Abstract
The pathogenesis of listeriosis results mainly from the ability of Listeria monocytogenes to attach, invade, replicate and survive within various cell types in mammalian tissues. In this work, the effect of two bacteriocin-producing Carnobacterium (C. divergens V41 and C. maltaromaticum V1) and three non-bacteriocinogenic strains: (C. divergens V41C9, C. divergens 2763, and C. maltaromaticum 2762) was investigated on the reduction of L. monocytogenes Scott A plaque-forming during human infection using the HT-29 in vitro model. All Carnobacteria tested resulted in a reduction in the epithelial cell invasion caused by L. monocytogenes Scott A. To understand better the mechanism underlying the level of L. monocytogenes infection inhibition by Carnobacteria, infection assays from various pretreatments of Carnobacteria were assessed. The results revealed the influence of bacteriocin production combined with a passive mechanism of mammalian cell monolayers protection by Carnobacteria. These initial results showing a reduction in L. monocytogenes virulence on epithelial cells by Carnobacteria would be worthwhile analyzing further as a promising probiotic tool for human health.
Keywords: foodborne pathogens, Carnobacterium divergens, Carnobacterium maltaromaticum, bacteriocin, HT29, mucus layer
Introduction
Listeria monocytogenes is one of the major foodborne pathogens responsible for listeriosis. The infections in high-risk individuals, such as pregnant women, newborn infants and immunocompromised people may cause meningitis, encephalitis, septicemia or spontaneous late-term abortions with a mortality rate as high as 30% (Farber and Peterkin, 1991; Rocourt et al., 2000; Vázquez-Boland et al., 2001; Swaminathan and Gerner-Smidt, 2007). In 2013, 1763 human cases of listeriosis were reported in Europe by the European Food Safety Authority and the European Center for Disease Prevention and Control (EFSA and ECDC, 2015). The notification rate was 0.44 cases per 100,000 population which represented an 8.6% increase compared with 2012 (EFSA and ECDC, 2015). There was a statistically significant increase in the incidence of listeriosis over the period 2009–2013. In total, 191 deaths due to listeriosis were reported in 2013, with the highest number (64 cases) occurring in France. L. monocytogenes is therefore of public concern in terms of food safety and regulations to control this microorganism. Currently, antibiotics are the most accepted treatment option for listeriosis infections. As vaccination is unavailable and the use of antibiotics is declining due to an increase in resistance and allergies, there is a need for innovative alternative ways of reducing L. monocytogenes infections in humans.
L. monocytogenes is wide spread in the environment and has been isolated from various sources such as dairy products, fresh vegetables, and meats (Beresford et al., 2001; Guerrieri et al., 2009). Isolations performed in the food processing environment reveal that L. monocytogenes can adhere to inert surfaces and grow as biofilms in diverse areas, such as dead ends, crevices and corner cracks (Kim and Frank, 1995; Tresse et al., 2006, 2007; Shi and Zhu, 2009; Pilchová et al., 2014; Guilbaud et al., 2015). Due to the ability of this pathogenic bacterium to grow in foodstuffs at refrigerated temperatures, efficient control methods are required to limit the risk in ready-to-eat food products with a long shelf life. As increasing numbers of consumers prefer foods without chemical preservatives (Cleveland et al., 2001; Devlieghere et al., 2004), there is an opportunity for methods focusing on the use of a protective culture such as lactic acid bacteria (LAB). The protective LAB could produce antimicrobial metabolites such as lactic acid, diacetyl, hydrogen peroxide and bacteriocin or bacteriocin-like compounds (Lindgren and Dodrogosz, 1990).
Some bacteriocin-producing LAB have demonstrated their efficiency in limiting the growth of L. monocytogenes (Harris et al., 1989; Rodriguez et al., 1997; Laukova et al., 1999; Ennahar et al., 2000; Sabia et al., 2002; Tyopponen et al., 2003; Todorov et al., 2011; Amado et al., 2012). Carnobacterium spp. are among the bacteriocin-producing bacteria recently studied as protective cultures in foodstuffs. The Carnobacterium genus belongs to the family Carnobacteriaceae within the order of Lactobacillales and currently consists of 12 species (Euzéby, 1997). Two of the Carnobacterium species, C. divergens and C. maltaromaticum, are mainly isolated from the environment and foods (Leisner et al., 2007). Both have been shown to exhibit a wide spectrum of activity against L. monocytogenes which has been attributed in some cases to the production of bacteriocins (Pilet et al., 1995; Buchanan and Bagi, 1997; Duffes et al., 1999; Nilsson et al., 1999; Schöbitz et al., 1999; Yamazaki et al., 2003; Józefiak et al., 2010). Attempts to apply extracted bacteriocins against L. monocytogenes have been limited due to (i) the loss of activity following bacteriocin purification steps, (ii) variations in bacteriocin production depending on Carnobacterium spp. and (iii) variations in susceptibility among L. monocytogenes strains (Richard et al., 2003; Brillet et al., 2004). Non-bacteriocinogenic strains have also demonstrated inhibitory activity toward L. monocytogenes (Nilsson et al., 2005). Some in vitro experiments on the antagonism of various LAB against pathogenic bacteria have been reported (Alemka et al., 2010; Garnier et al., 2010; Messaoudi et al., 2012). Although, the active ingredients were mainly attributed to the production of bacteriocins, the inhibitory effect of Carnobacteria on L. monocytogenes virulence has not yet been explored.
In this study, we examined the in vitro potential of protective Carnobacterium strains to assess their effect on the virulence of L. monocytogenes. Bacteriocin- and non-bacteriocin-producing Carnobacterium strains were tested on cell line models using HT29 and its mucin-producing counterpart HT29-MTX.
Materials and methods
Bacterial strains
Two clinical strains of L. monocytogenes and five strains of Carnobacterium spp. were used in this study. L. monocytogenes Scott A (serotype 4b) and L. monocytogenes LO28 (serotype 1/2c) were previously isolated from listeriosis outbreaks, C. divergens V41, and C. maltaromaticum V1 (formerly C. piscicola) were isolated from salmon and trout intestine and characterized by Pilet et al. (1995), C. divergens V41C9 is a C. divergens V41 mutant deficient in divercin production (Richard et al., 2003), C. divergens NCDO 2763 and C. maltaromaticum (formerly C. piscicola) NCDO 2762 (type strain) were obtained from the National Collection of Dairy Organisms (Reading, UK). All strains were stored in cryotubes at −80°C in brain heart infusion broth (BHI) for L. monocytogenes strains and in Elliker broth for Carnobacterium strains supplemented with 20% glycerol as a cryoprotectant.
Antimicrobial activity determination
The antimicrobial activity of Carnobacterium strains was tested on two L. monocytogenes strains using the agar diffusion assay (Pilet et al., 1995). Briefly, the L. monocytogenes subculture was grown in BHI broth for 8 h at 37°C and culture was incubated overnight at 37°C. A concentration of 3.5 × 107 cfu ml−1 was mixed to the BHI agar. Carnobacterium strains were subcultured in Elliker broth for 24 h at 20°C. Cultures were then incubated overnight at 20° and 30°C. Similar concentrations of bacteria were obtained after cultivation at 20° and 30°C (2.6 × 109 cfu ml−1 and 1.8 × 109 cfu ml−1). The cell-free supernatant of each Carnobacterium strain was obtained by centrifugation (8200 g, 10 min at 4°C) and 10 μl of the filtered supernatant (untreated supernatant and treated culture supernatant adjusted to pH 6.5) was then spotted onto indicator plates of BHI agar (1%) seeded with 106 cfu ml−1 of the target strain. After overnight incubation at 37°C, the presence of a translucent halo corresponding to the absence of L. monocytogenes growth was observed.
Cell line cultures
The human adenocarcinoma cell line HT29 and the mucus-secreting HT29 cells selected by adaptation to methotrexate (HT29-MTX) were used. HT29-MTX cells were obtained from Dr Thécla Lesuffleur (INSERM UMR S 938, Paris France) (Lesuffleur et al., 1993). Cells were routinely grown in 25 cm2 plastic tissue culture flasks (Nunc, Life Technologies) in 5 ml of culture medium (Dulbecco's modified Eagle's medium; DMEM; Eurobio, Courtaboeuf, France) supplemented with 10% (v/v) fetal calf serum (Eurobio, Courtaboeuf, France), 2 mM L-glutamine (Eurobio, Courtaboeuf, France) and antibiotics—penicillin 100 IU ml−1 and streptomycin 100 μg ml−1 (Sigma, France). Antibiotics were routinely added to the culture medium except for virulence assays. Cells were maintained in a humidified incubator (at least 90% RH) at 37°C under 5% (v/v) CO2 (SANYO Electric Co., Ltd., Osaka, Japan). The medium was changed every other day.
L. monocytogenes plaque-forming assay (PFA)
The ability of L. monocytogenes Scott A to form lysis plaques on cell lines HT29 and HT29-MTX was assessed using the PFA as previously described by Roche et al. (2001). Briefly, cell monolayers were grown until they reached 90% confluence in DMEM supplemented with antibiotics and then without antibiotics for another 24 h. The overnight L. monocytogenes cultures, grown in BHI broth, were appropriately diluted in DMEM without antibiotics. The 96-wells were inoculated with 2 to 8 log CFU L. monocytogenes per well in triplicate and incubated for 2 h at 37°C in a humidified incubator and treated with 100 μg ml−1 gentamicin (Sigma, France). After 1.5 h of incubation, cell monolayers were overlaid with an agarose gel containing 0.48% indubiose (Bio-Rad Laboratories, France) in DMEM supplemented with 10 μg ml−1 of gentamicin. The number of plaques was counted with a microscope after 48 h of incubation at 37°C in a humidified incubator. Each experiment was repeated three times from independent cultures for each strain and the results were expressed as the number of plaques obtained for 7 or 8 log L. monocytogenes loaded per well.
Ability of C. divergens to adhere to epithelial cells HT29
After cultivation, C. divergens V41 and V41C9 were harvested by centrifugation, resuspended in DMEM without antibiotics and serum at a concentration of 108 cfu ml−1 and loaded on confluent HT29 and HT29-MTX cell line monolayers. After 1 h or 4 h of incubation (37°C, 5% CO2), monolayers were washed three times with PBS to remove nonadherent bacteria and lysed with 0.1% Triton X100 for 15 min. The lysate was then diluted and plated on Elliker agar plates to determine the number of adherent bacteria. Each experiment was performed in duplicate.
PFA in the presence of carnobacterium
Carnobacterium strains were grown as described above. Cultures were pelleted by centrifugation at 8200 g for 10 min and resuspended in DMEM medium without antibiotics. A total of 100 μl of Carnobacterium culture in tissue culture medium was used to coat the cells with 109 cfu ml−1 for 1 h or 4 h at 37°C. After the initial incubation period, 100 μl of L. monocytogenes culture at 107 or 108 cfu ml−1 was added on the cell monolayers and incubated for 2 h at 37°C. Then, the medium was replaced with 100 μl of fresh sterile culture medium containing 100 μg ml−1 gentamicin and incubated for another 1.5 h at 37°C. Next, the same steps as mentioned above were performed to count the number of lysis plaques. As controls, L. monocytogenes and Carnobacterium strains were also tested separately on HT29 and HT29-MTX cell lines. Each experiment was repeated three times from independent cultures for each strain.
Pretreatment of carnobacterium before infection assays
Cell monolayers were originally incubated with probiotic cultures at 109 cfu ml−1 in antibiotic-free medium. To cover wide range of active compounds that could potentially affect the listerial invasion, six different pretreatments were applied to the Carnobacterium culture: (a) 1 ml of an overnight culture of Carnobacteria strains was centrifuged (8200 g for 10 min) and the cells obtained in the pellet were resuspended in 1 ml of DMEM (namely—resuspended cells—RS); (b) 1 ml of an overnight culture of Carnobacterium was centrifuged (8200 g for 10 min) and the cells obtained in the pellet were washed in 1 ml of DMEM, centrifuged once more and subsequently resuspended in 1 ml of DMEM (namely—washed cells—WS); (c) 1 ml of an overnight culture was heated at 100°C for 5 min, centrifuged (8200 g for 10 min) and the cells in the pellet resuspended in 1 ml of DMEM (namely—resuspended heated cells—RHC); (d) 1 ml of an overnight culture was heated at 100°C for 5 min, centrifuged (8200 g for 10 min) and the cells were washed in 1 ml of DMEM, centrifuged once more time and then resuspended in 1 ml of DMEM (namely—washed heated cells—WHC); (e) overnight cultures were centrifuged (8200 g for 10 min) and cell-free supernatant was adjusted to pH 6.5 and then either untreated (namely—non-filtered supernatant—NFS), or filtered (0.2 μm filter; namely—filtered supernatant—FS).
Statistical analyses
The data were analyzed using Statgraphics Centurion XVI software (StatPoint Inc., Herndon, Virginia, USA). With the confirmation of a normal distribution for each data set, significant differences were determined using two-sided Student's t-test comparisons at a 5% significance level.
Results
Effect of bacteriocin-producing C. divergens V41 on L. monocytogenes using in vitro virulence models
First, the antimicrobial activity of the divercin-producing C. divergens V41 (div+) and its non-bacteriocinogenic mutant C. divergens V41C9 (div−) against L. monocytogenes was confirmed (Table 1). As expected, the growth of Scott A and LO28 was inhibited by supernatants of the div+ strain cultivated at 20° and 30°C while no inhibition zone was observed for the div− strain in all conditions. Identical results observed with pH-neutralized supernatants confirmed the divercin action of V41 against L. monocytogenes (Table 1). In the following experiments, the effect of Carnobacteria against L. monocytogenes virulence was assessed using Scott A as it is more virulent than LO28. Plaque-forming on confluent monolayer epithelial cells by L. monocytogenes Scott A was evaluated after 1 h of C. divergens V41 inoculation (Figure 1). No plaque was observed when C. divergens V41 was loaded alone indicating the absence of a cytotoxic effect by C. divergens on HT29 and HT29 MTX cell lines. In contrast, L. monocytogenes Scott A was able to form plaques on both cell lines with a higher level on HT29 cells (log 3.27 ± 0.12 plaques) than on their mucus-secreting counterparts (log 2.80 ± 0.0.08). The significant difference between the two cell lines indicates a role of mucus in the prevention of L. monocytogenes plaque-forming. When Scott A was inoculated on epithelial cells previously coated with C. divergens V41, the plaque-forming ability of Scott A was reduced dramatically on both cell lines. At 108 cfu ml−1, plaque-forming by Scott A decreased to log 0.44 on HT29 and to log 0.29 on HT29 MTX while at 107 cfu ml−1, the plaque-forming of Scott A was reduced to an undetectable level on both lines. Furthermore, when the supernatant (filtered-FS and not filtered-NFS) of V41 culture was used, a similar inhibitory effect on Scott A plaque-forming was obtained on both cell lines.
Table 1.
Growth inhibition of L. monocytogenes Scott A and LO28 by C. divergens.
 | ||
|---|---|---|
| Scott A | LO28 | |
| Untreated supernatant | ||
| V41 (20°C) | + | + |
| V41 (30°C) | + | + |
| V41C9 (20°C) | − | − |
| V41C9 (30°C) | − | − |
| Supernatant adjusted to pH 6.5 | ||
| V41 (20°C) | + | + |
| V41 (30°C) | + | + |
| V41C9 (20°C) | − | − |
| V41C9 (30°C) | − | − |
Supernatants of cultures at 20° and 30°C of the bacteriocin-producing strain C. divergens V41 and its non-bacteriocinogenic variant V41C9 were tested. Growth inhibition was measured using the agar diffusion test as shown in the picture for Scott A with the cell-free culture supernatant of V41 at 20°C (lane I) and at 30°C (lane II). Neutralized supernatants were obtained by adjusting the pH to 6.5.
+, inhibition zone ≥ 3 mm; −, inhibition zone < 3 mm.
Figure 1.
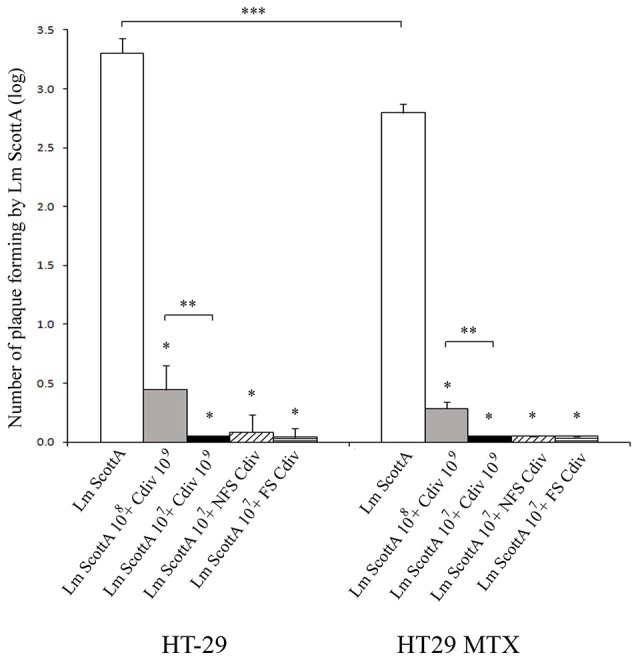
Inhibition of L. monocytogenes Scott A plaque-forming by C. divergens V41 using epithelial cells HT29 and mucus-secreting HT29 MTX. C. divergens (Cdiv) was loaded at 109 cfu ml−1 1 h before L. monocytogenes Scott A inoculation at 107 or 108 cfu ml−1 and incubated for 2 h on HT29 confluent cells. Supernatants (filtered FS or non-filtered NFS) of C. divergens culture were also tested. No plaque-forming was observed when Cdiv was loaded alone. Results represent the mean PFA of L. monocytogenes Scott A (Lm Scott A) ± SD from at least three independent experiments. Asterisks indicate significant differences (P < 0.05) compared to Scott A alone (*), between L. monocytogenes inocula (**) and between HT29 and HT29 MTX (***). As no significant difference in plaque-forming was observed after loading Scott A alone at 107 or 108 cfu ml−1, these results were pooled (n = 6).
Comparative effect of bacteriocinogenic and non-bacteriocinogenic C. divergens strains on L. monocytogenes Scott A in vitro virulence
In order to determine the contribution of the bacteriocin (divercin) secreted by C. divergens V41 to the inhibition of L. monocytogenes plaque-forming, the div− mutant, defective in bacteriocin synthesis, was tested. When the HT29 cells were precoated with the div− strain, the inhibitory level of L. monocytogenes plaque-forming only decreased to 91.5% indicating a major contribution by another factor besides that of bacteriocin (Table 2). From now on, the antimicrobial effects will be named “the bacteriocin effect” for those due to bacteriocins and “protective effect” for those independent of bacteriocins. The inhibition of plaque-forming by the div− strain was significantly lower than that of div+ on both cell lines confirming a slight but significant contribution of the bacteriocin effect in the div+ strain (Table 2). In addition, a lower plaque-forming inhibition was observed on HT29 MTX for Scott A at 108 cfu/mL which confirms the role of the mucus observed in Figure 1 in preventing L. monocytogenes plaque-forming (Table 2).
Table 2.
Comparison of the inhibiting efficiency of bacteriocin-producing strain C. divergens 41 (div−) and the non-bacteriocin-producing strain V41C9 (div−) on L. monocytogenes Scott A plaque-forming on HT29 and mucus-secreting HT29 MTX.
| C. divergens | L. monocytogenes (cfu ml−1) | HT29 | HT29-MTX |
|---|---|---|---|
| Mean ± SD (%) | Mean ± SD (%) | ||
| V41 (div+) | Scott A (107) | 100.0 ± 0.0 | 100.0 ± 0.0 |
| V41 (div+) | Scott A (108) | 86.5 ± 6.3 | 89.1 ± 1.7 |
| V41C9 (div−) | Scott A (107) | 91.5 ± 6.6* | 98.7 ± 2.7 |
| V41C9 (div−) | Scott A (108) | 74.9 ± 4.4* | 81.7 ± 3.2* |
Carnobacteria were inoculated at 109 cfu ml-1. HT29 and HT29 MTX epithelial cells were precoated with Carnobacteria 1 h before the inoculation of L. monocytogenes at 107 or 108cfu ml-1. Results were normalized to the plaques obtained with L. monocytogenes Scott A alone (log 3.27 ± 0.12, n = 4 on HT29 and log 2.80 ± 0.08, n = 4 on HT29 MTX). The means and standard deviations (SD) were calculated from at least three independent experiments. Asterisks indicate significant differences (P < 0.05) between div+ and div−.
Influence of precoating time C. divergens on the inhibition of L. monocytogenes Scott A infection using HT29 and HT29-MTX cell lines
The ability of strains div+ and div− to adhere to host cells was assessed by incubating the culture for 1 h and 4 h on confluent monolayers of HT29 and HT29-MTX (Figure 2). The initial C. divergens concentration loaded onto cell monolayers was 109 cfu ml−1. Overall, approximately 5 × 107 cfu ml−1 of viable C. divergens cells were recovered from cell lysates. A significantly greater number of adherent cells was observed on HT29-MTX than on HT29 indicating a positive effect of mucus on C. divergens adhesion (Figure 2). In addition, a decrease, although moderate, was observed in the adhesion of div+ after 4 h of contact on both cell lines compared to 1 h while adhesion tended to increase for div− on HT29-MTX. When assessed in the presence of L. monocytogenes Scott A, there was no significant difference between 1 and 4 h of contact except for div− after 4 h of contact on HT29 MTX which showed less inhibition of Scott A plaque-forming compared to 1 h (Table 3). Taken together, these results indicate that C. divergens V41 remains efficient against Scott A after 4 h of contact with both cell lines by maintaining both its protective and bacteriocin effects.
Figure 2.
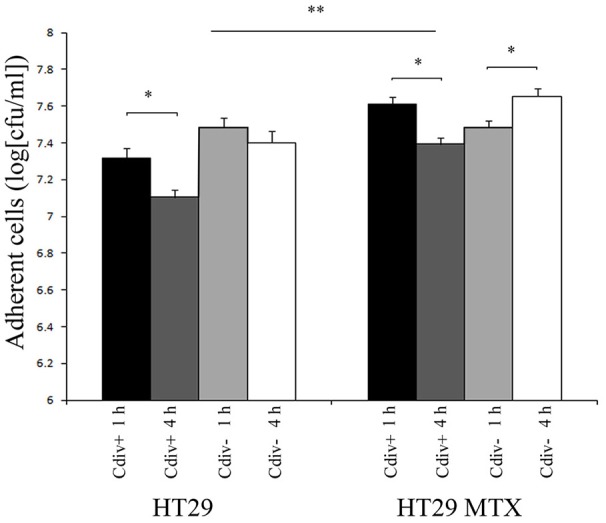
Viability of Carnobacterium strains on epithelial cells after 4 h of contact time with HT29 and HT29-MTX epithelial cell model. The bacteriocin-producing strain C. divergens V41 (div+) and the non-bacteriocinogenic strain V41C9 (div−) were inoculated at 109 cfu ml−1. Results represent the mean ± SD from three independent experiments. Asterisks indicate significant differences (P < 0.05) between 1 and 4 h of precoating (*) and between HT29 and HT29 MTX (**).
Table 3.
Effect of precoating contact time of Carnobacterium strains on the inhibition of L. monocytogenes plaque-forming on HT29 and HT29 MTX.
| Contact time of C. divergens (h) | HT29 | HT29 MTX | ||
|---|---|---|---|---|
| 1 | 4 | 1 | 4 | |
| V41 (div+)/Scott A | 100.0 ± 0.0 | 97.0 ± 3.1 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| V41C9 (div−)/Scott A | 91.5 ± 6.6 | 82.8 ± 9.9 | 98.7 ± 2.7 | 82.4 ± 2.6* |
L. monocytogenes cells were inoculated at 107 cfu ml-1 1h or 4 h after the inoculation of C. divergens V41 (div+) or V41C9 (div−) at 109 cfu ml-1. Results were normalized to the plaques obtained with L. monocytogenes Scott A (log 3.29 ± 0.13, n = 4 on HT29 and log 2.79 ± 0.08, n = 4 on HT29 MTX). The means and standard deviations (SD) were calculated from at least three independent experiments. Asterisks indicate significant difference (P < 0.05) between 1 and 4 h of precoating.
Effect of various pretreatments of C. divergens V41 on the inhibition of L. monocytogenes Scott A plaque-forming on HT29
To understand further the protective mode of action of C. divergens V41, additional infection assays with various pretreatments were carried out (Figure 3). The results showed no significant difference in plaque-forming by L. monocytogenes when cells were washed and/or heated before inoculation on HT29, indicating that no extracellular divercin remained after the preparation of the div+ culture and that dead cells were able to achieve the same efficient protective effect.
Figure 3.
Inhibition of L. monocytogenes Scott A plaque-forming by pretreated C. divergens V41 using the HT29 epithelial cell model. C. divergens V41 was inoculated at 109 cfu ml−1 1 h before inoculating L. monocytogenes at 107 cfu ml−1. (RC) control without pretreatment; (WC) washed cells; (RHC) resuspended heated cells and (WHC) washed heated cells. The results are expressed as mean ± SD from two independent experiments. Asterisks indicate significant differences (P < 0.05) compared to Scott A alone. No significant difference was observed between treatments and the control (P > 0.05).
Effect of carnobacterium spp. on the inhibition of L. monocytogenes plaque-forming on HT29
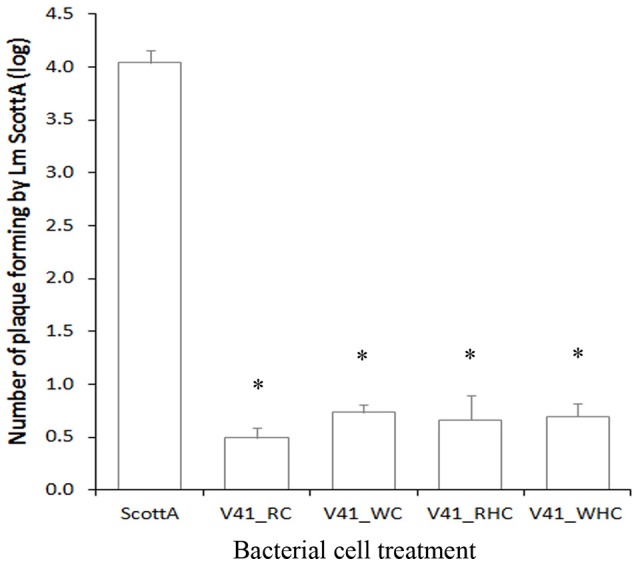
In order to determine if the protective effect of Carnobacterium against L. monocytogenes is specific to C. divergens V41, additional strains or species were tested (Figure 4). The anti-listerial activity of cell-free neutralized supernatants of C. maltaromaticum V1 was confirmed (inhibition zone = 10 mm ± 0; n = 3 at 20°C and 9 mm ± 1; n = 3 at 30°C) while the supernatants of non-bacteriocinogenic strains C. divergens 2763 and C. maltaromaticum 2762 did not exhibit any growth inhibition of L. monocytogenes Scott A (inhibition zone < 3 mm; n = 3 at 20° and 30°C; Figure 4A). All five Carnobacterium strains tested, bacteriocinogenic or not, significantly inhibited the number of plaques formed by L. monocytogenes Scott A (Figure 4B). However, the decrease in plaque-forming was lower for all strains compared to that of div+ indicating a better potential of C. divergens V41 to prevent L. monocytogenes infection. In line with what was observed with V41 and its div− mutant, the bacteriocin-producing C. maltaromaticum V1 was significantly more efficient at limiting the number of plaques formed by L. monocytogenes than the non-bacteriocin-producing strain C. maltaromaticum 2762. Similar results obtained with washed or preheated cells confirmed that inhibition of Scott A plaque-forming by Carnobacteria can utilize both bacteriocin and protective effects.
Figure 4.
Inhibition of L. monocytogenes Scott A plaque-forming by Carnobacteria using the HT29 epithelial cell model. Two bacteriocin-producing strains (C. divergens V41 and C. maltaromaticum V1 and three non-bacteriocinogenic strains (C. divergens V41C9, C. divergens 2763, C. maltaromaticum 2762) were compared. (A) Capabilities to inhibit growth of L. monocytogenes Scott A by C. divergens V1, C. divergens 2763, C. maltaromaticum 2762. Growth inhibition was measured using the agar diffusion test at 20° and at 30°C. Neutralized supernatants were obtained by adjusting pH to 6.5. (B) Each Carnobacterium strain was inoculated at 109 cfu ml−1 on HT29 cells 1 h before loading L. monocytogenes at 107 cfu ml−1. Pretreatments were as follow: control without pretreatment (RC); washed cells (WC); resuspended heated cells (RHC); and washed heated cells (WHC). The results are expressed as mean ± SD from two independent experiments. Plaque-forming by L. monocytogenes Scott A alone was log 3.52 ± 0.21, n = 4. All Carnobacteria significantly inhibited plaque-forming by L. monocytogenes Scott A. Asterisks indicate significant differences (P < 0.05) compared to C. divergens V41.
Discussion
The inhibitory effect of two bacteriocin-producing Carnobacteria (C. divergens V41 and C. maltaromaticum V1) and three non-bacteriocin-producing Carnobacteria (C. divergens V41C9, C. divergens 2763 and C. maltaromaticum 2762) against pathogenic L. monocytogenes strains has previously been investigated in food (Pilet et al., 1995; Richard et al., 2003; Brillet et al., 2004, 2005). The use of C. divergens V41 and C. maltaromaticum V1 could represent an alternative strategy to control the growth of L. monocytogenes in cold-smoked salmon (Brillet et al., 2004). Overall, LAB isolated from salmon intestine resulted in growth inhibition of Aeromonas salmonicida with no reduction in the mortality rate of the fish (Gildberg et al., 1995).
Several methods have been proposed to assess the virulence of L. monocytogenes. Mouse infection models have demonstrated their efficiency in differentiating virulent from non-virulent strains (Roche et al., 2001); nonetheless their use is limited due to ethical considerations. Expression of the main virulence genes hlyA, actA, inlA, and prfA using RT-qPCR has also been investigated to show the effect of environmental conditions on virulence factor transcript levels of L. monocytogenes (Duodu et al., 2010). However, the plaque-forming assay using HT29 cells is usually proposed as the best alternative to animal models as it takes into account the epithelial cell invasion capability of the pathogen (Roche et al., 2001). In this work, HT29 cell assays were completed by mucus-secreting HT29 MTX cells in order to include the potential role of mucus in bacterial invasion capability.
A significant reduction in L. monocytogenes virulence on epithelial cells was observed when the cell monolayers were precoated with C. divergens V41 cultures during 1 or 4 h. The capability to limit foodborne pathogen virulence has previously been tested for probiotic LAB and found to be strain-specific. For instance, Garriga et al. (2015) reported that only bacteriocinogenic Lactobacillus sakei of 5 other LAB tested significantly reduced the adhesion of L. monocytogenes. Lactobacillus and Bifidobacterium were also shown to inhibit significantly the subsequent listerial infection using the in vitro C2Bbe1 epithelial cell model (Corr et al., 2007). Pretreatment of intestinal cells T 84 with LAB prevents injury of Escherichia coli O157:H7 and E. coli O157:H6 induced by attaching-effacing-Lactobacillus species (Sherman et al., 2005). In the case of Campylobacter jejuni, the leading cause of bacterial foodborne diseases (EFSA and ECDC, 2015; Turonova et al., 2015), adhesion, internalization and translocation of HT29 cells were attenuated by strains of Lactobacillus rhamnosus, Lactobacillus helveticus, and Lactobacillus salivarius (Alemka et al., 2010). The authors reported that live LAB and prolonged precolonization of mammalian cells with probiotics is a prerequisite for probiotic action against Campylobacter virulence. More recently, Srividya et al. (2015) demonstrated an in vitro inhibition of 70% of Shigella dysentariae by a probiotic lactic acid bacterial lysate.
In this study, to investigate the mechanism that could be involved in the efficiency of counteracting L. monocytogenes Scott A by Carnobacterium, comparisons were made between the divercin-producing C. divergens V41 (div+) and C. divergens V41C9 (div−), a mutant defective in divercin production (Richard et al., 2003). As expected, div− did not show any inhibitory effect on L. monocytogenes cultured on plates in accordance with previous studies (Pilet et al., 1995; Richard et al., 2003). In addition, div+ supernatants resulted in a similar inhibitory effect on plaque-forming by L. monocytogenes indicating the efficiency of divercin on L. monocytogenes during invasion assays. Nonetheless, both div+ and div− were able to reduce dramatically L. monocytogenes plaque-forming on HT29 and HT29 MTX cell lines. The effect of the div+ strain was slightly but significantly higher than that obtained with div− indicating the contribution of bacteriocin activity by the div+ strain since the adhesion rate of both protective cultures was similar on both cell line models. Another bacteriocin-producing C. maltaromaticum V1 was also more efficient at limiting the number of plaques formed by L. monocytogenes than the non-bacteriocinogenic strain counterpart (C. maltaromaticum 2762). This work indicates that non-bacteriocinogenic Carnobacteria strains are also effective candidates for limiting the pathogenicity of L. monocytogenes using the combined effects of bacteriocin activity and mammalian cell protection. The preheated cell treatment suggests that the protective effect of Carnobacteria could be attributed to a passive mechanism. A significant inhibition of L. monocytogenes adhesion, invasion and transepithelial translocation was obtained using Lactobacillus paracasei but only if this strain was recombined to obtain the expression of Listeria adhesion protein (LAP, Lmo1634) in order to interact specifically with the host cell receptor Hsp60 (Koo et al., 2012). In our study, an inhibitory effect of L. monocytogenes virulence by Carnobacterium was obtained without genetically engineered strains. This could be explained by an increase in epithelial barrier functions due to an interaction with secreted components (Shen et al., 2005). Inhibition mechanisms could also involve specific proteins that accumulate on the cell-surface as described for Streptococcus pneumoniae (Guiral et al., 2005). However, further analyses are required to unravel the protective effect of mammalian cells by Carnobacteria.
We also observed that the mucus layer enhanced the impact of the protective effect of Carnobacteria. This was correlated to a significantly higher number of adherent cells. Similar results were obtained by Alemka et al. (2010) who reported the contribution of the mucus layer to the potential efficacy of probiotic treatment for the attenuation of C. jejuni pathogenicity. Mucus constitutes a physical and chemical protective barrier of epithelial cells. Its complex composition includes electrolytes, plasma proteins, lipids, nucleic acids and a large variety of high molecular weight glycoproteins called mucins that contribute to the viscoelasticity of mucus (Johansson et al., 2011). The mucus is a sheltering interspace for bacteria protecting them from shearing motions due to intestinal peristalsis. In addition, commensal bacteria trapped in the mucus are less motile and could be organized into biofilms reinforcing epithelial cell protection (Zoetendal et al., 2002). Weak interactions between mucus and bacterial cell surfaces such as hydrophilic/hydrophobic bonds or cell appendages such as pili could also contribute to maintaining cells in sheltering interspace (Douillard et al., 2013).
Carnobacteria did not alter HT29 cells indicating the absence of cytotoxicity. For their potential use as protective cultures in food, the question of human the safety of these LAB arises. Carnobacteria are not known as members of the human gastrointestinal microbial community like several other LAB. Previous studies have shown that they do not present an imminence for human illnesses, nor for nosocomial infections in hospitals (Leisner et al., 2007). The genome sequence of the type strain of C. maltaromaticum NCDO2762 (ATCC35586) has shown potential virulence factors but none of the specific virulence factors that are present in L. monocytogenes strains and it was thus concluded that there are no human safety concerns for this species (Leisner et al., 2012). The whole genome sequence of C. divergens V41 is now being annotated and analyzed in our laboratory for further research on its harmlessness. In addition, C. maltaromaticum and C. divergens are considered microorganisms with technological beneficial use according to Bourdichon et al. (2012). Recently, C. divergens was added to the authoritative list of microorganisms with a QPS status (qualified presumption of safety) validated by EFSA for the safety risk assessment of microorganisms intentionally added to food and feed (EFSA, 2014). In the USA, the Food and Drug Administration (FDA) provides a notice inventory of substances that have been approved in terms of safety namely substances generally recognized as safe (GRAS) based the on specific usage and dosage for each substance. This inventory lists viable bacteria including C. maltaromaticum (FDA, 2015).
In conclusion, this work demonstrates the potential probiotic effect of Carnobacterium strains to attenuate the pathogenesis of L. monocytogenes Scott A. The probiotic mechanism results from a bacteriocin effect combined to a protective effect of mammalian cells. Probiotics have positive effects on human health and general well-being. They have been historically associated with cultured milk and dairy products. More recently, they have been analyzed for their potential to inhibit pathogenic and spoilage microorganisms (Klaenhammer, 2000; Gill and Guarner, 2004; Grover et al., 2012).
Author contributions
OT conceived the work; MP, JC and OT designed the work; TP performed the experimental work; TP and OT analyzed and interpreted the data; TP drafted the manuscript; OT, MP and JC contributed to the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
TP was a recipient of funding support from UCT Prague (MSMT No 21/2012) and the French Embassy in Czech Republic, L'UNAM in France and the Cost Action BacFoodNet FA1202 in Europe. We thank Valérie Anthoine and Nicolas Moriceau for their technical support.
References
- Alemka A., Clyne M., Shanahan F., Tompkins T., Corcionivoschi N., Bourke B. (2010). Probiotic colonization of the adherent mucus layer of HT29MTXE12 cells attenuates Campylobacter jejuni virulence properties. Infect. Immun. 78, 2812–2822. 10.1128/IAI.01249-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amado I. R., Fuciños C., Fajardo P., Guerra N. P., Pastrana L. (2012). Evaluation of two bacteriocin-producing probiotic lactic acid bacteria as inoculants for controlling Listeria monocytogenes in grass and maize silages. Anim. Feed Sci. Technol. 175, 137–149. 10.1016/j.anifeedsci.2012.05.006 [DOI] [Google Scholar]
- Beresford M. R., Andrew P. W., Shama G. (2001). Listeria monocytogenes adheres to many materials found in food-processing environments. J. Appl. Microbiol. 90, 1000–1005. 10.1046/j.1365-2672.2001.01330.x [DOI] [PubMed] [Google Scholar]
- Bourdichon F., Casaregola S., Farrokh C., Frisvad J. C., Gerds M. L., Hammes W. P., et al. (2012). Food fermentations: microorganisms with technological beneficial use. Int. J. Food Microbiol. 154, 87–97. 10.1016/j.ijfoodmicro.2011.12.030 [DOI] [PubMed] [Google Scholar]
- Brillet A., Pilet M. F., Prévost H., Bouttefroy A., Leroi F. (2004). Biodiversity of Listeria monocytogenes sensitivity to bacteriocin-producing Carnobacterium strains and application in sterile cold-smoked salmon. J. Appl. Microbiol. 97, 1029–1037. 10.1111/j.1365-2672.2004.02383.x [DOI] [PubMed] [Google Scholar]
- Brillet A., Pilet M. F., Prévost H., Cardinal M., Leroi F. (2005). Effect of inoculation of Carnobacterium divergens V41, a biopreservative strain against Listeria monocytogenes risk, on the microbiological, chemical and sensory quality of cold-smoked salmon. Int. J. Food Microbiol. 104, 309–324. 10.1016/j.ijfoodmicro.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Buchanan R. L., Bagi L. K. (1997). Microbial competition: effect of culture conditions on the suppression of Listeria monocytogenes Scott A by Carnobacterium piscicola. J. Food Prot. 60, 254–261. [DOI] [PubMed] [Google Scholar]
- Cleveland J., Montville T. J., Nes I. F., Chikindas M. L. (2001). Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71, 1–20. 10.1016/S0168-1605(01)00560-8 [DOI] [PubMed] [Google Scholar]
- Corr S. C., Li Y., Riedel C. U., O'Toole P. W., Hill C., Gahan C. G. (2007). Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. U.S.A. 104, 7617–7621. 10.1073/pnas.0700440104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlieghere F., Vermeiren L., Debevere J. (2004). New preservation technologies: possibilities and limitations. Int. Dairy J. 14, 273–285. 10.1016/j.idairyj.2003.07.002 [DOI] [Google Scholar]
- Douillard F. P., Ribbera A., Kant R., Pietila T. E., Jarvinen H. M., Messing M., et al. (2013). Comparative genomic and functional analysis of 100 Lactobacillus rhamnosus strains and their comparison with strain GG. PLoS Genet. 9:e1003683. 10.1371/journal.pgen.1003683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffes F., Leroi F., Boyaval P., Dousset X. (1999). Inhibition of Listeria monocytogenes by Carnobacterium spp. strains in a simulated cold smoked fish system stored at 4°C. Int. J. Food Microbiol. 47, 33–42. 10.1016/S0168-1605(98)00206-2 [DOI] [PubMed] [Google Scholar]
- Duodu S., Holst-Jensen A., Skjerdal T., Cappelier J. M., Pilet M. F., Loncarevic S. (2010). Influence of storage temperature on gene expression and virulence potential of Listeria monocytogenes strains grown in a salmon matrix. Food Microbiol. 27, 795–801. 10.1016/j.fm.2010.04.012 [DOI] [PubMed] [Google Scholar]
- EFSA (2014). The 2013 updated list of QPS Status recommended biological agents in support of EFSA risk assessments – 1st revision (new additions). EFSA J. 12, 3938. [Google Scholar]
- EFSA ECDC (2015). European food safety authority, European centre for disease prevention and control: the European union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 13, 3991. [Google Scholar]
- Ennahar S., Sashihara T., Sonomoto K., Ishizaki A. (2000). Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24, 85–106. 10.1111/j.1574-6976.2000.tb00534.x [DOI] [PubMed] [Google Scholar]
- Euzéby J. (1997). List of bacterial names with standing in nomenclature: a folder available on the Internet. Int. J. Syst. Bact. 47, 590–592. 10.1099/00207713-47-2-590 [DOI] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. (1991). Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55, 476–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2015). Generally Recognized as Safe (GRAS) Notifications. Avaliable online at: www.fda.gov/food/ingredientspackaginglabeling/gras/default.htm
- Garnier M., Matamoros S., Chevret D., Pilet M. F., Leroi F., Tresse O. (2010). Adaptation to cold and proteomic responses of the psychrotrophic biopreservative Lactococcus piscium strain CNCM I-4031. Appl. Environ. Microbiol. 76, 8011–8018. 10.1128/AEM.01331-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga M., Rubio R., Aymerich T., Ruas-Madiedo P. (2015). Potentially probiotic and bioprotective lactic acid bacteria starter cultures antagonise the Listeria monocytogenes adhesion to HT29 colonocyte-like cells. Benef. Microbes 6, 337–343. 10.3920/BM2014.0056 [DOI] [PubMed] [Google Scholar]
- Gildberg A., Johansen A., Bogwald J. (1995). Growth and survival of Atlantic salmon (Salmo salar) fry given diets supplemented with fish protein hydrolysate and lactic acid bacteria during a challenge trial with Aeromonas salmonicida. Aquaculture 138, 23–34. 10.1016/0044-8486(95)01144-7 [DOI] [Google Scholar]
- Gill H. S., Guarner F. (2004). Probiotics and human health: a clinical perspective. Postgrad. Med. J. 80, 516–526. 10.1136/pgmj.2003.008664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S., Rashmi H. M., Srivastava A. K., Batish V. K. (2012). Probiotics for human health -new innovations and emerging trends. Gut. Pathog. 4:15. 10.1186/1757-4749-4-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri E., de Niederhäusern S., Messi P., Sabia C., Iseppi R., Anacarso I., et al. (2009). Use of lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes in a small-scale model. Food Control 20, 861–865. 10.1016/j.foodcont.2008.11.001 [DOI] [Google Scholar]
- Guilbaud M., Piveteau P., Desvaux M., Brisse S., Briandet R. (2015). Exploring the diversity of Listeria monocytogenes biofilm architecture by high-throughput confocal laser scanning microscopy and the predominance of the honeycomb-like morphotype. Appl. Environ. Microbiol. 81, 1813–1819. 10.1128/AEM.03173-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiral S., Mitchell T. J., Martin B., Claverys J. P. (2005). Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc. Natl. Acad. Sci. U.S.A. 102, 8710–8715. 10.1073/pnas.0500879102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. J., Daeschel M. A., Stiles M. E., Klaenhammer T. R. (1989). Antimicrobial activitz of lactic acid bacteria against Listeria monocytogenes. J. Food Prot. 52, 384–387. [DOI] [PubMed] [Google Scholar]
- Johansson M. E., Ambort D., Pelaseyed T., Schutte A., Gustafsson J. K., Ermund A., et al. (2011). Composition and functional role of the mucus layers in the intestine. Cell. Mol. Life Sci. 68, 3635–3641. 10.1007/s00018-011-0822-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józefiak D., Sip A., Kaczmarek S., Rutkowski A. (2010). The effects of Carnobacterium divergens AS7 bacteriocin on gastrointestinal microflora in vitro and on nutrient retention in broiler chickens. J. Anim. Feed Sci. 19, 460–467. [Google Scholar]
- Kim K. Y., Frank J. F. (1995). Effects of nutrients on biofilm formation by Listeria monocytogenes on stainless steel. J. Food Prot 58, 24–28. [DOI] [PubMed] [Google Scholar]
- Klaenhammer T. R. (2000). Probiotic bacteria: today and tomorrow. J. Nutr. 130, 415–416. [DOI] [PubMed] [Google Scholar]
- Koo O., Amalaradjou M., Bhunia A. (2012). Recombinant probiotic expressing Listeria adhesion protein attenuates Listeria monocytogenes virulence in vitro. PLoS ONE 7:e29277. 10.1371/journal.pone.0029277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukova A., Czikkova S., Laczkova S., Turek P. (1999). Use of enterocin CCM 4231 to control Listeria monocytogenes in experimentally contaminated dry fermented Hornád salami. Int. J. Food Microbiol. 52, 115–119. 10.1016/S0168-1605(99)00125-7 [DOI] [PubMed] [Google Scholar]
- Leisner J. J., Hansen M. A., Larsen M. H., Hansen L., Ingmer H., Sorensen S. J. (2012). The genome sequence of the lactic acid bacterium, Carnobacterium maltaromaticum ATCC 35586 encodes potential virulence factors. Int. J. Food Microbiol. 152, 107–115. 10.1016/j.ijfoodmicro.2011.05.012 [DOI] [PubMed] [Google Scholar]
- Leisner J. J., Laursen B. G., Prévost H., Drider D., Dalgaard P. (2007). Carnobacterium: positive and negative effects in the environment and in foods. FEMS Microbiol. Rev. 31, 592–613. 10.1111/j.1574-6976.2007.00080.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuffleur T., Porchet N., Aubert J. P., Swallow D., Gum J. R., Kim Y. S., et al. (1993). Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 106, 771–783. [DOI] [PubMed] [Google Scholar]
- Lindgren S. W., Dodrogosz W. J. (1990). Antagonistic activities of lactic acid bacteria in food and feed fermentation. FEMS Microbiol. Rev. 87, 149–164. 10.1111/j.1574-6968.1990.tb04885.x [DOI] [PubMed] [Google Scholar]
- Messaoudi S., Madi A., Prévost H., Feuilloley M., Manai M., Dousset X., et al. (2012). In vitro evaluation of the probiotic potential of Lactobacillus salivarius SMXD51. Anaerobe 18, 584–589. 10.1016/j.anaerobe.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Nilsson L., Gram L., Huss H. H. (1999). Growth control of Listeria monocytogenes on cold-smoked salmon using a competitive Lactic Acid Bacteria Flora. J. Food Prot. 62, 336–342. [DOI] [PubMed] [Google Scholar]
- Nilsson L., Hansen T. B., Garrido P., Buchrieser C., Glaser P., Knochel S., et al. (2005). Growth inhibition of Listeria monocytogenes by a nonbacteriocinogenic Carnobacterium pisciola. J. Appl. Microbiol. 98, 172–183. 10.1111/j.1365-2672.2004.02438.x [DOI] [PubMed] [Google Scholar]
- Pilchová T., Hernould M., Prévost H., Demnerová K., Pazlarová J., Tresse O. (2014). Influence of food processing environments on structure initiation of static biofilm of Listeria monocytogenes. Food Control 35, 366–372. 10.1016/j.foodcont.2013.07.021 [DOI] [Google Scholar]
- Pilet M. F., Dousset X., Barré R., Novel G., Desmazeaud M., Piard J. C. (1995). Evidence for two bacteriocins produced by Carnobacterium pisciola and Carnobacterium divergens isolated from fish and active against Listeria monocytogenes. J. Food Prot. 58, 256–262. [DOI] [PubMed] [Google Scholar]
- Richard C., Brillet A., Pilet M. F., Prévost H., Drider D. (2003). Evidence on inhibition of Listeria monocytogenes by divercin V41 action. Lett. Appl. Microbiol. 36, 288–292. 10.1046/j.1472-765X.2003.01310.x [DOI] [PubMed] [Google Scholar]
- Roche S. M., Velge P., Bottreau E., Durier C., Marquet-van der Mee N., Pardon P. (2001). Assessment of the virulence of Listeria monocytogenes: agreement between a plaque-forming assay with HT-29 cells and infection of immunocompetent mice. Int. J. Food Microbiol. 68, 33–44. 10.1016/S0168-1605(01)00460-3 [DOI] [PubMed] [Google Scholar]
- Rocourt J., Jacquet C., Reilly A. (2000). Epidemiology of human listeriosis and seafoods. Int. J. Food Microbiol. 62, 197–209. 10.1016/S0168-1605(00)00336-6 [DOI] [PubMed] [Google Scholar]
- Rodriguez E., Tomillo J., Nunez M., Medina M. (1997). Combined effect of bacteriocin-producing lactic acid bacteria and lactoperoxidase system activation on Listeria monocytogenes in refrigerated raw milk. J. Appl. Microbiol. 83, 389–395. 10.1046/j.1365-2672.1997.00243.x [DOI] [PubMed] [Google Scholar]
- Sabia C., Manicardi G., Messi P., de Niederhäusern S., Bondi M. (2002). Enterocin 416 K1, an antilisterial bacteriocin produced by Enterococcus casseliflavus IM 416K1 isolated from Italian sausages. Int. J. Food Microbiol. 75, 163–170. 10.1016/S0168-1605(01)00741-3 [DOI] [PubMed] [Google Scholar]
- Schöbitz R., Zaror T., León O., Costa M. (1999). A bacteriocin from Carnobacterium pisciola for the control of Listeria monocytogenes in vacuum-packaged meat. Food Microbiol. 16, 249–255. 10.1006/fmic.1998.0241 [DOI] [Google Scholar]
- Shen T. Y., Qin H. L., Gao Z. G., Fan X. B., Hang X. M., Jiang Y. Q. (2005). [Influences of enteral nutrition combined with probiotics on the gut microecology and barrier function of the rats with abdominal infection]. Zhonghua Wei Chang Wai Ke Za Zhi 8, 443–446. [PubMed] [Google Scholar]
- Sherman P. M., Johnson-Henry K. C., Yeung H. P., Ngo P. S., Goulet J., Tompkins T. A. (2005). Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect. Immun. 73, 5183–5188. 10.1128/IAI.73.8.5183-5188.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Zhu X. (2009). Biofilm formation and food safety in food industries. Trends Food Sci. Technol. 20, 407–413. 10.1016/j.tifs.2009.01.054 [DOI] [Google Scholar]
- Srividya D., Prakash S., Dharmesh S., Agrawal R. (2015). Anti-Shigella dysenteriae activity by probiotic lactic acid bacteria (Pediococcus pentosaceus); an in vitro study. J. Microbiol. Biotechnol. Food Sci. 4, 317–320. 10.15414/jmbfs.2015.4.4.317-320 [DOI] [Google Scholar]
- Swaminathan B., Gerner-Smidt P. (2007). The epidemiology of human listeriosis. Microbes Infect. 9, 1236–1243. 10.1016/j.micinf.2007.05.011 [DOI] [PubMed] [Google Scholar]
- Todorov S. D., Furtado D. N., Saad S. M., Tome E., Franco B. D. (2011). Potential beneficial properties of bacteriocin-producing lactic acid bacteria isolated from smoked salmon. J. Appl. Microbiol. 110, 971–986. 10.1111/j.1365-2672.2011.04950.x [DOI] [PubMed] [Google Scholar]
- Tresse O., Lebret V., Benezech T., Faille C. (2006). Comparative evaluation of adhesion, surface properties, and surface protein composition of Listeria monocytogenes strains after cultivation at constant pH of 5 and 7. J. Appl. Microbiol. 101, 53–62. 10.1111/j.1365-2672.2006.02968.x [DOI] [PubMed] [Google Scholar]
- Tresse O., Shannon K., Pinon A., Malle P., Vialette M., Midelet-Bourdin G. (2007). Variable adhesion of Listeria monocytogenes isolates from food-processing facilities and clinical cases to inert surfaces. J. Food Prot. 70, 1569–1578. [DOI] [PubMed] [Google Scholar]
- Turonova H., Briandet R., Rodrigues R., Hernould M., Hayek N., Stintzi A., et al. (2015). Biofilm spatial organization by the emerging pathogen Campylobacter jejuni: comparison between NCTC 11168 and 81-176 strains under microaerobic and oxygen-enriched conditions. Front. Microbiol. 6:709. 10.3389/fmicb.2015.00709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyopponen S., Markkula A., Petaja E., Suihko M. L., Mattila-Sandholm T. (2003). Survival of Listeria monocytogenes in North European type dry sausages fermented by bioprotective meat starter cultures. Food Control 14, 181–185. 10.1016/S0956-7135(02)00086-5 [DOI] [Google Scholar]
- Vázquez-Boland J. A., Kuhn M., Berche P., Chakraborty T., Domínguez-Bernal G., Goebel W., et al. (2001). Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14, 584–640. 10.1128/CMR.14.3.584-640.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K., Suzuki M., Kawai Y. (2003). Inhibition of Listeria monocytogenes in cold-smoked Salmon by Carnobacterium piscicola CS526 isolated from frozen surimi. J. Food Prot. 66, 20–25. [DOI] [PubMed] [Google Scholar]
- Zoetendal E. G., von Wright A., Vilpponen-Salmela T., Ben-Amor K., Akkermans A. D., de Vos W. M. (2002). Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl. Environ. Microbiol. 68, 3401–3407. 10.1128/AEM.68.7.3401-3407.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]



