Abstract
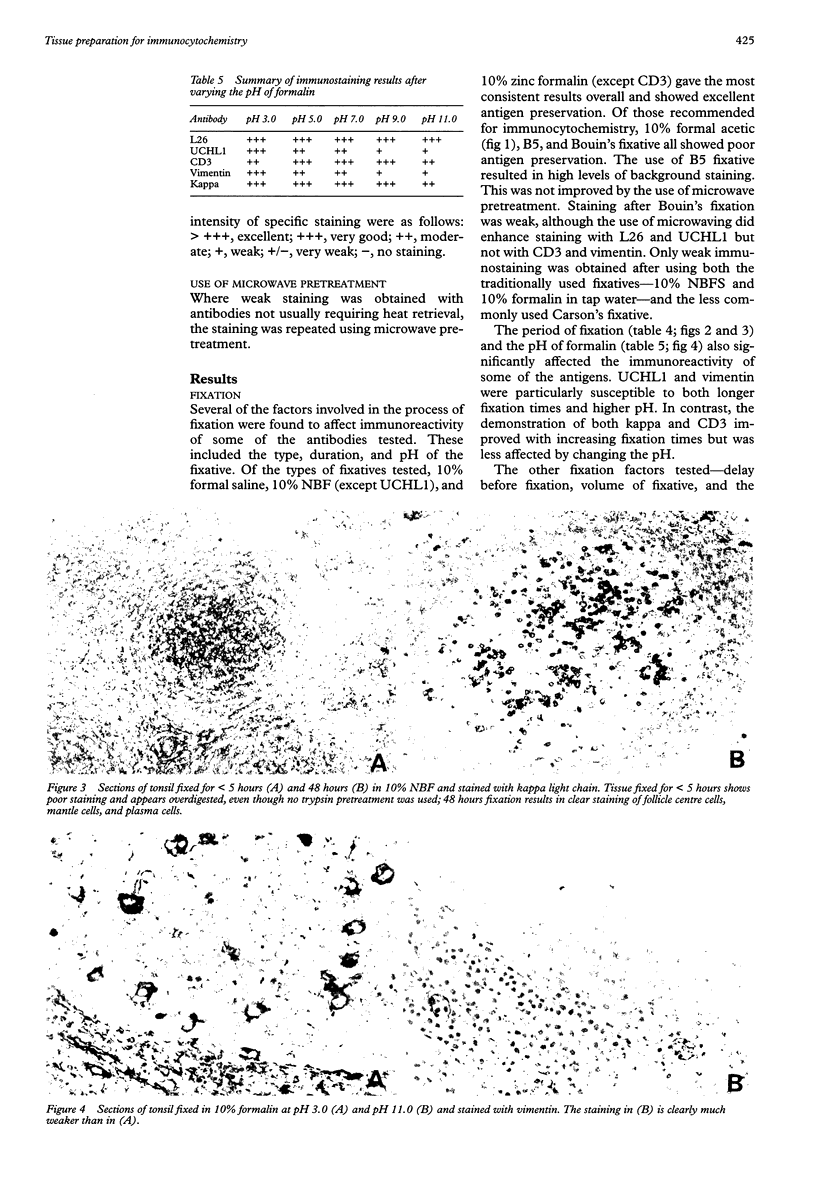
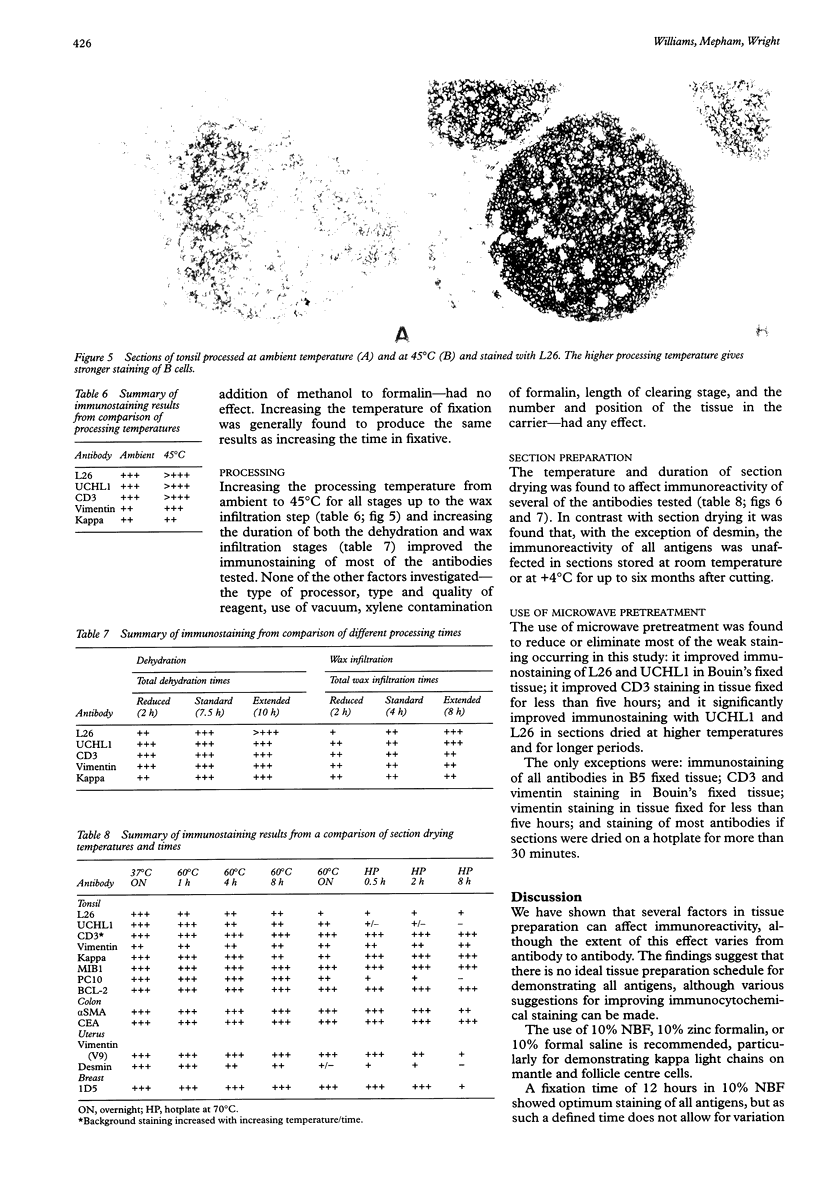
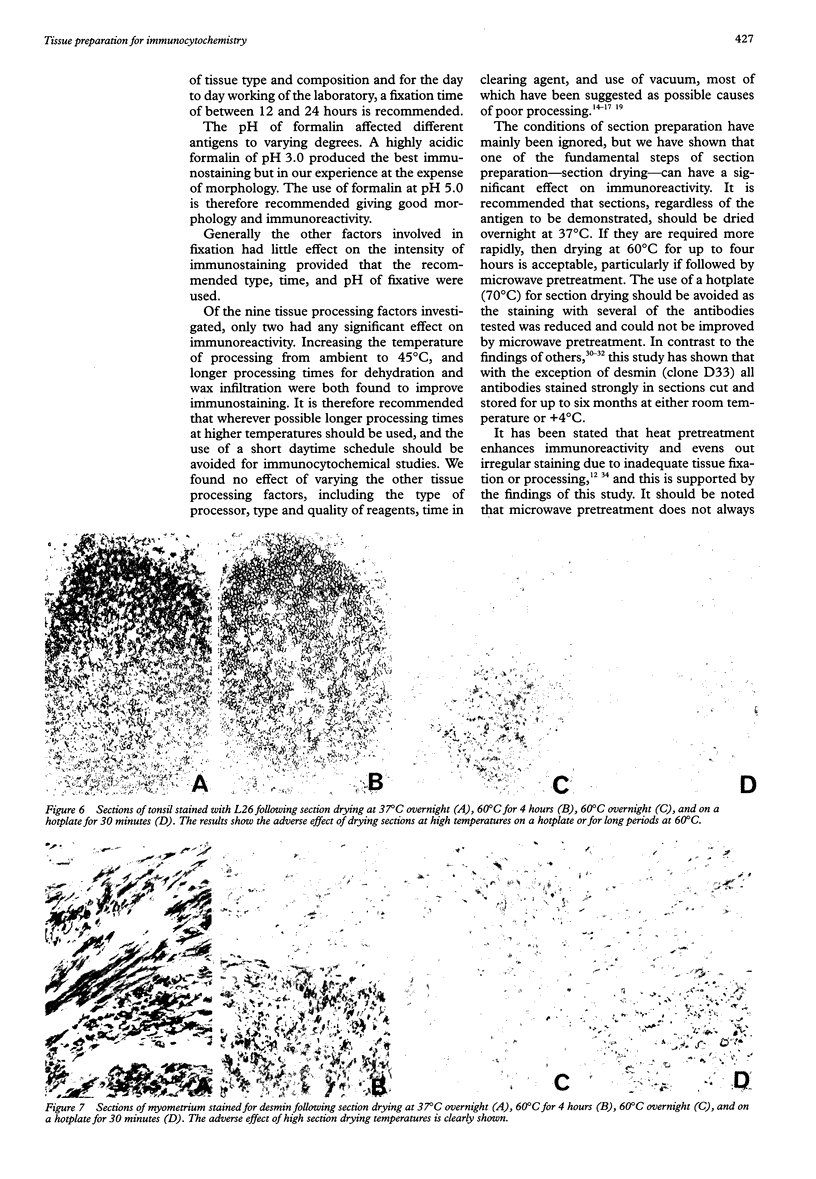
AIMS: To investigate the effect of tissue preparation on immunostaining and to establish whether there is a standard tissue preparation schedule that allows optimal demonstration of all antigens. METHODS: Blocks of tonsil were subjected to variations to a standard fixation, processing, and section preparation schedule. The sections were stained with five antibodies-L26 (CD20), UCHL1 (CD45RO), CD3, vimentin, and anti-kappa light chain--using the streptavidinbiotin immunostaining technique. When further investigation was necessary, other tissues and antibodies were used and where weak immunostaining was obtained the use of microwave pretreatment to improve staining was tested. RESULTS: Several factors involved in fixation were found to affect immunoreactivity. These included the duration, pH, and type of fixative used. In tissue processing only temperature and the duration of the dehydration and wax infiltration steps affected immunoreactivity. Of all the factors investigated, the temperature and duration of the section drying had the greatest effect. In contrast, long term storage of cut sections before immunostaining had no effect on the reactivity of the antibodies tested. Antibodies were found to be affected by alterations to tissue preparation by varying degrees, UCHL1 and vimentin being the most susceptible to changes in fixation and L26 to changes in processing. Where weak staining occurred, microwave pretreatment was generally found to eliminate the problem. CONCLUSIONS: There is no standard tissue preparation schedule for the optimal demonstration of all antigens. Factors involved in all aspects of tissue preparation can affect immunoreactivity, so it is important that precise details of the preparation schedule are given when reporting immunocytochemical studies, rather than using the general term "routinely fixed and processed".
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battifora H. Assessment of antigen damage in immunohistochemistry. The vimentin internal control. Am J Clin Pathol. 1991 Nov;96(5):669–671. doi: 10.1093/ajcp/96.5.669. [DOI] [PubMed] [Google Scholar]
- Carson F. L., Martin J. H., Lynn J. A. Formalin fixation for electron microscopy: a re-evaluation. Am J Clin Pathol. 1973 Mar;59(3):365–373. doi: 10.1093/ajcp/59.3.365. [DOI] [PubMed] [Google Scholar]
- Cerio R., MacDonald D. M. Effect of routine paraffin wax processing on cell membrane immunoreactivity in cutaneous tissue. J Clin Lab Immunol. 1986 Jun;20(2):97–100. [PubMed] [Google Scholar]
- Collings L. A., Poulter L. W., Janossy G. The demonstration of cell surface antigens on T cells, B cells and accessory cells in paraffin-embedded human tissues. J Immunol Methods. 1984 Dec 31;75(2):227–239. doi: 10.1016/0022-1759(84)90106-6. [DOI] [PubMed] [Google Scholar]
- Cross S. S., Start R. D., Smith J. H. Does delay in fixation affect the number of mitotic figures in processed tissue? J Clin Pathol. 1990 Jul;43(7):597–599. doi: 10.1136/jcp.43.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. C., Gregory J. Effects of fixation and processing on immunohistochemical demonstration of immunoglobulin in paraffin sections of tonsil and bone marrow. J Clin Pathol. 1980 Nov;33(11):1047–1057. doi: 10.1136/jcp.33.11.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donhuijsen K., Schmidt U., Hirche H., van Beuningen D., Budach V. Changes in mitotic rate and cell cycle fractions caused by delayed fixation. Hum Pathol. 1990 Jul;21(7):709–714. doi: 10.1016/0046-8177(90)90030-9. [DOI] [PubMed] [Google Scholar]
- EDWARDS J. L., DONALSON J. T. THE TIME OF FIXATION AND THE MITOTIC INDEX. Am J Clin Pathol. 1964 Feb;41:158–162. doi: 10.1093/ajcp/41.2.158. [DOI] [PubMed] [Google Scholar]
- Elias J. M., Gown A. M., Nakamura R. M., Wilbur D. C., Herman G. E., Jaffe E. S., Battifora H., Brigati D. J. Quality control in immunohistochemistry. Report of a workshop sponsored by the Biological Stain Commission. Am J Clin Pathol. 1989 Dec;92(6):836–843. doi: 10.1093/ajcp/92.6.836. [DOI] [PubMed] [Google Scholar]
- Fisher C. J., Gillett C. E., Vojtesek B., Barnes D. M., Millis R. R. Problems with p53 immunohistochemical staining: the effect of fixation and variation in the methods of evaluation. Br J Cancer. 1994 Jan;69(1):26–31. doi: 10.1038/bjc.1994.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graem N., Helweg-Larsen K. Mitotic activity and delay in fixation of tumour tissue. The influence of delay in fixation on mitotic activity of a human osteogenic sarcoma grown in athymic nude mice. Acta Pathol Microbiol Scand A. 1979 Sep;87A(5):375–378. [PubMed] [Google Scholar]
- Horikawa M., Chisaka N., Yokoyama S., Onoé T. Effect of stirring during fixation upon immunofluorescence. Results with distribution of albumin-producing cells in liver. J Histochem Cytochem. 1976 Aug;24(8):926–932. doi: 10.1177/24.8.60440. [DOI] [PubMed] [Google Scholar]
- Matthews J. B. Influence of clearing agent on immunohistochemical staining of paraffin-embedded tissue. J Clin Pathol. 1981 Jan;34(1):103–105. doi: 10.1136/jcp.34.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Norton A. J., Jordan S., Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994 Aug;173(4):371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- Prioleau J., Schnitt S. J. p53 antigen loss in stored paraffin slides. N Engl J Med. 1995 Jun 1;332(22):1521–1522. doi: 10.1056/NEJM199506013322217. [DOI] [PubMed] [Google Scholar]
- Raymond W. A., Leong A. S. Oestrogen receptor staining of paraffin-embedded breast carcinomas following short fixation in formalin: a comparison with cytosolic and frozen section receptor analyses. J Pathol. 1990 Apr;160(4):295–303. doi: 10.1002/path.1711600405. [DOI] [PubMed] [Google Scholar]
- Start R. D., Flynn M. S., Cross S. S., Rogers K., Smith J. H. Is the grading of breast carcinomas affected by a delay in fixation? Virchows Arch A Pathol Anat Histopathol. 1991;419(6):475–477. doi: 10.1007/BF01650675. [DOI] [PubMed] [Google Scholar]
- Tahan S. R., Wei Y., Ling P., Bistrian B. R. Influence of formalin fixation time and tissue processing method on immunoreactivity of monoclonal antibody PC10 for proliferating cell nuclear antigen. Mod Pathol. 1995 Feb;8(2):177–182. [PubMed] [Google Scholar]
- Taylor C. R., Burns J. The demonstration of plasma cells and other immunoglobulin-containing cells in formalin-fixed, paraffin-embedded tissues using peroxidase-labelled antibody. J Clin Pathol. 1974 Jan;27(1):14–20. doi: 10.1136/jcp.27.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan A., Gudat F., Busachi C., Stöcklin E., Bianchi L. An improved method for HBcAg demonstration in paraffin-embedded liver tissue. Liver. 1982 Dec;2(4):331–339. doi: 10.1111/j.1600-0676.1982.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Wakins J., Kellock D., Gillet C., Egan M., Pontin J. E., Millis R. R., Levinson D. A. Enhancement of immunostaining. Histopathology. 1990 Aug;17(2):185–185. doi: 10.1111/j.1365-2559.1990.tb00700.x. [DOI] [PubMed] [Google Scholar]
- von Wasielewski R., Werner M., Nolte M., Wilkens L., Georgii A. Effects of antigen retrieval by microwave heating in formalin-fixed tissue sections on a broad panel of antibodies. Histochemistry. 1994 Sep;102(3):165–172. doi: 10.1007/BF00268892. [DOI] [PubMed] [Google Scholar]









