Fatigue is high in localized colorectal cancer (CRC) patients soon after diagnosis. It peaks immediately after adjuvant chemotherapy, but remains more common at 12 and 24 months in CTh+ versus CTh− patients. There was no significant difference between CTh− and healthy controls at 6 and 12 months. Cognitive and affective symptoms, QoL, comorbidities, CTh, and baseline fatigue are predictors of longer-term fatigue.
Keywords: cancer-related fatigue, colorectal cancer, chemotherapy, quality of life
Abstract
Background
Fatigue is associated with cancer and chemotherapy and may be sustained. Here, we describe a prospective longitudinal study evaluating fatigue and putative mechanisms in people with colorectal cancer (CRC).
Patients and methods
People with localized CRC completed the Functional Assessment of Cancer Treatment-Fatigue (FACT-F) questionnaire at baseline (before chemotherapy, if given), 6, 12, and 24 months. Healthy controls (HCs) were assessed at the first three time points. Fatigue was defined by standardized FACT-F scores ≤68/100. Quality-of-life (QoL, assessed by the FACT-G questionnaire), affective, and cognitive symptoms were evaluated. Associations were sought between fatigue, baseline factors, and blood tests (including hemoglobin, cytokines, and sex hormones). Regression analyses, Fisher's exact tests, and Wilcoxon rank-sum tests assessed levels of fatigue at each time point and change in fatigue from baseline. A repeated-measures analysis investigated prognostic factors of fatigue across all time points.
Results
A total of 289 subjects with localized CRC (173 received chemotherapy) and 72 HCs were assessed. More CRC patients had fatigue than HCs at baseline (52% versus 26%, P < 0.001). Fatigue was increased in the chemotherapy (CTh) group at 6 months [CTh+ 70% versus CTh− 31% (P < 0.001), HCs 22%] and remained more common at 12 [CTh+ 44% versus CTh− 31% (P = 0.079)] and 24 months [CTh+ 39% versus CTh− 24% (P = 0.047)]. There was no significant difference between those not receiving chemotherapy and HCs at follow-up assessments. Fatigue was associated with poor QoL, affective and cognitive symptoms, but not consistently with cytokine levels. Predictors for sustained fatigue were baseline fatigue, treatment group, cognitive and affective symptoms, poorer QoL, and comorbidities.
Conclusions
CRC patients have more fatigue than HCs at baseline. Fatigue peaks immediately after adjuvant chemotherapy, but remains common for 2 years in those who receive chemotherapy. Cognitive and affective symptoms, QoL, comorbidities, chemotherapy, and baseline fatigue predict for longer term fatigue.
introduction
Cancer-related fatigue (CRF) has been defined as: ‘a distressing, persistent, subjective sense of tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning’ [1]. It is common and impacts on physical and social function, psychological distress, and quality-of-life (QoL) [2]; it results in tiredness and weakness despite adequate rest or sleep [1].
While fatigue is most prevalent in those undergoing chemotherapy and/or radiotherapy, or those with advanced cancer, some survivors report fatigue long after completion of treatment, despite no evidence of disease recurrence [3, 4]. Fatigue is more common in breast cancer (BC) survivors 1–3 years after chemotherapy than in HCs [5], and in women who received chemotherapy compared with those who did not. A cross-sectional population-based study of fatigue in colorectal cancer (CRC) survivors up to 10 years post-diagnosis found 39% reported fatigue compared with 22% of the normative population [6]. There have been few longitudinal studies of fatigue in patients receiving chemotherapy for diseases other than BC, and particularly for cancers that affect both men and women.
Mechanisms leading to CRF are likely multifactorial. Putative mechanisms include cytokine dysregulation, dysregulation of the hypothalamic–pituitary–adrenal axis, alterations in the autonomic nervous system, anemia, neurotransmitter dysregulation, as well as patient-related factors such as psychosocial states, and demographic and medical factors [7].
The primary goals of the present study were to characterize the severity and duration of fatigue and cognitive function in men and women with CRC and to evaluate underlying mechanisms. The cognitive results have been reported elsewhere [8, 9]. Here, we report the longitudinal evaluation of fatigue.
patients and methods
Details of patient characteristics and methods are described elsewhere [8, 9]. The main study included subjects with localized CRC recruited from eight hospitals in Toronto, Canada, and six in Sydney, Australia. Participants were assessed prospectively at baseline (pre-chemotherapy, if given), 6 (post-chemotherapy, if given), 12, and 24 months. Subjects were treated with surgery and adjuvant or neoadjuvant chemotherapy (CTh+) [generally 5-fluorouracil (5-FU) or capecitabine ± oxaliplatin] or underwent surgery without adjuvant chemotherapy (CTh−). A substudy included subjects with newly diagnosed limited metastatic or locally recurrent CRC who received chemotherapy (generally oxaliplatin or irinotecan regimens). A group of age-matched healthy controls (HCs), with no history of invasive cancer, were recruited from the Sydney hospitals. Most were family or friends of the CRC patients.
Inclusion criteria included age ≤75 years, no prior malignancy or chemotherapy (except the metastatic/recurrent group who could have received adjuvant chemotherapy ≥12 months previously), and no major psychiatric or neurological illness [8, 9]. Subjects with localized CRC who developed recurrent cancer were not assessed subsequently.
Research Ethics Board approval was obtained for each participating hospital. All subjects gave their written informed consent.
assessments
Participants in all groups completed paper-based patient-reported outcomes (PROs) and neuropsychological tests in person at each assessment. Questionnaires included the Functional Assessment of Cancer Therapy-Fatigue (FACT-F) [10] evaluating fatigue, the FACT-G (G = general) evaluating QoL, and the 12-item General Health Questionnaire (GHQ) evaluating symptoms of anxiety and depression [11]. Cognitive function was assessed with neuropsychiatric tests and FACT-Cognition (FACT-Cog) v2 [12] evaluating cognitive symptoms [8]. Comorbidities were based on subject self-report. Participants with localized CRC and HCs donated blood at each assessment to evaluate 10 cytokines, complete blood count, creatinine, liver function tests, carcino-embryonic antigen, sex hormones, and blood clotting markers [8, 9]. Blood draws were not standardized by time of day.
statistical analysis
A priori primary end points were fatigue and cognitive function at 12 months. Fatigue was defined as a standardized FACT-F fatigue score of ≤68/100 and severe fatigue as ≤45/100 [13]. We designated a 10% change in the normalized (0–100) scale from baseline as a meaningful change. Secondary end points included QoL, symptoms of anxiety/depression, potential causative factors, and associations between primary and secondary end points. A patient with a score of <50% on the GHQ was regarded as having symptoms of anxiety/depression [11]. Missing data in the PRO were handled according to the FACT guidelines.
Comparison between groups on the raw scores, and the change from baseline, was carried out using the Kruskal–Wallis test for continuous variables, Cochran–Armitage test for trend for ordinal variables, and exact χ2 tests for categorical variables. Analyses were carried out initially at each time point, given that changes in PRO were expected to occur at different times depending on treatment received. Subsequently, repeated-measures analysis was carried out to investigate predictive factors for standardized fatigue scores across all time points, adjusting for treatment group, time point, and interaction effect. The Spearman rank-sum correlation coefficients were used to determine associations between outcomes. All P values are two-sided and unadjusted. Analyses were carried out in SAS v9.0 (SAS Institute, Cary, NC).
sample size
The sample size was powered for the (joint primary) cognitive end point, which required a larger sample size [8]. A sample of 170 subjects with localized CRC who received chemotherapy and 120 who did not was sufficient to detect or rule out differences of ≥10 units in the standardized scores (0–100) for the fatigue subscale between the CTh+ and CTh− groups, with >90% power and α 0.05.
results
We recruited 289 subjects with localized CRC (173 CTh+, 116 CTh−) and 72 HCs [8, 9]. Participant demographics are summarized in supplementary Table S1, available at Annals of Oncology online. The median age of those with localized CRC was 59 (range 23–75) years. There were more men (63%) than women with CRC, although 57% of HCs were women. Seventy-three subjects with recurrent or metastatic CRC were recruited to the substudy.
fatigue
Table 1 and Figure 1 outline the FACT-F scores and percentage with fatigue. At the primary end point of 12 months, fatigue was greatest in the CTh+ group: 44% compared with 31% of CTh− (P = 0.079), and 30% of HCs. At baseline, 52% of subjects with localized CRC reported fatigue, compared with 26% of HCs (P < 0.001). There was no significant difference in baseline fatigue rates by stage of disease, or in CTh+ in those scheduled for adjuvant (i.e. post-surgery) versus neoadjuvant treatment (i.e. pre-surgery) (n = 46). Fatigue peaked at completion of chemotherapy with 70% reporting fatigue at 6 months compared with 31% of CTh− (P < 0.001), and 22% of HCs. At 24 months, fatigue remained higher in CTh+ (39%) compared with that in CTh− (24%, P = 0.047). Rates of fatigue were greater in CTh+ at 6 months in subjects who received oxaliplatin (75.4%) or chemoradiotherapy (77.4%), compared with those who received 5-FU/capecitabine (51.4%) (P = 0.023), but this was not significantly different at 12 or 24 months.
Table 1.
Patient-reported outcomes (PROs) by group at each assessment time: (a) mean (standard deviation) and (b) number and percent reporting fatigue or affective symptoms
| PRO | Visit | Localized CRC |
Metastatic CRC | Healthy controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ch+ | Ch− | Total | ||||||||||||
| (a) | Mean (SD) | Mean (SD) | P valuea | Mean (SD) | Mean (SD) | Mean (SD) | P valueb | P valuec | ||||||
| FACT-F (scaled) | Baseline | 69.5 | 13.7 | 68.3 | 14.5 | 0.45 | 69.1 | 14.0 | 64.7 | 15.0 | 76.8 | 12.0 | 0.034 | <0.001 |
| 6 month | 64.8 | 13.4 | 74.6 | 14.5 | <0.001 | 68.8 | 14.6 | 62.9 | 14.7 | 75.3 | 11.1 | 0.017 | <0.001 | |
| 12 month | 71.4 | 13.7 | 77.6 | 14.8 | 0.007 | 74.1 | 14.4 | 67.7 | 15.0 | 75.4 | 11.6 | 0.007 | 0.33 | |
| 24 month | 72.9 | 15.1 | 78.8 | 12.9 | 0.007 | 75.4 | 14.5 | |||||||
| FACT-G | Baseline | 86.0 | 14.4 | 87.2 | 13.9 | 0.51 | 86.5 | 14.2 | 76.5 | 17.5 | 92.8 | 9.8 | <0.001 | 0.001 |
| 6 month | 85.6 | 15.4 | 92.2 | 12.5 | <0.001 | 88.3 | 14.6 | 79.0 | 15.1 | 90.2 | 12.4 | <0.001 | 0.56 | |
| 12 month | 90.7 | 13.7 | 92.9 | 11.7 | 0.32 | 91.7 | 12.9 | 81.9 | 14.4 | 89.8 | 12.0 | <0.001 | 0.13 | |
| 24 month | 90.6 | 15.3 | 95.1 | 10.9 | 0.10 | 92.5 | 13.8 | |||||||
| GHQ | Baseline | 11.3 | 4.8 | 11.2 | 5.5 | 0.59 | 11.3 | 5.0 | 15.0 | 6.3 | 9.1 | 2.7 | <0.001 | 0.002 |
| 6 month | 10.9 | 5.4 | 9.4 | 4.2 | 0.036 | 10.3 | 5.0 | 11.6 | 4.9 | 9.4 | 3.4 | 0.022 | 0.55 | |
| 12 month | 9.6 | 4.6 | 9.7 | 4.5 | 0.68 | 9.6 | 4.6 | 10.5 | 4.7 | 9.6 | 4.2 | 0.27 | 0.84 | |
| 24 month | 9.5 | 4.4 | 8.8 | 3.6 | 0.57 | 9.2 | 4.1 | |||||||
| (b) | n | % | n | % | P valuea | n | % | n | % | n | % | P valueb | P valuec | |
| n (%) fatigued | Baseline | 87/172 | 50.6 | 61/113 | 54.0 | 0.63 | 148/285 | 51.9 | 47/66 | 68.1 | 19/72 | 26.4 | 0.016 | <0.001 |
| 6 months | 92/132 | 69.7 | 28/90 | 31.1 | <0.001 | 120/222 | 54.1 | 33/50 | 66.0 | 16/72 | 22.2 | 0.16 | <0.001 | |
| 12 months | 50/114 | 43.9 | 27/87 | 31.0 | 0.079 | 77/201 | 38.3 | 24/39 | 61.5 | 21/70 | 30.0 | 0.008 | 0.25 | |
| 24 months | 39/99 | 39.4 | 17/72 | 24.3 | 0.047 | 56/169 | 33.1 | |||||||
| n (%) severe fatigue | Baseline | 5 | 2.9 | 4 | 3.5 | 0.74 | 9 | 3.2 | 4 | 5.8 | 0 | 0.0 | 0.29 | 0.21 |
| 6 months | 12 | 9.1 | 2 | 2.2 | 0.049 | 14 | 6.3 | 6 | 12.0 | 1 | 1.4 | 0.22 | 0.13 | |
| 12 months | 2 | 1.8 | 0 | 0.0 | 0.51 | 2 | 1.0 | 1 | 2.6 | 0 | 0.0 | 0.41 | 1.00 | |
| 24 months | 1 | 1.0 | 0 | 0.0 | 1.00 | 1 | 0.6 | – | – | – | – | |||
| n (%) symptoms of anxiety/depression | Baseline | 13/169 | 7.7 | 13/111 | 11.7 | 0.30 | 26/280 | 9.3 | 12/68 | 17.7 | 0/71 | 0.0 | 0.054 | 0.004 |
| 6 months | 14/130 | 10.8 | 4/90 | 4.4 | 0.13 | 18/220 | 8.2 | 3/47 | 6.4 | 2/72 | 2.8 | 1.00 | 0.18 | |
| 12 months | 5/110 | 4.6 | 5/86 | 5.8 | 0.75 | 10/196 | 5.1 | 2/37 | 5.4 | 2/70 | 2.9 | 1.00 | 0.74 | |
| 24 months | 5/97 | 5.2 | 1/68 | 1.5 | 0.40 | 6/165 | 3.6 | |||||||
F-test was used to determine P values.
CRC, colorectal cancer; CTh, chemotherapy; n, number; %, percent; FACT, Functional Assessment of Cancer Treatment; F, fatigue; G, general; GHQ, General Health Questionnaire 12.
aLocalized CRC CTh+ versus CTh−.
bLocalized CRC versus metastatic CRC.
cLocalized CRC versus healthy controls.
Figure 1.
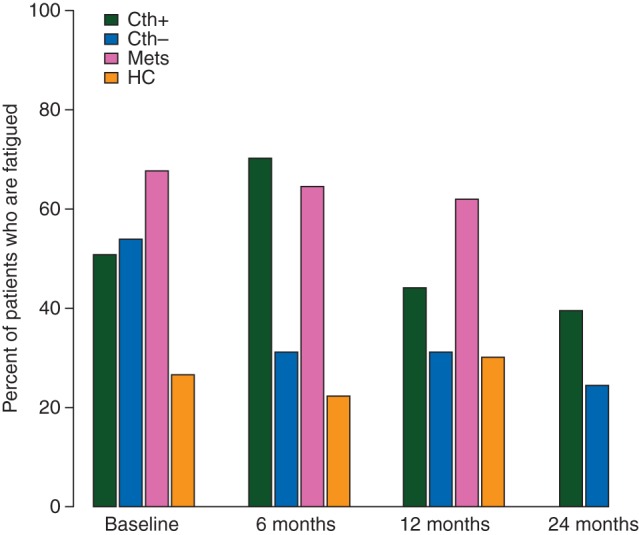
Rates of fatigue by group and assessment. CTh+, localized colorectal cancer (CRC) who received adjuvant/neoadjuvant chemotherapy; CTh−, localized CRC who did not receive chemotherapy; Mets, metastatic or recurrent CRC; HCs, healthy controls.
Subjects who were fatigued (FACT-F ≤ 68/100) at baseline were more likely to be fatigued at subsequent visits (69% versus 32% at 6 months, 59% versus 21% at 12 months, and 50% versus 17% at 24 months, all P < 0.001). Results were similar within each separate cohort (supplementary Table S2, available at Annals of Oncology online). This was particularly evident in women. Fatigue at 24 months in those with localized CRC was reported by 55% of women who were fatigued at baseline and 12% without baseline fatigue (P < 0.001), compared with 45% of men with baseline fatigue and 28% without baseline fatigue (P = 0.17). However overall, men with localized CRC had more fatigue than women (70.5% versus 66.5%; P = 0.005). There was no significant difference in the prevalence of fatigue in those <60 compared with ≥60 years.
Assessment of change in fatigue from baseline to 6 months showed that 44% of CTh+ had worse fatigue compared with 10% of CTh− (P < 0.001), with 19.5% versus 9% (P = 0.071) deterioration from baseline to 12 months. There were no significant differences for change in fatigue between baseline and 24 months (Table 2).
Table 2.
Number (percent) of patients with colorectal cancer with longitudinal changes in fatigue, quality of life, and symptoms of anxiety and depression
| Baseline to 6 months |
Baseline to 12 months |
Baseline to 24 months |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTh+ (n = 130) | CTh− (n = 89) | Mets (n = 46) | HC (n = 71) | CTh+ (n = 111) | CTh− (n = 86) | Mets (n = 36) | HC (n = 66) | CTh+ (n = 96) | CTh− (n = 67) | |
| Fatigue | ||||||||||
| Improvement | 23 (17.4) | 39 (43.8) | 7 (14.3) | 11 (15.3) | 34 (30.1) | 46 (53.5) | 10 (26.3) | 12 (17.1) | 37 (37.8) | 33 (47.1) |
| No change | 51 (38.6) | 41 (46.1) | 18 (36.7) | 45 (62.5) | 57 (50.4) | 32 (37.2) | 17 (44.7) | 43 (61.4) | 42 (42.9) | 26 (37.1) |
| Deterioration | 58 (43.9) | 9 (10.1) | 24 (49.0) | 16 (22.2) | 22 (19.5) | 8 (9.3) | 11 (29.0) | 15 (21.4) | 19 (19.4) | 11 (15.7) |
| P values | <0.001a | 0.071a | 0.68a | |||||||
| <0.001b | 0.044b | |||||||||
| QoL (FACT-G) | ||||||||||
| Improvement | 25 (18.9) | 23 (25.6) | 13 (26.5) | 5 (7.5) | 38 (34.2) | 28 (32.6) | 11 (30.6) | 6 (9.1) | 36 (37.1) | 24 (34.3) |
| No change | 80 (60.6) | 62 (68.9) | 25 (51.0) | 48 (71.6) | 63 (56.8) | 49 (57.0) | 17 (47.2) | 48 (72.7) | 48 (49.5) | 41 (58.6) |
| Deterioration | 27 (20.5) | 5 (5.6) | 11 (22.5) | 14 (20.9) | 10 (9.0) | 9 (10.5) | 8 (22.2) | 12 (18.2) | 13 (13.4) | 5 (7.1) |
| P values | 0.002a | 0.81a | 0.22a | |||||||
| 0.010b | 0.098b | |||||||||
| Anxiety/depression (GHQ) | ||||||||||
| Improvement | 56 (43.1) | 44 (50.0) | 29 (63.0) | 24 (33.8) | 60 (54.6) | 40 (47.6) | 25 (69.4) | 22 (31.9) | 53 (55.2) | 28 (41.8) |
| No change | 27 (20.8) | 25 (28.4) | 11 (23.9) | 21 (29.6) | 19 (17.3) | 18 (21.4) | 7 (19.4) | 20 (29.0) | 15 (15.6) | 19 (28.4) |
| Deterioration | 47 (36.2) | 19 (21.6) | 6 (13.0) | 26 (36.6) | 31 (28.2) | 26 (31.0) | 4 (11.1) | 27 (39.1) | 28 (29.2) | 20 (29.9) |
| P values | 0.025a | 0.75a | 1.00a | |||||||
| 0.004b | 0.028b | |||||||||
n, total number of subjects with localized colorectal cancer included in the analysis.
Improvement defined as ≥10% increase in the fatigue score compared with baseline; worsening defined as ≥10% decrease in the fatigue score.
CTh+, localized colorectal cancer (CRC) who received adjuvant/neoadjuvant chemotherapy; CTh−, localized CRC who did not receive chemotherapy; Mets, metastatic or recurrent CRC; HC, healthy controls; QoL, quality of life; FACT-G, Functional Assessment of Cancer Therapy-General; GHQ, General Health Questionnaire 12 item.
aComparing percent deterioration rates of fatigue between CTh+ with CTh−.
bComparing percent deterioration rates of fatigue between all four groups.
quality of life, anxiety, and depression
The distribution of FACT-G scores indicated that QoL was poorer in subjects with localized CRC than in HCs at baseline (P < 0.001) and remained lower in CTh+ at 6 months, but CTh− patients were not significantly different from HCs. There was no significant difference in FACT-G scores at 12 months between those with localized CRC and HCs.
Subjects with CRC had more symptoms of anxiety/depression than HCs at baseline. CTh+ scored worse than CTh− on the GHQ at 6 months (P = 0.036), with the CTh− scores similar to those of HCs. At 12 months, there was no difference between groups (P = 0.75), with only 3–6% reporting anxiety/depression.
blood results
Although the mean hemoglobin level remained within the normal range, subjects with localized CRC had significantly lower hemoglobin at baseline and 6 months compared with that in HCs; hemoglobin was lowest in CTh+ patients at 6 months, but there was no difference by 12 months. There were minor differences between subjects with localized CRC and HCs on some liver function tests, albumin, neutrophils, lymphocytes, and clotting factors. Testosterone levels were marginally lower in HC men than those with localized CRC, but estradiol was significantly higher in HC women.
Subjects with localized CRC had significantly higher levels of most cytokines than HCs at each assessment [8, 9]. At baseline and 6 months, cytokine levels were higher in CTh+ than in CTh− patients, and in those with a higher stage of disease. There was no significant difference between patients assessed before or after surgery.
associations
In patients with localized CRC, there was a moderately strong association at each assessment of fatigue with lower QoL, cognitive symptoms, and symptoms of anxiety/depression (supplementary Table S3, available at Annals of Oncology online); each of these individual variables was correlated with each of the others (ρ 0.37–0.69).
At baseline, fatigue was associated with lower hemoglobin (ρ = 0.21) and increased platelets (ρ = −0.23). At baseline, there was no association between any of the cytokines and fatigue, QoL, or anxiety/depression. At 12 months, there was no association between fatigue and any of the cytokine levels. There was a weak association between fatigue and elevated interleukins-6, -8, -10 at 6 months (ρ −0.16 to −0.20), and between interleukins-1β, -2, -4, -6, -8, -10 and GM-CSF at 24 months (ρ −0.16 to −0.30) (supplementary Table S3, available at Annals of Oncology online).
Body mass index (BMI) in patients with localized CRC was not associated with fatigue (ρ = 0.04), or predictive of fatigue (odds ratio = 1.02, 95% confidence interval 0.95–1.09, P = 0.60).
prognostic factors for fatigue
Adjusted for group, time point, and interaction effect, younger patients, men, those not married/common-law, more comorbidities, lower FACT-G (worse QoL), more anxiety/depression), lower FACT-COG (greater cognitive symptoms), lower albumin, higher neutrophils, higher platelet count, and higher interleukin-1β were associated with increased levels of fatigue (supplementary Table S4, available at Annals of Oncology online). A multivariable analysis was carried out, adjusting for group, visit, and interaction effect, by selecting factors hypothesized a priori to be prognostic for fatigue. Treatment group, GHQ, FACT-G, FACT-COG, and number of comorbidities remained statistically significant (supplementary Table S5, available at Annals of Oncology online). Patients with metastatic disease, followed by patients with localized disease who received chemotherapy, had greater fatigue.
substudy in patients with metastatic/recurrent CRC
Subjects with metastatic/recurrent disease had a high prevalence of fatigue (62–68%) at every time point. At 12 months, they scored on average 7.7 points lower on the standardized FACT-F subscale compared with HCs. They had significantly worse QoL than those with localized CRC at every assessment (all P < 0.001). Except at baseline, there were no significant differences in rates of anxiety/depression between those with localized CRC and those with metastatic disease (Table 1).
discussion
In the present study, about half of the participants with localized CRC and two-thirds of those with metastatic/recurrent CRC reported fatigue before any chemotherapy, compared with one-quarter of the HCs. Immediately following adjuvant chemotherapy, 70% reported fatigue compared with 31% of CTh− patients. Consistent with other studies (predominantly in BC) [5, 14], fatigue remained present beyond the treatment period in a subset of people, particularly in those who received chemotherapy: 44% of CTh+ patients reported fatigue at 12 months, despite completing chemotherapy 6 months earlier, whereas there were no differences between CTh− patients and HCs (∼30%). This indicates that fatigue levels for most CTh− survivors return to that of the general population.
Comparing fatigue across studies is inherently difficult due to differences in the criteria used to define fatigue, measurement instruments used, time points selected, and heterogeneity in tumor site, disease stage, and treatment modalities. Most prior studies have been cross-sectional, and limited to women with BC, who receive more toxic adjuvant chemotherapy regimens than 5-FU/folinic acid (5FU/FA), which was the standard adjuvant treatment for CRC until 2004. In general, these studies have shown greater fatigue in survivors who received adjuvant chemotherapy, and that fatigue can remain prevalent for many years when compared with that in HCs [3, 5, 6, 14, 15].
Two large adjuvant studies for people with localized CRC (MOSAIC and NSABP C-07), and subsequent population-based data found that the addition of oxaliplatin to 5FU/FA resulted in improved survival [16–18], and this is now the standard of care in good-performance patients <70 years of age. In the NSABP C-07 study, severe fatigue during chemotherapy was not significantly different between groups when measured by the NCI toxicity criteria [18]. FDA drug approval summaries for oxaliplatin in patients with metastatic CRC after progression on 5FU/FA reported fatigue of all grades of 52% in the 5FU/FA arm, 61% in oxaliplatin, and 68% in the 5FU/FA/oxaliplatin arm. Rates of grade 3 or 4 fatigue were 6%, 9%, and 7%, respectively. Our results suggest higher rates of severe fatigue after adjuvant chemotherapy than described in the NSABP study [18] or in the FDA drug approval summaries for oxaliplatin [19].
Long-term fatigue at 5 and 10 years post-treatment has been reported in a population-based heterogeneous cohort of CRC survivors, compared with HCs [20]. Fatigue scores improved from year 1 to 3, but then declined out to 10 years. However, the study did not assess fatigue before chemotherapy or compare results between CTh+ and CTh− patients, and was limited by the use of historical controls. Furthermore, it predates the use of oxaliplatin.
Goedendorp et al. reported that 14%–28% of patients with a variety of cancers had severe fatigue before receiving potentially curative chemotherapy. They found that lower rates of physical activity (P = 0.013), more affective symptoms (P = 0.014), disturbed sleep patterns (P = 0.045), and fatigue in the year preceding diagnosis (P = 0.005) contributed to severe fatigue [21]. In BC survivors, predictors of fatigue 6 months post-treatment were fatigue before and on completion of adjuvant treatment; and at 42 months, fatigue on completion of treatment, higher BMI and obesity, and poorer baseline coping tendencies [14]. We found, particularly in women, that those who had fatigue at baseline were more likely to have fatigue at subsequent assessments. This has clinical implications and suggests that we should assess for early fatigue and treat where appropriate. We found that treatment group, presence of cognitive symptoms, anxiety and depression, poorer QoL, and increased number of comorbidities were predictors of fatigue. Lower hemoglobin at baseline was associated with greater fatigue, although hemoglobin levels were often within the normal range.
A number of studies of ‘sickness behavior’ in both animal models and humans have reported a role for cytokines in CRF [7, 22]. The premise is that cancer and/or cancer treatments up-regulate proinflammatory cytokines, which in turn affects the central nervous system, leading to fatigue [7]. While some studies support an association between fatigue in cancer survivors and proinflammatory markers, others have failed to find an association. Some investigators have reported an association of fatigue with C-reactive protein (CRP) but not with cytokines [23]. One study in 644 BC survivors found an association between fatigue and CRP in univariable analysis, but this became non-significant after controlling for comorbidities, BMI, and use of antidepressant/anti-anxiolytic medication [24].
Consistent with studies of BC survivors [25], we found that CRC survivors have sustained elevation of cytokines in comparison to HCs, despite no evidence of cancer recurrence. We did not, however, find a consistent association between fatigue and proinflammatory cytokines, except for a weak association with interleukins-6, -8, -10 at 6 months, and with interleukins-1β, -2, -4, -6, -8, -10 and GM-CSF at 24 months. Those with sustained elevation of cytokines might have more fatigue at 24 months or beyond, but our sample size at 24 months was smaller, and longer follow-up is required to determine the duration of fatigue.
Reasons for disparate conclusions between studies relating cytokines with fatigue may be due to differences in how they are measured. We evaluated peripheral cytokine levels using a multiplex platform, whereas Bower's group measured cytokine receptors using ELISA [25]. Also fatigue and inflammation may vary with different treatment regimens [7] and tumor sites, and some cytokines are known to vary with circadian rhythm.
Major strengths of our study are the longitudinal design, with pre-chemotherapy assessments, inclusion of participants who do and do not receive chemotherapy, and analysis of purported mechanisms of fatigue. Inclusion of a group of HCs was important, as fatigue is common in the general population. There are several limitations, including missing data and attrition, and the greater number of women in the HC group, with men self-reporting worse fatigue than women. Blood samples for cytokines were not collected at standardized times of the day. We have investigated several variables and associations and elected not to adjust P values, but to regard secondary end points as exploratory.
Fatigue is frequently underreported in clinical practice and the main treatment found to be effective in randomized, controlled trials is exercise [26], although management of associated conditions such as anxiety and depression can be helpful. Our results concur with the NCCN guidelines [1] that fatigue is common and should be evaluated at baseline, during, and after completion of treatment. Particular attention should be directed to treating women who report fatigue at baseline, as they are most likely to continue to suffer from fatigue in the longer term. In view of the strong association between fatigue and symptoms of anxiety and depression seen in our study, all patients presenting with fatigue should be screened for affective disorders.
funding
This work was supported by: National Cancer Institute of Canada (grant number #15261, 2004); American Society of Clinical Oncology Young Investigator Award to JLV (2004); National Health Medical Research Council (grant number 457386, 2007); and the Cancer Institute New South Wales (grant number 05/CRF/1-06, 2006; grant number 09/RIG1-13, 2010) to JLV. The study was registered with clinicaltrials.gov (Trial registration: NCT00188331).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank David Laurence for his work as data manager for the study, and collaborators, study coordinators, and participants from the following hospitals: Toronto: Princess Margaret, Toronto General, Toronto Western, Mt Sinai, Sunnybrook, Credit Valley, Humber River, St Michael's, Toronto East General. Sydney: Concord Repatriation and General, Royal Prince Alfred, Bankstown, Royal North Shore, Prince of Wales, Nepean
references
- 1.NCCN. NCCN Clinical Practice Guidelines in Oncology: Cancer-Related Fatigue. Fort Washington, PA: NCCN; 2011. [Google Scholar]
- 2.Hofman M, Ryan JL, Figueroa-Moseley CD et al. Cancer-related fatigue: the scale of the problem. Oncologist 2007; 12(Suppl 1): 4–10. [DOI] [PubMed] [Google Scholar]
- 3.Servaes P, Gielissen MF, Verhagen S, Bleijenberg G. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psychooncology 2007; 16: 787–795. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein D, Bennett BK, Webber K et al. Cancer-related fatigue in women with breast cancer: outcomes of a 5-year prospective cohort study. J Clin Oncol 2012; 30: 1805–1812. [DOI] [PubMed] [Google Scholar]
- 5.Goedendorp MM, Andrykowski MA, Donovan KA et al. Prolonged impact of chemotherapy on fatigue in breast cancer survivors: a longitudinal comparison with radiotherapy-treated breast cancer survivors and noncancer controls. Cancer 2012; 118: 3833–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thong MS, Mols F, Wang XS et al. Quantifying fatigue in (long-term) colorectal cancer survivors: a study from the population-based patient reported outcomes following initial treatment and long term evaluation of survivorship registry. Eur J Cancer 2013; 49: 1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun 2013; 30 (Suppl): S48–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vardy J, Dhillon HM, Pond GR et al. Cognitive function and fatigue after diagnosis of colorectal cancer. Ann Oncol 2014; 25: 2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardy JL, Dhillon HM, Pond GR et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J Clin Oncol 2015; 33: 4085–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yellen SB, Cella DF, Webster K et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997; 13: 63–74. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg DP. A User's Guide to the General Health Questionnaire. Windsor: NFER-Nelson; 1991. [Google Scholar]
- 12.Wagner L, Sweet J, Butt Z et al. Measuring patient self-reported cognitive function: development of the Functional Assessment of Cancer Therapy-Cognitive Function instrument. J Support Oncol 2009; 7: W32–W39. [Google Scholar]
- 13.Cella D, Lai JS, Chang CH et al. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002; 94: 528–538. [DOI] [PubMed] [Google Scholar]
- 14.Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer 2010; 116: 5740–5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bower JE, Ganz PA, Desmond KA et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer 2006; 106: 751–758. [DOI] [PubMed] [Google Scholar]
- 16.Andre T, Boni C, Mounedji-Boudiaf L et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–2351. [DOI] [PubMed] [Google Scholar]
- 17.Sanoff HK, Carpenter WR, Martin CF et al. Comparative effectiveness of oxaliplatin vs non-oxaliplatin-containing adjuvant chemotherapy for stage III colon cancer. J Natl Cancer Inst 2012; 104: 211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuebler JP, Wieand HS, O'Connell MJ et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007; 25: 2198–2204. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim A, Hirschfeld S, Cohen MH et al. FDA drug approval summaries: oxaliplatin. Oncologist 2004; 9: 8–12. [DOI] [PubMed] [Google Scholar]
- 20.Jansen L, Herrmann A, Stegmaier C et al. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: a population-based study. J Clin Oncol 2011; 29: 3263–3269. [DOI] [PubMed] [Google Scholar]
- 21.Goedendorp MM, Gielissen MF, Verhagen CA et al. Severe fatigue and related factors in cancer patients before the initiation of treatment. Br J Cancer 2008; 99: 1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleeland CS, Bennett GJ, Dantzer R et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer 2003; 97: 2919–2925. [DOI] [PubMed] [Google Scholar]
- 23.Orre IJ, Reinertsen KV, Aukrust P et al. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res 2011; 71: 136–141. [DOI] [PubMed] [Google Scholar]
- 24.Alfano CM, Imayama I, Neuhouser ML et al. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J Clin Oncol 2012; 30: 1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med 2002; 64: 604–611. [DOI] [PubMed] [Google Scholar]
- 26.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012; 11: CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


