Abstract
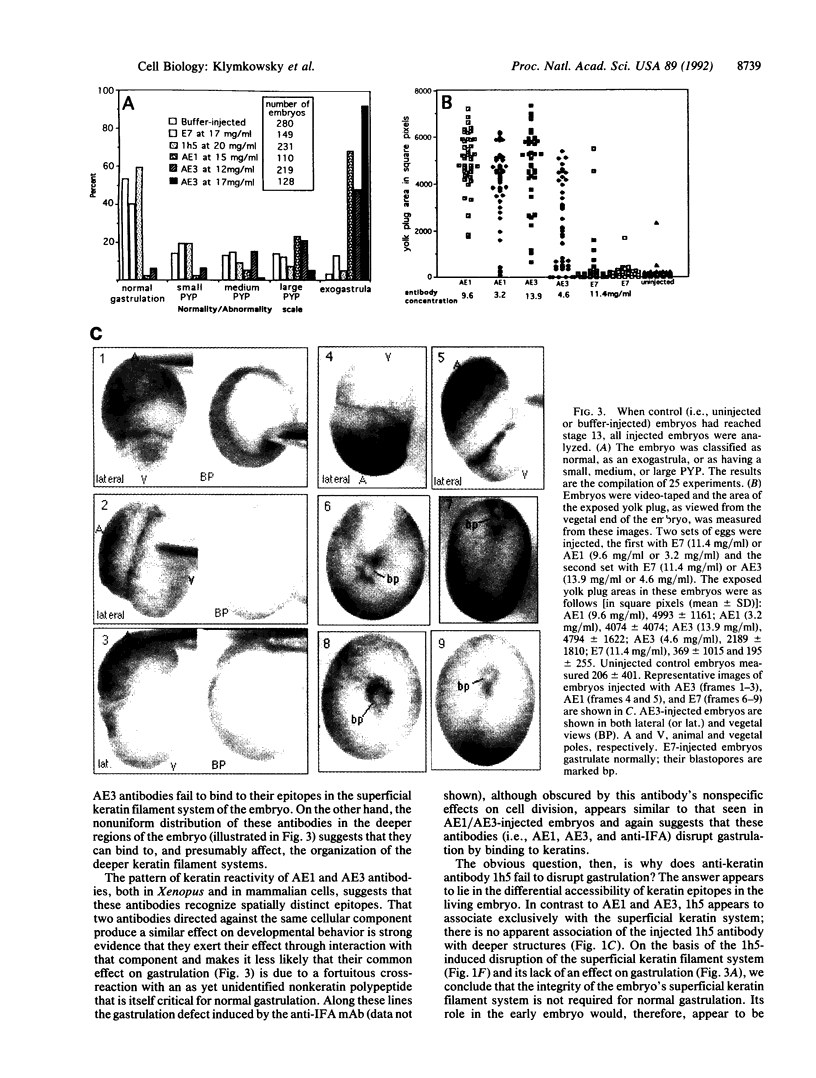
To study the role of keratin filaments in Xenopus development, fertilized eggs were injected with anti-keratin monoclonal antibodies. The anti-keratin monoclonal antibodies AE1 and AE3 induce abnormal gastrulation; in the most severely affected embryos gastrulation fails completely. In contrast, embryos injected with the anti-keratin antibody 1h5 develop normally. Immunocytochemical data indicate that injected 1h5 binds to the dense superficial keratin filament system of the embryo but not to the deeper keratin filament networks of ectodermal and subectodermal cells. Injected AE1 and AE3 do not bind to the superficial keratin system but appear to interact preferentially with the deep keratin filament systems of the embryo. We conclude that the superficial keratin filament system is not involved in the process of gastrulation per se but may protect the embryo from mechanical damage. On the other hand, our results suggest that the integrity of the deeper keratin filament systems is required for the mechanical integration of the morphogenetic movements that underlie gastrulation in Xenopus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emerson J. A. Disruption of the cytokeratin filament network in the preimplantation mouse embryo. Development. 1988 Oct;104(2):219–234. doi: 10.1242/dev.104.2.219. [DOI] [PubMed] [Google Scholar]
- Franz J. K., Franke W. W. Cloning of cDNA and amino acid sequence of a cytokeratin expressed in oocytes of Xenopus laevis. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6475–6479. doi: 10.1073/pnas.83.17.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz J. K., Gall L., Williams M. A., Picheral B., Franke W. W. Intermediate-size filaments in a germ cell: Expression of cytokeratins in oocytes and eggs of the frog Xenopus. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6254–6258. doi: 10.1073/pnas.80.20.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Tyner A. L., Giudice G. J., Marchuk D., RayChaudhury A., Rosenberg M. The human keratin genes and their differential expression. Curr Top Dev Biol. 1987;22:5–34. doi: 10.1016/s0070-2153(08)60097-6. [DOI] [PubMed] [Google Scholar]
- Jamrich M., Sargent T. D., Dawid I. B. Cell-type-specific expression of epidermal cytokeratin genes during gastrulation of Xenopus laevis. Genes Dev. 1987 Apr;1(2):124–132. doi: 10.1101/gad.1.2.124. [DOI] [PubMed] [Google Scholar]
- Jonas E., Sargent T. D., Dawid I. B. Epidermal keratin gene expressed in embryos of Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5413–5417. doi: 10.1073/pnas.82.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Early embryonic development of Xenopus laevis. Methods Cell Biol. 1991;36:61–113. doi: 10.1016/s0091-679x(08)60273-3. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Bachant J. B., Domingo A. Functions of intermediate filaments. Cell Motil Cytoskeleton. 1989;14(3):309–331. doi: 10.1002/cm.970140302. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Hanken J. Whole-mount staining of Xenopus and other vertebrates. Methods Cell Biol. 1991;36:419–441. doi: 10.1016/s0091-679x(08)60290-3. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W. Intermediate filaments. Getting under the skin. Nature. 1991 Nov 28;354(6351):264–264. doi: 10.1038/354264a0. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Maynell L. A., Nislow C. Cytokeratin phosphorylation, cytokeratin filament severing and the solubilization of the maternal mRNA Vg1. J Cell Biol. 1991 Aug;114(4):787–797. doi: 10.1083/jcb.114.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky M. W., Maynell L. A., Polson A. G. Polar asymmetry in the organization of the cortical cytokeratin system of Xenopus laevis oocytes and embryos. Development. 1987 Jul;100(3):543–557. doi: 10.1242/dev.100.3.543. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Miller R. H., Lane E. B. Morphology, behavior, and interaction of cultured epithelial cells after the antibody-induced disruption of keratin filament organization. J Cell Biol. 1983 Feb;96(2):494–509. doi: 10.1083/jcb.96.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Cytoplasmic intermediate filament proteins and the nuclear lamins A, B and C share the IFA epitope. Exp Cell Res. 1987 May;170(1):195–203. doi: 10.1016/0014-4827(87)90129-7. [DOI] [PubMed] [Google Scholar]
- Perry M. M. Microfilaments in the external surface layer of the early amphibian embryo. J Embryol Exp Morphol. 1975 Feb;33(1):127–146. [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Winkles J. A., Sargent T. D., Parry D. A., Jonas E., Dawid I. B. Developmentally regulated cytokeratin gene in Xenopus laevis. Mol Cell Biol. 1985 Oct;5(10):2575–2581. doi: 10.1128/mcb.5.10.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Eichner R., Nelson W. G., Sun T. T. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]