Abstract
Although accumulating evidence suggests that microglia-mediated neuroinflammation may be crucial for the initiation and progression of Parkinson's disease (PD), and that the control of neuroinflammation may be a useful strategy for preventing the degeneration of nigrostriatal dopaminergic (DA) projections in the adult brain, it is still unclear what kinds of endogenous biomolecules initiate microglial activation, consequently resulting in neurodegeneration. Recently, we reported that the increase in the levels of prothrombin kringle-2 (pKr-2), which is a domain of prothrombin that is generated by active thrombin, can lead to disruption of the nigrostriatal DA projection. This disruption is mediated by neurotoxic inflammatory events via the induction of microglial Toll-like receptor 4 (TLR4) in vivo , thereby resulting in less neurotoxicity in TLR4-deficient mice. Moreover, inhibition of microglial activation following minocycline treatment, which has anti-inflammatory activity, protects DA neurons from pKr-2-induced neurotoxicity in the substantia nigra (SN) in vivo. We also found that the levels of pKr-2 and microglial TLR4 were significantly increased in the SN of PD patients compared to those of age-matched controls. These observations suggest that there may be a correlation between pKr-2 and microglial TLR4 in the initiation and progression of PD, and that inhibition of pKr-2-induced microglial activation may be protective against the degeneration of the nigrostriatal DA system in vivo. To describe the significance of pKr-2 overexpression, which may have a role in the pathogenesis of PD, we have reviewed the mechanisms of pKr-2-induced microglial activation, which results in neurodegeneration in the SN of the adult brain.
Keywords: Prothrombin kringle-2, Parkinson's disease, Microglia, Toll-like receptor 4
INTRODUCTION
Parkinson's disease (PD) is the second-most common neurodegenerative disorder and is characterized by the progressive degeneration of dopaminergic (DA) neurons and a decrease in striatal dopamine. PD is associated with clinical movement disorders, including a tremor at rest, rigidity of the limbs, bradykinesia (slowness and paucity of voluntary movement), and postural instability (a tendency to fall even in the absence of weakness or cerebellar balance disturbance) [1,2,3]. Although we do not fully understand the etiology of PD, accumulating evidence suggests that microglia, which are the resident immune cells of the brain, are crucial mediators of the brain inflammatory processes that lead to neurotoxicity, and that excessive microglial activation contributes to the initiation and progression of PD [3,4,5]. However, it is largely unknown what endogenous biomolecules initiate and stimulate microglial activation, even though the control of microglial activators, which stimulate neurotoxic inflammation, may be a useful strategy for the prevention of the degeneration of the nigrostriatal DA projection in the adult brain.
Toll-like receptors (TLRs) are pattern recognition receptors that recognize specific pathogen-associated molecular signatures and subsequently initiate inflammatory and immune responses [3,6]. TLR4 recognizes various ligands, such as lipopolysaccharide (LPS), envelope proteins, heat-shock proteins, fibrinogen, and hyaluronan [3,6]. The activation of TLR4 in immune cells induces increases in the levels of inflammatory cytokines [3,7]. Although the pattern of TLR expression in the brain is controversial, there are many reports suggesting that microglia are important cells for TLR4-mediated immune responses, which may be involved in neurodegenerative diseases such as Alzheimer's disease (AD) and PD [8,9,10]. Moreover, increases in TLR4 expression have been observed in α-synuclein-overexpressing transgenic mice and in patients with multiple system atrophy [11], although alterations in TLR4 expression in patients with PD are still unclear. These results suggest that an increase in microglial TLR4 may be crucial for the pathogenesis of PD, and that the discovery of endogenous molecules involved in the induction of microglial TLR4 may be useful in guiding the development of knowledge-based targeted therapeutics for PD.
We previously reported that prothrombin kringle-2 (pKr-2), which is a domain of prothrombin that is generated by active thrombin, is able to induce the death of DA neurons in the rat SN through microglial activation, even though pKr-2 itself was not directly toxic to neurons [5]. Moreover, we recently found that patients with PD have increased pKr-2 expression in the SN, and that nigrostriatal DA projections might degenerate due to neurotoxic inflammation following pKr-2 upregulation-induced production of microglial TLR4 in the SN of adult murine brain [3]. These results suggest that pKr-2 might be a potential pathogenic factor in PD, and that limiting pKr-2-induced microglial activation may be an effective therapeutic strategy for protecting DA neurons in the adult brain.
Parkinson's disease and microglial activation
The histopathological features of PD are the idiopathic degeneration of DA neurons in the pars compacta of the SN and loss of DA nerve terminals in the striatum [12]. This progressive neurodegeneration, which consequently results in the reduction of dopamine in the nigrostriatal DA system [12], is generally accompanied by both motor and non-motor symptoms. The non-motor symptoms of PD include olfactory dysfunction, cognitive impairment, psychiatric symptoms, sleep disorders, pain, depression, and rapid eye movement sleep behavior disorders [13]. The motor symptoms of PD include movement disorders such as resting tremor, muscular rigidity, bradykinesia, akinesia, and postural instability [13]. Although the maintenance of the dopamine concentration is considered to be a useful target in the development of therapeutics against PD progression, clinical trials focusing on dopamine production have not been successful [14,15]. Levodopa (L-3,4-dihydroxyphenylalanine), which is a precursor of dopamine, is one of the main drugs used to treat PD symptoms. However, the long-term use of levodopa is associated with complications, such as abnormal involuntary movements called dyskinesias and dystonias [14,15]. Moreover, no treatment has been identified that forestalls deterioration attributable to progressive neurodegeneration [1,2]. These findings indicate that sustained dopamine supplementation alone is unable to protect or restore DA systems during PD progression. Thus, the control of PD pathogenesis using approaches such as inhibition of mitochondrial dysfunction and reduction of activated microglia-derived oxidative stress and/or neuroinflammation, may be more important in treating PD progression than the maintenance of dopamine production [16,17,18,19], even though this is the major therapeutic strategy currently used to treat patients with PD.
Accumulating evidence suggests that microglial activation, which is an important neurotoxic mechanism, plays important roles in the initiation and progression of PD [20,21]. Imamura et al. have previously reported that the accumulation of CR3/43-positive cells (activated microglia) is increased in the SN and putamen in the post-mortem brains of patients with PD [20]. Furthermore, [11C](R)-PK11195, which is a radiotracer used to detect activated microglia, noticeably accumulates in the brains of patients diagnosed with PD [21]. These findings strongly suggest that microglial activation negatively affects neuronal cell survival and consequently leads to neurodegeneration in PD.
Microglia and neuroinflammation
Microglia are the resident immune cells in the central nervous system (CNS). In the resting state, microglia have small cell bodies and numerous processes and can support neuronal function and survival [22]. In pathological conditions, microglia stimulated by various activators undergo phagocytic morphological changes, which are characterized by enlarged cell bodies and short processes, and these altered microglia exert beneficial effects that repair tissue by releasing anti-inflammatory cytokines and neurotrophic factors [22,23]. However, the major cause of neurotoxic inflammation in the CNS is the response of microglia to a variety of stimuli, such as infection, trauma, and toxins [19], and activated microglia produce neurotoxic inflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6 [22,24]. In addition, activated microglia can produce reactive oxygen species (ROS), such as O2- and O2--derived oxidants, via the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which is also involved in neuroinflammatory processes [25]. Moreover, under proinflammatory conditions, microglia can release more ROS than neurons [26]. Excessive ROS production is implicated in neurodegenerative diseases [27]. Accordingly, many activated microglia are observed in post-mortem tissue from patients with PD [3]. In addition, highly increased levels of proinflammatory cytokines, such as TNF-α, IL-1β and interferon-γ, are also found in the SN of PD patients [3,19,20]. Similar to the increases in proinflammatory cytokines, the levels of NADPH oxidase are upregulated in microglia in the post-mortem midbrain tissue of PD patients [28].
Consistent with the observation of increases in NADPH oxidase and neurotoxic cytokines in the brains of patients with PD [3,19,20,28], there are many reports indicating the significance of these factors involved in the degeneration of the nigrostriatal DA system in animal models of PD. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was discovered to be a neurotoxic compound in drug addicts who showed parkinsonian symptoms [29] and has been widely used in the production of PD animal models [30]. In the SN of adult mice, MPTP induces microglial activation, which is involved in neurotoxicity [31]. The blockade of microglial activation following treatment with minocycline, a broad-spectrum tetracycline antibiotic, inhibits the production of microglial-derived deleterious factors, and exerts neuroprotective effects in the MPTP-lesioned SN [30]. These suggest that microglial activation is involved in the PD pathogenesis related to DA neuronal damage. In addition, the increased expression of gp91phox, which is an NADPH oxidase subunit, is observed in the activated microglia of MPTP-injected mice [28]. In fact, the upregulation of gp91phox within activated microglia is markedly reduced by minocycline treatment, which leads to the protection of DA neurons in vivo [28].
6-hydroxydopamine (6-OHDA) is also often used to produce PD models for the testing symptomatic therapies and the study of the mechanisms of DA neuronal death [1]. Since the structure of 6-OHDA is similar to that of dopamine, it is taken up into DA neurons by the dopamine transporter, where it acts as a neuronal toxin by generating ROS [30]. In addition to direct neuronal toxicity, 6-OHDA-induced neurotoxicity can promote microglial activation in nigrostriatal DA projection areas [32,33], resulting in the production of neurotoxic proinflammatory cytokines [34,35]. In addition, microglial activation induced by 6-OHDA treatment parallels the activation of NADPH oxidase, including the p47phox and gp91phox subunits, in nigral microglia [36]. Similar to the effects of MPTP, the 6-OHDA-induced toxic effects in DA neurons are attenuated by minocycline treatment [37]. Taken together, these results suggest that microglial-derived neuroinflammation, which includes the production of neurotoxic cytokines and activation of NADPH oxidase within microglia, may play a crucial role in DA neuronal degeneration in animal models of PD. In addition to MPTP and 6-OHDA, studies using rotenone, which is an odorless, colorless, crystalline isoflavone used as a broad-spectrum insecticide, piscicide, and pesticide, have also indicated that the production of neurotoxic cytokines and activation of NADPH oxidase in microglia play important roles in PD pathogenesis [38,39].
The above observations indicate that excessive increases in proinflammatory mediators and ROS production following microglial activation lead to severe neurotoxicity, resulting in the deterioration of the DA system in the adult brain. While numerous studies have examined the development of therapeutic and preventive agents against PD, there are currently no cures that stop or slow down the progressive degeneration of DA neurons and PD symptoms. Therefore, efforts to develop therapeutic and preventive agents for PD need to aim at inhibiting microglial-derived neuroinflammation, which would suppress microglial activation and its pathogenic mechanisms.
Control of neuroin flammation by micro glial TLR4
TLR4 initiates the activation of the innate immune response by recognizing pathogens as a pattern-recognition receptor. LPS is a representative ligand for TLR4 [40]. The TLR4-mediated activation of NF-κB and mitogen-activated protein kinases that occurs via myeloid differentiation factor 88-dependent and -independent pathways induces the expression of proinflammatory cytokines and chemokines [6,41,42]. TLR4, which is highly expressed in the brain, is stimulated by LPS in microglia [43,44]. Neuroinflammation following microglial TLR4 activation has been implicated in neurodegenerative disease. The knockout of TLR4 protects against traumatic brain injury [45], focal cerebral ischemic injury [46], and pertussis toxin-induced experimental autoimmune encephalomyelitis [47]. In AD, betaamyloid (Aβ) deposition promotes inflammatory reactions, and TLR4 is upregulated in the glial cells surrounding the Aβ plaques in the brains of patients with AD [48]. The destructive mutation of TLR4 inhibits microglial activation by Aβ deposition, which results in a significant decrease in the levels of proinflammatory cytokines and chemokines [9,48]. These findings suggest that TLR4 is essential for the Aβ-induced release of proinflammatory cytokines and chemokines, which is a hallmark of microglial activation and neurotoxicity [8,9,48,49]. Microglial TLR4 is also associated with neuroinflammation in PD. Recent reports indicate that increased expression of microglial TLR4 is found in the post-mortem tissue of patients with PD [3]. In addition, TLR4 deficiency attenuates MPTP-induced neurotoxicity and is correlated with the inhibition of microglial activation in the SN [50]. Alpha-synuclein contributes to microglial activation during PD progression. The generation of ROS and proinflammatory cytokines is reduced in TLR4-deficient microglia after treatment with α-synuclein [10]. Moreover, LPS-induced ROS generation can be mediated by direct interaction between microglial TLR4 and NADPH oxidase, suggesting that microglial TLR4 may be a beneficial target in inhibiting NADPH oxidase activity [51]. Therefore, the control of microglial TLR4 expression may be a potential and important target for therapeutic intervention in neurodegenerative disease.
Production of pKr-2 and its general roles in vivo
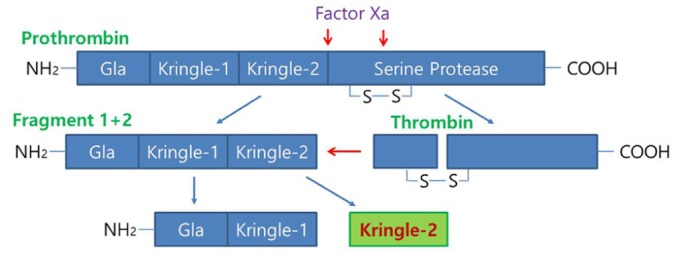
Prothrombin, which is known to be synthesized mainly in the liver and then secreted into the bloodstream [52], is cleaved to produce fragment 1-2 (kringle regions) and thrombin during activation [53,54]. Activated thrombin, a serine protease which converts soluble fibrinogen into insoluble fibrin for blood coagulation, can cleave pKr-1 and -2 (Fig. 1) [55]. The functions of pKr-2 are well-established in the field of angiogenesis. For instance, pKr-2, which is purified from LPS-treated rabbit serum, acts as an angiogenic inhibitor during bovine capillary endothelial cell proliferation [56]. Treatment with pKr-2 induces the suppression of basic fibroblast growth factor-triggered endothelial cell growth and angiogenesis in the chorioallantoic membrane of chick embryos [57]. Moreover, treatment with pKr-2 inhibits endothelial cell proliferation and angiogenesis by inactivating the cyclin D1/cyclin-dependent kinase 4 (CDK4) complex through the induction of ROS production and upregulation of nuclear CDK inhibitors [58]. It also inhibits fibrin formation and platelet aggregation by binding to thrombin and inducing conformational changes at its active site, resulting in a reduction in the clotting activity of thrombin [59]. In addition, recombinant human pKr-2 reduces the immunoreactivity of matrix metalloproteinases 2 and 9 and the expression of vascular endothelial growth factor, which results in the inhibition of B16F10 melanoma cell metastasis [60].
Fig. 1. Schematic representation of prothrombin kringle-2 (pKr-2) generation from prothrombin. Prothrombin consists of Gla, Kringle-1, Kringle-2, and the serine protease domain (thrombin). Prothrombin is cleaved by Factor Xa into two fragments: fragment 1 plus 2, which includes Gla, Kringle-1, and Kringle-2; and a protease catalytic domain, thrombin. Active thrombin then cleaves fragment 1 plus 2 into fragment 1, which includes Gla and Kringle-1, and fragment 2, which consists of Kringle-2, which then produces pKr-2. The red arrows indicate the proteolytic activity of Factor Xa and thrombin, and the blue arrows indicate the domains of proteolytic activity.
Although many studies have investigated the functions of pKr-2 [56,57,58,59,60] and prothrombin is expressed in brain tissues [52], few reports have examined the roles of pKr-2 in the CNS. The accumulation of prothrombin and thrombin, which might be due to blood-brain barrier leakage, has been shown in the brains of patients with PD and AD [61,62,63], which suggests a possible increase in pKr-2 expression. Moreover, we previously reported that the upregulation of pKr-2 could contribute to microglial activation, resulting in neurodegeneration in the SN of murine brains [3]. Therefore, these results suggest that pKr-2 expression is increased in the lesioned brain and that its upregulation is involved in neurotoxic effects in the adult brain.
pKr-2 as an endogenous pathogen in the nigrostriatal DA system
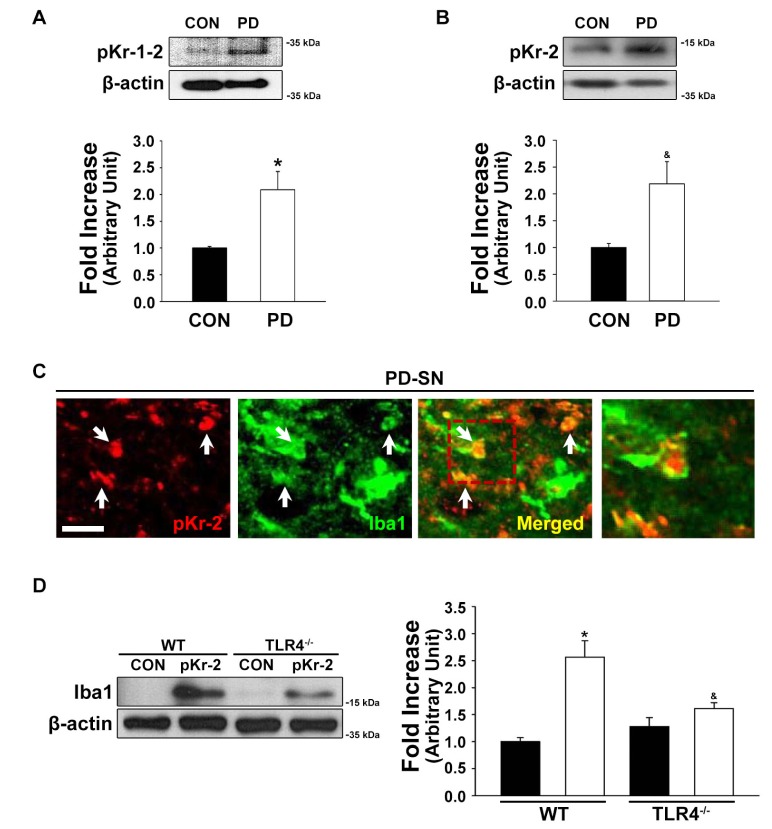
pKr-2 can trigger microglial activation, resulting in the production of neurotoxic cytokines such as TNF-α and IL-1β, and inflammatory mediators such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 [5,64,65]. In addition, pKr-2-activated microglia produce O2- and O2--derived oxidants through the activation of NADPH oxidase, which is also involved in neurotoxic events in the murine cortex [64]. Moreover, we recently suggested that pKr-2 upregulation is involved in the pathogenesis of PD, and that the control of microglial pKr-2 expression and pKr-2-induced microglial TLR4 overexpression might be important for protecting the nigrostriatal DA system against PD [3]. These results help the understanding of the relationships among pKr-2, microglia, and TLR4 (Fig. 2). To ascertain whether pKr-2 is involved in PD via the activation of microglial and microglial TLR4-mediated neuroinflammatory responses, we quantitatively analyzed pKr-2 levels in the SN of patients with PD and found that TLR4 was involved in pKr-2-induced microglial activation (Fig. 2). The expression levels of pKr-1-2, which is a precursor of active thrombin-cleaved pKr-2, and pKr-2 were significantly increased in the post-mortem brain tissue of patients with PD [*p=0.018 and &p=0.008 vs. age-matched controls (CON), respectively; Fig. 2A and 2B]. As previously reported [3], 41% of the pKr-2 expression was localized within ionized calcium binding adaptor molecule 1 (Iba1)-positive microglia in the SN of patients with PD (Fig. 2C; data not shown). Moreover, the levels of Iba1 expression were significantly increased in the SN of pKr-2-treated wild-type (WT) mice [*p<0.001 vs. phosphate buffered saline (PBS)-treated WT mice; Fig. 2D]. However, this increase was significantly reduced by TLR4 deficiency (&p=0.011 vs. pKr-2-treated WT mice; Fig. 2D). Taken together, these results suggest that an increase in pKr-2 expression is involved in microglial activation via TLR4 upregulation in the SN of adult brain (Fig. 2), even though it is unclear whether pKr-2-specific receptors exist in microglia and how pKr-2 induces an increase in microglial TLR4.
Fig. 2. The expression of pKr-2 in the substantia nigra (SN) of patients with Parkinson's disease (PD). (A, B) Western blot analysis for pKr-1-2 and pKr-2, respectively, in the human SN. Patients with PD had increases in the levels of pKr-1-2 (A) and pKr-2 (B) in the SN compared to age-matched controls (CON). *p=0.018 vs. CON (t-test; n=4, each group); &p=0.008 vs. CON (t-test; n=3, each group). (C) The immunofluorescence staining for pKr-2 (red) and Iba1 (green) indicates that the expression of pKr-2 is co-localized within Iba1-positive cells in the SN of patients with PD. Scale bar, 10 µm. (D) Western blot analysis of Iba1 expression in the pKr-2-treated SN. Three days after treatment with pKr-2 (24 µg/2 µl) or phosphate-buffered saline (PBS, vehicle control), the levels of pKr-2-increased Iba1 expression were significantly decreased in TLR4 knockout (TLR4-/-) mice compared to wild type (WT) mice. *p<0.001 vs. PBS-treated mice (CON); &p=0.011 vs. pKr-2-treated WT mice (one-way ANOVA and Tukey's post hoc analysis; n=4, each group).
Control of pKr-2-induced neurotoxicity
Since inhibiting inflammation is an important strategy for the prevention of neuronal damage in neurodegenerative disease, therapies for the control of the activation of microglia have been proposed. Although the exact mechanisms are unknown, our recent results strongly suggest that TLR4 and pKr-2 are closely associated with neuroinflammation in PD [3]. Similar to our results involved in neurodegeneration in the DA system, previous studies have shown that TLR4 expression is upregulated in the MPTP-treated animal model of PD [66] and that microglial TLR4 is directly activated by α-synuclein treatment [10]. Taken together, these results suggest that the modulation of microglial TLR4 by controlling pKr-2 production may provide us with important clues regarding the mechanisms responsible for inflammation-associated neurodegeneration in PD and open innovative therapeutic perspectives for the treatment of PD. For instance, treatment with minocycline has neuroprotective effects in preclinical studies of neurodegenerative diseases [2,30,67,68,69,70,71]. In particular, treatment with minocycline protects DA neurons against pKr-2-induced neurotoxicity through the inhibition of inflammatory responses in the brains of adult mice [2]. Moreover, its treatment reduces the expression of proinflammatory cytokines and iNOS, which is significantly increased by activated microglia following pKr-2 upregulation. These results suggest that the development of efficient anti-inflammatory drugs against pKr-2 may be useful for protecting DA neurons in the SN of lesioned adult brain.
Conclusion
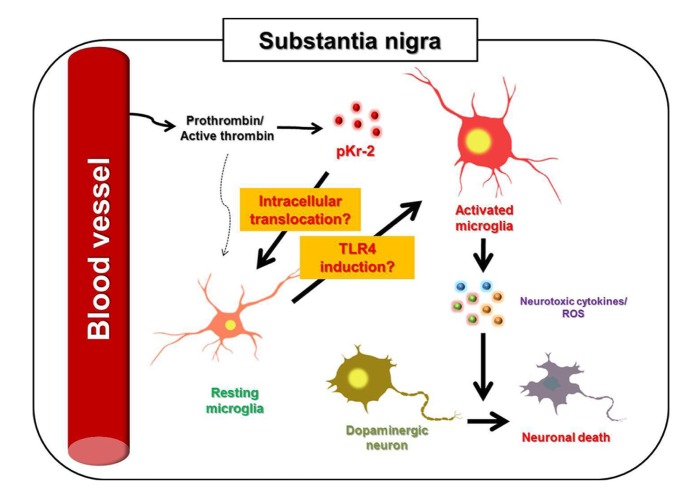
The discovery of endogenous biomolecules that initiate and stimulate microglial activation and result in neurodegeneration via neurotoxic inflammatory events in the adult brain is very important, as the control of microglial activators may be a useful strategy in preventing the degeneration of the nigrostriatal DA projection in the adult brain [3,5]. In addition, the discovery of endogenous molecules involved in the induction of microglial TLR4, which may be crucial for the pathogenesis of PD, may also be useful in guiding the development of knowledge-based targeted therapeutics for PD. pKr-2 upregulation may lead to the disruption of the nigrostriatal DA projection by microglial activation, resulting in the production of neurotoxic inflammatory biomolecules, such as ROS following NADPH oxidase activation, and proinflammatory cytokines, such as TNF-α and IL-1β [3,5,64]. Moreover, pKr-2 expression is significantly increased and co-localized in activated microglia in the SN of patients with PD, and the upregulation of microglial TLR4 might be a key mechanism for pKr-2-induced neurotoxic inflammation in the nigrostriatal DA system of murine brain [3]. Although it is still unclear how pKr-2 is translocated within microglia and further studies are needed to clarify the relationship between pKr-2 and the transcription factors involved in the induction of microglial TLR4 (Fig. 3), our results suggest that pKr-2 upregulation in the SN may be a potential pathogenic mechanism in PD, and that the induction of microglial TLR4 following pKr-2 overexpression may be an important target mechanism in the development of therapeutics against pKr-2-induced neurodegeneration in the nigrostriatal DA system of the adult brain.
Fig. 3. Schematic representation of pKr-2-mediated neurotoxicity in the SN. Breakdown of the blood-brain barrier may be involved in an influx of prothrombin, which consequently resulting in the production of thrombin and pKr-2 in the SN. The increased pKr-2 may be translocated within microglia, and microglial activation may be mediated by the induction of microglial TLR4 following pKr-2 translocation. Activated microglia can produce neurotoxic cytokines and reactive oxygen species (ROS). These neurotoxic factors induce dopaminergic (DA) neuronal death.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (No. 2014R1A1A2056508), and also by grants from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare (HI15C1928).
References
- 1.Kim SR, Kareva T, Yarygina O, Kholodilov N, Burke RE. AAV transduction of dopamine neurons with constitutively active Rheb protects from neurodegeneration and mediates axon regrowth. Mol Ther. 2012;20:275–286. doi: 10.1038/mt.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nam JH, Leem E, Jeon MT, Jeong KH, Park JW, Jung UJ, Kholodilov N, Burke RE, Jin BK, Kim SR. Induction of GDNF and BDNF by hRheb(S16H) transduction of SNpc neurons: neuroprotective mechanisms of hRheb(S16H) in a model of Parkinson's disease. Mol Neurobiol. 2015;51:487–499. doi: 10.1007/s12035-014-8729-2. [DOI] [PubMed] [Google Scholar]
- 3.Shin WH, Jeon MT, Leem E, Won SY, Jeong KH, Park SJ, McLean C, Lee SJ, Jin BK, Jung UJ, Kim SR. Induction of microglial toll-like receptor 4 by prothrombin kringle-2: a potential pathogenic mechanism in Parkinson's disease. Sci Rep. 2015;5:14764. doi: 10.1038/srep14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 5.Kim SR, Chung ES, Bok E, Baik HH, Chung YC, Won SY, Joe E, Kim TH, Kim SS, Jin MY, Choi SH, Jin BK. Prothrombin kringle-2 induces death of mesencephalic dopaminergic neurons in vivo and in vitro via microglial activation. J Neurosci Res. 2010;88:1537–1548. doi: 10.1002/jnr.22318. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 8.Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer's disease. J Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song M, Jin J, Lim JE, Kou J, Pattanayak A, Rehman JA, Kim HD, Tahara K, Lalonde R, Fukuchi K. TLR4 mutation reduces microglial activation, increases Aβ deposits and exacerbates cognitive deficits in a mouse model of Alzheimer's disease. J Neuroinflammation. 2011;8:92. doi: 10.1186/1742-2094-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefanova N, Reindl M, Neumann M, Kahle PJ, Poewe W, Wenning GK. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord. 2007;22:2196–2203. doi: 10.1002/mds.21671. [DOI] [PubMed] [Google Scholar]
- 12.Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 13.Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 14.Katzenschlager R, Lees AJ. Treatment of Parkinson's disease: levodopa as the first choice. J Neurol. 2002;249(Suppl 2):II19–II24. doi: 10.1007/s00415-002-1204-4. [DOI] [PubMed] [Google Scholar]
- 15.Thanvi BR, Lo TC. Long term motor complications of levodopa: clinical features, mechanisms, and management strategies. Postgrad Med J. 2004;80:452–458. doi: 10.1136/pgmj.2003.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson's disease. Biochim Biophys Acta. 2010;1802:29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson's disease: molecular mechanisms and pathophysiological consequences. EMBO J. 2012;31:3038–3062. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui K, Luo X, Xu K, Ven Murthy MR. Role of oxidative stress in neurodegeneration: recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:771–799. doi: 10.1016/j.pnpbp.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson's disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 21.Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 22.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 23.Gomes-Leal W. Microglial physiopathology: how to explain the dual role of microglia after acute neural disorders? Brain Behav. 2012;2:345–356. doi: 10.1002/brb3.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 26.Haslund-Vinding J, McBean G, Jaquet V, Vilhardt F. NADPH oxidases in microglia oxidant production: activating receptors, pharmacology, and association with disease. Br J Pharmacol. 2016 doi: 10.1111/bph.13425. (in presss) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RL, James-Kracke M, Sun GY, Sun AY. Oxidative and inflammatory pathways in Parkinson's disease. Neurochem Res. 2009;34:55–65. doi: 10.1007/s11064-008-9656-2. [DOI] [PubMed] [Google Scholar]
- 28.Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci U S A. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bové J, Perier C. Neurotoxin-based models of Parkinson's disease. Neuroscience. 2012;211:51–76. doi: 10.1016/j.neuroscience.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 30.Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Członkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Członkowski A. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson's disease mice model. Neurodegeneration. 1996;5:137–143. doi: 10.1006/neur.1996.0020. [DOI] [PubMed] [Google Scholar]
- 32.Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci. 2002;15:991–998. doi: 10.1046/j.1460-9568.2002.01938.x. [DOI] [PubMed] [Google Scholar]
- 33.Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K, Laurie C, Gendelman HE. Neuroinflammation, oxidative stress and the pathogenesis of Parkinson's disease. Clin Neurosci Res. 2006;6:261–281. doi: 10.1016/j.cnr.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-α) increases both in the brain and in the cerebrospinal fluid from Parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 35.Kim HD, Jeong KH, Jung UJ, Kim SR. Myricitrin ameliorates 6-hydroxydopamine-induced dopaminergic neuronal loss in the substantia nigra of mouse brain. J Med Food. 2016;19:374–382. doi: 10.1089/jmf.2015.3581. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Pallares J, Parga JA, Muñoz A, Rey P, Guerra MJ, Labandeira-Garcia JL. Me chanism of 6-hydroxydopamine neurotoxicity: the role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J Neurochem. 2007;103:145–156. doi: 10.1111/j.1471-4159.2007.04699.x. [DOI] [PubMed] [Google Scholar]
- 37.Hernandes MS, Santos GD, Café-Mendes CC, Lima LS, Scavone C, Munhoz CD, Britto LR. Microglial cells are involved in the susceptibility of NADPH oxidase knockout mice to 6-hydroxy-dopamine-induced neurodegeneration. PLoS One. 2013;8:e75532. doi: 10.1371/journal.pone.0075532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23:6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molteni M, Gemma S, Rossetti C. The role of toll-like receptor 4 in infectious and noninfectious inflammation. Mediators Inflamm. 2016;2016:6978936. doi: 10.1155/2016/6978936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 42.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 43.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 44.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmad A, Crupi R, Campolo M, Genovese T, Esposito E, Cuzzocrea S. Absence of TLR4 reduces neurovascular unit and secondary inflammatory process after traumatic brain injury in mice. PLoS One. 2013;8:e57208. doi: 10.1371/journal.pone.0057208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 47.Kerfoot SM, Long EM, Hickey MJ, Andonegui G, Lapointe BM, Zanardo RC, Bonder C, James WG, Robbins SM, Kubes P. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J Immunol. 2004;173:7070–7077. doi: 10.4049/jimmunol.173.11.7070. [DOI] [PubMed] [Google Scholar]
- 48.Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- 49.Gambuzza ME, Sofo V, Salmeri FM, Soraci L, Marino S, Bramanti P. Toll-like receptors in Alzheimer's disease: a therapeutic perspective. CNS Neurol Disord Drug Targets. 2014;13:1542–1558. doi: 10.2174/1871527313666140806124850. [DOI] [PubMed] [Google Scholar]
- 50.Noelker C, Morel L, Lescot T, Osterloh A, Alvarez-Fischer D, Breloer M, Henze C, Depboylu C, Skrzydelski D, Michel PP, Dodel RC, Lu L, Hirsch EC, Hunot S, Hartmann A. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep. 2013;3:1393. doi: 10.1038/srep01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 52.Dihanich M, Kaser M, Reinhard E, Cunningham D, Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron. 1991;6:575–581. doi: 10.1016/0896-6273(91)90060-d. [DOI] [PubMed] [Google Scholar]
- 53.Mann KG. Prothrombin. Methods Enzymol. 1976;45:123–156. doi: 10.1016/s0076-6879(76)45016-4. [DOI] [PubMed] [Google Scholar]
- 54.Taneda H, Andoh K, Nishioka J, Takeya H, Suzuki K. Blood coagulation factor Xa interacts with a linear sequence of the kringle 2 domain of prothrombin. J Biochem. 1994;116:589–597. doi: 10.1093/oxfordjournals.jbchem.a124565. [DOI] [PubMed] [Google Scholar]
- 55.Shikamoto Y, Morita T. Expression of factor X in both the rat brain and cells of the central nervous system. FEBS Lett. 1999;463:387–389. doi: 10.1016/s0014-5793(99)01657-9. [DOI] [PubMed] [Google Scholar]
- 56.Lee TH, Rhim T, Kim SS. Prothrombin kringle-2 domain has a growth inhibitory activity against basic fibroblast growth factor-stimulated capillary endothelial cells. J Biol Chem. 1998;273:28805–28812. doi: 10.1074/jbc.273.44.28805. [DOI] [PubMed] [Google Scholar]
- 57.Rhim TY, Park CS, Kim E, Kim SS. Human prothrombin fragment 1 and 2 inhibit bFGF-induced BCE cell growth. Biochem Biophys Res Commun. 1998;252:513–516. doi: 10.1006/bbrc.1998.9682. [DOI] [PubMed] [Google Scholar]
- 58.Kim TH, Oh S, Kim SS. Recombinant human prothrombin kringle-2 induces bovine capillary endothelial cell cycle arrest at G0-G1 phase through inhibition of cyclin D1/CDK4 complex: modulation of reactive oxygen species generation and up-regulation of cyclin-dependent kinase inhibitors. Angiogenesis. 2005;8:307–314. doi: 10.1007/s10456-005-9020-y. [DOI] [PubMed] [Google Scholar]
- 59.Dasgupta SK, Thiagarajan P. Inhibition of thrombin activity by prothrombin activation fragment 1.2. J Thromb Thrombolysis. 2007;24:157–162. doi: 10.1007/s11239-007-0018-8. [DOI] [PubMed] [Google Scholar]
- 60.Kim TH, Ahn S, Kim J, Kim I, Yang MZ, Lee JE, Kim SS. Recombinant human prothrombin kringle-2 inhibits B16F10 melanoma metastasis through inhibition of neovascularization and reduction of matrix metalloproteinase expression. Clin Exp Metastasis. 2006;23:391–399. doi: 10.1007/s10585-006-9048-4. [DOI] [PubMed] [Google Scholar]
- 61.Ishida Y, Nagai A, Kobayashi S, Kim SU. Upregulation of protease-activated receptor-1 in astrocytes in Parkinson disease: astrocyte-mediated neuroprotection through increased levels of glutathione peroxidase. J Neuropathol Exp Neurol. 2006;65:66–77. doi: 10.1097/01.jnen.0000195941.48033.eb. [DOI] [PubMed] [Google Scholar]
- 62.Berzin TM, Zipser BD, Rafii MS, Kuo-Leblanc V, Yancopoulos GD, Glass DJ, Fallon JR, Stopa EG. Agrin and microvascular damage in Alzheimer's disease. Neurobiol Aging. 2000;21:349–355. doi: 10.1016/s0197-4580(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 63.Sokolova E, Reiser G. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: localization, expression and participation in neurodegenerative diseases. Thromb Haemost. 2008;100:576–581. [PubMed] [Google Scholar]
- 64.Won SY, Choi SH, Jin BK. Prothrombin kringle-2-induced oxidative stress contributes to the death of cortical neurons in vivo and in vitro : role of microglial NADPH oxidase. J Neuroimmunol. 2009;214:83–92. doi: 10.1016/j.jneuroim.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Ryu J, Min KJ, Rhim TY, Kim TH, Pyo H, Jin B, Kim SU, Jou I, Kim SS, Joe EH. Prothrombin kringle-2 activates cultured rat brain microglia. J Immunol. 2002;168:5805–5810. doi: 10.4049/jimmunol.168.11.5805. [DOI] [PubMed] [Google Scholar]
- 66.Ros-Bernal F, Hunot S, Herrero MT, Parnadeau S, Corvol JC, Lu L, Alvarez-Fischer D, Carrillo-de Sauvage MA, Saurini F, Coussieu C, Kinugawa K, Prigent A, Höglinger G, Hamon M, Tronche F, Hirsch EC, Vyas S. Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in Parkinsonism. Proc Natl Acad Sci U S A. 2011;108:6632–6637. doi: 10.1073/pnas.1017820108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi SH, Lee DY, Chung ES, Hong YB, Kim SU, Jin BK. Inhibition of thrombin-induced microglial activation and NADPH oxidase by minocycline protects dopaminergic neurons in the substantia nigra in vivo. J Neurochem. 2005;95:1755–1765. doi: 10.1111/j.1471-4159.2005.03503.x. [DOI] [PubMed] [Google Scholar]
- 68.Noble W, Garwood C, Stephenson J, Kinsey AM, Hanger DP, Anderton BH. Minocycline reduces the development of abnormal tau species in models of Alzheimer's disease. FASEB J. 2009;23:739–750. doi: 10.1096/fj.08-113795. [DOI] [PubMed] [Google Scholar]
- 69.Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 70.Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 2002;52:54–61. doi: 10.1002/ana.10242. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393–1399. doi: 10.1097/00006123-200106000-00051. [DOI] [PubMed] [Google Scholar]