Abstract
In the current era of the early diagnosis of prostate cancer (PCa) and the development of minimally invasive surgical techniques, erectile dysfunction (ED) represents an important issue, with up to 68% of patients who undergo radical prostatectomy (RP) complaining of postoperative erectile function (EF) impairment. In this context, it is crucial to comprehensively consider all factors possibly associated with the prevention of post-RP ED throughout the entire clinical management of PCa patients. A careful assessment of both oncological and functional baseline characteristics should be carried out for each patient preoperatively. Baseline EF, together with age and the overall burden of comorbidities, has been strongly associated with the chance of post-RP EF recovery. With this goal in mind, internationally validated psychometric instruments are preferable for ensuring proper baseline EF evaluations, and questionnaires should be administered at the proper time before surgery. Careful preoperative counselling is also required, both to respect the patient's wishes and to avoid false expectations regarding eventual recovery of baseline EF. The advent of robotic surgery has led to improvements in the knowledge of prostate surgical anatomy, as reflected by the formal redefinition of nerve-sparing techniques. Overall, comparative studies have shown significantly better EF outcomes for robotic RP than for open techniques, although data from prospective trials have not always been consistent. Preclinical data and several prospective randomized trials have demonstrated the value of treating patients with oral phosphodiesterase 5 inhibitors (PDE5is) after surgery, with the concomitant potential benefit of early re-oxygenation of the erectile tissue, which appears to be crucial for avoiding the eventual penile structural changes that are associated with postoperative neuropraxia and ultimately result in severe ED. For patients who do not properly respond to PDE5is, proper counselling regarding intracavernous treatment should be considered, along with the further possibility of surgical treatment for ED involving the implantation of a penile prosthesis.
Keywords: Erectile dysfunction, Phosphodiesterase 5 inhibitors, Prostatectomy, Prostatic neoplasms, Robotics
INTRODUCTION
Prostate cancer (PCa) is one of the most frequently diagnosed cancers in Western countries [1]. Currently, radical prostatectomy (RP) has been demonstrated to be the only therapeutic approach associated with improved patient survival in comparison to conservative management [2], and RP has emerged as one of the most commonly used first-line treatment modalities in men with localized PCa [3].
Although many advances have been made in terms of both our knowledge of the surgical anatomy of the prostate and the development of minimally invasive surgical techniques, erectile dysfunction (ED) after RP still represents a troublesome issue for both patients and physicians, with reported incidence rates ranging widely between 6% and 68% [4]. In this context, over the last decades, PCa has become more commonly diagnosed in younger men, which has clearly influenced the increasing importance of erectile function (EF) recovery after PCa treatment, as well as leading to a consequent focus on the preservation of patients' quality of life (QoL) [5,6]. Similarly, the steady increase in life expectancy that has been observed in most developed countries due to generally healthier lifestyles has underscored the importance of these considerations for individuals undergoing RP [7]. Finally, the tendency for disease to pose reduced levels of risk at the time of diagnosis, along with the advent of robotic surgical techniques and the promising oncological and functional results of potentially less invasive treatments such as focal therapy, has progressively increased patients' expectations regarding surgical treatment for PCa. As a consequence of this, rates of postoperative dissatisfaction and regret have been reported to be as high as 19%, partially due to unexpected decreases in overall QoL [8].
With all of these considerations in mind, postoperative ED should be properly managed, with a careful consideration of all factors that influence the preservation of EF after surgery, including the preoperative patient assessment, precise operative techniques, and finally, implementing a comprehensive plan for postoperative ED management. The aim of this review was to critically assess the current evidence available on this topic, providing a reader-friendly expert viewpoint useful for assisting physicians in planning postoperative approaches to ED for PCa patients in real-life settings.
PREOPERATIVE SETTING: THE IMPORTANCE OF A GOOD START
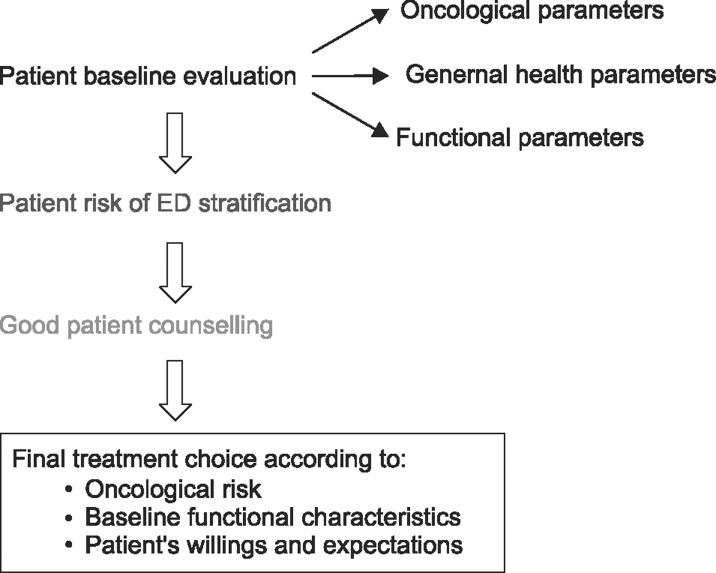
The preoperative assessment of a candidate for RP is the first compulsory step in preventing postoperative ED. Indeed, a flowchart (Fig. 1) should be followed starting from the overall baseline evaluation of a patient, with assessments of both oncological and functional parameters. This allows a correct estimation of the potential risk of postsurgical ED in each individual, allowing the physician to correctly counsel the patient regarding the optimal treatment modality in an attempt to match his wishes and expectations with the need for an oncologically safe procedure.
Fig. 1. Practical flowchart for preoperative patient assessment. ED: erectile dysfunction.
Evaluating the clinical and pathological characteristics of the disease is of paramount importance in decision-making regarding treatment. According to the guidelines of the European Association of Urology [3] nerve-sparing (NS) procedures are a safe surgical approach in the majority of men with localised PCa [9], whereas NS techniques are clearly contraindicated in men with well-known high risk factors for extracapsular extension (ECE), such as clinical stage (cT) T2c or T3 disease and/or a biopsy Gleason score greater than 7. In this context, useful predictive tools have been developed and validated for predicting ECE before surgery, showing an accuracy as high as 89% in a robot-assisted RP (RARP) series [10]. In a recent meta-analysis conducted on a total of 9,796 patients aimed to assess the diagnostic accuracy of preoperative magnetic resonance imaging (MRI) for the local staging of PCa, the authors showed a sensitivity and specificity of 57% and 91% for predicting ECE, respectively, and of 61% and 88% for predicting overall cT3 disease, respectively, thus demonstrating poor sensitivity mainly in detecting microscopic ECE; for these reasons, MRI is currently not routinely recommended for local preoperative staging [11]. Regardless of international clinical recommendations, Recabal et al [12] showed that in a cohort of 584 patients with high-risk characteristics, bilateral NS RP (BNSRP) was eventually feasible in 72% of the cases, with only 24% having a positive surgical margin in the final pathology and up to 47% reporting a recovery of EF 2 years after surgery.
After the decision to treat a patient with or without a BNSRP, a comprehensive clinical and functional assessment should be conducted. Overall, comorbidities such as cardiovascular diseases and diabetes mellitus (DM), in combination with advanced patient age, have emerged as well-recognized risk factors for EF impairment in the general population, regardless of surgery for PCa [13,14]; likewise, these predictors have also demonstrated a detrimental effect on postoperative EF recovery. In this regard, Rabbani et al [15] clearly demonstrated the effect of patient age on the probability of EF recovery after surgery, showing rates of recovery of 70%, 45%, and 30% for patients ≤60, 60~65, and >65 years of age, respectively. Similar results were presented in a larger series of 1,288 patients treated with BNSRP [16]. More recently, in a cohort of 3,241 patients undergoing RARP, Kumar et al [17] demonstrated that those ≥70 years of age had significantly lower EF recovery rates than a matched subgroup of younger patients (33.5% vs. 52.3%, respectively). Moreover, Salomon et al [18] examined the overall burden of comorbidities and patient age, showing that body mass index, type 2 DM, and depression were significantly associated with baseline ED in candidates for RP. Similarly, overall vascular risk factors, including hypertension, hypercholesterolemia, DM, coronary diseases, and cigarette smoking, emerged as independent predictors of impaired EF recovery 24~30 months after RP in a cohort of 984 patients, irrespective of NS status and baseline EF [19]. More recently, Gandaglia et al [20] described the role of non-surgical causes of ED after BNSRP and found that, in addition to baseline EF, preoperative depressive status defined using the Center for Epidemiologic Studies-Depression questionnaire was significantly related to postoperative ED.
In an attempt to comprehensively evaluate the impact of preoperative patient characteristics on postoperative EF recovery, Briganti et al [21] developed a risk stratification tool including patient's age, preoperative EF measured with the International Index of Erectile Function (IIEF) scores, and the Charlson Comorbidity Index (CCI) as a proxy for general health status; they demonstrated that the risk of post-RP ED could be stratified into 3 groups of risk: low risk for ED (≤65 years of age, IIEF-EF≥26, and CCI≤1), intermediate risk for ED (66~69 years of age or IIEF-EF of 11~25, CCI≤1), and high risk for ED (>70 years of age, IIEF-EF≤10, or CCI≥2). The 3-year EF recovery rates were 85%, 59%, and 37% for patients in the low-, intermediate-, and high-risk categories, respectively (p<0.001). Novara et al [22] applied the same stratification system in a series of RARP patients, showing that age at surgery (hazard ratio [HR]: 2.8; p<0.001), CCI (HR: 2.9; p=0.007), and baseline EF (HR: 0.8; p<0.001) were independent predictors of EF recovery, with 12-month EF recovery rates of 82%, 57%, and 29% in low-, intermediate-, and high-risk patients, respectively [21].
Preoperative EF status was found to be the main predictor of post-RP EF recovery [23]. Indeed, up to 48% of patients with some degree of ED before RP showed postoperative ED [18,24]. For these reasons, a critical and complete assessment of baseline EF is a fundamental part of the preoperative patient evaluation [25]. In this context, two important issues should be considered: the timing and modality of the baseline EF assessment. Kim et al [26] attempted to assess the optimal timing for administering a number of psychometric tools to evaluate baseline EF in 54 candidates to RARP; all patients were asked to complete the IIEF-5 questionnaire before a prostate biopsy, 1 day before RARP, and 1 month after RARP. The results showed that the IIEF-5 scores obtained before the biopsy exhibited greater agreement with the results obtained after surgery than the scores gathered one day prior to RARP [26]. Moreover, in an evaluation of the baseline IIEF scores of 234 patients undergoing RP, Salonia et al [25] showed that as many as 28% of them had preoperative scores suggestive of severe ED, with more than one-third of the patients with severe ED not reporting any sexual attempts during the 4 weeks prior to surgery. These data imply that temporal proximity to surgery may reduce the sexual activity and/or desire of the patient and/or the couple; likewise, overall cancer-related psychological distress may also negatively impact real-time assessments of EF immediately before surgery.
As a second major element, EF assessment should rely on the use of validated psychometric instruments [7], including the IIEF and the Sexual Health Inventory for Men questionnaires, which are the validated tools that are most widely used worldwide. Still, confusion exists in terms of a clear modality of EF assessment in this specific subset of patients; Mulhall [27], for instance, showed that a correct modality of baseline EF evaluation was provided by only 16 of 24 studies (66.7%) from large-volume centres.
Overall, comprehensive information on patients' oncological risks and baseline functional status are essential for proper preoperative counselling aiming to provide every patient with realistic expectations of his own post-RP EF recovery. In a survey conducted on 336 consecutive patients submitted to either open RP (ORP) or RARP, Deveci et al [28] sought to characterize the sexuality-related information received preoperatively by all patients at a 3-month post-RP assessment, finding that RARP patients expected a shorter EF recovery time and a higher likelihood of recovering their own baseline EF. Importantly, 50% of the subjects were unaware of the occurrence of postoperative anejaculation. Similarly, previous data showed that among RP patients, only 45% were actually aware of the NS status of the operation that they underwent [29]. Interestingly, Imbimbo et al [30] assessed patients' desire to preserve post-RP EF and matched their preferences to the actual feasibility of a NS procedure in a cohort of 2,408 men; they found that as many as 13% of patients were not interested to NS despite being suitable candidates, whereas 31% were interested but unsuitable. Taken together, these findings underscore the need for comprehensive preoperative counselling in every patient, with a major focus on the concept of "going back to baseline EF" [7,23]. Indeed, it appears extremely important to reduce the risk of false expectations through a critical and realistic discussion about the eventual probability of regaining erections equivalent to those experienced prior to RP, especially in light of the results of each institution. Indeed, spontaneous recovery of baseline EF has been demonstrated only in up to 30% of patients after RP [23]. Moreover, the experience of satisfactory sexual function after surgery does not always correspond to the achievement of baseline conditions. With this in mind, Briganti et al [31] conducted a survey on a cohort of preoperatively fully potent (IIEF-EF≥26) patients treated with BNSRP, assessing postoperative scores of the IIEF domains of intercourse satisfaction (IS) and overall satisfaction (OS). They found that after a mean follow-up of 26.7 months, patients with an IIEF-EF of 22~25 had comparable results in terms of IS and OS scores to those with an IIEF-EF≥26, thus concluding that IIEF-EF scores ≥22 could be a reliable cut-off for defining post-RP EF recovery, regardless of a patient's baseline condition.
INTRAOPERATIVE SETTING: HOW CAN THE RISK OF ERECTILE DYSFUNCTION BE REDUCED?
1. Physiopathology of postoperative erectile dysfunction
Penile erection is defined as a neurovascular event modulated by psychological factors and hormonal status, where both neuronal and vascular components are essential in the physiological pathway [32]. During sexual stimulation, neurotransmitters responsible for the relaxation of the smooth muscle in the arteries and arterioles supplying the erectile tissue are released by the cavernous nerve (CN) terminals, which provide parasympathetic innervation to the corpora cavernosa; these CN terminals originate from a dense neural network known as the pelvic plexus that is located in the fibro-fatty plane between the bladder and the rectum [33]. These fibres are normally accompanied by vascular structures, and are thus comprehensively defined as neurovascular bundles (NVBs).
From a pathophysiological point of view, post-RP ED has been described as neurogenic, arterogenic, venogenic, or a combination thereof. Since the original identification of the correct location of the CNs laterally to the prostate by Walsh [34], post-RP ED has been related to injuries of the pelvic plexus and the CNs during the lateral and apical dissection of the prostate. However, in addition to direct injuries to the nerves, ED can occur as a consequence of neuropraxia caused by traction, compression, and coagulation [23,24]. This type of injury induces Wallerian degeneration of the nerves, thus leading to the denervation of the corpora cavernosa and the consequent loss of nocturnal EF activity, with penile hypoxia and fibrosis that can finally result in venous leakage responsible for ED [35]. With this in mind, the postoperative length of time preceding EF recovery has been associated with the risk of venous leakage, with previous findings showing incidence rates of venous leakage of 14% and 35% in patients showing EF recovery at less than 4 months and at 9~12 months post-RP, respectively [36]. Finally, it has been postulated that the primary mechanism responsible for postoperative arterogenic ED may be the transection of the accessory pudendal arteries (APAs), which have been described in up to 75% of patients, and could lead to penile hypoxia independently of the status of the CNs [37].
2. Surgical anatomy
Our knowledge of prostate anatomy has dramatically improved over the last three decades. This has led to significant changes in surgical techniques, with the specific goal of achieving better postoperative functional outcomes. In the context of EF recovery, two main aspects must be considered: prostate vascular supply and the anatomy of the NVBs.
1) Prostate arterial supply: artery-sparing surgery
The prostate arteries arise from the internal pudendal artery in 35% to 56% of cases, from the gluteal-pudendal trunk in 15% to 28% of cases, and less frequently from the obturator artery (10% to 12% of cases) [38]. Two main bifurcations of the artery can be bilaterally recognized: a posterior pedicle, surrounding the seminal vesicles and the vas deferens and reaching the prostate base, and an anterior pedicle at the level of the lateral side of the prostate, reaching the prostate apex. At this level, the preservation of small anterior capsular prostate branches may be associated with EF recovery, as they are responsible for ancillary penile blood supply [39].
APAs have been described in 4% to 75% of men [37]; they may originate from the internal or the external iliac or obturator arteries and usually run along the fascial tendinous arch of the pelvis or on the anterolateral aspect of the prostate apex [40]. Several published studies have shown that damage to the APAs can lead to penile arterial insufficiency after surgery [36,37]. Mulhall et al [37], in a cohort of men undergoing open BNSRP, showed that up to 59% of patients with postoperative ED had arterial insufficiency; likewise, a number of further observations showed that the APAs may be solely responsible for arterial blood supply to the corpora cavernosa [41]. Conversely, Box et al [42] recently assessed the effect of sacrificing the APAs in a series of 200 patients treated with RARP; they showed that ligation of the APAs occurred in 19 patients, with 95% of them reporting EF recovery after surgery.
2) Neurovascular bundles: nerve-sparing technique
The nerve fibres originating from the pelvic plexus and innervating the corpora cavernosa reach the lateral side of the bladder neck and are located posterolaterally to the seminal vesicles, running very close to their tips; indeed, careful dissection of the seminal vesicles during RP may reduce the risk of postoperative ED [43]. Proximally to the prostate, these fibres present a "spray-like" distribution on the posterolateral and anterolateral surface of the gland, up to the level of the 2 o'clock and 10 o'clock positions [44]. Most of these fibres have been reported at the posterolateral level of the gland, with only 19% to 40% of them located on the anterolateral aspect, where they are mostly at the level of the apex [45]. Costello et al [33] showed that the fibres running anteriorly in NVBs mainly innervate the levator ani muscle and the prostate, while nerve branches located more posterolaterally innervate the corpora cavernosa [33]. Moreover, Ganzer et al [46] showed that only 1.5% of the parasympathetic nerves, which are mainly involved in EF, are located on the anterolateral aspect of the prostate apex, thus suggesting that the influence of these fibres on EF may be uncertain. Conversely, using a three-dimensional reconstruction, Alsaid et al [45] showed that the nerves extending to the corpora cavernosa are mainly a continuation of the fibres running anteriorly at the apex level, concluding that an ideal NS operation should include the preservation of the anterolateral tissue and fascia covering the prostate. Overall, NVBs are included in a multi-layered fascia that is either fused or separated from the prostatic capsule, covering the outer surface of the prostate, and is known as the peri-prostatic fascia (PPF) [40]. The relationship between the NVB and the PPF has been variably described, especially in reports of the wide range of NS techniques that have been proposed over the last two decades. Indeed, several dissection planes can be recognized within the PPF, allowing different "degrees" of NS procedures and leading to the novel concept of the incremental NS approach. Previously, three possible dissection planes had been described: an intrafascial dissection plane, following a plane on the pseudocapsule of the prostate, internal to the PPF and anterior to the fascia covering the seminal vesicles, allowing safe complete sparing of the NVB; an interfascial dissection plane within the thickness of the PPF, allowing a complete or partial NS procedure according to individual variations in the locations of NVBs; and an extrafascial dissection plane that extends laterally to the levator ani fascia and is used for complete resection of the NVBs [40]. Subsequently, a different terminology was proposed, identifying full, partial, and minimal NS approaches as corresponding to intrafascial, interfascial, and "sub" extrafascial dissections, respectively [47]. More recently, with the advent of robotic surgery allowing for optic magnification, Tewari et al [48] described a 4-degree NS approach, taking as a vascular landmarks the veins located on the lateral aspects of the prostate. In this approach, a dissection plane running between the pseudocapsule and the periprostatic veins is defined as grade 1, corresponding to the maximum level of NS dissection. However, moving laterally from the veins towards the levator ani fascia, NS approaches of grades 2, 3, and 4 can be identified, with a progressively less NS to non-NS (NNS) technique. In a cohort of 2,536 patients treated with RARP, the 1-year postoperative potency rates were 90.6%, 76.2%, 60.5%, and 57.1% for patients undergoing NS grade 1, 2, 3, and 4 dissections, respectively [49]. Similarly, Schatloff et al [50] described a 5-grade scale of dissection, with grade 5 representing optimal NS and grade 1 representing NNS; as a landmark for the different dissection planes, they identified a prostatic artery lying on the lateral side of the gland that has been recognized in up to 73% of cases. They evaluated the amount of residual nerve tissue found on the surgical specimens, showing that it was significantly different according to the grade of the NS approach, with a wider area of residual tissue associated with NS 1. Importantly, careful preservation of the nervous tissue involved in EF control should be also pursued when performing pelvic lymph node dissections (PLNDs); the pelvic plexus lies within an area of fibro-fatty tissue located between the bladder and the rectum that could be included in PLND, especially during the dissection of the area medial to the internal iliac artery or in the presacral area [44]. However, no consensus exists regarding the possibility of a higher incidence of postoperative ED associated with more extended PLNDs [51,52].
3. Reported outcomes after radical prostatectomy: comparison of techniques
Data about the incidence of post-RP ED have been widely reported over the last two decades, with considerable differences found among reports. Indeed, factors dealing with the different definitions and measures of ED applied in each study, the characteristics of surgery and patient selection criteria, and the different postoperative rehabilitative protocols adopted over time have been found to play an important role in determining the wide variability of reported EF outcomes [23]. Potency rates ranging from 31% to 86% have been shown after ORP at a minimum of 12 months of follow-up [53]; similarly, potency rates after laparoscopic RP (LRP) have been reported to range from 42% to 76% [54]. More recently, a meta-analysis of RARP series reported potency recovery rates of 32% to 68%, 50% to 86%, 54% to 90%, and 63% to 94% at 3, 6, 12, and 24 months after surgery, respectively [4]. Given concerns regarding variable methodology among studies, a comparison of EF outcomes between open and minimally invasive surgery, rather than between laparoscopic and robotic techniques, appears even more difficult. Moreover, most data come from retrospective series (level of evidence [LE] 4) with only few prospective studies and randomized clinical trials reporting a LE of 2 or 3 for the comparison of EF outcomes among surgical techniques (Table 1) [55,56,57,58,59,60]. In order to assess possible differences in EF recovery rates according to different surgical approaches, Ficarra et al [4] performed a comprehensive analysis of published data on RP series up to 2012, incorporating a cumulative analysis of data from ORP versus RARP series. Their study showed a statistically significant advantage in favour of RARP, with an absolute risk reduction for ED of 23.6% at 12 months after surgery. Similar data were reported in a unique study reporting functional outcomes at a longer (24-month) follow-up [55]. Turning to prospective studies, a significant advantage in terms of post-RARP 3-month EF recovery was demonstrated by Tewari et al [56] in a single-institution series. Haglind et al [57] recently reported data from a multicentre prospective controlled non-randomised study including 778 ORP patients and 1,847 RARP patients; according to patients' IIEF-5 scores, a slightly significant advantage in favour of RARP (odds ratio=0.75; 95% confidence interval=0.58~0.96) was seen at a 12-month postoperative assessment after adjusting for confounding variables. Of clinical relevance, they reported overall poor EF outcomes after both procedures, with only 30% and 25% of men being potent after RARP and ORP, respectively, as indicated by a validated instrument that was sent to a third party for evaluation [57]. Interestingly, Stolzenburg et al [61] assessed the effect of different surgical approaches on EF after NSRP using data from the multicentre randomised, double-blind REACTT trial conducted to compare once-daily tadalafil, on-demand tadalafil, and placebo for penile rehabilitation. They showed that the odds of achieving EF recovery at the end of the drug-free washout period were twice as high for RARP compared to ORP, but no difference was observed between LRP and ORP patients. Moreover, recently published large population-based studies comparing ORP and RARP have shown controversial results [62,63,64].
Table 1. Prospective trials comparing the functional outcomes of different radical prostatectomy techniques.
| Study (year) | Case (n) | Study design | Patient characteristic | Definition of EF recovery | Potency rate | Level of evidence |
|---|---|---|---|---|---|---|
| Tewari et al (2003) [56] | ORP: 100 RARP: 200 |
Prospective comparison | Life expectancy >10 years | Erection sufficient for intercourse | ORP: 50% at 36 months RARP: 50% at 6 months |
3 |
| Ficarra et al (2009) [60] | ORP: 41 RARP: 64 |
Prospective comparison | Mean age of 61 years Preoperatively potent BNS |
SHIM>17 | 12 months ORP: 49% RARP: 81% |
3 |
| Kim et al (2011) [55] | ORP: 122 RARP: 373 |
Prospective comparison | Mean age of 64 years Preoperatively potent UNS/BNS |
Erection sufficient for intercourse | 12 months: ORP: 28% RARP: 57% 24 months: ORP: 47% RARP: 84% |
3 |
| Di Pierro et al (2011) [59] | ORP: 47 RARP: 22 |
Prospective comparison | Mean age of 62 years Preoperatively potent BNS |
Erection sufficient for intercourse | 12 months ORP: 26% RARP: 55% |
3 |
| Asimakopoulos et al (2011) [58] | LRP: 64 RARP: 52 |
RCT | Age<70 years Preoperatively potent BNS |
Erection sufficient for intercourse | 12 months: LRP: 32% RARP: 77% |
2 |
| Haglind et al (2015) [57] | ORP: 144 RARP: 366 |
RCT | Age<75 years Operated on by experienced surgeons (surgical volume ≥100 operations) |
Erection sufficient for intercourse | 12 months: ORP: 25% RARP: 29% |
2 |
EF: erectile function, ORP: open radical prostatectomy, RARP: robot-assisted radical prostatectomy, LRP: laparoscopic radical prostatectomy, RCT: randomized clinical trial, BNS: bilateral nerve-sparing procedure, UNS: unilateral nerve-sparing procedure, SHIM: Sexual Health Inventory for Men.
Asimakopoulos et al [58] reported the results of a prospective randomised study conducted on 128 patients treated with either LRP or RARP using a BNS approach; they showed that RARP patients regained capability for intercourse significantly more quickly and exhibited a higher rate of return to baseline IIEF-EF scores than LRP patients. In contrast, Ficarra et al [4] showed only a non-significant trend in favour of RARP when comparing EF outcomes between LRP and RARP, with an overall 55.6% incidence of ED after LRP compared to 39.8% after RARP; these findings were probably related to the influence of the retrospective series included in the meta-analysis, which mainly reported non-significant advantages of one technique in comparison to the other. Magheli et al [65] recently published a comparative analysis between LRP and ORP showing no difference between the two groups in terms of postoperative potency rates, despite significant methodological bias due to the lack of a preoperative EF assessment for both groups.
Taken together, these data suggest an advantage in terms of EF recovery for patients treated with a robotic approach in comparison to those treated with either a purely laparoscopic technique or open surgery; however, the lack of strong evidence from randomized clinical trials (RCTs), together with the important role played by the surgeon's surgical experience and personal skill, impedes the possibility of drawing definitive conclusions regarding the gold-standard technique for RP.
POSTOPERATIVE MANAGEMENT
Regardless of the type of surgery, the postoperative setting represents an extremely important step for preventing ED or for treating ED symptoms in patients who have undergone RP. In this context, the postoperative management of ED is mainly based on the much-debated concept of penile rehabilitation, with the possibility of incorporating different therapeutic tools.
1. Penile rehabilitation
The surgical removal of the prostate is almost invariably associated with a sometimes temporary period of dormancy of the nerves controlling EF, which can lead to an impairment of erectile tissue oxygenation and eventually definitive damage of the corpora cavernosa, thereby hampering any chance of EF recovery [66]. From a pathophysiologic standpoint, the chronic absence of oxygenation linked to neuropraxia would lead to the production of fibrogenic factors (e.g., transforming growth factor-β1, endothelin-1, nerve growth factor, and hypoxia-inducible factor-1α) responsible for structural changes in the erectile tissue, including impairment of the elasticity of the corpora cavernosa and the irreversible loss of smooth muscle cells, finally resulting in veno-occlusive dysfunction [67,68,69]. In this context, all treatments aiming to preserve adequate functional oxygenation of the erectile tissue in the early phase after surgery can be expected to help prevent the onset of permanent ED, as well as promoting EF recovery.
The concept of penile rehabilitation was introduced to the clinical setting by Montorsi et al [70], who showed in a small cohort of patients that the early postoperative intracavernous administration of alprostadil improved EF recovery rates [70]. Thereafter, the advent of phosphodiesterase 5 inhibitors (PDE5is) in clinical applications led to several RCTs assessing the role of different oral compounds in the context of post-RP rehabilitation (Table 2). These studies were encouraged by strong preclinical animal data showing that PDE5is were able to decrease erectile tissue fibrosis, to prevent the degeneration of nerves, and to stimulate neuroregeneration [66,71,72,73,74]. Padma-Nathan et al [75] randomized 76 patients treated with ORP to receive sildenafil nightly or placebo for 36 weeks. After a drug-free period of 8 weeks, they showed that patients treated with sildenafil more frequently recovered EF, showing higher mean IIEF-EF scores and improvements in nocturnal penile erections compared to those treated with placebo. Montorsi et al [76] first presented data assessing the effect of as-needed oral treatment compared to a nightly treatment for penile rehabilitation in a double-blind RCT on vardenafil. Patients were randomized to placebo or either 10 mg of vardenafil nightly or 10 mg vardenafil as needed after BNSRP, showing that on-demand dosing was associated with significantly greater IIEF-EF scores and higher positive response rates to the Sexual Encounter Profile question 3 (SEP3) than placebo after 9 months of treatment. However, the results after a 2-month drug washout period showed that EF recovery rates did not significantly improve in either vardenafil group [76]. Similarly, in a more recent trial assessing the effect of nightly versus as-needed sildenafil after BNRSP, Pavlovich et al [77] failed to confirm previous data and did not demonstrate a significant improvement in terms of EF recovery for either treatment protocol.
Table 2. Randomized clinical trials assessing the outcomes of penile rehabilitation with PDE5is.
| Study (year) | Case (n) | Study design | Patient characteristic | Rehabilitation protocol | Primary outcome |
|---|---|---|---|---|---|
| Padma-Nathan et al (2008) [75] | Sil, 50 mg, OaD: 23 Sil, 100 mg, OaD: 28 Placebo: 25 |
Double-blind RCT | Age, 18~70 years Preoperatively potent BNS |
Started 4 weeks after RP EDT at 36 weeks 8 weeks of DFW |
EF recoverya 27% for Sil 4% for placebo |
| Montorsi et al (2008) [76] | Vard, OaD: 137 Vard, PRN: 141 Placebo: 145 |
Double-blind Double-dummy RCT |
Age, 18~64 years Preoperatively potent BNS |
Started 14 days after RP EDT at 9 months 2 months of DFW 2 months of Vard, OaD, OL |
IIEF-EF score ≥22 at EDT 48.2% for Vard, OaD 32% for Vard, PRN 24.8% for placebo IIEF-EF score ≥22 at DFW 29.1% for Vard, OaD 24.1% for Vard, PRN 29.1% for placebo |
| Mulhall et al (2013) [81] | Ava, 200 mg: 94 Ava, 100 mg: 90 Placebo: 87 |
Double-blind RCT | Age, 18~70 years History of ED after BNS |
Started≥6 months after RP EDT at 12 weeks |
IIEF-EF score change at EDT (points) 5.2 for Ava, 200 mg 3.6 for Ava, 100 mg 0.1 for placebo |
| Pavlovich et al (2013) [77] | Sil, OaD+placebo PRN: 50 Sil, PRN+placebo OaD: 50 |
Double-blind RCT | Age, <65 years Preoperatively potent UNS/BNS |
Started 1 day after RP EDT at 12 months 1 month of DFW |
Recovery of baseline IIEF-EF at EDT 63% for Sil, PRN 57% for Sil, OaD Recovery of baseline IIEF-EF at DFW 65% for Sil, PRN 47% for Sil, OaD |
| Montorsi et al (2014) [78] | Tad, OaD: 139 Tad, PRN: 143 Placebo: 141 |
Double-blind Double-dummy RCT |
Age, <68 years Baseline IIEF-EF ≥2 BNS |
Started within 6 weeks after RP EDT at 9 months 6 weeks of DFW 3 months of OL |
IIEF-EF score ≥22 at DFW 20.9% for Tad, OaD 16.9% for Tad, PRN 19.1% for placebo |
PDE5is: phosphodiesterase 5 inhibitors, Sil: sildenafil, OaD: once daily, Vard: vardenafil, PRN: on-demand, Ava: avanafil, Tad: tadalafil, RCT: randomized clinical trial, BNS: bilateral nerve-sparing procedure, ED: erectile dysfunction, UNS: unilateral nerve-sparing procedure, IIEF: International Index of Erectile Function, EF: erectile function, EDT: end-of-study treatment, DFW: drug-free washout period, OL: open-label treatment, RP: radical prostatectomy.
aDefined as a score >8 on Q3 and Q4 of the IIEF and a 'yes' response to the question 'Over the past 4 weeks, have your erections been good enough for satisfactory sexual activity?'
The effect of tadalafil as an active compound throughout the post-RP rehabilitative period was tested in a large RCT including 423 patients, aiming to compare 5 mg of tadalafil taken once daily, 20 mg of tadalafil as needed, and placebo after NSRP [78]. At the end of 9 months of treatment, IIEF-EF scores ≥22 were significantly more common in patients treated with tadalafil once daily than in the placebo group; likewise, IIEF-EF scores significantly improved, exceeding the criteria for minimal clinical important differences, in both tadalafil groups, but a significant improvement was only found for once-daily tadalafil compared to placebo. Moreover, at the end of treatment protocol, the SEP3 positive response rate was significantly higher only for the once-daily group than for placebo. In contrast, data collected after a 6-week washout period showed no difference in men treated with both active treatments compared to those in the placebo arm for all measured outcomes. Finally, after an open-label treatment phase, patients randomised to once-daily tadalafil had a significantly higher positive response rate for SEP3 than the placebo group. Overall, the authors concluded that although tadalafil was not able to improve drug-unassisted EF recovery after RP, once-daily treatment could be responsible for the maintenance of cavernosal tissue integrity [78]. Moncada et al [79] conducted a sub-analysis of the same data, showing that the administration of once-daily tadalafil was associated with a shorter time to EF recovery during the 9-month course of treatment than was observed in the other groups. More recently, Montorsi et al [80] published the results of a further analysis devoted to understanding predictors for EF recovery after NSRP with the goal of helping clinicians and patients in preoperative counselling and expectation management regarding EF rehabilitation strategies. Interestingly, they concluded that high presurgery sexual desire, confidence, and IS were key predictors of EF recovery. They suggested that patients meeting these criteria might benefit the most from NS surgery and early postsurgery EF rehabilitation. Of clinical importance, for patients meeting these criteria, additional non-IIEF-related predictors included RARP, the quality of NS surgery, and treatment with once-daily tadalafil [80].
Additionally, the effect of avanafil after BNSRP was tested in a RCT with patients randomised to receive 100 mg of avanafil, 200 mg of avanafil on demand, or placebo, with avanafil treatment showing higher IIEF-EF scores and greater SEP3 positive response rates after 12 weeks of treatment [81].
Overall, these data suggest that PDE5is have a positive effect terms of penile rehabilitation in patients treated with RP, clearly supporting the idea that treatment is better than doing nothing [82], although it has not yet been established whether a specific drug or, of even greater clinical relevance, a daily versus an as-needed protocol is most advantageous. Similarly, the need to start the rehabilitation protocol as soon as possible after surgery has been clearly demonstrated, underscoring the importance of timing for the development of irreversible structural changes of the erectile tissue as a consequence of postoperative neuropraxia [7,83].
In addition to PDE5is, intracavernous injections (ICIs) in the context of penile rehabilitation protocols have shown positive results in terms of EF recovery [70,84,85,86]. In this context, high patient motivation and adherence to protocol have been stressed as required aspects for this kind of treatment. Yiou et al [86], for instance, reported data from a prospective study conducted on a cohort of men treated with laparoscopic NSRP who underwent a twice-weekly treatment protocol of 2.5 µg of alprostadil, showing that up to 11% discontinued the therapy because of pain and that the pain scores were negatively correlated with IIEF-EF scores at 6 months of follow-up. Mulhall et al [85] assessed the outcome of ICI treatment in patients who were non-responders to postoperative sildenafil and had been treated with BNS, unilateral NS, or NNS RP. Patients treated with a trimix formulation (papaverine, phentolamine, and prostaglandine E1) had higher response rates than those who received no treatment after RP, thus supporting the role of ICIs in the rehabilitation flowchart of non-responders to PDE5is [7].
In addition to pharmacological treatments, the effects of vacuum devices (VEDs) on penile rehabilitation after RP have been evaluated. Indeed, preclinical studies have demonstrated that VED therapy was responsible for the preservation of endothelial and smooth muscle integrity due to a transient increase in arterial flow and oxygenation in the corpora cavernosa [87]. However, studies assessing the effect of VEDs in the post-RP setting have shown contradictory results [88,89,90]. Basal et al [90] assessed EF recovery rates in 200 patients randomized to VEDs, PDE5is, VEDs and PDE5is, or placebo after RARP. They showed that only PDE5is alone and the combination of VEDs and PDE5is significantly improved postoperative EF recovery. Overall, robust clinical data supporting the use of VEDs for penile rehabilitation post-RP are still lacking, even if it may have a role in selected patients, especially in combination with oral therapy.
Finally, the importance of sexual counselling should not be undervalued in the postoperative setting. In this regard, it was previously demonstrated that up to 49% of patients not adequately counselled throughout an 18-month postoperative period decided not to begin any ED treatment, although before surgery they were highly motivated to preserve EF [91]. Therefore, these and other findings support the proposal that, just as in the preoperative setting, patients must be carefully counselled postoperatively regarding the need to find the optimal rehabilitation treatment to increase the possibility of re-gaining adequate EF.
2. Penile prostheses
Penile prosthesis implantation is currently considered a third-line treatment for patients with ED, after other non-invasive therapies [3,82]. Indeed, patients undergoing NNS surgery for PCa, but still desiring a sexually active life, may benefit from penile prostheses after the failure of other treatment modalities [82]. However, despite numerous demonstrations of an excellent efficacy profile and high satisfaction rates in up to 98% of implanted patients and 96% of patients' partners [92,93,94], penile prostheses are currently underused in the setting of post-RP ED. Tal et al [95], for instance, reported data from the Surveillance Epidemiology and End Results cancer registry, showing that only 0.78% of patients treated with either RP or radiation therapy eventually received a penile implant. Recently, the Fourth International Consultation on Sexual Medicine revised its recommendations for penile prosthesis surgery, stating that with improvements in the design and safety of new implantable devices, this kind of treatment presents high efficacy rates with a lower risk for mechanical failure and infection, although post-RP patients have frequently reported complaints regarding the loss of penile length [94]. In this context, prospective data from the Memorial Sloan Kettering Cancer Center showed no significant objective changes in penile length after prosthesis implantation, despite a subjectively reported loss of length in 72% of cases [96]. All of these observations support the need for comprehensive patient counselling about prosthetic surgery, including the high probability of achieving excellent results.
CONCLUSIONS
In the current era of early diagnosis of PCa and excellent oncological outcomes of surgery, the preservation of adequate postoperative sexual function has become even more important. In this context, clinicians should be aware of the correct strategies to apply in order to increase the probability of post-RP EF recovery, never forgetting to emphasize the challenging fact that baseline EF is very difficult, if not almost impossible, to regain. Pathways to prevent postoperative ED clearly encompass all steps of the comprehensive clinical management of every PCa patient, including preoperative, intraoperative, and postoperative settings. Indeed, candidates for various surgical strategies should be carefully selected according to baseline oncological and functional factors. In this regard, a comprehensive assessment of the patient's preoperative general health profile as well as preoperative sexual function, as objectively scored with validated psychometric tools, are of tremendous importance in providing the patient with realistic expectations in terms of regaining adequate EF after surgery. Moreover, the advent of minimally invasive RP procedures has led to improved general anatomic knowledge and to the development of more conservative surgical techniques, thus facilitating a significant overall improvement in functional postoperative outcomes over the last two decades. Finally, according to the available preclinical and clinical data, patients should be carefully counselled on the need to undergo the optimal postoperative rehabilitation treatment, with the aim of achieving faster EF recovery and avoiding irreversible penile structural changes leading to severe ED, without promising miraculous EF recoveries. Therefore, a proper treatment regimen including oral, local, and/or surgical therapies should be suggested, according to each patient's overall characteristics and surgically related aspects of treatment.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–942. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 4.Ficarra V, Novara G, Ahlering TE, Costello A, Eastham JA, Graefen M, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012;62:418–430. doi: 10.1016/j.eururo.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 5.Merrill RM, Sloan A. Risk-adjusted incidence rates for prostate cancer in the United States. Prostate. 2012;72:181–185. doi: 10.1002/pros.21419. [DOI] [PubMed] [Google Scholar]
- 6.Sidana A, Hernandez DJ, Feng Z, Partin AW, Trock BJ, Saha S, et al. Treatment decision-making for localized prostate cancer: what younger men choose and why. Prostate. 2012;72:58–64. doi: 10.1002/pros.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulhall JP, Bella AJ, Briganti A, McCullough A, Brock G. Erectile function rehabilitation in the radical prostatectomy patient. J Sex Med. 2010;7:1687–1698. doi: 10.1111/j.1743-6109.2010.01804.x. [DOI] [PubMed] [Google Scholar]
- 8.Schroeck FR, Krupski TL, Sun L, Albala DM, Price MM, Polascik TJ, et al. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54:785–793. doi: 10.1016/j.eururo.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 9.Roethke MC, Lichy MP, Kniess M, Werner MK, Claussen CD, Stenzl A, et al. Accuracy of preoperative endorectal MRI in predicting extracapsular extension and influence on neurovascular bundle sparing in radical prostatectomy. World J Urol. 2013;31:1111–1116. doi: 10.1007/s00345-012-0826-0. [DOI] [PubMed] [Google Scholar]
- 10.Zorn KC, Gallina A, Hutterer GC, Walz J, Shalhav AL, Zagaja GP, et al. External validation of a nomogram for prediction of side-specific extracapsular extension at robotic radical prostatectomy. J Endourol. 2007;21:1345–1351. doi: 10.1089/end.2007.0044. [DOI] [PubMed] [Google Scholar]
- 11.de Rooij M, Hamoen EH, Witjes JA, Barentsz JO, Rovers MM. Accuracy of magnetic resonance imaging for local staging of prostate cancer: a diagnostic meta-analysis. Eur Urol. 2016;70:233–245. doi: 10.1016/j.eururo.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Recabal P, Assel M, Musser JE, Caras RJ, Sjoberg DD, Coleman JA, et al. Erectile function recovery after radical prostatectomy in men with high risk features. J Urol. 2016 doi: 10.1016/j.juro.2016.02.080. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, Montorsi P, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65:968–978. doi: 10.1016/j.eururo.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Salonia A, Castagna G, Saccà A, Ferrari M, Capitanio U, Castiglione F, et al. Is erectile dysfunction a reliable proxy of general male health status? The case for the International Index of Erectile Function-Erectile Function domain. J Sex Med. 2012;9:2708–2715. doi: 10.1111/j.1743-6109.2012.02869.x. [DOI] [PubMed] [Google Scholar]
- 15.Rabbani F, Stapleton AM, Kattan MW, Wheeler TM, Scardino PT. Factors predicting recovery of erections after radical prostatectomy. J Urol. 2000;164:1929–1934. [PubMed] [Google Scholar]
- 16.Penson DF, McLerran D, Feng Z, Li L, Albertsen PC, Gilliland FD, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2008;179:S40–S44. doi: 10.1016/j.juro.2008.03.136. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Samavedi S, Bates AS, Giedelman Cuevas CA, Coelho RF, Rocco B, et al. Age stratified comparative analysis of perioperative, functional and oncologic outcomes in patients after robot assisted radical prostatectomy: a propensity score matched study. Eur J Surg Oncol. 2015;41:837–843. doi: 10.1016/j.ejso.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Salomon G, Isbarn H, Budaeus L, Schlomm T, Briganti A, Steuber T, et al. Importance of baseline potency rate assessment of men diagnosed with clinically localized prostate cancer prior to radical prostatectomy. J Sex Med. 2009;6:498–504. doi: 10.1111/j.1743-6109.2008.01089.x. [DOI] [PubMed] [Google Scholar]
- 19.Teloken PE, Nelson CJ, Karellas M, Stasi J, Eastham J, Scardino PT, et al. Defining the impact of vascular risk factors on erectile function recovery after radical prostatectomy. BJU Int. 2013;111:653–657. doi: 10.1111/j.1464-410X.2012.11321.x. [DOI] [PubMed] [Google Scholar]
- 20.Gandaglia G, Lista G, Fossati N, Suardi N, Gallina A, Moschini M, et al. Non-surgically related causes of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Prostate Cancer Prostatic Dis. 2016;19:185–190. doi: 10.1038/pcan.2016.1. [DOI] [PubMed] [Google Scholar]
- 21.Briganti A, Gallina A, Suardi N, Capitanio U, Tutolo M, Bianchi M, et al. Predicting erectile function recovery after bilateral nerve sparing radical prostatectomy: a proposal of a novel preoperative risk stratification. J Sex Med. 2010;7:2521–2531. doi: 10.1111/j.1743-6109.2010.01845.x. [DOI] [PubMed] [Google Scholar]
- 22.Novara G, Ficarra V, D'Elia C, Secco S, De Gobbi A, Cavalleri S, et al. Preoperative criteria to select patients for bilateral nerve-sparing robotic-assisted radical prostatectomy. J Sex Med. 2010;7:839–845. doi: 10.1111/j.1743-6109.2009.01589.x. [DOI] [PubMed] [Google Scholar]
- 23.Salonia A, Burnett AL, Graefen M, Hatzimouratidis K, Montorsi F, Mulhall JP, et al. Prevention and management of postprostatectomy sexual dysfunctions. Part 1: choosing the right patient at the right time for the right surgery. Eur Urol. 2012;62:261–272. doi: 10.1016/j.eururo.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 24.Salonia A, Zanni G, Gallina A, Saccà A, Sangalli M, Naspro R, et al. Baseline potency in candidates for bilateral nervesparing radical retropubic prostatectomy. Eur Urol. 2006;50:360–365. doi: 10.1016/j.eururo.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Salonia A, Gallina A, Briganti A, Zanni G, Saccà A, Dehò F, et al. Remembered International Index of Erectile Function domain scores are not accurate in assessing preoperative potency in candidates for bilateral nerve-sparing radical retropubic prostatectomy. J Sex Med. 2008;5:677–683. doi: 10.1111/j.1743-6109.2007.00711.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim DS, Chung YG, Kim DJ, Park KK, Chung MS, Lee DH, et al. Optimal timing to evaluate prediagnostic baseline erectile function in patients undergoing robot-assisted radical prostatectomy. J Sex Med. 2012;9:602–607. doi: 10.1111/j.1743-6109.2011.02465.x. [DOI] [PubMed] [Google Scholar]
- 27.Mulhall JP. Defining and reporting erectile function outcomes after radical prostatectomy: challenges and misconceptions. J Urol. 2009;181:462–471. doi: 10.1016/j.juro.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 28.Deveci S, Gotto GT, Alex B, O'Brien K, Mulhall JP. A survey of patient expectations regarding sexual function following radical prostatectomy. BJU Int. 2015 doi: 10.1111/bju.13398. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skeldon SC, Gani J, Radomski SB. Do patients know their nerve-sparing status after radical prostatectomy? Urology. 2014;83:1099–1103. doi: 10.1016/j.urology.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Imbimbo C, Creta M, Gacci M, Simonato A, Gontero P, de Cobelli O, et al. Patients' desire to preserve sexual activity and final decision for a nerve-sparing approach: results from the MIRROR (Multicenter Italian Report on Radical Prostatectomy Outcomes and Research) Study. J Sex Med. 2011;8:1495–1502. doi: 10.1111/j.1743-6109.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- 31.Briganti A, Gallina A, Suardi N, Capitanio U, Tutolo M, Bianchi M, et al. What is the definition of a satisfactory erectile function after bilateral nerve sparing radical prostatectomy. J Sex Med. 2011;8:1210–1217. doi: 10.1111/j.1743-6109.2010.02179.x. [DOI] [PubMed] [Google Scholar]
- 32.Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 33.Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 2004;94:1071–1076. doi: 10.1111/j.1464-410X.2004.05106.x. [DOI] [PubMed] [Google Scholar]
- 34.Walsh PC. The discovery of the cavernous nerves and development of nerve sparing radical retropubic prostatectomy. J Urol. 2007;177:1632–1635. doi: 10.1016/j.juro.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Weyne E, Mulhall J, Albersen M. Molecular pathophysiology of cavernous nerve injury and identification of strategies for nerve function recovery after radical prostatectomy. Curr Drug Targets. 2015;16:459–473. doi: 10.2174/1389450116666150316224456. [DOI] [PubMed] [Google Scholar]
- 36.Mulhall JP, Slovick R, Hotaling J, Aviv N, Valenzuela R, Waters WB, et al. Erectile dysfunction after radical prostatectomy: hemodynamic profiles and their correlation with the recovery of erectile function. J Urol. 2002;167:1371–1375. doi: 10.1016/s0022-5347(05)65303-7. [DOI] [PubMed] [Google Scholar]
- 37.Mulhall JP, Secin FP, Guillonneau B. Artery sparing radical prostatectomy--myth or reality? J Urol. 2008;179:827–831. doi: 10.1016/j.juro.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Bilhim T, Pisco JM, Rio Tinto H, Fernandes L, Pinheiro LC, Furtado A, et al. Prostatic arterial supply: anatomic and imaging findings relevant for selective arterial embolization. J Vasc Interv Radiol. 2012;23:1403–1415. doi: 10.1016/j.jvir.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Patel VR, Schatloff O, Chauhan S, Sivaraman A, Valero R, Coelho RF, et al. The role of the prostatic vasculature as a landmark for nerve sparing during robot-assisted radical prostatectomy. Eur Urol. 2012;61:571–576. doi: 10.1016/j.eururo.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 40.Walz J, Burnett AL, Costello AJ, Eastham JA, Graefen M, Guillonneau B, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010;57:179–192. doi: 10.1016/j.eururo.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Secin FP, Touijer K, Mulhall J, Guillonneau B. Anatomy and preservation of accessory pudendal arteries in laparoscopic radical prostatectomy. Eur Urol. 2007;51:1229–1235. doi: 10.1016/j.eururo.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 42.Box GN, Kaplan AG, Rodriguez E, Jr, Skarecky DW, Osann KE, Finley DS, et al. Sacrifice of accessory pudendal arteries in normally potent men during robot-assisted radical prostatectomy does not impact potency. J Sex Med. 2010;7:298–303. doi: 10.1111/j.1743-6109.2009.01459.x. [DOI] [PubMed] [Google Scholar]
- 43.John H, Hauri D. Seminal vesicle-sparing radical prostatectomy: a novel concept to restore early urinary continence. Urology. 2000;55:820–824. doi: 10.1016/s0090-4295(00)00547-1. [DOI] [PubMed] [Google Scholar]
- 44.Walz J, Epstein JI, Ganzer R, Graefen M, Guazzoni G, Kaouk J, et al. A critical analysis of the current knowledge of surgical anatomy of the prostate related to optimisation of cancer control and preservation of continence and erection in candidates for radical prostatectomy: an update. Eur Urol. 2016;70:301–311. doi: 10.1016/j.eururo.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Alsaid B, Bessede T, Diallo D, Moszkowicz D, Karam I, Benoit G, et al. Division of autonomic nerves within the neurovascular bundles distally into corpora cavernosa and corpus spongiosum components: immunohistochemical confirmation with three-dimensional reconstruction. Eur Urol. 2011;59:902–909. doi: 10.1016/j.eururo.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 46.Ganzer R, Stolzenburg JU, Wieland WF, Bründl J. Anatomic study of periprostatic nerve distribution: immunohistochemical differentiation of parasympathetic and sympathetic nerve fibres. Eur Urol. 2012;62:1150–1156. doi: 10.1016/j.eururo.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 47.Montorsi F, Wilson TG, Rosen RC, Ahlering TE, Artibani W, Carroll PR, et al. Best practices in robot-assisted radical prostatectomy: recommendations of the pasadena consensus panel. Eur Urol. 2012;62:368–381. doi: 10.1016/j.eururo.2012.05.057. [DOI] [PubMed] [Google Scholar]
- 48.Tewari AK, Srivastava A, Huang MW, Robinson BD, Shevchuk MM, Durand M, et al. Anatomical grades of nerve sparing: a risk-stratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP) BJU Int. 2011;108:984–992. doi: 10.1111/j.1464-410X.2011.10565.x. [DOI] [PubMed] [Google Scholar]
- 49.Tewari AK, Ali A, Metgud S, Theckumparampil N, Srivastava A, Khani F, et al. Functional outcomes following robotic prostatectomy using athermal, traction free risk-stratified grades of nerve sparing. World J Urol. 2013;31:471–480. doi: 10.1007/s00345-012-1018-7. [DOI] [PubMed] [Google Scholar]
- 50.Schatloff O, Chauhan S, Sivaraman A, Kameh D, Palmer KJ, Patel VR. Anatomic grading of nerve sparing during robot-assisted radical prostatectomy. Eur Urol. 2012;61:796–802. doi: 10.1016/j.eururo.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 51.van der Poel HG, Tillier C, de Blok W, van Muilekom E. Extended nodal dissection reduces sexual function recovery after robot-assisted laparoscopic prostatectomy. J Endourol. 2012;26:1192–1198. doi: 10.1089/end.2012.0011. [DOI] [PubMed] [Google Scholar]
- 52.Gandaglia G, Suardi N, Gallina A, Abdollah F, Capitanio U, Salonia A, et al. Extended pelvic lymph node dissection does not affect erectile function recovery in patients treated with bilateral nerve-sparing radical prostatectomy. J Sex Med. 2012;9:2187–2194. doi: 10.1111/j.1743-6109.2012.02812.x. [DOI] [PubMed] [Google Scholar]
- 53.Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50:711–718. doi: 10.1016/j.eururo.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–1063. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 55.Kim SC, Song C, Kim W, Kang T, Park J, Jeong IG, et al. Factors determining functional outcomes after radical prostatectomy: robot-assisted versus retropubic. Eur Urol. 2011;60:413–419. doi: 10.1016/j.eururo.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Tewari A, Srivasatava A, Menon M Members of the VIP Team. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003;92:205–210. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 57.Haglind E, Carlsson S, Stranne J, Wallerstedt A, Wilderäng U, Thorsteinsdottir T, et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol. 2015;68:216–225. doi: 10.1016/j.eururo.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 58.Asimakopoulos AD, Pereira Fraga CT, Annino F, Pasqualetti P, Calado AA, Mugnier C. Randomized comparison between laparoscopic and robot-assisted nerve-sparing radical prostatectomy. J Sex Med. 2011;8:1503–1512. doi: 10.1111/j.1743-6109.2011.02215.x. [DOI] [PubMed] [Google Scholar]
- 59.Di Pierro GB, Baumeister P, Stucki P, Beatrice J, Danuser H, Mattei A. A prospective trial comparing consecutive series of open retropubic and robot-assisted laparoscopic radical prostatectomy in a centre with a limited caseload. Eur Urol. 2011;59:1–6. doi: 10.1016/j.eururo.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 60.Ficarra V, Novara G, Fracalanza S, D'Elia C, Secco S, Iafrate M, et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int. 2009;104:534–539. doi: 10.1111/j.1464-410X.2009.08419.x. [DOI] [PubMed] [Google Scholar]
- 61.Stolzenburg JU, Graefen M, Kriegel C, Michl U, Martin Morales A, Pommerville PJ, et al. Effect of surgical approach on erectile function recovery following bilateral nerve-sparing radical prostatectomy: an evaluation utilising data from a randomised, double-blind, double-dummy multicentre trial of tadalafil vs placebo. BJU Int. 2015;116:241–251. doi: 10.1111/bju.13030. [DOI] [PubMed] [Google Scholar]
- 62.O'Neil B, Koyama T, Alvarez J, Conwill RM, Albertsen PC, Cooperberg MR, et al. The comparative harms of open and robotic prostatectomy in population based samples. J Urol. 2016;195:321–329. doi: 10.1016/j.juro.2015.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alemozaffar M, Sanda M, Yecies D, Mucci LA, Stampfer MJ, Kenfield SA. Benchmarks for operative outcomes of robotic and open radical prostatectomy: results from the health professionals follow-up study. Eur Urol. 2015;67:432–438. doi: 10.1016/j.eururo.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ong WL, Evans SM, Spelman T, Kearns PA, Murphy DG, Millar JL. Comparison of oncological and health related quality of life (HRQOL) outcomes between open (ORP) and robotic-assisted radical prostatectomy (RARP) for localized prostate cancer - findings from the population-based Victorian Prostate Cancer Registry (PCR) BJU Int. 2015 doi: 10.1111/bju.13380. [Epub] [DOI] [PubMed] [Google Scholar]
- 65.Magheli A, Busch J, Leva N, Schrader M, Deger S, Miller K, et al. Comparison of surgical technique (open vs. laparoscopic) on pathological and long term functional outcomes following radical prostatectomy. BMC Urol. 2014;14:18. doi: 10.1186/1471-2490-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55:334–347. doi: 10.1016/j.eururo.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 67.Burnett AL. Rationale for cavernous nerve restorative therapy to preserve erectile function after radical prostatectomy. Urology. 2003;61:491–497. doi: 10.1016/s0090-4295(02)02271-9. [DOI] [PubMed] [Google Scholar]
- 68.Moreland RB. Is there a role of hypoxemia in penile fibrosis: a viewpoint presented to the Society for the Study of Impotence. Int J Impot Res. 1998;10:113–120. doi: 10.1038/sj.ijir.3900328. [DOI] [PubMed] [Google Scholar]
- 69.Weyne E, Castiglione F, Van der Aa F, Bivalacqua TJ, Albersen M. Landmarks in erectile function recovery after radical prostatectomy. Nat Rev Urol. 2015;12:289–297. doi: 10.1038/nrurol.2015.72. [DOI] [PubMed] [Google Scholar]
- 70.Montorsi F, Guazzoni G, Strambi LF, Da Pozzo LF, Nava L, Barbieri L, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol. 1997;158:1408–1410. [PubMed] [Google Scholar]
- 71.Mulhall JP, Müller A, Donohue JF, Mullerad M, Kobylarz K, Paduch DA, et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med. 2008;5:1126–1136. doi: 10.1111/j.1743-6109.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 72.Müller A, Akin-Olugbade Y, Deveci S, Donohue JF, Tal R, Kobylarz KA, et al. The impact of shock wave therapy at varied energy and dose levels on functional and structural changes in erectile tissue. Eur Urol. 2008;53:635–642. doi: 10.1016/j.eururo.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 73.Lagoda G, Jin L, Lehrfeld TJ, Liu T, Burnett AL. FK506 and sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J Sex Med. 2007;4:908–916. doi: 10.1111/j.1743-6109.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 74.Kovanecz I, Rambhatla A, Ferrini MG, Vernet D, Sanchez S, Rajfer J, et al. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int. 2008;101:203–210. doi: 10.1111/j.1464-410X.2007.07223.x. [DOI] [PubMed] [Google Scholar]
- 75.Padma-Nathan H, McCullough AR, Levine LA, Lipshultz LI, Siegel R, Montorsi F, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20:479–486. doi: 10.1038/ijir.2008.33. [DOI] [PubMed] [Google Scholar]
- 76.Montorsi F, Brock G, Lee J, Shapiro J, Van Poppel H, Graefen M, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54:924–931. doi: 10.1016/j.eururo.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 77.Pavlovich CP, Levinson AW, Su LM, Mettee LZ, Feng Z, Bivalacqua TJ, et al. Nightly vs on-demand sildenafil for penile rehabilitation after minimally invasive nerve-sparing radical prostatectomy: results of a randomized double-blind trial with placebo. BJU Int. 2013;112:844–851. doi: 10.1111/bju.12253. [DOI] [PubMed] [Google Scholar]
- 78.Montorsi F, Brock G, Stolzenburg JU, Mulhall J, Moncada I, Patel HR, et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT) Eur Urol. 2014;65:587–596. doi: 10.1016/j.eururo.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 79.Moncada I, de Bethencourt FR, Lledó-García E, Romero-Otero J, Turbi C, Büttner H, et al. Effects of tadalafil once daily or on demand versus placebo on time to recovery of erectile function in patients after bilateral nerve-sparing radical prostatectomy. World J Urol. 2015;33:1031–1038. doi: 10.1007/s00345-014-1377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Montorsi F, Oelke M, Henneges C, Brock G, Salonia A, d'Anzeo G, et al. Exploratory decision-tree modeling of data from the randomized REACTT trial of tadalafil versus placebo to predict recovery of erectile function after bilateral nerve-sparing radical prostatectomy. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.02.036. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mulhall JP, Burnett AL, Wang R, McVary KT, Moul JW, Bowden CH, et al. A phase 3, placebo controlled study of the safety and efficacy of avanafil for the treatment of erectile dysfunction after nerve sparing radical prostatectomy. J Urol. 2013;189:2229–2236. doi: 10.1016/j.juro.2012.11.177. [DOI] [PubMed] [Google Scholar]
- 82.Salonia A, Burnett AL, Graefen M, Hatzimouratidis K, Montorsi F, Mulhall JP, et al. Prevention and management of postprostatectomy sexual dysfunctions part 2: recovery and preservation of erectile function, sexual desire, and orgasmic function. Eur Urol. 2012;62:273–286. doi: 10.1016/j.eururo.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 83.Mulhall JP, Bivalacqua TJ, Becher EF. Standard operating procedure for the preservation of erectile function outcomes after radical prostatectomy. J Sex Med. 2013;10:195–203. doi: 10.1111/j.1743-6109.2012.02885.x. [DOI] [PubMed] [Google Scholar]
- 84.Polito M, d'Anzeo G, Conti A, Muzzonigro G. Erectile rehabilitation with intracavernous alprostadil after radical prostatectomy: refusal and dropout rates. BJU Int. 2012;110:E954–E957. doi: 10.1111/j.1464-410X.2012.11484.x. [DOI] [PubMed] [Google Scholar]
- 85.Mulhall J, Land S, Parker M, Waters WB, Flanigan RC. The use of an erectogenic pharmacotherapy regimen following radical prostatectomy improves recovery of spontaneous erectile function. J Sex Med. 2005;2:532–540. doi: 10.1111/j.1743-6109.2005.00081_1.x. [DOI] [PubMed] [Google Scholar]
- 86.Yiou R, Cunin P, de la Taille A, Salomon L, Binhas M, Lingombet O, et al. Sexual rehabilitation and penile pain associated with intracavernous alprostadil after radical prostatectomy. J Sex Med. 2011;8:575–582. doi: 10.1111/j.1743-6109.2010.02002.x. [DOI] [PubMed] [Google Scholar]
- 87.Broderick GA, McGahan JP, Stone AR, White RD. The hemodynamics of vacuum constriction erections: assessment by color Doppler ultrasound. J Urol. 1992;147:57–61. doi: 10.1016/s0022-5347(17)37132-x. [DOI] [PubMed] [Google Scholar]
- 88.Köhler TS, Pedro R, Hendlin K, Utz W, Ugarte R, Reddy P, et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU Int. 2007;100:858–862. doi: 10.1111/j.1464-410X.2007.07161.x. [DOI] [PubMed] [Google Scholar]
- 89.Raina R, Agarwal A, Ausmundson S, Lakin M, Nandipati KC, Montague DK, et al. Early use of vacuum constriction device following radical prostatectomy facilitates early sexual activity and potentially earlier return of erectile function. Int J Impot Res. 2006;18:77–81. doi: 10.1038/sj.ijir.3901380. [DOI] [PubMed] [Google Scholar]
- 90.Basal S, Wambi C, Acikel C, Gupta M, Badani K. Optimal strategy for penile rehabilitation after robot-assisted radical prostatectomy based on preoperative erectile function. BJU Int. 2013;111:658–665. doi: 10.1111/j.1464-410X.2012.11487.x. [DOI] [PubMed] [Google Scholar]
- 91.Salonia A, Gallina A, Zanni G, Briganti A, Dehò F, Saccà A, et al. Acceptance of and discontinuation rate from erectile dysfunction oral treatment in patients following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;53:564–570. doi: 10.1016/j.eururo.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 92.Hellstrom WJ, Montague DK, Moncada I, Carson C, Minhas S, Faria G, et al. Implants, mechanical devices, and vascular surgery for erectile dysfunction. J Sex Med. 2010;7:501–523. doi: 10.1111/j.1743-6109.2009.01626.x. [DOI] [PubMed] [Google Scholar]
- 93.Menard J, Tremeaux JC, Faix A, Pierrevelcin J, Staerman F. Erectile function and sexual satisfaction before and after penile prosthesis implantation in radical prostatectomy patients: a comparison with patients with vasculogenic erectile dysfunction. J Sex Med. 2011;8:3479–3486. doi: 10.1111/j.1743-6109.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- 94.Levine LA, Becher E, Bella A, Brant W, Kohler T, Martinez-Salamanca JI, et al. Penile prosthesis surgery: current recommendations from the international consultation on sexual medicine. J Sex Med. 2016;13:489–518. doi: 10.1016/j.jsxm.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 95.Tal R, Jacks LM, Elkin E, Mulhall JP. Penile implant utilization following treatment for prostate cancer: analysis of the SEER-Medicare database. J Sex Med. 2011;8:1797–1804. doi: 10.1111/j.1743-6109.2011.02240.x. [DOI] [PubMed] [Google Scholar]
- 96.Deveci S, Martin D, Parker M, Mulhall JP. Penile length alterations following penile prosthesis surgery. Eur Urol. 2007;51:1128–1131. doi: 10.1016/j.eururo.2006.10.026. [DOI] [PubMed] [Google Scholar]