Novel immunotherapeutics that target tumor PD-L1 expression are emerging treatment options for patients with advanced cancer. In this study, we show that the majority of penile squamous cell carcinomas (SqCC) express PD-L1, which is associated with high-risk clinicopathologic features and poor clinical outcome. These data provide a rational basis for immunotherapeutics in advanced penile SqCC.
Keywords: penile cancer, squamous cell carcinoma, PD-L1, immunohistochemistry, immunotherapy
Abstract
Background
Despite aggressive multimodal therapy, locally advanced and/or metastatic penile squamous cell carcinoma (SqCC) is associated with significant morbidity and mortality, indicating a need for new therapeutic options. Given the emerging clinical utility of immunotherapeutics, we sought to assess the incidence and potential clinical significance of PD-L1 expression in penile SqCC.
Patients and methods
Using an anti-PD-L1 primary antibody (clone 5H1), immunohistochemistry was carried out on whole tumor sections from 37 patients with penile SqCC treated at our institution between 2005 and 2013. PD-L1-positive tumors were defined as those with membranous staining in ≥5% of tumor cells. Association between PD-L1 expression and clinicopathologic parameters was examined using Fisher's exact test. Correlation between PD-L1 expression in primary tumors and matched metastases was assessed using the Spearman rank correlation coefficient (ρ). The difference in cancer-specific mortality between PD-L1-positive and -negative groups was examined using the log-rank test.
Results
Twenty-three (62.2%) of 37 primary tumors were positive for PD-L1 expression, and there was strong positive correlation of PD-L1 expression in primary and metastatic samples (ρ = 0.72; 0.032 < P < 0.036). Primary tumor PD-L1 expression was significantly associated with usual type histology (P = 0.040) and regional lymph node metastasis (P = 0.024), as well as decreased cancer-specific survival (P = 0.011).
Conclusions
The majority of primary penile SqCC tumors express PD-L1, which is associated with high-risk clinicopathologic features and poor clinical outcome. These data provide a rational basis for further investigation of anti-PD-1 and anti-PD-L1 immunotherapeutics in patients with advanced penile SqCC.
introduction
Squamous cell carcinoma (SqCC) is the most common primary neoplasm of the penis [1]. The vast majority of penile SqCC present as clinically localized disease, for which organ-preserving surgical excision and/or radiation are usually sufficient for primary treatment and management of subsequent local recurrences, and overall, low-stage tumors have a good prognosis with high possibility of cure [2]. In contrast, locally advanced penile SqCC, particularly those with spread to regional lymph nodes, may require a multimodal approach, including primary lymphadenectomy, adjuvant radiation, and/or chemotherapy; despite these aggressive treatment strategies, however, high-stage tumors have a poor prognosis with significant morbidity and mortality [2]. Thus, there is a clear need for additional therapeutic options for patients with locally advanced and/or metastatic penile SqCC.
Recent advances in immunotherapy offer promising new treatments for some solid tumors [3]. The PD-1/PD-L1 immune checkpoint pathway, for example, is one of the major targets of a new generation of immunotherapeutics. PD-1, a co-inhibitory receptor present on a subset of CD8-positive cytotoxic T cells, interacts with its ligand PD-L1 on tumor cell membranes, resulting in suppression of T-cell activation and proliferation, and thereby, dampening of the host anti-tumor immune response [4]; inhibiting the PD-1/PD-L1 interaction, therefore, should augment tumor cell killing by cytotoxic T cells. Indeed, immunotherapeutic approaches targeting PD-1 or PD-L1 have been shown to enhance anti-tumor activity in preclinical models [5, 6], and anti-PD-1 and anti-PD-L1 immunotherapeutics have yielded promising results in early and late phase clinical trials [7–13], with recent Food and Drug Administration (FDA) approval for use in melanoma and lung SqCC.
Membranous PD-L1 expression has been described in a diverse assortment of human malignancies, including breast cancer and melanoma [14, 15]. In addition, PD-L1 expression is frequently reported in primary SqCC of a variety of other organs, including lung, head and neck, and cervix [16–18]. Despite intensive study of PD-1/PD-L1 pathway molecules and possible anti-PD1 and anti-PD-L1 immunotherapies in other urologic malignancies, including bladder cancer, kidney cancer, and prostate cancer [19, 20], to our knowledge, PD-L1 expression has not been previously examined in penile SqCC. Thus, in this study, we sought to assess the prevalence and clinicopathologic significance of membranous PD-L1 expression by immunohistochemistry in primary and metastatic penile SqCC.
materials and methods
patient selection
This cohort of penile SqCC patients has been described previously [21]. Briefly, penile SqCC cases diagnosed between 2005 and 2013 were retrospectively identified from the surgical pathology records database at the University of Michigan Health System. Tissue from 37 patients, including 37 primary tumors and 9 matched metastases, was available for the purposes of this study. HPV DNA genotyping, p16 immunohistochemistry (IHC), and targeted next-generation sequencing was carried out previously (see supplementary Materials, available at Annals of Oncology online for details) [21].
PD-L1 IHC
For all primary and metastatic tumors, PD-L1 IHC was carried out on representative whole tissue sections using an anti-PD-L1 primary antibody (clone 5H1; 1:500 dilution), as described previously [20], and the percentage of tumor cells with membranous staining was assessed together by two study pathologists (MJM and RM) blinded to patient clinicopathologic parameters. Primary tumors were considered positive for PD-L1 expression if ≥5% of tumor cells showed membranous staining. The relative intensity (moderate or severe) of host inflammatory response was recorded, as well as the presence or absence of PD-L1 IHC staining in tumor-infiltrating mononuclear cells (using a ≥5% cut-off).
statistical analysis
Association between PD-L1 IHC staining and clinicopathologic parameters or common molecular alterations was examined using Fisher's exact test. Correlation between PD-L1 expression in primary tumors and matched metastases was assessed using the Spearman rank correlation coefficient (ρ). The difference in cancer-specific mortality between PD-L1-positive and -negative groups was examined using the log-rank test. Cancer-specific mortality was measured from the time of diagnosis to death or date of last follow-up (at which point the patient was censored). All statistical analyses were carried out using SAS, version 9.4 (Cary, NC), and statistical significance was defined as P < 0.05.
results
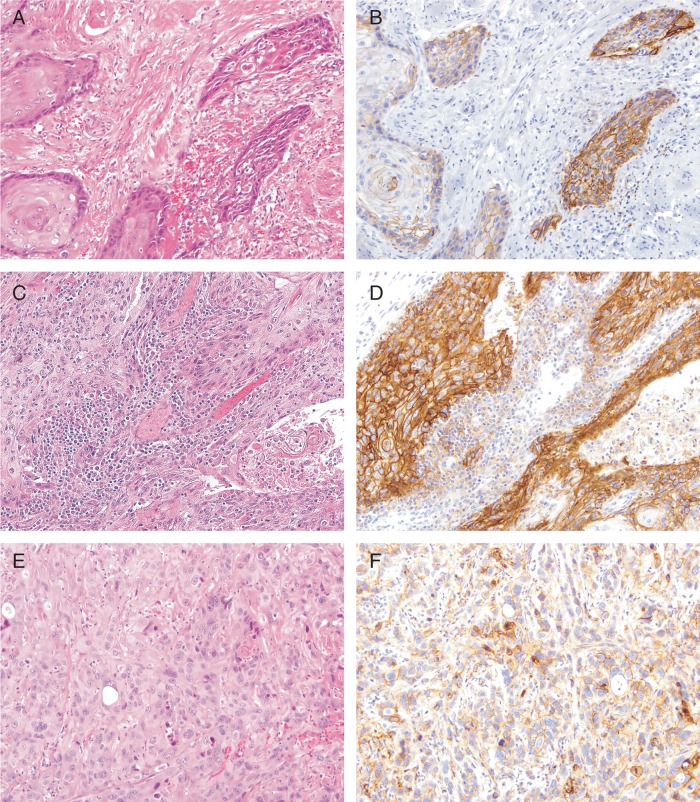
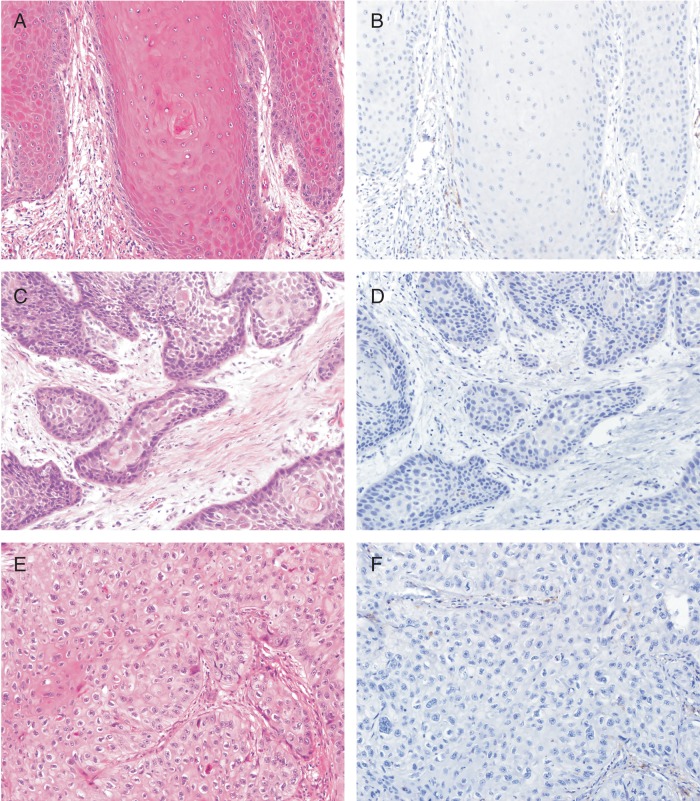
Of the 37 primary penile SqCC tumors examined, 23 (62.2%) were positive for PD-L1 expression (see Materials and methods); essentially, all positive samples showed moderate to strong membranous staining (Figure 1), and the percentage of tumor cells staining ranged from 5% to 70% (median = 20%). The other 14 primary penile SqCC tumors were negative for PD-L1 expression (Figure 2), although very focal weak to moderate membranous staining (<5% of tumor cells) was seen in four cases. Clinicopathologic characteristics of PD-L1-positive and PD-L1-negative primary penile SqCC tumors are presented in Table 1.
Figure 1.
Positive PD-L1 expression in primary penile squamous cell carcinoma tumors. (B, D, and F) PD-L1 IHC demonstrates frequent moderate to strong membranous staining in primary penile squamous cell carcinoma, including well differentiated (A and B), moderately differentiated (C and D), and poorly differentiated (E and F) tumors. Magnification, ×20.
Figure 2.
Negative PD-L1 expression in primary penile squamous cell carcinoma tumors. (B, D, and F) PD-L1 IHC is negative in a subset of primary penile squamous cell carcinoma, including well differentiated (A and B), moderately differentiated (C and D), and poorly differentiated (E and F) tumors. Magnification, ×20.
Table 1.
Association of primary tumor PD-L1 expression with clinicopathologic parameters
| Parameter | PD-L1-positive (n = 23) | PD-L1-negative (n = 14) | P value |
|---|---|---|---|
| Age | |||
| ≤65 years | 11 (47.8%) | 9 (64.3%) | 0.498 |
| >65 years | 12 (52.2%) | 5 (35.7%) | |
| Circumcision status | |||
| Yes | 4 (22.2%) | 2 (18.2%) | 1.000 |
| No | 14 (77.8%) | 9 (81.8%) | |
| Histologic subtype | |||
| Usual | 18 (78.3%) | 6 (42.9%) | 0.040 |
| Othera | 5 (21.7%) | 8 (57.1%) | |
| Histologic grade | |||
| Well | 4 (17.4%) | 6 (42.9%) | 0.197 |
| Moderate | 11 (47.8%) | 6 (42.9%) | |
| Poor | 8 (34.8%) | 2 (14.2%) | |
| HPV status | |||
| Positive | 2 (10.5%) | 3 (21.4%) | 0.629 |
| Negative | 17 (89.5%) | 11 (78.6%) | |
| p16 expression | |||
| Positive | 5 (22.7%) | 5 (38.5%) | 0.444 |
| Negative | 17 (77.3%) | 8 (61.5%) | |
| Pathologic stage | |||
| pT1 | 4 (19.0%) | 6 (46.2%) | 0.130 |
| pT2–4 | 17 (81.0%) | 7 (53.8%) | |
| Lymph node status | |||
| pN0/NX | 11 (52.4%) | 12 (92.3%) | 0.024 |
| pN1–3 | 10 (47.6%) | 1 (7.7%) | |
| Clinical stage | |||
| I | 4 (19.0%) | 7 (50.0%) | 0.073 |
| II-IV | 17 (81.0%) | 7 (50.0%) | |
| Local recurrence | |||
| Yes | 2 (10.0%) | 0 (0.0%) | 0.501 |
| No | 18 (90.0%) | 14 (100.0%) | |
| Distant progression | |||
| Yes | 5 (25.0%) | 0 (0.0%) | 0.063 |
| No | 15 (75.5%) | 14 (100.0%) | |
| Cancer-specific mortality | |||
| Yes | 7 (31.8%) | 0 (0.0%) | 0.029 |
| No | 15 (68.2%) | 14 (100.0%) | |
HPV, human papillomavirus.
aOther includes warty, papillary, verrucous, warty/basaloid, and basaloid subtypes.
Statistically significant associations are in bold.
Twenty-four primary penile SqCC tumors were of usual type histology; the remainder of tumors were of varied histologic subtypes, including warty (five), papillary (four), verrucous (two), warty/basaloid (one), and basaloid (one). Primary tumors with usual type histology were significantly more likely to express PD-L1 (P = 0.040), with an odds ratio of 4.8 relative to other histologic subtypes (95% confidence interval = 1.13–20.46). In contrast, among primary tumors with warty or verrucous type histology, none were PD-L1 positive (compared with 76.7% of primary tumors with other histologic subtypes; P < 0.001).
Ten primary penile SqCC tumors were well differentiated, 17 were moderately differentiated, and 10 were poorly differentiated. Overall, there was a non-statistically significant trend toward association between higher histologic grade (i.e. more poorly differentiated) and PD-L1 expression (P = 0.197), with 80.0% of poorly differentiated tumors being PD-L1-positive (compared with 40.0% of well-differentiated tumors). Similarly, PD-L1 expression by primary tumors showed a non-statistically significant trend toward association with higher pathologic stage (T2–4 versus T1, P = 0.130), with 70.8% of T2–4 tumors being PD-L1-positive (compared with 40.0% of T1 tumors).
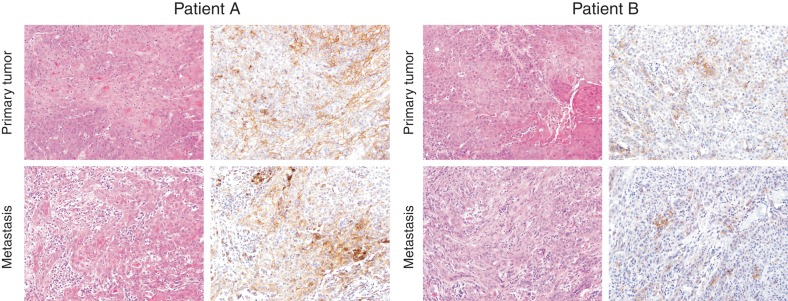
In contrast to histologic grade and pathologic stage, there was a clear association between lymph node status and PD-L1 expression in primary penile SqCC tumors. Primary tumors in patients with lymph node metastases were significantly more likely to express PD-L1 (P = 0.024), with an odds ratio of 10.91 relative to primary tumors in patients without lymph node metastases (95% confidence interval = 1.19–100). Furthermore, in the nine patients with matched primary and metastatic penile SqCC tumors, there was strong positive correlation of PD-L1 membranous staining in primary and metastatic samples (ρ = 0.72; 0.032 < P < 0.036; Figure 3). All nine matched primary tumors were PD-L1-positive; seven (77.8%) matched metastatic tumors were PD-L1-positive, and two (22.2%) matched metastatic tumors were PD-L1-negative.
Figure 3.
Concordant PD-L1 expression in matched primary and metastatic penile squamous cell carcinoma tumors. PD-L1 IHC (right panels) in matched primary and metastatic penile squamous cell carcinoma from two patients. For patient A, both the primary and metastatic tumor show high positive PD-L1 expression, while for patient B, both the primary and metastatic tumor show low positive PD-L1 expression. The percentage of cells with membranous staining shows strong positive correlation (ρ = 0.72; 0.032 < P < 0.036) between matched primary and metastatic penile squamous cell carcinoma tumors. Magnification, ×20.
Overall, there was a non-statistically significant trend toward association between higher clinical stage and primary penile SqCC tumor PD-L1 expression (clinical stage I versus II–IV, P = 0.073), with 70.8% of stage II–IV tumors being PD-L1-positive (compared with 36.4% of stage I tumors). No other significant associations were identified between primary tumor PD-L1 expression and other examined clinicopathologic parameters, including age, circumcision status, HPV status, and p16 expression.
Thirty-six of the primary penile SqCC tumors examined in this study were previously interrogated by our group for recurrent molecular alterations (somatic mutations and copy number alterations) using a targeted next-generating sequencing approach [21]. In that prior study, the five most common prioritized somatic mutations involved TP53, CDK2NA, PIK3CA, HRAS, and NFE2L2, and the five most frequent prioritized copy number alterations involved CDK2NA, MYC, CCND1, SOX2, and EGFR. In the current study, we sought to integrate our primary penile SqCC tumor PD-L1 expression results with these prior molecular data. As shown in supplementary Table S1, available at Annals of Oncology online, there were no significant associations between primary tumor PD-L1 expression and specific molecular alterations, although by logistic regression, there was a non-statistically significant trend toward association between increased total number of molecular alterations and PD-L1 expression (P = 0.16), with an odds ratio of 1.36 compared with a sample with one fewer alteration (95% confidence interval = 0.88–2.11).
Of the patients with primary penile SqCC tumors examined in this study, two experienced local recurrence, five had distant progression, and seven died of disease. Importantly, although primary tumor PD-L1 expression was not significantly associated with local recurrence (10.0% versus 0.0% of PD-L1-positive and -negative tumors, respectively, P = 0.501), patients with PD-L1-positive primary tumors were more likely to have distant progression (25.0% versus 0.0% of PD-L1-positive and -negative tumors, respectively, P = 0.063) and die of disease (31.8% versus 0.0% of PD-L1-positive and -negative tumors, respectively, P = 0.029). In addition to pathologic stage (T2–4 versus T1), histologic subtype (usual versus other), and lymph node status (N1–3 versus N0/NX), primary tumor PD-L1 expression (positive versus negative) was significantly associated with decreased cancer-specific survival [log-rank test statistic = 6.54, P = 0.011; supplementary Table S2, available at Annals of Oncology online]. (Hazard ratios from proportional hazards models were not estimable because there were no observed lethal events for patients with T1 pathologic stage, non-usual histologic subtype, or negative PD-L1 expression.)
Finally, no significant associations were detected between the intensity of host inflammatory response or presence of PD-L1 staining on tumor-infiltrating mononuclear cells and primary penile or metastatic SqCC tumor PD-L1 expression, examined clinicopathologic factors, or clinical outcome (data not shown).
discussion
To our knowledge, this is the first study to examine PD-L1 expression in penile SqCC, and we found that nearly two-thirds of primary tumors are PD-L1-positive, with strong positive correlation of membranous PD-L1 staining in primary and metastatic tumors. PD-L1 expression is more frequent in primary tumors with usual type histology and regional lymph node metastasis (both relatively poor prognostic factors), and furthermore, PD-L1-positive primary tumors are significantly associated with decreased cancer-specific survival. These results suggest that PD-L1-positive tumors may define a subset of clinically aggressive penile SqCC and provide a rational basis for subsequent investigation of anti-PD-1 and anti-PD-L1 immunotherapeutics in the treatment of patients with locally advanced and/or metastatic penile SqCC. Indeed, based on the results of monotherapy clinical trials in other malignancies [7–11], which suggest that patients with PD-L1 expression by tumor cells have a greater response to PD-1 and/or PD-L1 inhibition, our findings suggest that a subset of penile SqCC tumors may be susceptible to enhanced immune-mediated killing with anti-PD-1 and/or anti-PD-L1 immunotherapeutics.
In our study, PD-L1-positive primary penile SqCC tumors are associated with several high-risk clinicopathologic features, including usual type histology and regional lymph node metastasis, as well as decreased cancer-specific survival. The relatively small sample size and lack of observed lethal events in certain strata, however, limit further analysis with proportional hazards models; we cannot entirely exclude, therefore, that the detected association between primary penile SqCC tumor PD-L1 expression and decreased cancer-specific survival is due to the effect of covariates (i.e. lymph node status, etc.). In this regard, it is interesting to note the strong association between lack of primary tumor PD-L1 expression and warty or verrucous type histology, as these subtypes typically belie better clinical outcomes [2]. Importantly, the incidence of HPV infection was relatively low in our cohort (15.2%), as was the frequency of HPV-related histologic subtypes (i.e. warty and basaloid). Therefore, additional independent studies are needed to clarify the relationship between HPV status and PD-L1 expression in penile SqCC.
Recently published work from our group utilized targeted next-generation sequencing to identify recurrent molecular alterations (somatic mutations and copy number alterations) in a large cohort of penile SqCC [21], including the majority of tumors evaluated for PD-L1 expression in the current study. Although no significant association was detected between primary tumor PD-L1 expression and specific molecular alterations, there was a non-statistically significant trend toward increased total number of molecular alterations in PD-L1-positive tumors. These results conform to the emerging paradigm of increased PD-L1 expression and/or response to PD-1/PD-L1 pathway inhibition in tumors with high mutational load [22–25]. In addition, in our previous study, we demonstrated that penile SqCC shares a similar spectrum of molecular alterations with lung SqCC [21], which provides additional circumstantial support for the potential efficacy of PD-1/PD-L1 inhibition in penile SqCC.
Before implementation of clinical trials for anti-PD1 or anti-PD-L1 therapeutics in penile SqCC, however, several additional considerations are necessary. For anti-PD-L1 immunotherapy, for example, there are several compounds currently in clinical trials [26, 27], and each compound is linked to a specific companion diagnostic PD-L1 IHC assay. These assays utilize different primary antibodies and have unique, predefined cut-offs for determining positive PD-L1 expression [28]. In our study, we utilized the anti-PD-L1 clone 5H1, which has been validated in early phase clinical trials (e.g. the initial phase I trials with the anti-PD-1 immunotherapeutic BMS-936558). Overall, from a clinical workflow perspective, however, PD-L1 IHC was relatively straightforward to evaluate in penile SqCC, as the majority of tumors were either completely negative or showed moderate to strong membranous staining in a significant proportion (≥20%) of tumor cells (Figures 1 and 2). In borderline cases (i.e. those samples with weak to moderate membranous staining in 1%–10% of tumor cells), digital quantitation and/or internal consultation are recommended to reach a consensus. Finally, several recently published clinical trials, including two evaluating the efficacy of the anti-PD-1 immunotherapeutic nivolumab for treatment of advanced tumors [8, 9], have demonstrated clinical response that is independent of tumor PD-L1 expression. These results suggest that PD-L1 expression may not be a reliable predictive biomarker for therapeutic response, and this possibility should be considered when designing clinical trials for compounds that target the PD-1/PD-L1 immune checkpoint pathway.
The major strengths of this study include: its novelty, the use of whole tissue sections, the use of a previously published/validated anti-PD-L1 antibody, and the integration of PD-L1 expression with pre-existing detailed molecular data. In particular, utilizing whole tissue sections has significant advantages over tissue microarray (TMA) approaches (which are commonly used in biomarker investigation studies), as whole tissue sections allow for a more comprehensive assessment of tumor protein expression. This is especially important for PD-L1 IHC, which can show patchy, non-uniform distribution of membranous staining. The major weakness of this study is the relatively low rate of observed lethal events, which limits the conclusions that can be drawn from survival analysis and may indicate a need for subsequent large, multi-institutional validation.
In conclusion, the majority of penile SqCC show membranous PD-L1 expression, and there is strong positive correlation of PD-L1 expression in primary and metastatic tumors. PD-L1 expression is more common in primary tumors with usual type morphology and regional lymph node metastasis, and PD-L1-positive primary tumors are significantly associated with decreased cancer-specific survival. These data provide a rational basis for further exploration of targeted anti-PD-1 and anti-PD-L1 immunotherapeutics as new treatment options for advanced penile SqCC.
funding
This work was supported in part by the Early Detection Research Network of the National Cancer Institute (UO1 CA113913 to AMC). AMC is also supported by the A. Alfred Taubman Medical Research Institute and The Howard Hughes Medical Institute.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We would like to thank Eugene D. Kwon (Mayo Clinic, Rochester, MN, USA) for graciously providing the PD-L1 antibody, as well as Jack D. Kalbfleisch, Ph.D. (University of Michigan School of Public Health, Ann Arbor, MI, USA), for help with survival analysis.
references
- 1.Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, Giuliano AR. Incidence trends in primary malignant penile cancer. Urol Oncol 2007; 25: 361–367. [DOI] [PubMed] [Google Scholar]
- 2.Hakenberg OW, Comperat EM, Minhas S et al. EAU guidelines on penile cancer: 2014 update. Eur Urol 2015; 67: 142–150. [DOI] [PubMed] [Google Scholar]
- 3.Bordon Y. Immunotherapy: checkpoint parley. Nat Rev Cancer 2015; 15: 3. [DOI] [PubMed] [Google Scholar]
- 4.Littman DR. Releasing the brakes on cancer immunotherapy. Cell 2015; 162: 1186–1190. [DOI] [PubMed] [Google Scholar]
- 5.Iwai Y, Ishida M, Tanaka Y et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 2002; 99: 12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 2010; 107: 4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghaei H, Paz-Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–330. [DOI] [PubMed] [Google Scholar]
- 11.Robert C, Schachter J, Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 12.Brahmer JR, Tykodi SS, Chow LQ et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghebeh H, Mohammed S, Al-Omair A et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 2006; 8: 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massi D, Brusa D, Merelli B et al. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol 2014; 25: 2433–2442. [DOI] [PubMed] [Google Scholar]
- 16.Boland JM, Kwon ED, Harrington SM et al. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer 2013; 14: 157–163. [DOI] [PubMed] [Google Scholar]
- 17.Badoual C, Hans S, Merillon N et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013; 73: 128–138. [DOI] [PubMed] [Google Scholar]
- 18.Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol 2015; 28: 1594–1602. [DOI] [PubMed] [Google Scholar]
- 19.Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol 2015; 68: 267–279. [DOI] [PubMed] [Google Scholar]
- 20.McDaniel AS, Alva A, Zhan T et al. Expression of PDL1 (B7-H1) before and after neoadjuvant chemotherapy in urothelial carcinoma. Eur Urol Focus 2016; 1: 265–268. [DOI] [PubMed] [Google Scholar]
- 21.McDaniel AS, Hovelson DH, Cani AK et al. Genomic profiling of penile squamous cell carcinoma reveals new opportunities for targeted therapy. Cancer Res 2015; 75: 5219–5227. [DOI] [PubMed] [Google Scholar]
- 22.Le DT, Uram JN, Wang H et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong SQ, Waldeck K, Vergara IA et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res 2015; 75: 5228–5234. [DOI] [PubMed] [Google Scholar]
- 24.Rizvi NA, Hellmann MD, Snyder A et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howitt BE, Shukla SA, Sholl LM et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol 2015; 1: 1319–1323. [DOI] [PubMed] [Google Scholar]
- 26.Wolchok JD, Kluger H, Callahan MK et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbst RS, Soria JC, Kowanetz M et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apolo AB. PDL1: the illusion of an ideal biomarker. Eur Urol Focus 2016; 1: 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.