ABSTRACT
The human intestine harbors diverse communities of bacteria and bacteriophages. Given the specificity of phages for their bacterial hosts, there is growing interest in using phage therapies to combat the rising incidence of multidrug-resistant bacterial infections. A significant barrier to such therapies is the rapid development of phage-resistant bacteria, highlighting the need to understand how bacteria acquire phage resistance in vivo. Here we identify novel lytic phages in municipal raw sewage that kill Enterococcus faecalis, a Gram-positive opportunistic pathogen that resides in the human intestine. We show that phage infection of E. faecalis requires a predicted integral membrane protein that we have named PIPEF (for phage infection protein from E. faecalis). We find that PIPEF is conserved in E. faecalis and harbors a 160-amino-acid hypervariable region that determines phage tropism for distinct enterococcal strains. Finally, we use a gnotobiotic mouse model of in vivo phage predation to show that the sewage phages temporarily reduce E. faecalis colonization of the intestine but that E. faecalis acquires phage resistance through mutations in PIPEF. Our findings define the molecular basis for an evolutionary arms race between E. faecalis and the lytic phages that prey on them. They also suggest approaches for engineering E. faecalis phages that have altered host specificity and that can subvert phage resistance in the host bacteria.
IMPORTANCE
Bacteriophage therapy has received renewed attention as a potential solution to the rise in antibiotic-resistant bacterial infections. However, bacteria can acquire phage resistance, posing a major barrier to phage therapy. To overcome this problem, it is necessary to understand phage resistance mechanisms in bacteria. We have unraveled one such resistance mechanism in Enterococcus faecalis, a Gram-positive natural resident of the human intestine that has acquired antibiotic resistance and can cause opportunistic infections. We have identified a cell wall protein hypervariable region that specifies phage tropism in E. faecalis. Using a gnotobiotic mouse model of in vivo phage predation, we show that E. faecalis acquires phage resistance through mutations in this cell wall protein. Our findings define the molecular basis for lytic phage resistance in E. faecalis. They also suggest opportunities for engineering E. faecalis phages that circumvent the problem of bacterial phage resistance.
INTRODUCTION
Enterococcus faecalis is a Gram-positive bacterium that is a natural resident of the mammalian gastrointestinal tract (1). In addition to living a commensal lifestyle, E. faecalis is an opportunistic pathogen that causes intestinal dysbiosis and bloodstream infections (2, 3). The enterococci, including E. faecalis, have emerged as prevalent hospital-acquired pathogens and have increasingly acquired pathogenic and antibiotic resistance traits (4, 5). Antibiotic resistance in E. faecalis is especially troubling considering the emergence of organisms that are resistant to last resort antibiotics such as vancomycin and daptomycin (6–8). In addition, E. faecalis can be a conduit for the horizontal transfer of DNA between other opportunistic pathogens such as Clostridium difficile and Staphylococcus aureus (9, 10). Therefore, new therapeutic strategies are needed to control enterococcal populations both in native intestinal habitats and during hospital outbreaks.
Bacteriophages (phages), viruses that infect and kill bacteria, have long held promise as potential therapeutics (11). With the critical need for novel therapeutics in the fight against multidrug-resistant bacteria, phages are receiving renewed interest for their use as bactericidal agents. Phage therapy has several advantages over broad-spectrum antibiotics. First, phages are highly specific and can be tailored to target a narrow spectrum of bacteria. Second, phage replication is restricted to the environment where the host bacterium resides, and thus phages are self-resolving upon exhaustion of their host reservoir (12). Third, obligate lytic phages cannot integrate into the host bacterial genome as prophages, which limits the chance of introducing phage-carried virulence factors and antibiotic resistance genes into the bacterial genome.
A number of phages infect E. faecalis and Enterococcus faecium and include both obligate lytic phages and prophages (13). The E. faecalis genome harbors multiple prophages that have been implicated in virulence, interspecies competition, and biofilm dispersal (14–16). There are also numerous obligate lytic phages that infect E. faecalis and rapidly kill their bacterial hosts (13). These phages show a remarkable degree of specificity for certain E. faecalis strains. This specificity suggests that there is an evolutionary “arms race” between E. faecalis and its lytic phages, with underlying mechanisms that promote the evolution of phage resistance in the targeted strains and a corresponding ability of the phage to evolve new host strain specificities.
Despite the therapeutic promise of E. faecalis phages (17, 18), little is known about E. faecalis phage receptors, the molecular basis for phage strain specificity, or how E. faecalis develops phage resistance. Phage resistance is an especially formidable barrier for phage therapy, and thus, it is imperative to understand resistance mechanisms in order to develop phage therapies that sidestep this problem. Here we have isolated novel lytic phages from municipal raw sewage that infect E. faecalis. We have used these phages to identify an E. faecalis membrane protein named PIPEF (for phage infection protein from E. faecalis) that promotes lytic phage infection. We find that a variable region in PIPEF specifies phage tropism for distinct E. faecalis strains and that mutations in this variable region confer E. faecalis phage resistance. We also find that the PIPEF variable region in enterococci from raw sewage is diversified, suggesting that phage-bacterium interactions drive the accumulation of PIPEF variation in environmental E. faecalis. Last, we use a gnotobiotic mouse model of phage predation to show that E. faecalis acquires phage resistance in vivo through mutations in PIPEF. Our findings define the molecular basis for an evolutionary arms race between E. faecalis and its lytic phages that leads to E. faecalis phage resistance.
RESULTS
Genome sequence analysis of novel lytic E. faecalis bacteriophages.
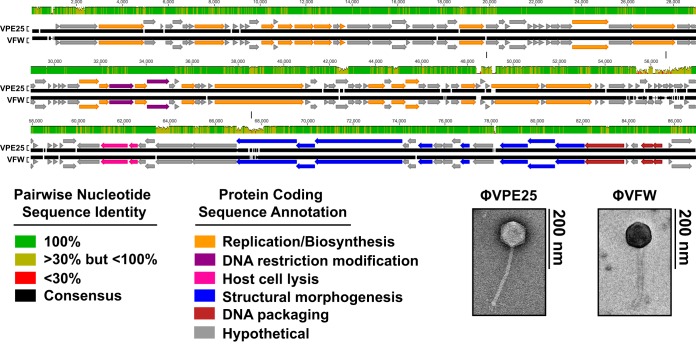
Municipal raw sewage was screened for phages that formed plaques on E. faecalis V583. Two phages were isolated and clonally purified by successive agar overlays. These phages formed clear plaques on E. faecalis V583. Transmission electron microscopy revealed that the phage morphologies were consistent with the Siphoviridae family of noncontractile tailed phages (Fig. 1) (19). We designated these phages φVPE25 and φVFW, where V stands for E. faecalis strain V583, on which these phages were isolated, followed by the source of raw sewage (PE25 for primary effluent pump 25 and FW for flocculated water).
FIG 1 .
Genome organization of lytic phages φVPE25 and φVFW. Whole-genome alignments were performed using MAFTT version 1.3 (61). Open reading frames for φVPE25 and φVFW were determined using RAST version 2.0, and the resulting data were imported into Geneious 6.0.6. Modular gene organization based on predicted function is color coded. Vertical lines indicate regions with a high degree of nucleotide heterogeneity between φVPE25 and φVFW. Transmission electron microscopy revealed that φVPE25 and φVFW are noncontractile tailed siphophages.
The φVPE25 and φVFW genomes are double-stranded DNA consisting of 86,524 bp and 85,865 bp, respectively. Each genome assembled into one large contig with multiple ambiguous nucleotide assignments and low read mapping coverage at the 5′ and 3′ ends suggesting no clear edges at the ends of the contigs. This was consistent with a circularly permuted genome that is terminally redundant. The two genomes are highly congruent, sharing ~95% nucleotide identity, suggesting that these phages recently diverged. A comparative analysis was performed to determine the degree of φVPE25 and φVFW genomic DNA similarity to seven recently characterized siphophages that infect E. faecalis (see Fig. S1A in the supplemental material) (20–23). φVPE25 and φVFW have short regions of similarity to these phages but are largely dissimilar at the nucleotide level. The genomes of φVPE25 and φVFW were then compared to the NCBI nonredundant nucleotide database using BLASTn (24). This confirmed that φVPE25 and φVFW have short regions of nucleotide identity to enterococcal phages AUEF3, IME-EF3, EfaCPT1, and EFAP1 and also revealed regions of similarity to Lactococcus lactis phage KSY1 and Bacillus sp. plasmid pBUYP1 (Fig. S1B) (25). These comparative analyses show that although some nucleotide similarity is observed between φVPE25 and φVFW and other enterococcal siphophages, they are mostly composed of previously unidentified DNA sequence.
Open reading frames (ORFs) were identified and annotated by rapid annotation using subsystem technology (RAST) and BLASTp (24, 26). Approximately 130 ORFs were predicted for both phage genomes. Thirty-seven percent of the ORFs were either assigned a function or were related to other predicted phage genes. The remaining 63% of the ORFs were categorized as hypothetical (see Table S1 in the supplemental material). Similar to other siphophages, the genomes of φVPE25 and φVFW are modular, consisting of genes organized by predicted function (27). These include genes involved in nucleotide biosynthesis and modification, phage particle morphogenesis and DNA packaging, and host cell lysis (Fig. 1 and Table S1). Analysis of these phage gene clusters revealed the presence of a putative recombinase with homology to phage integrase protein family members (Pfam, PF00589). However, integrated phage genomes were not detected in the genomic DNA of E. faecalis V583 that had evolved resistance to φVPE25 and φVFW as determined by Southern blotting (Fig. S2). This suggests that φVPE25 and φVFW are obligate lytic phages that are incapable of lysogeny. However, it is possible that these phages emerged from a common prophage ancestor.
The φVPE25 and φVFW genomes are modified to avoid restriction digestion.
Phages frequently deploy strategies that limit restriction digestion of their genomes by host bacterial endonucleases. This includes chemical modification of specific genomic sequences. Analysis of the φVPE25 and φVFW genomes revealed two ORFs predicted to encode phage DNA-modifying proteins. Both ORFs encode proteins resembling nucleotide sugar synthetase-like enzymes, including a β-glucosyltransferase.
Phage-encoded β-glucosyltransferases modify hydroxymethylated DNA (28). Cytosine residues are first converted to 5-methylcytosine (5mC) followed by hydroxylation of the methyl group to 5-hydroxymethylcytosine (5hmC). β-Glucosyltransferase then adds a glucose moiety from uridine diphosphoglucose to 5hmC, creating 5-glucose-hydroxymethylcytosine (5ghmC) (Fig. 2A) (29–31). In Escherichia coli T-even phages, β-glucosyltransferase activity protects cytoplasmic phage DNA from destruction by restriction endonucleases (32).
FIG 2 .
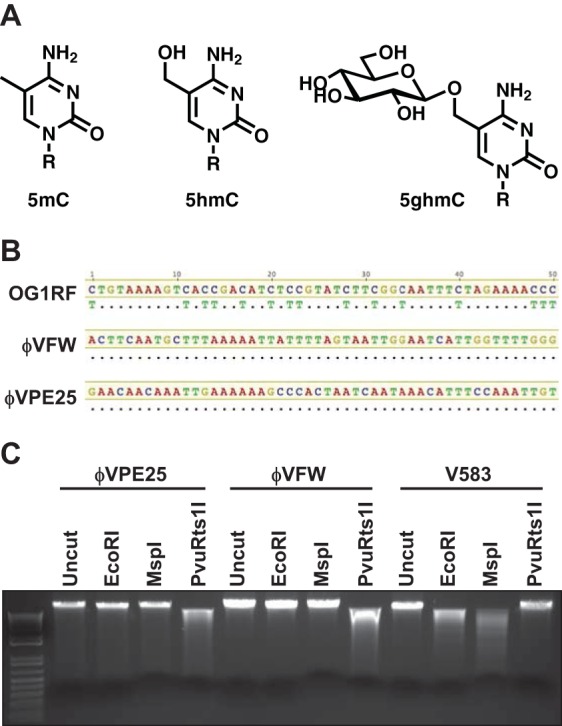
φVPE25 and φVFW DNA is modified at cytosine residues. (A) Structure of methylation and glycosylation modifications that occur at cytosine residues in DNA. Cytosine can be methylated in the form of a single methyl group (5mC) or hydroxyl-methylated (5hmC). 5hmC can be converted to a glucose-linked cytosine (5ghmC) by glucosyltransferase. (B) DNA sequencing analysis of sodium bisulfite-treated E. faecalis OG1RF genomic DNA or φVPE25 and φVFW genomic DNA. Unmodified cytosine in E. faecalis genomic DNA is converted to uracil after bisulfite treatment and when sequenced appears as thymidine. φVPE25 and φVFW genomic DNA resists bisulfite conversion, confirming cytosine modification. Dots indicate that these nucleotides match those in the consensus sequence. (C) Restriction endonuclease digestion of genomic DNA from φVPE25, φVFW, and E. faecalis V583. Incomplete digestion by PvuRts1I may be due to a minimum number of glycosylation sites or to inefficient DNA cleavage.
To determine whether φVPE25 and φVFW chromosomal DNA is modified, we treated phage and E. faecalis genomic DNA with sodium bisulfite which deaminates cytosine, converting it to uracil. However, modified cytosines are protected from conversion (33). After sodium bisulfite treatment, the converted cytosine residues appear as thymine in Sanger sequencing reactions. Both φVPE25 and φVFW genomic DNAs were protected from sodium bisulfite conversion, suggesting that the phage DNAs are modified at cytosine residues (Fig. 2B). Both φVPE25 and φVFW DNAs were resistant to digestion with the restriction enzyme EcoRI, further suggesting that the phage DNAs are modified (Fig. 2C).
To determine whether the DNA modification was methylation or glycosylation, we performed restriction digestions using enzymes that recognize methylated and glycosylated DNA. MspI cleaves unmethylated DNA or DNA containing 5mC and 5hmC but not 5ghmC and was unable to digest the phage DNAs, consistent with the presence of glycosylated cytosine (Fig. 2C). Conversely, PvuRts1I, which can cleave 5ghmC-containing DNA (34), digested both φVPE25 and φVFW DNAs but not the E. faecalis genomic DNA control (Fig. 2C). These data suggest that the phage DNAs are modified by glycosylation, likely in the form of glucose moieties.
The protein EF0858 is essential for phage infection of E. faecalis.
We next sought to identify E. faecalis genes involved in lytic phage infection. We added φVPE25 and φVFW to E. faecalis V583 and assessed infection using a confluent lysis agar overlay assay. The emergence of colonies within zones of lysis suggested that these bacteria were phage resistant. This was confirmed by cross streaking pure cultures of the E. faecalis isolates against both φVPE25 and φVFW (see Table S2 in the supplemental material). To determine the nature of the phage resistance, we sequenced the genomes of nine isolates using Illumina HiSeq. The sequencing reads of these nine isolates were mapped to the E. faecalis V583 reference genome to identify potential polymorphisms. The mutations in each of the nine isolates mapped to the open reading frame EF0858.
EF0858 encodes an 888-amino-acid protein that is a predicted transmembrane protein in E. faecalis V583. EF0858 is 68% identical and 81% similar to the Lactococcus lactis subsp. lactis Il1403 and L. lactis subsp. cremoris MG1363 phage infection protein (PIP), and the N terminus is distantly related (21% identity, 42% similarity) to the Bacillus subtilis 168 protein YueB, both of which are integral membrane proteins involved in phage adsorption and infection (35–37). Due to the high similarity of EF0858 to the L. lactis phage infection protein, we will refer to EF0858 as PIPEF for phage infection protein from E. faecalis. Various mutation types were observed in PIPEF, including deletion or insertion polymorphisms (DIPs), single nucleotide polymorphisms (SNPs), and an IS256 insertion element positioned in the 5′ region of the PIPEF coding sequence (see Table S2 in the supplemental material) (38). In the case of the DIPs and SNPs, each of these mutations produced a frameshift or a nonsense mutation in the form of a premature stop codon (Table S2).
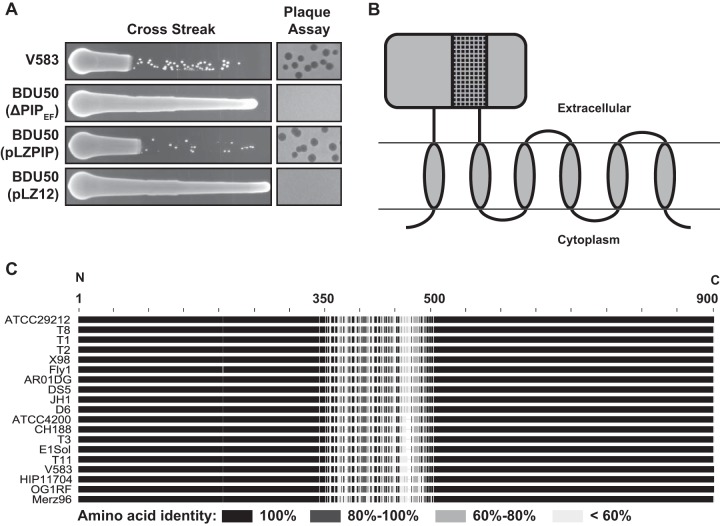
To confirm that PIPEF alone is responsible for the phage resistance phenotype, we constructed an in-frame deletion of PIPEF on the E. faecalis V583 chromosome using allelic replacement. Like the phage-resistant isolates in Table S2, the in-frame PIPEF deletion mutant BDU50 was resistant to phage infection as measured by both cross streak and plaque assays (Fig. 3A). Importantly, phage sensitivity could be restored by adding PIPEF on a multicopy plasmid to strain BDU50. Thus, PIPEF is sufficient to promote phage infection in E. faecalis V583.
FIG 3 .
EF0858 encodes PIPEF, promotes phage infection, and harbors a hypervariable region. (A) Cross streak and plaque assays using φVPE25 show the susceptibility or resistance profiles of E. faecalis V583 and the isogenic PIPEF deletion strain BDU50. Introduction of the pLZPIP plasmid, which contains the entire open reading frame of PIPEF restores phage infectivity of E. faecalis BDU50. pLZ12 is the empty vector. (B) Topological cartoon of E. faecalis V583 PIPEF generated using TOPCONS (62). The cartoon depicts PIPEF as an integral membrane protein that spans the membrane six times. The ~160-amino-acid variable region of PIPEF is represented as the black box with a black netting pattern in the large extracellular domain. (C) Pairwise amino acid sequence alignments of 19 PIPEF homologs were performed using Geneious 6.0.6. The N and C termini are indicated.
A PIPEF variable region determines lytic phage tropism.
A homolog of E. faecalis V583 PIPEF is present in multiple E. faecalis strains whose genomes have been sequenced. Amino acid alignment of PIPEF proteins from 19 sequenced E. faecalis strains showed a high degree of amino acid identity at the N and C termini, whereas the central region of PIPEF, which encompasses approximately 160 amino acids, is highly variable (Fig. 3C). This variable region covers amino acids 342 to 494 in E. faecalis V583 PIPEF and is part of a large predicted extracellular domain positioned between the first and second transmembrane domains (Fig. 3B and 4A). On the basis of the positioning of the variable region in an extracellular facing domain of PIPEF, we hypothesized that this region might play a biological role during phage infection. Indeed, truncations within the variable region of PIPEF rendered E. faecalis V583 resistant to infection by φVPE25 (Fig. 4B).
FIG 4 .
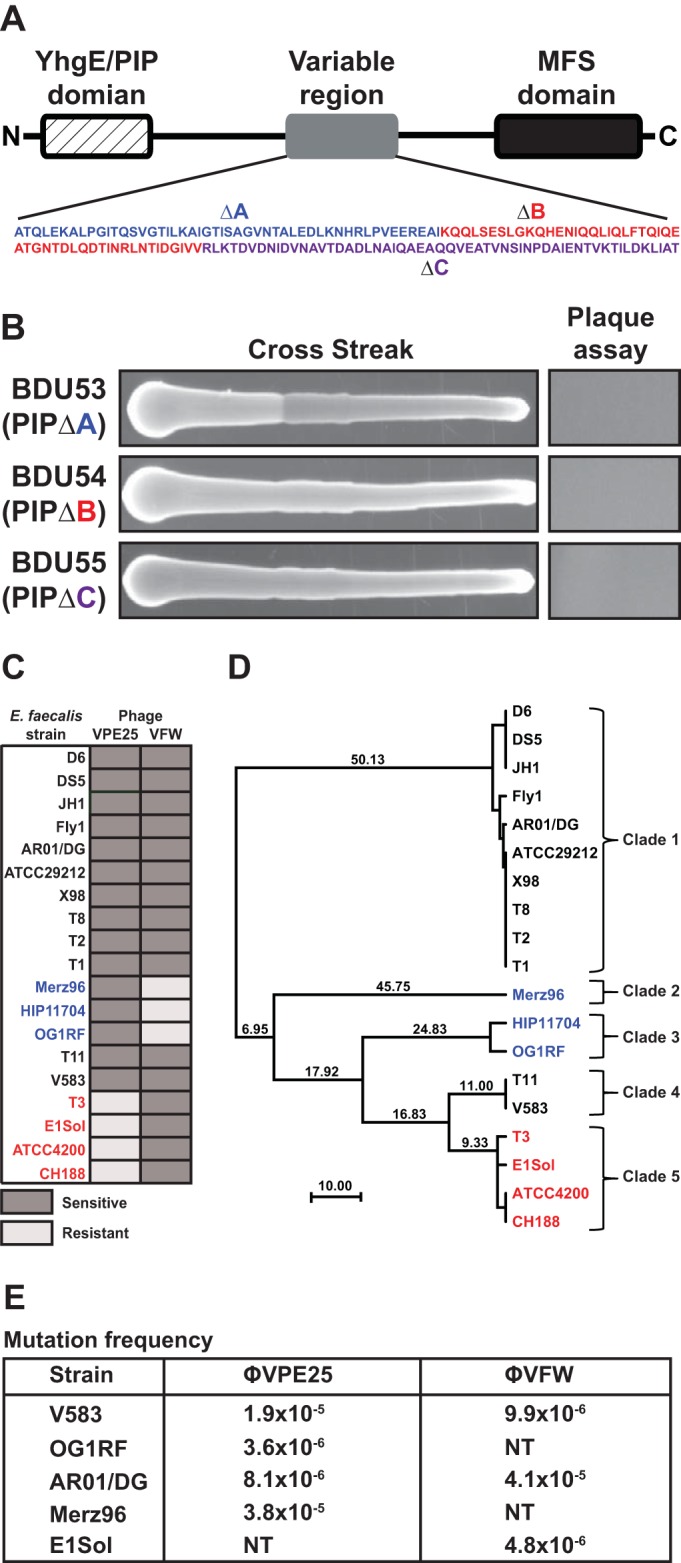
A variable region in PIPEF determines phage tropism in E. faecalis. (A) Schematic of PIPEF from E. faecalis V583. A variable region covering ~160 amino acids is located in the center of the PIPEF coding sequence. (B) Both φVPE25 cross streak and plaque assays show that truncations in the PIPEF variable region abolish phage infectivity regardless of location. The deletions (ΔA, ΔB, and ΔC) correspond to the colored amino acids highlighted in the magnified area of the variable region shown in panel A. (C) Susceptibility profiles of 19 E. faecalis strains for phages φVPE25 and φVFW. (D) Clustering of PIPEF variable region amino acid alignments from 19 strains of E. faecalis. Strains cluster according to their susceptibility patterns as determined in panel C. These strains are indicated by color coding as follows: strains sensitive to killing by both phages (black), strains sensitive to only φVPE25 (blue), and strains sensitive only to φVFW (red). Strains can be further grouped into five specific clades based on PIPEF variable region amino acid identity. (E) Representative clade-specific mutation frequencies for phages φVPE25 and φVFW. NT, not tested (due to natural resistance to the phage of interest).
We next queried the spectrum of φVPE25 and φVFW bactericidal activity against 19 E. faecalis strains using a cross streak assay. Unique patterns of host tropism emerged for both φVPE25 and φVFW. Twelve of the E. faecalis strains were sensitive to killing by both φVPE25 and φVFW, three strains (Merz96, HIP11704, and OG1RF) were killed by only φVPE25 and not φVFW, and four strains (T3, E1Sol, ATCC 4200, and CH188) were killed by φVFW but resisted killing by φVPE25 (Fig. 4C). Interestingly, we noted a relationship between the amino acid relatedness of the PIPEF variable region and the phage infectivity profile. PIPEF variable region sequences clustered into three distinct groups according to phage susceptibility patterns that included strains that were either exclusively killed by φVPE25, exclusively killed by φVFW, or those that were killed by both phages (Fig. 4D). These data suggested that the PIPEF variable region is a phage specificity determinant.
The PIPEF variable region amino acid sequence serves as a predictor of phage susceptibility and allows E. faecalis strains to be grouped into clades based on these sensitivity patterns. We propose the following typing scheme based on the PIPEF variable region amino acid sequence for predicting phage sensitivity in E. faecalis strains: clade 1, strains D6, JH1, DS5, AR01/DG, T8, ATCC 29212, T2, T1, X98, and Fly1; clade 2, strain Merz96; clade 3, strains OG1RF and HIP11704; clade 4, strains V583 and T11; clade 5, strains T3, CH188, ATCC 4200, and E1Sol. The PIPEF variable region sequence identity cutoff for clade grouping was 95% at the nucleic acid level. The frequency for resistance to φVPE25 and φVFW was determined using a representative E. faecalis strain from each phage sensitivity clade (Fig. 4E). All five phage sensitivity clades showed resistance frequencies of 10−5 to 10−6, and for φVPE25, it appears that clades 2 and 3 are less prone to develop resistance than clades 1 and 4. These data show that spontaneous phage resistance in E. faecalis arises at a frequency similar to that of other bacteria (39–41).
PIPEF swapping alters bacteriophage tropism.
To further test the idea that PIPEF is an E. faecalis phage tropism determinant, we introduced the pLZPIP plasmid, which carries PIPEF from E. faecalis V583 (clade 4), into E. faecalis E1Sol (clade 5). Strain E1Sol is resistant to infection by φVPE25 (Fig. 4C). However, when carrying pLZPIP, the phage tropism of E1Sol was altered, rendering the strain susceptible to infection by φVPE25 (Fig. 5A). This was confirmed by replacing in-frame the entire portion of the E1Sol PIPEF coding sequence with the V583 PIPEF (PIPV583) coding sequence using allelic exchange (Fig. 5A).
FIG 5 .
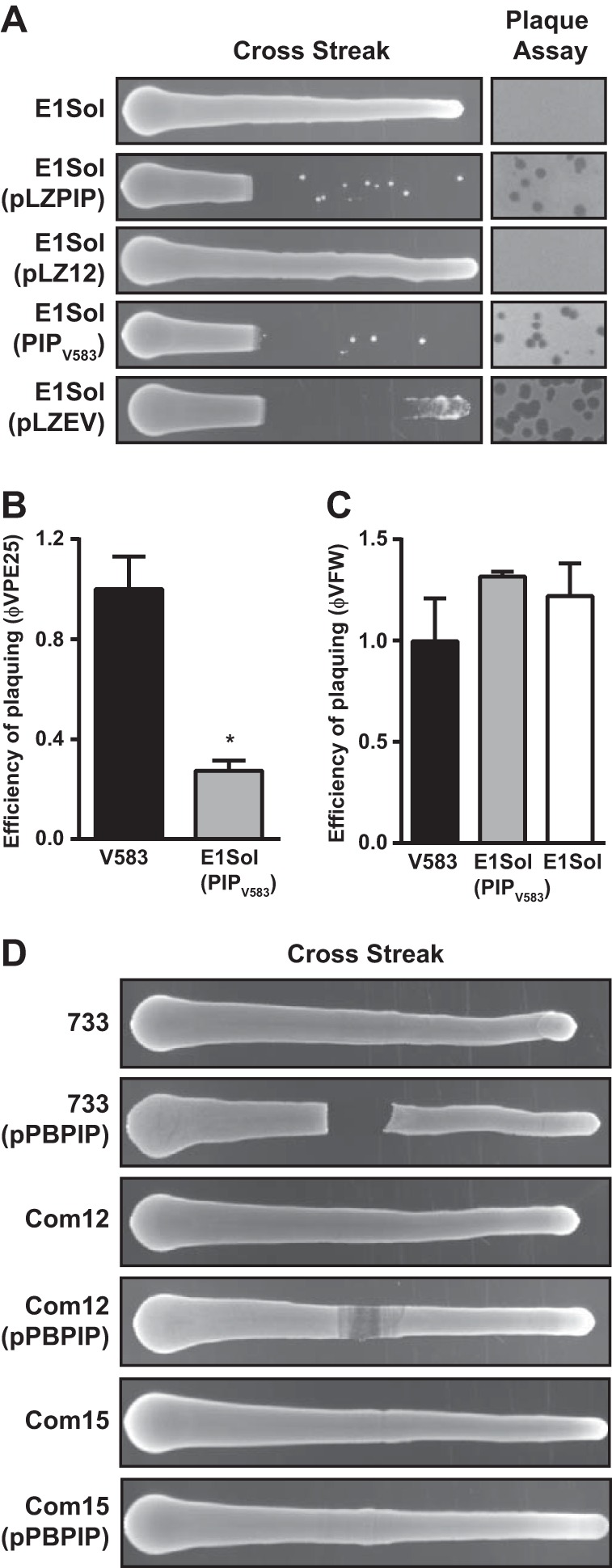
PIPEF swapping alters phage tropism. (A) Using the E. faecalis strain E1Sol, which is naturally resistant to infection by φVPE25, both cross streak and plaque assays showed that E1Sol can acquire φVPE25 susceptibility by expressing the E. faecalis V583 PIPEF gene (pLZPIP). Single-copy replacement of the E. faecalis E1Sol PIPEF homolog with V583 PIPEF in the E1Sol chromosome also confers φVPE25 sensitivity (PIPV583). A chimera of E. faecalis E1Sol PIPEF and the variable region of V583 PIPEF show that the PIPEF variable region determines phage tropism (pLZEV). (B and C) Plaquing efficiency of φVPE25 (B) and φVFW (C) on E. faecalis V583, E1Sol, and PIPV583 transgenic E1Sol strains. The value that was significantly different (P < 0.01) by Student’s t test from the value for E. faecalis V583 is indicated by an asterisk. (D) Cross streak assay showing that expression of E. faecalis V583 PIPEF from plasmid pPBPIP can confer φVPE25 sensitivity on E. faecium strains 1,141,733 and Com12, but not strain Com15.
To determine whether the PIPEF variable region specified the altered phage tropism of the transgenic E1Sol strain, we constructed the pLZEV plasmid, which contains the entire coding sequence of E1Sol PIPEF except that the variable region is replaced in frame with the V583 PIPEF variable region. Upon introduction of pLZEV into E. faecalis E1Sol, phage tropism was altered, resulting in the sensitivity of E1Sol to φVPE25 infection (Fig. 5A). These data show that the PIPEF variable region determines phage specificity for the bacterial host strain. However, we observed that φVPE25 was less efficient at plaquing on the transgenic E. faecalis E1Sol-PIPV583 strain than it was on its preferred host E. faecalis V583, whereas φVFW’s ability to form plaques on E1Sol-PIPV583 was unaltered (Fig. 5B and C). This suggests that other factors, such as differences in cell wall composition, help to determine maximal phage infectivity.
We next asked whether PIPEF could direct phage tropism toward a species other than E. faecalis. We chose the related species E. faecium, focusing on three strains, Com12, Com15, and 1,141,733, that each encode a PIP homolog that is ~65% identical to V583 PIPEF. All three strains are resistant to infection by φVPE25 (Fig. 5D). To test whether the tropism of φVPE25 could be redirected by introducing E. faecalis V583 PIPEF into these strains, we created the pPBPIP plasmid, which contains the entire coding sequence of V583 PIPEF controlled by its native promoter. Expression of E. faecalis V583 PIPEF in E. faecium Com12 and 1,141,733 conferred sensitivity to φVPE25, albeit to a lesser extent than that observed when V583 PIPEF was expressed in E. faecalis E1Sol. This finding was reinforced by the observation of a zone of clearance in the high phage titer region of cross streak plates for strains Com12 and 1,141,733 (Fig. 5D). Expression of V583 PIPEF in E. faecium Com15 did not render this strain sensitive to φVPE25 infection (Fig. 5D). These data show that PIP swapping in the related species E. faecium can alter the tropism of lytic enterococcal phages.
Ectopic expression of E. faecalis V583 PIPEF in E. faecium altered φVPE25 tropism, yet we did not observe visible plaques during the infection when performing an agar overlay experiment. Using E. faecium 1,141,733 carrying pPBPIP, we confirmed that the addition of φVPE25 to logarithmically growing bacterial cells retarded growth, suggesting that these phages successfully infect the bacteria (see Fig. S3A in the supplemental material). However, after 2 h of growth in the presence of φVPE25, an accumulation of φVPE25 particles in the culture fluid was not observed (Fig. S3B). We detected phage transcripts after infection in both wild-type and transgenic PIPEF E. faecium 1,141,733, suggesting that replicated phage particles were trapped within the bacteria due to inefficient host cell lysis (Fig. S3C). To test for trapped viable phage particles, we treated the cells with lysozyme and sonication. Indeed, both E. faecium strains released viable phage particles upon sonication, whereas the control strain E. faecalis E1Sol did not (Fig. S3D). These data show that φVPE25 infects E. faecium 1,141,733 and to a greater extent if the bacterium is expressing E. faecalis V583 PIPEF from plasmid pPBPIP; however, the phages cannot lyse the E. faecium cells.
PIPEF is dispensable for initial phage attachment and is implicated in DNA entry.
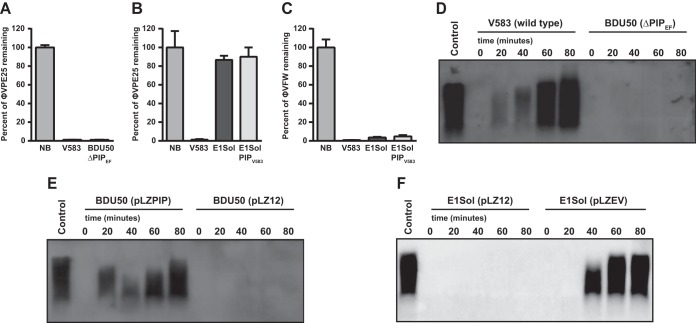
To determine the role of PIPEF during phage adsorption, we used whole bacterial cell pulldown assays to test the ability of phages φVPE25 and φVFW to bind to the E. faecalis cell surface. φVPE25 bound to wild-type E. faecalis V583 and the isogenic PIPEF mutant BDU50 equally well (Fig. 6A). However, φVPE25 did not bind to the surface of E. faecalis E1Sol, even when E1Sol had its PIPEF replaced with E. faecalis V583 PIPEF on the chromosome (Fig. 6B). This is in contrast to φVFW which adsorbed to E1Sol, transgenic PIPV583 E1Sol, and V583 similarly (Fig. 6C). These data suggest that PIPEF is not required for initial phage adsorption to E. faecalis.
FIG 6 .
PIPEF promotes DNA entry, but not initial phage adsorption. (A to C) Initial phage adsorption to E. faecalis cells was measured by determining the percentage of phages remaining in the supernatant after the addition of various E. faecalis strains. NB, no bacteria added. (D to F) Southern blotting was performed using an φVPE25 whole-genome probe on DNA isolated from whole cells infected with φVPE25. (D) E. faecalis V583 compared to the isogenic PIPEF mutant strain BDU50. (E) φVPE25 replication can be restored in strain BDU50 when PIPEF is provided in trans. (F) The variable region of strain V583 is sufficient to allow φVPE25 DNA entry into E. faecalis, because φVPE25 DNA can replicate in strain E1Sol only if the strain expresses a PIPEF chimera carrying the V583 PIPEF variable region (pLZEV) in the large extracellular facing domain. pLZ12 is the empty vector control.
We next sought to determine whether PIPEF is involved in phage DNA entry into E. faecalis cells. We infected E. faecalis strains with φVPE25 and monitored intracellular phage replication over time. As expected, φVPE25 replication was observed in E. faecalis V583 but not the PIPEF mutant strain BDU50 (Fig. 6D). φVPE25 replication could be restored in strain BDU50 by plasmid-encoded PIPEF (Fig. 6E). However, φVPE25 could replicate in E. faecalis E1Sol only when a plasmid-encoded chimeric PIPEF was introduced into the strain (Fig. 6F). This chimeric PIPEF was comprised of the E1Sol PIPEF sequence except for the variable region, which was from V583 PIPEF. These data support the conclusion that the PIPEF variable region facilitates phage DNA entry.
An environmental reservoir harbors E. faecalis with PIPEF diversity.
To characterize PIPEF diversity in a natural environment where E. faecalis is endemic, we used culture-dependent and -independent techniques to query the PIPEF variable region in enterococcal isolates from raw sewage. DNA was isolated from total raw sewage and from pooled enterococci that were first isolated from raw sewage by growth on selective agar. PIPEF variable-region-specific PCR was performed, and the resulting amplicons were sequenced using Illumina MiSeq. We first mapped the sequencing reads to a representative from each PIPEF-specific clade in order to quantify PIPEF diversity (see Table S3 in the supplemental material). Regardless of whether the PIPEF variable region was directly amplified from total raw sewage DNA or from genomic DNA after selective plating of enterococcal isolates, all five PIPEF clades were present; however, the majority of the PIPEF-specific reads matched clades 4 and 5, which include PIP from strains V853 and E1Sol, respectively (Table S3). Interestingly, clade 1 was more highly represented in the sequencing reads from pooled enterococci isolated by selective plating than in those generated in the absence of selection, suggesting that growth on selective agar may allow for the propagation of minority community members that otherwise go undetected by PCR in the absence of selection. These data show that raw sewage is a robust habitat for enterococci harboring diverse PIPEF genes representing all five identified phage sensitivity clades.
We next assessed the amino acid conservation of the PIPEF variable region sequences obtained from total raw sewage and raw sewage enterococcal isolates grown from selective plating. We first performed de novo assemblies of the sequencing reads for the PIPEF variable region and aligned the resulting contigs to the E. faecalis V583 PIPEF variable region as a reference (see Fig. S4A in the supplemental material). The majority of the PIPEF variable region amino acid content from both total raw sewage and raw sewage enterococcal isolates was identical to the amino acid sequence of the E. faecalis V583 PIPEF variable region. This is consistent with the observation that most PIPEF variable region reads mapped to strain V583 at the nucleotide level (Table S3). However, assemblies carrying a number of amino acid variants were identified in a region that is divergent at the amino acid level between clade 4 and clade 5 within the PIPEF variable region (Fig. S4B). These residues may be responsible for directing phage specificity within PIPEF and suggest that PIPEF diversity is maintained in the environment.
Phage-mediated killing of E. faecalis drives the emergence of PIPEF mutants in vivo.
Enterococcal overgrowth within the human intestinal tract can occur in response to broad-spectrum antibiotics or immunosuppressive drugs (2, 42). This overgrowth can result in intestinal dysbiosis that puts patients at risk of acquiring a secondary septic enterococcal infection. With the rise of multidrug-resistant strains of E. faecalis and E. faecium, novel therapeutic approaches to combat such infections are needed.
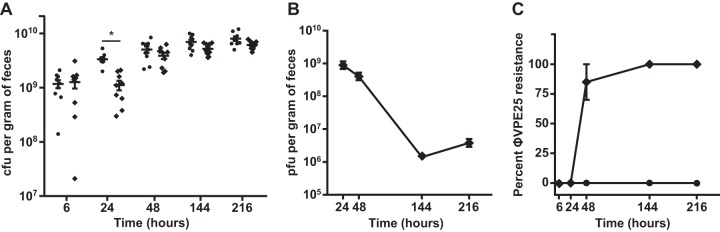
We sought to test the ability of phage φVPE25 to kill E. faecalis growing in the intestinal tract. We established a gnotobiotic mouse model of E. faecalis intestinal colonization in which germfree C57BL6/J mice were orally inoculated with E. faecalis V583. After a 6-h colonization period, φVPE25 was orally administered to the mice. Starting at 6 h and extending to 216 h (9 days) after φVPE25 administration, feces were collected from each mouse, and viable E. faecalis cells were enumerated by colony counting. After 24 h of φVPE25 treatment, there was a threefold drop in total E. faecalis intestinal loads compared to non-φVPE25-treated animals. This suggests that φVPE25 can modestly reduce the E. faecalis numbers in the mouse intestine (Fig. 7A). However, as early as 48 h and through day 9 of the experiment, the E. faecalis community rebounded to the level of colonization observed for untreated animals, even when the phages were added to the drinking water for continuous administration (Fig. 7A). The number of phage particles recovered from feces was the highest at 24 and 48 h after φVPE25 treatment (Fig. 7B). Phage particles were maintained in the intestines of these mice for the duration of the experiment, but the number of recoverable phage particles significantly decreased by 144 h (6 days) and 216 h (9 days) of treatment (Fig. 7B). Thus, the intestinal E. faecalis population became φVPE25 resistant.
FIG 7 .
In vivo phage predation selects for E. faecalis PIPEF mutants. Germfree C57BL6/J mice were orally inoculated with E. faecalis V583 followed by an oral treatment of φVPE25. (A) Fecal burden of E. faecalis from mice with and without phage treatment over a 9-day period. Each symbol represents the value for an individual mouse, and the short black bar represents the mean of the group of mice. The means that are significantly different (P < 0.001) by Student’s t test are indicated by a bar and asterisk. (B) φVPE25 particle numbers from gnotobiotic mouse feces as determined by a plaque assay. (C) Percentage of φVPE25-resistant E. faecalis clones isolated from gnotobiotic mice following phage treatment. The percentage of φVPE25 resistance was calculated by determining the number of phage-resistant isolates by cross streaking and then dividing the number of resistant isolates by the total number of isolates acquired at each time point and multiplying by 100. Symbols: ♦, treated with φVPE25; ●, not treated with phage.
To test for the acquisition of phage resistance at each time point, we screened a total of 40 E. faecalis fecal isolates from gnotobiotic mice that received either an φVPE25 or saline control inoculation. After 6 h of φVPE25 treatment, 100% of isolates remained sensitive to φVPE25 infection as determined by cross streaking (Fig. 7C). By 24 h after φVPE25 treatment, the number of E. faecalis isolates that were φVPE25 resistant rose to approximately 85%, and the number of resistant clones increased to 100% by day 9 (Fig. 7C). Thus, E. faecalis acquired widespread phage resistance after exposure to lytic phage in the gastrointestinal tract.
We next determined whether these phage-resistant isolates had intact PIPEF ORFs. At 48 h after colonization, a total of 20 E. faecalis fecal isolates were collected from both φVPE25-treated and saline-treated mice. Genomic DNA was purified from these isolates and used in PCRs that amplified the PIPEF coding sequence starting175 bp upstream of the predicted translational start site. The integrity of the PIPEF coding sequence was determined using automated Sanger DNA sequencing. PIPEF from all 20 fecal isolates collected from the saline-treated control group had a sequence identical to PIPEF from wild-type E. faecalis V583 (data not shown) and remained φVPE25 sensitive (Fig. 7C). However, the 20 fecal isolates from φVPE25-treated mice all had polymorphisms that localized to the PIPEF open reading frame. The polymorphisms that occurred in PIPEF from the fecal isolates included insertion/deletion mutations, frameshift mutations, and insertion sequence mutations (see Table S4 in the supplemental material).
These data show that phage predation on E. faecalis in the intestine exerts a strong selective pressure for the development of phage resistance. More importantly, our findings establish that E. faecalis acquires phage resistance in vivo through mutations in PIPEF.
DISCUSSION
Novel antimicrobial strategies are urgently needed to control increasingly frequent antibiotic-resistant bacterial infections. Phages have long held promise as potential antibacterial therapeutics, and thus, a revival in phage therapy has begun over the last several years. This has led to a renaissance in an understanding of the basic molecular biology of phages and their contributions to host-pathogen interactions (43). This is especially relevant for Gram-positive pathogens, such as E. faecalis, that place a heavy burden on the health care system. However, before phages can be routinely used as antienterococcal therapeutics, a detailed understanding of how they interact with their bacterial hosts and the mechanisms that enterococci use to avoid phage infection need to be delineated.
In this study, we have discovered two previously unidentified lytic siphophages, φVPE25 and φVFW, which infect E. faecalis. These phages are unique and have little genomic similarity to previously identified siphophages that infect E. faecalis. We found that φVPE25 and φVFW harbor genes encoding DNA-modifying enzymes that allow the phages to glycosylate their DNA at cytosine residues as a possible mechanism to avoid host restriction. This is inferred from the presence of a phage-encoded β-glucosyltransferase that is ~40% identical to the enterobacterial phage T4 β-glucosyltransferase, which modifies T4 DNA at 5hmC residues with glucose (31). Our findings are among the first to suggest that Gram-positive phages can modify their DNA by glycosylation (44).
Interestingly, there is a second open reading frame, encoded in the φVPE25 and φVFW genomes, that is annotated as a nucleotide sugar synthetase-like protein. The protein has 28% identity to β-1,6-galactofuranosyltransferase, a member of a class of enzyme that catalyzes the transfer of galactofuranose from UDP-galactofuranose to α-d-glucopyranosides (45). It is possible that the φVPE25/φVFW-encoded enzyme could further modify the glycosylated phage DNA. Genome sequence analysis did not uncover any putative virulence factors or antibiotic resistance genes, suggesting that φVPE25 and φVFW should be considered candidate phages to be further studied and possibly modified for therapeutic applications.
Our studies of φVPE25 and φVFW led us to identify PIPEF as an E. faecalis integral membrane protein that is essential for phage infection. We used the phages to select for phage-resistant mutants of E. faecalis V583 and found that genomic mutations associated with resistance were clustered in the PIPEF-encoding reading frame EF0858. PIPEF is orthologous to L. lactis PIP, which promotes phage binding and infection (35, 36). PIPEF harbors two distinctive domains: an N-terminal YhgE/PIP domain that is conserved in all Firmicutes that harbor a PIP ortholog and lacks a known function and a C-terminal major facilitator superfamily (MFS) domain that might play a role in small-molecule transport.
PIPEF is conserved among E. faecalis strains, suggesting that PIPEF performs an important biological function in E. faecalis. Interestingly, a second protein containing the YhgE/PIP domain was identified in 8 of the 19 E. faecalis strains used in this study. This protein has 21% sequence identity to the N-terminal YhgE/PIP domain of PIPEF and resides in a cluster of genes annotated to function as a type VIIb secretion system (46). This protein is an ortholog of the S. aureus EsaA type VIIb secretion system protein and has a similar predicted topology of six transmembrane domains and a C-terminal MFS domain as observed for PIPEF (47). We therefore speculate that PIPEF may be involved in the transport of a small molecule or function in concert with a type VIIb secretion system to secrete effector proteins across the bacterial membrane.
PIPEF harbors a 160-amino-acid region with marked sequence diversity. This protein region is centrally located within the first predicted extracellular domain of PIPEF and can be used to group E. faecalis strains based on their phage susceptibility profile. We found that this variable region is both necessary and sufficient to drive phage tropism for specific host bacteria. Raw sewage harbored E. faecalis with diverse PIPEF variable regions, suggesting that sewage phages coevolved with diverse E. faecalis strains. The changing abundance of such strains due to phage predation may drive the emergence of phages with altered tropisms for PIPEF variants.
Variable regions of cell wall-embedded proteins have been implicated in phage specificity for Gram-negative bacteria (48–50). In Gram-positive bacteria, the study of how variation in bacterium-encoded phage receptors impacts phage tropism has been limited to cell wall-associated polysaccharides (51, 52). To our knowledge, our findings constitute the first description of a diversifying region of a bacterial membrane protein phage receptor in Gram-positive bacteria.
A key remaining question is the identity of the phage antireceptor that interacts with PIPEF. Known phage antireceptors are components of the phage tail (53–55). Several phage tail genes are clustered together in the φVPE25 and φVFW genomes. One particular gene, represented by orf_112 in φVPE25 and orf_110 in φVFW, is a candidate for the antireceptor as it is orthologous to Streptococcus thermophilus phage antireceptors with hypervariable regions that specify phage tropism (56).
The PIPEF variable region sequences allowed clustering of E. faecalis strains into clades based on PIPEF homology and phage sensitivity. With the continued classification of lytic phages that target E. faecalis through a PIPEF-dependent mechanism and their assignment to the infection of specific clades, we envision the use of PIPEF variable region sequencing as a precursor for the selection of potential therapeutic phages that could be used to selectively kill E. faecalis outbreak strains.
Using a gnotobiotic mouse model of in vivo phage predation, we found that E. faecalis acquires phage resistance through mutations in PIPEF. Lytic phages can only modestly reduce E. faecalis numbers in the intestines of gnotobiotic mice, and E. faecalis numbers rebound due to the selective overgrowth of PIPEF mutants. Thus, prophylactic phage administration provides a strong selective pressure for the emergence of PIPEF mutations in E. faecalis.
The rapid development of phage resistance is just one of several barriers to deploying phages therapeutically (57). Although these phages are specific killers of E. faecalis, their exquisite selectivity for a target host enables the development of phage infection resistance and highlights a potential barrier to phage monotherapies against E. faecalis. We suggest that our identification of a key driver of phage resistance in E. faecalis could facilitate the engineering of phages with altered tropism. Likewise, once identified, the phage antireceptor specific for PIPEF could be modified by site-directed mutagenesis in hopes of loosening its specificity for cell wall targets. Chemical mutagenesis could also be performed on the phage genomes to create phages with altered tropisms. These approaches could enable the development of phage cocktails that circumvent the problem of resistance and could thus be used to treat E. faecalis infections.
MATERIALS AND METHODS
Bacterial strains and bacteriophages.
A complete list of bacterial strains and phages used in this study can be found in Table S5 in the supplemental material. E. faecalis and E. faecium were grown statically in brain heart infusion (BHI) broth or on BHI agar at 37°C. Escherichia coli was grown in Lennox L broth (LB) with shaking or on LB agar at 37°C. Chloramphenicol was added at 15 µg/ml for E. faecalis and E. faecium or 8 µg/ml for E. coli when needed. Enterococci from municipal raw sewage were isolated using Enterococcosel agar (Becton Dickinson). Growth conditions for the generation of mutant strains of E. faecalis by allelic exchange were as specified by Thurlow et al. (58). For a more detailed description of bacterial growth conditions and for plasmid construction, see Text S1 and Table S5 in the supplemental material, respectively.
Phages φVPE25 and φVFW were isolated from untreated raw sewage obtained from a Dallas-Fort Worth water reclamation facility in Texas. Fifty milliliters of raw sewage was centrifuged at 3,220 × g for 10 min at room temperature (RT) to sediment large particles. The supernatant was decanted and passed through a 0.45-µm filter. One hundred microliters of clarified sewage was mixed with 130 µl of a 1:10 dilution of an overnight (O/N) culture of E. faecalis V583 and then added to Todd-Hewitt broth (THB) top agar (0.35% agar) and poured over a 1.5% agar THB plate. Both top agar and base agar were supplemented with 10 mM MgSO4. After O/N growth at 37°C, the resulting plaques were recovered using a sterile Pasteur pipette, and phages were eluted from the agar plugs in 500 µl of SM-plus buffer (100 mM NaCl, 50 mM Tris-HCl, 8 mM MgSO4, 5 mM CaCl2 [pH 7.4]) O/N at 4°C. Phages were replaqued on E. faecalis V583 two more times to ensure that the phages were clonal isolates.
High-titer phage stocks were propagated by infecting 300 ml of logarithmically growing E. faecalis V583 at a multiplicity of infection of 0.1 in BHI broth containing 10 mM MgSO4. Lysis was allowed to proceed for 4 h at 37°C with shaking. The remaining bacterial cells and debris were pelleted at 4,400 × g for 10 min at RT. The culture supernatant was filtered through a 0.45-µm membrane and treated with 5 µg/ml each of DNase and RNase at RT for 1 h, and phages were precipitated by adding 1 M NaCl and 10% (wt/vol) polyethylene glycol 8000 (PEG 8000) and incubated on ice O/N at 4°C. Phages were pelleted by centrifugation at 11,270 × g and resuspended in 2 ml of SM-plus buffer. One-third volume of chloroform was added with shaking, and the phases were separated by centrifugation at 16,300 × g. The aqueous phase containing the phages was subjected to further purification using cesium chloride centrifugation as described previously (59). Phages were enumerated using the THB agar overlay plaque assay described above.
Phage cross streak assays.
We used cross streaking to determine the sensitivity of various E. faecalis and E. faecium strains to phages φVPE25 and φVFW. A total of 109 to 1010 phage particles were streaked down the center of a THB agar plate containing 10 mM MgSO4 with or without 15 µg/ml chloramphenicol when necessary. Ten microliters of an O/N bacterial culture was spread horizontally across the phage streak. The plates were incubated at 37°C O/N, and bacterial strain sensitivity to a particular phage was indicated by limited to no bacterial growth within and beyond the phage streak area.
Whole-genome sequencing.
The genomes of phages φVPE25 and φVFW and E. faecalis phage-resistant isolates were sequenced by Tufts University Core Facility (TUCF) Genomics, Tufts University. The phage genomes were sequenced using Illumina MiSeq paired-end 250-bp DNA sequencing with an average coverage depth of 881× for φVPE25 and 903× for φVFW. The E. faecalis phage-resistant isolates were sequenced using Illumina HiSeq2000 single-end 100-bp DNA sequencing. Variant coverage information for these strains can be found in Table S2. Libraries were prepared with the Nextera XT library preparation kit. All assemblies were performed using CLC Workbench (Qiagen). For additional information about DNA sequencing and for explanations of bioinformatic applications, refer to Text S1.
Animals.
Germfree C57BL6/J mice were reared at University of Texas (UT) Southwestern Medical Center under sterile conditions as previously described (60). Gnotobiotic E. faecalis-colonized mice were established by orally inoculating male C57BL6/J mice with 5 × 107 CFU of E. faecalis V583. Intestinal colonization levels were determined by homogenizing fresh fecal pellets in 1 ml of sterile phosphate-buffered saline (PBS) and performing colony counting on Enterococcosel agar. When appropriate, φVPE25 was administered by orally gavaging mice with 1 × 1010 PFU and by administering phage in drinking water at a concentration of 5 × 108 PFU/ml. All animal protocols were approved by the Institutional Animal Care and Use Committee of UT Southwestern Medical Center.
Accession numbers.
All sequences generated for this study have been deposited in the European Nucleotide Archive. The following accession numbers have been assigned: PRJEB13004 (φVPE25 assembled genome), PRJEB13155 (φVFW assembled genome), PRJEB13005 (E. faecalis phage-resistant isolates), and PRJEB13161 (PIPEF raw sewage amplicons).
SUPPLEMENTAL MATERIAL
Supplemental Materials and Methods. Download
Comparative analysis of the φVPE25 and φVFW genomes. (A) Comparative whole-genome alignments of φVPE25 and φVFW and seven other siphophages that infect E. faecalis were performed using Mauve 2.3.1 (A. E. Darling, B. Mau, and N. T. Perna, PLoS One 5:e11147, 2010, http://dx.doi.org/10.1371/journal.pone.0011147). Lines indicate genomic regions that have connectivity based on nucleotide sequence similarity. (B) A BLASTn analysis was performed on φVPE25 and φVFW to identify genome relatedness to phages outside of known enterococcal siphophages. φVPE25 was set as the reference sequence for circular alignment using Brig 0.95 (N. F. Alikhan, N. K. Petty, N. L. Ben Zakour, and S. A. Beatson, BMC Genomics 12:402, 2011, http://dx.doi.org/10.1186/1471-2164-12-402). Download
Phage-resistant E. faecalis isolates do not harbor integrated prophages. Genomic DNA isolated from E. faecalis cells that developed spontaneous resistance to φVPE25 and φVFW infection (see Table S2 in the supplemental material) lacks detectable phage DNA as determined by Southern blotting. The NS-mix lane contains a pool of E. faecalis V583 phage-resistant isolates recovered from scraping the soft agar of a semiconfluent φVPE25 lysis plate that was serially passaged in BHI three times prior to extraction of total genomic DNA for Southern blot analysis. The V583 lane contains genomic DNA from phage-sensitive wild-type E. faecalis V583. Purified φVPE25 and φVFW DNAs are included as controls. Download
φVPE25 infection of transgenic E. faecium 1,141,733. (A) Growth kinetics of E. faecium 1,141,733 carrying the E. faecalis V583 PIPEF expression plasmid pPBPIP in the presence (●) and absence (♦) of φVPE25. The arrow indicates the time of φVPE25 addition to the culture. (B) φVPE25 particle numbers from infected E. faecalis V583 cells carrying pPBPIP immediately after phage addition (Input) or 2 h after phage infection (2 hours post). (C) Quantitative real-time PCR of the φVPE25 transcripts orf_106 (lysin), orf_117 (major tail protein), and orf_123 (major capsid protein) isolated from E. faecium 1,141,733 or E. faecium 1,141,733 carrying plasmid pPBPIP. E. faecalis V583 and the PIPEF mutant strain BDU50 are included as controls. Transcript abundances are plotted on a logarithmic scale. (D) Viable phage particles recovered from wild-type and PIPEF transgenic E. faecium 1,141,733 after cell disruption using lysozyme and sonication. E. faecalis E1Sol was included as a control strain that is resistant to φVPE25 infection. ND, none detected. Download
Sequence variation among the PIPEF variable regions of E. faecalis sewage isolates. (A) Schematic of the variable region of PIPEF (amino acids 342 to 494). The amino acids where variation was detected by direct PCR from raw sewage (EBOX) or from pooled enterococcal isolates grown on selective agar (P1) are indicated in red and green, respectively. For both EBOX and P1 samples, the majority of the amino acid content of the PIPEF variable region matched E. faecalis V583 (52.00% of contigs for P1 and 41.61% for EBOX). The top four or five representative contigs containing variant amino acid composition compared to the E. faecalis V583 PIPEF variable region sequence as a reference are indicated. (B) Alignment of the E. faecalis V583 (clade 4) and E1Sol (clade 5) PIPEF variable regions. Download
Enterococcal bacteriophage genome organization and features.
Spontaneous mutations in EF0858 (PIPEF) result in phage resistance.
Sewage PIP read mapping to clade-specific PIPEF variable region.
Mutations conferring φVPE25 resistance in E. faecalis from gnotobiotic mouse feces.
Bacterial strains, phages, plasmids, and primers used in this study.
ACKNOWLEDGMENTS
We thank C. Boyd, B. Hassell, and T. Leal for assistance with gnotobiotic animal husbandry, H. Zhang for assistance with electron microscopy, B. Zhang for preliminary screening of PIPEF mutants and phage host tropism, and D. Propheter for providing nucleotide art shown in Fig. 2A.
Footnotes
Citation Duerkop BA, Huo W, Bhardwaj P, Palmer KL, Hooper LV. 2016. Molecular basis for lytic bacteriophage resistance in enterococci. mBio 7(4):e01304-16. doi:10.1128/mBio.01304-16.
REFERENCES
- 1.Lebreton F, Willems RJL, Gilmore MS. 2014. Enterococcus diversity, origins in nature, and gut colonization. In Gilmore MS, Clewell DB, Ike Y, Shankar N (ed), Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston, MA. [PubMed] [Google Scholar]
- 2.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, Pamer EG. 2010. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 4.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities . 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 5.Agudelo Higuita NI, Huycke MM. 2014. Enterococcal disease, epidemiology, and implications for treatment. In Gilmore MS, Clewell DB, Ike Y, Shankar N (ed), Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston, MA. [Google Scholar]
- 6.Cattoir V, Leclercq R. 2013. Twenty-five years of shared life with vancomycin-resistant enterococci: is it time to divorce? J Antimicrob Chemother 68:731–742. doi: 10.1093/jac/dks469. [DOI] [PubMed] [Google Scholar]
- 7.Arias CA, Panesso D, McGrath DM, Qin X, Mojica MF, Miller C, Diaz L, Tran TT, Rincon S, Barbu EM, Reyes J, Roh JH, Lobos E, Sodergren E, Pasqualini R, Arap W, Quinn JP, Shamoo Y, Murray BE, Weinstock GM. 2011. Genetic basis for in vivo daptomycin resistance in enterococci. N Engl J Med 365:892–900. doi: 10.1056/NEJMoa1011138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer KL, Daniel A, Hardy C, Silverman J, Gilmore MS. 2011. Genetic basis for daptomycin resistance in enterococci. Antimicrob Agents Chemother 55:3345–3356. doi: 10.1128/AAC.00207-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jasni AS, Mullany P, Hussain H, Roberts AP. 2010. Demonstration of conjugative transposon (Tn5397)-mediated horizontal gene transfer between Clostridium difficile and Enterococcus faecalis. Antimicrob Agents Chemother 54:4924–4926. doi: 10.1128/AAC.00496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, Kolonay JF, Shetty J, Killgore GE, Tenover FC. 2003. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302:1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 11.Sulakvelidze A, Alavidze Z, Morris JG Jr.. 2001. Bacteriophage therapy. Antimicrob Agents Chemother 45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nobrega FL, Costa AR, Kluskens LD, Azeredo J. 2015. Revisiting phage therapy: new applications for old resources. Trends Microbiol 23:185–191. doi: 10.1016/j.tim.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Duerkop BA, Palmer KL, Horsburgh MJ. 2014. Enterococcal bacteriophages and genome defense. In Gilmore MS, Clewell DB, Ike Y, Shankar N (ed), Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston, MA. [PubMed] [Google Scholar]
- 14.Matos RC, Lapaque N, Rigottier-Gois L, Debarbieux L, Meylheuc T, Gonzalez-Zorn B, Repoila F, Lopes MDF, Serror P. 2013. Enterococcus faecalis prophage dynamics and contributions to pathogenic traits. PLoS Genet 9:e1003539. doi: 10.1371/journal.pgen.1003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossmann FS, Racek T, Wobser D, Puchalka J, Rabener EM, Reiger M, Hendrickx AP, Diederich AK, Jung K, Klein C, Huebner J. 2015. Phage-mediated dispersal of biofilm and distribution of bacterial virulence genes is induced by quorum sensing. PLoS Pathog 11:e1004653. doi: 10.1371/journal.ppat.1004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duerkop BA, Clements CV, Rollins D, Rodrigues JL, Hooper LV. 2012. A composite bacteriophage alters colonization by an intestinal commensal bacterium. Proc Natl Acad Sci U S A 109:17621–17626. doi: 10.1073/pnas.1206136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalifa L, Brosh Y, Gelman D, Coppenhagen-Glazer S, Beyth S, Poradosu-Cohen R, Que YA, Beyth N, Hazan R. 2015. Targeting Enterococcus faecalis biofilms with phage therapy. Appl Environ Microbiol 81:2696–2705. doi: 10.1128/AEM.00096-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Letkiewicz S, Miedzybrodzki R, Fortuna W, Weber-Dabrowska B, Gorski A. 2009. Eradication of Enterococcus faecalis by phage therapy in chronic bacterial prostatitis—case report. Folia Microbiol (Praha) 54:457–461. doi: 10.1007/s12223-009-0064-z. [DOI] [PubMed] [Google Scholar]
- 19.Ackermann HW. 2007. 5500 phages examined in the electron microscope. Arch Virol 152:227–243. doi: 10.1007/s00705-006-0849-1. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Ding P, Han C, Fan H, Wang Y, Mi Z, Feng F, Tong Y. 2014. Genome analysis of Enterococcus faecalis bacteriophage IME-EF3 harboring a putative metallo-beta-lactamase gene. Virus Genes 49:145–151. doi: 10.1007/s11262-014-1079-3. [DOI] [PubMed] [Google Scholar]
- 21.Lee YD, Park JH. 2012. Complete genome sequence of enterococcal bacteriophage SAP6. J Virol 86:5402–5403. doi: 10.1128/JVI.00321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son JS, Jun SY, Kim EB, Park JE, Paik HR, Yoon SJ, Kang SH, Choi YJ. 2010. Complete genome sequence of a newly isolated lytic bacteriophage, EFAP-1 of Enterococcus faecalis, and antibacterial activity of its endolysin EFAL-1. J Appl Microbiol 108:1769–1779. doi: 10.1111/j.1365-2672.2009.04576.x. [DOI] [PubMed] [Google Scholar]
- 23.Fard RM, Barton MD, Arthur JL, Heuzenroeder MW. 2010. Whole-genome sequencing and gene mapping of a newly isolated lytic enterococcal bacteriophage EFRM31. Arch Virol 155:1887–1891. doi: 10.1007/s00705-010-0800-3. [DOI] [PubMed] [Google Scholar]
- 24.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 25.Chopin A, Deveau H, Ehrlich SD, Moineau S, Chopin MC. 2007. KSY1, a lactococcal phage with a T7-like transcription. Virology 365:1–9. doi: 10.1016/j.virol.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 26.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brüssow H, Canchaya C, Hardt WD. 2004. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev 68:560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkin E. 1954. The linkage of glucose in coliphage nucleic acids. J Am Chem Soc 76:5892–5893. doi: 10.1021/ja01651a117. [DOI] [Google Scholar]
- 29.Josse J, Kornberg A. 1962. Glucosylation of deoxyribonucleic acid. III. α- and β-glucosyl transferases from T4-infected Escherichia coli. J Biol Chem 237:1968–1976. [PubMed] [Google Scholar]
- 30.Jesaitis MA. 1956. Differences in the chemical composition of the phage nucleic acids. Nature 178:637. doi: 10.1038/178637a0. [DOI] [PubMed] [Google Scholar]
- 31.Morera S, Imberty A, Aschke-Sonnenborn U, Ruger W, Freemont PS. 1999. T4 phage β-glucosyltransferase: substrate binding and proposed catalytic mechanism. J Mol Biol 292:717–730. [DOI] [PubMed] [Google Scholar]
- 32.Huang LH, Farnet CM, Ehrlich KC, Ehrlich M. 1982. Digestion of highly modified bacteriophage DNA by restriction endonucleases. Nucleic Acids Res 10:1579–1591. doi: 10.1093/nar/10.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Pastor WA, Shen Y, Tahiliani M, Liu DR, Rao A. 2010. The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PLoS One 5:e8888. doi: 10.1371/journal.pone.0008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janosi L, Yonemitsu H, Hong H, Kaji A. 1994. Molecular cloning and expression of a novel hydroxymethylcytosine-specific restriction enzyme (PvuRts1I) modulated by glucosylation of DNA. J Mol Biol 242:45–61. doi: 10.1006/jmbi.1994.1556. [DOI] [PubMed] [Google Scholar]
- 35.Geller BL, Ivey RG, Trempy JE, Hettinger-Smith B. 1993. Cloning of a chromosomal gene required for phage infection of Lactococcus lactis subsp. lactis C2. J Bacteriol 175:5510–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monteville MR, Ardestani B, Geller BL. 1994. Lactococcal bacteriophages require a host cell wall carbohydrate and a plasma membrane protein for adsorption and ejection of DNA. Appl Environ Microbiol 60:3204–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.São-José C, Baptista C, Santos MA. 2004. Bacillus subtilis operon encoding a membrane receptor for bacteriophage SPP1. J Bacteriol 186:8337–8346. doi: 10.1128/JB.186.24.8337-8346.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankar N, Baghdayan AS, Gilmore MS. 2002. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 417:746–750. doi: 10.1038/nature00802. [DOI] [PubMed] [Google Scholar]
- 39.Demerec M, Fano U. 1945. Bacteriophage-resistant mutants in Escherichia coli. Genetics 30:119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le S, Yao X, Lu S, Tan Y, Rao X, Li M, Jin X, Wang J, Zhao Y, Wu NC, Lux R, He X, Shi W, Hu F. 2014. Chromosomal DNA deletion confers phage resistance to Pseudomonas aeruginosa. Sci Rep 4:4738. doi: 10.1038/srep04738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King WR, Collins EB, Barrett EL. 1983. Frequencies of bacteriophage-resistant and slow acid-producing variants of Streptococcus cremoris. Appl Environ Microbiol 45:1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donskey CJ, Chowdhry TK, Hecker MT, Hoyen CK, Hanrahan JA, Hujer AM, Hutton-Thomas RA, Whalen CC, Bonomo RA, Rice LB. 2000. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 343:1925–1932. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young R, Gill JJ. 2015. Phage therapy redux what is to be done? Science 350:1163–1164. doi: 10.1126/science.aad6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenberg E. 1965. d-Mannose as a constituent of the DNA of a mutant strain of bacteriophage Sp8. Proc Natl Acad Sci U S A 53:836–841. doi: 10.1073/pnas.53.4.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wing C, Errey JC, Mukhopadhyay B, Blanchard JS, Field RA. 2006. Expression and initial characterization of WbbI, a putative d-Galf:α-d-Glc β-1,6-galactofuranosyltransferase from Escherichia coli K-12. Org Biomol Chem 4:3945–3950. doi: 10.1039/b609455d. [DOI] [PubMed] [Google Scholar]
- 46.Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W. 2007. Type VII secretion mycobacteria show the way. Nat Rev Microbiol 5:883–891. doi: 10.1038/nrmicro1773. [DOI] [PubMed] [Google Scholar]
- 47.Burts ML, Williams WA, DeBord K, Missiakas DM. 2005. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc Natl Acad Sci U S A 102:1169–1174. doi: 10.1073/pnas.0405620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabsch W, Ma L, Wiley G, Najar FZ, Kaserer W, Schuerch DW, Klebba JE, Roe BA, Laverde Gomez JA, Schallmey M, Newton SM, Klebba PE. 2007. FepA- and TonB-dependent bacteriophage H8: receptor binding and genomic sequence. J Bacteriol 189:5658–5674. doi: 10.1128/JB.00437-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.German GJ, Misra R. 2001. The TolC protein of Escherichia coli serves as a cell-surface receptor for the newly characterized TLS bacteriophage. J Mol Biol 308:579–585. doi: 10.1006/jmbi.2001.4578. [DOI] [PubMed] [Google Scholar]
- 50.Nieweg A, Bremer E. 1997. The nucleoside-specific Tsx channel from the outer membrane of Salmonella typhimurium, Klebsiella pneumoniae and Enterobacter aerogenes: functional characterization and DNA sequence analysis of the tsx genes. Microbiology 143:603–615. doi: 10.1099/00221287-143-2-603. [DOI] [PubMed] [Google Scholar]
- 51.Ainsworth S, Sadovskaya I, Vinogradov E, Courtin P, Guerardel Y, Mahony J, Grard T, Cambillau C, Chapot-Chartier MP, van Sinderen D. 2014. Differences in lactococcal cell wall polysaccharide structure are major determining factors in bacteriophage sensitivity. mBio 5:e00880-14. doi: 10.1128/mBio.00880-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eugster MR, Morax LS, Hüls VJ, Huwiler SG, Leclercq A, Lecuit M, Loessner MJ. 2015. Bacteriophage predation promotes serovar diversification in Listeria monocytogenes. Mol Microbiol 97:33–46. doi: 10.1111/mmi.13009. [DOI] [PubMed] [Google Scholar]
- 53.Hashemolhosseini S, Holmes Z, Mutschler B, Henning U. 1994. Alterations of receptor specificities of coliphages of the T2 family. J Mol Biol 240:105–110. doi: 10.1006/jmbi.1994.1424. [DOI] [PubMed] [Google Scholar]
- 54.Vegge CS, Vogensen FK, McGrath S, Neve H, van Sinderen D, Brøndsted L. 2006. Identification of the lower baseplate protein as the antireceptor of the temperate lactococcal bacteriophages TP901-1 and Tuc2009. J Bacteriol 188:55–63. doi: 10.1128/JB.188.1.55-63.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stuer-Lauridsen B, Janzen T, Schnabl J, Johansen E. 2003. Identification of the host determinant of two prolate-headed phages infecting Lactococcus lactis. Virology 309:10–17. doi: 10.1016/S0042-6822(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 56.Duplessis M, Moineau S. 2001. Identification of a genetic determinant responsible for host specificity in Streptococcus thermophilus bacteriophages. Mol Microbiol 41:325–336. doi: 10.1046/j.1365-2958.2001.02521.x. [DOI] [PubMed] [Google Scholar]
- 57.Henein A. 2013. What are the limitations on the wider therapeutic use of phage? Bacteriophage 3:e24872. doi: 10.4161/bact.24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thurlow LR, Thomas VC, Hancock LE. 2009. Capsular polysaccharide production in Enterococcus faecalis and contribution of CpsF to capsule serospecificity. J Bacteriol 191:6203–6210. doi: 10.1128/JB.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sambrook J, Fritsch EF, Maniatis T. 1989. Bacteriophageλ vectors, p 2.1–2.125. In Nolan C (ed), Molecular cloning: a laboratory manual, vol 1, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 60.Cash HL, Whitham CV, Behrendt CL, Hooper LV. 2006. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsirigos KD, Peters C, Shu N, Käll L, Elofsson A. 2015. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res 43:W401–W407. doi: 10.1093/nar/gkv485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials and Methods. Download
Comparative analysis of the φVPE25 and φVFW genomes. (A) Comparative whole-genome alignments of φVPE25 and φVFW and seven other siphophages that infect E. faecalis were performed using Mauve 2.3.1 (A. E. Darling, B. Mau, and N. T. Perna, PLoS One 5:e11147, 2010, http://dx.doi.org/10.1371/journal.pone.0011147). Lines indicate genomic regions that have connectivity based on nucleotide sequence similarity. (B) A BLASTn analysis was performed on φVPE25 and φVFW to identify genome relatedness to phages outside of known enterococcal siphophages. φVPE25 was set as the reference sequence for circular alignment using Brig 0.95 (N. F. Alikhan, N. K. Petty, N. L. Ben Zakour, and S. A. Beatson, BMC Genomics 12:402, 2011, http://dx.doi.org/10.1186/1471-2164-12-402). Download
Phage-resistant E. faecalis isolates do not harbor integrated prophages. Genomic DNA isolated from E. faecalis cells that developed spontaneous resistance to φVPE25 and φVFW infection (see Table S2 in the supplemental material) lacks detectable phage DNA as determined by Southern blotting. The NS-mix lane contains a pool of E. faecalis V583 phage-resistant isolates recovered from scraping the soft agar of a semiconfluent φVPE25 lysis plate that was serially passaged in BHI three times prior to extraction of total genomic DNA for Southern blot analysis. The V583 lane contains genomic DNA from phage-sensitive wild-type E. faecalis V583. Purified φVPE25 and φVFW DNAs are included as controls. Download
φVPE25 infection of transgenic E. faecium 1,141,733. (A) Growth kinetics of E. faecium 1,141,733 carrying the E. faecalis V583 PIPEF expression plasmid pPBPIP in the presence (●) and absence (♦) of φVPE25. The arrow indicates the time of φVPE25 addition to the culture. (B) φVPE25 particle numbers from infected E. faecalis V583 cells carrying pPBPIP immediately after phage addition (Input) or 2 h after phage infection (2 hours post). (C) Quantitative real-time PCR of the φVPE25 transcripts orf_106 (lysin), orf_117 (major tail protein), and orf_123 (major capsid protein) isolated from E. faecium 1,141,733 or E. faecium 1,141,733 carrying plasmid pPBPIP. E. faecalis V583 and the PIPEF mutant strain BDU50 are included as controls. Transcript abundances are plotted on a logarithmic scale. (D) Viable phage particles recovered from wild-type and PIPEF transgenic E. faecium 1,141,733 after cell disruption using lysozyme and sonication. E. faecalis E1Sol was included as a control strain that is resistant to φVPE25 infection. ND, none detected. Download
Sequence variation among the PIPEF variable regions of E. faecalis sewage isolates. (A) Schematic of the variable region of PIPEF (amino acids 342 to 494). The amino acids where variation was detected by direct PCR from raw sewage (EBOX) or from pooled enterococcal isolates grown on selective agar (P1) are indicated in red and green, respectively. For both EBOX and P1 samples, the majority of the amino acid content of the PIPEF variable region matched E. faecalis V583 (52.00% of contigs for P1 and 41.61% for EBOX). The top four or five representative contigs containing variant amino acid composition compared to the E. faecalis V583 PIPEF variable region sequence as a reference are indicated. (B) Alignment of the E. faecalis V583 (clade 4) and E1Sol (clade 5) PIPEF variable regions. Download
Enterococcal bacteriophage genome organization and features.
Spontaneous mutations in EF0858 (PIPEF) result in phage resistance.
Sewage PIP read mapping to clade-specific PIPEF variable region.
Mutations conferring φVPE25 resistance in E. faecalis from gnotobiotic mouse feces.
Bacterial strains, phages, plasmids, and primers used in this study.