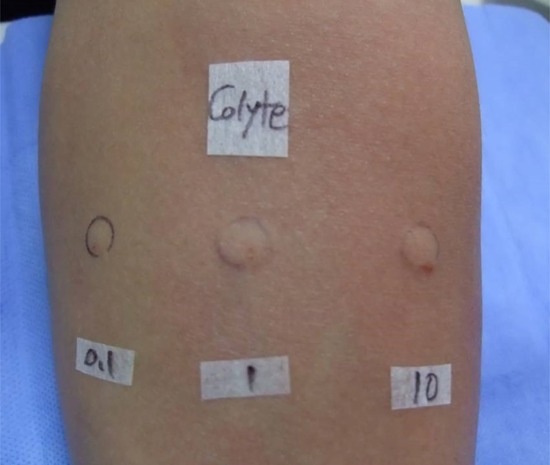
We performed skin prick and intradermal tests with different concentrations of Colyte® solutions, histamine as a positive control, and normal saline as a negative control 2 months after the event in 2013. As shown in Fig. 1, all skin prick test results were negative, but the intradermal test with 10 mg/mL Colyte® showed a 5 × 5 mm sized wheal. Next, basophil activation and histamine releasability tests were conducted to evaluate the non-IgE mediated mechanism of Colyte®; however, both tests gave negative results. Finally, oral provocation tests with cefixime were performed to evaluate drug allergy, but the result was also negative.
Fig. 1. Intradermal tests with different concentrations of diluted Colyte® solutions. The intradermal test with 10 mg/mL of Colyte® showed a 5 × 5 mm sized wheal. 0.1:0.1 mg/mL of Colyte®, 1:1 mg/mL of Colyte®, 10:10 mg/mL of Colyte®.
DISCUSSION
PEGs or macrogols are hydrophilic polyethers commonly used in pharmaceutical, cosmetic, food and household products. Various molecular weights of PEG have been used extensively for numerous applications such as injection solutions, pills, aqueous solutions, skin disinfectants, and toothpastes (1). PEG with a molecular mass of 3,350 g/mol is widely used as an osmotic laxative due to its lack of absorption from the gastrointestinal tract and chemically inertness in healthy people. Although there are a few reports of PEG induced anaphylaxis, the incidence is extremely rare (2).
Precise mechanisms of PEG-induced anaphylaxis have not yet been fully elucidated. We performed skin prick, intradermal, basophil activation, and histamine releasability tests with PEG, but there was only a weakly positive reaction in intradermal tests. In a previous report, Wenande et al. (3) reported the possibility of IgE medicated mechanism in PEG allergy after passive sensitization of IgE stripped donor basophil with patient serum without detection of PEG specific IgE, and other report showed that complement activation may play a key role in hypersensitivity reactions to pegylated liposomal doxorubicin (4).
However, there is a possibility that loss of mucosal integrity can promote systemic absorption of PEG to facilitate the immunologic reaction (5). A reported case with a history of ulcerative colitis presented with anaphylaxis after administration of PEG (6); other cases were referred for colonoscopy for rectal bleeding, and preparation with PEG solution resulted in angioedema and anaphylaxis (7,8). We reported the case of anaphylaxis to Colyte® in a patient with diverticulitis. Damage to gastrointestinal mucous membrane integrity may be a predisposing factor for anaphylaxis to Colyte®; therefore, caution should be exercised in its use in patients who have gastrointestinal disintegrity such as diverticulitis.
Although anaphylaxis to Colyte® is extremely rare, it should be considered in its use for patients who have damaged gastrointestinal mucous membrane integrity.
Footnotes
Funding: This research was supported by a grant of the Korea Health Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI14C2628).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Research conception & design: Shin YS, Park HS. Performing the experiments: Hwang SH. Data interpretation: Park JS. Drafting of the manuscript: Hwang SH, Park JS, Lee SH. Critical revision of the manuscript: Shin YS, Lee SH. Approval of final manuscript: all authors.
References
- 1.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 2.Lee SH, Cha JM, Lee JI, Joo KR, Shin HP, Baek IH, Jeon JW, Lim JU, Lee JL, Lee HM, et al. Anaphylactic shock caused by ingestion of polyethylene glycol. Intest Res. 2015;13:90–94. doi: 10.5217/ir.2015.13.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenande EC, Skov PS, Mosbech H, Poulsen LK, Garvey LH. Inhibition of polyethylene glycol-induced histamine release by monomeric ethylene and diethylene glycol: a case of probable polyethylene glycol allergy. J Allergy Clin Immunol. 2013;131:1425–1427. doi: 10.1016/j.jaci.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Chanan-Khan A, Szebeni J, Savay S, Liebes L, Rafique NM, Alving CR, Muggia FM. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol. 2003;14:1430–1437. doi: 10.1093/annonc/mdg374. [DOI] [PubMed] [Google Scholar]
- 5.Almer S, Franzén L, Olaison G, Smedh K, Ström M. Increased absorption of polyethylene glycol 600 deposited in the colon in active ulcerative colitis. Gut. 1993;34:509–513. doi: 10.1136/gut.34.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah S, Prematta T, Adkinson NF, Ishmael FT. Hypersensitivity to polyethylene glycols. J Clin Pharmacol. 2013;53:352–355. doi: 10.1177/0091270012447122. [DOI] [PubMed] [Google Scholar]
- 7.Stollman N, Manten HD. Angioedema from oral polyethylene glycol electrolyte lavage solution. Gastrointest Endosc. 1996;44:209–210. doi: 10.1016/s0016-5107(96)70150-5. [DOI] [PubMed] [Google Scholar]
- 8.Schuman E, Balsam PE. Probable anaphylactic reaction to polyethylene glycol electrolyte lavage solution. Gastrointest Endosc. 1991;37:411. doi: 10.1016/s0016-5107(91)70761-x. [DOI] [PubMed] [Google Scholar]


