Abstract
Objective
Friedreich's ataxia (FRDA) is a spinocerebellar degenerative disorder, in which cognitive deficits are sparsely explored. In this behavioral and multimodal magnetic resonance imaging (MRI) study, we investigated the neurocognitive profile and cortico‐cerebellar dysfunctions underlying executive functioning in individuals with FRDA.
Methods
22 FRDA patients and 22 controls were clinically and neuropsychologically examined. Fifteen of each underwent structural and functional MRI using a verbal‐fluency task with phonemic and semantic conditions. Gray (GM) and white matter (WM) alterations were assessed by means of voxel‐based morphometry and diffusion‐tensor imaging.
Results
The neuropsychological profile demonstrated deficits in verbal fluency, working memory and social cognition. Functional MRI data showed most pronounced group‐differences in phonemic fluency with patients exhibiting enhanced activity in the cerebellum (VI, Crus I), fronto‐insular, premotor and temporo‐occipital regions. The semantic condition only revealed reduced activity in the anterior cerebellum; for overt speech, we found increased activity in the motor cortex. Functional connectivity‐analysis showed higher co‐activation within cerebellar and cortical regions, respectively, and impaired interregional coupling between the cerebellum and fronto‐insular cortex for phonemic processing, which was also related to poorer task performance. GM reduction in FRDA was mainly found in lobule VI, whereas WM degeneration was more pronounced including brainstem, cerebellum, and cortex. Decreased cerebellar GM was associated with enhanced activity in the fronto‐insular cortex, while loss of WM integrity may translate cortico‐cerebellar pathway disruptions.
Interpretation
The pattern of increased neural response with both cerebellar and cortical involvement underlying executive functioning indicates functional reorganization driven by disease‐related structural damage in FRDA.
Introduction
Friedreich's ataxia (FRDA) is the most common inherited ataxia in the Caucasian population. For the vast majority of FRDA cases, this progressive spinocerebellar neurodegenerative disorder is caused by a homozygous pathological expansion of GAA‐triplet repeats in the first intron of the frataxin (FXN) gene. Neuronal loss afflicts the dorsal root ganglia, spinal cord, dorsal medulla, and within the cerebellum, especially the dentate nuclei and cerebellar white matter.1, 2, 3 The clinical phenotype, with a typical onset around puberty, presents with gait and limb ataxia, poor balance and coordination, leg weakness, sensory loss, areflexia, dysarthria, dysphagia, scoliosis, foot deformities, cardiomyopathy, and diabetes. However, the neurobiology of cognitive dysfunction in FRDA remains poorly understood. Recent data points to disturbances in information processing and executive functioning, including impaired verbal fluency, which are suggested to be caused by disrupted cerebro‐cerebellar circuits.4, 5, 6, 7 Tests of verbal fluency, in which subjects are asked to rapidly generate words, traditionally assess phonemic (letter) or semantic (category) modalities. Within the large cross‐sectional database of the European Friedreich's Ataxia Consortium for Translational Studies (EFACTS, www.e-facts.eu), we have previously shown that phonemic fluency worsens with disease progression, and is moderately associated with ataxia and nonataxia symptoms.8 Hence, investigations into cognitive impairment in FRDA may increase our understanding of cortico–cerebellar dysfunctions and effects of cerebellar damage in this severely debilitating disease.
Beside its traditional role in motor control, the cerebellum is involved in a wide range of higher cognitive functions. In particular, posterior cerebellar lobules (VI, VII) are considered as the ‘cognitive cerebellum’ and play a crucial role in language processing.9, 10 Evidence from neuroimaging studies has shown that verbal fluency performance relies on the coordinated activity of left prefrontal and temporal lobes, but also involves right‐lateralized (i.e., contralateral) cerebellar activity.10, 11, 12 In FRDA, cognitive deficits have been linked to cerebellar degeneration13; and only few functional imaging studies have investigated the functional activity pattern underlying cognitive functioning in FRDA.14, 15 However, the interdependence between disease‐related structural damage and neural dysfunction has not been studied before.
Therefore, we aimed to investigate (1) the cognitive profile of individuals with FRDA compared to controls using an extensive neuropsychological test battery. We hypothesized that phonemic verbal fluency performance in particular would be impaired, as cerebellar damage seems to affect phonological search strategies more than semantic fluency.16 Further, using a multimodal magnetic resonance imaging (MRI) approach, we were interested in (2) the functional activity and network connectivity profile underlying verbal fluency deficits in FRDA. For this, we applied functional MRI (fMRI) with a paced word‐generation task, and expected that FRDA patients would exhibit alterations not only in executive prefrontal areas, but particularly show cerebellar involvement associated with verbal fluency execution. Finally, we aimed to explore (3) the relationship between the functional profile and cerebellar degeneration in gray and white matter using voxel‐based morphometry (VBM) and diffusion tensor imaging (DTI). Here, we expected to find interference of cerebellar damage on functional response in cerebellar–cortical loops by showing significant associations between functional activity patterns and structural alterations in FRDA.
Methods
Study sample
22 German‐speaking patients with genetically confirmed FRDA were recruited from the EFACTS cohort. The control group comprised 22 healthy volunteers with no history of neurological or psychiatric diseases, matched for age, gender, education, and handedness (Table 1). From this sample, 15 patients underwent structural and functional MRI and were compared to a respective group of 15 controls (Table 1). All assessments were conducted at the Department of Neurology of the RWTH Aachen University, after subjects gave their written informed consent for participation in this study, which was approved by the local ethics committee.
Table 1.
Demographics and clinical characteristics of FRDA patients and controls
| All subjects | MRI subgroup | |||||
|---|---|---|---|---|---|---|
| FRDA patients (n = 22) Mean (SD) | Controls (n = 22) Mean (SD) | P | FRDA patients (n = 15) Mean (SD) | Controls (n = 15) Mean (SD) | P | |
| Demographics | ||||||
| Age (years) | 41.36 (13.57) | 42.41 (12.29) | 0.790 | 37.73 (13.57) | 39.20 (12.62) | 0.762 |
| Gender (female/male) | 12/10 | 11/11 | 0.763a | 7/8 | 7/8 | 1.000a |
| Education (ISCED) | 3.59 (1.01) | 3.77 (0.87) | 0.492b | 3.60 (0.99) | 3.73 (0.88) | 0.665b |
| Handedness (right/left)c | 19/3 | 19/3 | 1.00a | 12/3 | 12/3 | 1.000a |
| Clinical measures | ||||||
| Disease duration (years) | 20.41 (11.04) | n.a. | 18.33 (9.09) | n.a. | ||
| Age of onset (years) | 20.95 (9.64) | n.a. | 19.40 (7.98) | n.a. | ||
| GAA repeats: | ||||||
| Allele 1 | 433.86 (213.72) | n.a. | 469.60 (229.70) | n.a. | ||
| Allele 2 | 759.77 (247.32) | n.a. | 771.93 (252.09) | n.a. | ||
| SARA | ||||||
| Total score | 18.61 (8.64) | n.a. | 20.10 (7.34) | n.a. | ||
| Speech problems | 2.45 (0.96) | n.a. | 2.60 (0.91) | n.a. | ||
| INAS | ||||||
| Total score | 4.59 (2.56) | n.a. | 4.73 (2.37) | n.a. | ||
| Speech disturbances: | 1.50 (0.80) | n.a. | 1.67 (0.9) | n.a. | ||
| SCAFI: | ||||||
| 8 m walk (sec) | 10.08 (4.87)d | 4.17 (0.62) | 0.004 | 11.28 (5.41)d | 4.15 (0.46) | 0.013 |
| 9 hole peg, dominant hand (sec) | 60.96 (35.02)e | 17.40 (2.60) | <0.001 | 55.71 (22.25)e | 17.09 (2.44) | <0.001 |
| Non‐dominant hand (sec) | 61.76 (25.17)f | 17.27 (2.18) | <0.001 | 62.59 (25.19)f | 17.06 (2.15) | <0.001 |
| PATA (words in 10 sec) | 21.68 (5.23) | 36.27 (6.25) | <0.001 | 22.50 (5.20) | 37.37 (6.92) | <0.001 |
| HADS | ||||||
| Total score | 9.77 (6.61) | 7.50 (4.96) | 0.204 | 10.20 (6.86) | 6.27 (4.71) | 0.078 |
| Anxiety | 5.77 (4.0) | 4.0 (2.62) | 0.090 | 5.67 (4.08) | 3.67 (2.38) | 0.115 |
| Depression | 3.95 (2.97) | 3.45 (2.97) | 0.580 | 4.47 (3.07) | 2.60 (2.77) | 0.091 |
SD, standard deviation; ISCED, International Standard Classification of Education (1997); FRDA, Friedreich's ataxia; SARA, Scale for the Assessment and Rating for Ataxia; INAS, Inventory of Non‐Ataxia Symptoms; SCAFI, Spinocerebellar Ataxia Functional Index; HADS, Hospital Anxiety and Depression Scale; n.a., not applicable.
Chi‐square/Fisher's exact test.
Mann Whitney U‐test (remaining group comparisons assessed with two sample t‐tests).
Based on the Edinburgh Inventory (Oldfield, 1971): laterality quotients in left‐handers ≤−20 and ≥50 in right‐handers.
n = 10 patients (7 in MRI group).
n = 20 (13 in MRI group),
n = 19 (13 in MRI group).
All patients were homozygous for expanded GAA repeats in the FXN gene, with the shorter allele containing 145–900 GAA‐triplet repeats. Six patients had early‐onset FRDA (≤14 years of age; 4 in the MRI subgroup), nine patients intermediate‐onset (15–23 years; 7 in the MRI subgroup) and seven were late‐onset patients (≥27 years; 4 in the MRI subgroup). At the time of study participation, four patients were not receiving any medication and four were taking solely supplements (e.g. Coenzyme Q10, vitamin B, vitamin E or l‐Carnitin). Of the remaining patients, five were taking Idebenone and ten were receiving symptomatic treatments (e.g., against hypertension, urinary or prostate dysfunctions, anti‐diabetic or thyroid medication). Two patients were taking antidepressants, one of which underwent MRI. Participants were examined using the Scale for the Assessment and Rating of Ataxia (SARA)17, the Inventory of Non‐Ataxia Symptoms (INAS)18 and the Spinocerebellar Ataxia Functional Index (SCAFI)19, which includes an 8 m‐walk test, the nine‐hole‐peg test and the rate of repeating the syllables PATA within 10 sec. Within the SARA, speech disturbances were clinically categorized ranging from 0‐normal to 6‐anarthria. While 50% of patients had mild speech problems (score of 1‐'suggestion of speech disturbances’ or 2‐’impaired speech but easy to understand’), speech impairment was moderate in the remaining patients (3‐’occasional words difficult to understand’ or 4‐’many words difficult to understand’). Generally, clinical scores were within the characteristic range for FRDA8, and patients did not differ significantly from controls in depression or anxiety symptoms (Table 1).
Neuropsychological assessment
The neuropsychological test battery included the Montreal Cognitive Assessment (MoCA)20, a brief screening instrument for general cognitive impairment. As an estimation measure for the premorbid (crystallized) intelligence level, we used a multiple choice vocabulary test (MWT‐B).21 Verbal learning and memory were assessed with the short version of the California Verbal Learning Test (CVLT).22 Attention and working memory were measured with the digit‐span test (forward and backward) from the Wechsler Memory Scale and the Paced Auditory Serial Addition Test (PASAT)23 with a 3 sec interval pace. Selective attention and cognitive control were further tested with the Color‐Word‐Interference Test (Stroop) including a word‐reading, color‐naming, and interference condition.24 In order to account for speech slowness, we calculated a nomination index for naming speed using a regression‐based correction for reading speed, and an interference (selectivity) index corrected for naming speed.24 For the assessment of verbal fluency, participants were asked to generate rapidly words beginning with the letters A and F (phonemic fluency), and object names from the given category ‘food’ (semantic fluency). For each task, subjects were given two minutes to adapt for speech slowness and articulatory deficits in FRDA patients. In order to avoid practice effects, letters and categories chosen for the offline verbal fluency tasks were different from those applied in the fMRI task (see below). Finally, we tested social cognition abilities, specifically theory of mind, with a short version of the Faux‐Pas‐Recognition test.25
Analysis of behavioral data
Group differences between patients and controls in neuropsychological test scores were tested using two‐sample t‐tests or Mann–Whitney U‐tests where appropriate and after assessing significant violations of normal distribution (Kolmogorov–Smirnov test). To assess the impact of articulatory deficits on time‐constrained tests (verbal fluency, PASAT), we correlated test performances with the SARA‐speech disturbances rating and PATA repeats using Spearman's rho and Pearson's product‐moment coefficients, respectively. Subsequent analyses of covariance (ANCOVA) were performed to test the effect of SARA‐speech scores and PATA‐repeats as covariates. Additionally, we compared scores of patients categorized as having mild speech problems according to SARA (n = 11) to those with moderate speech impairment (n = 11). All statistical analyses were performed using IBM SPSS Statistics 21 (Armonk, NY, USA) with a P ≤ 0.05 set as the threshold for significance.
MRI data acquisition
A subgroup of 15 FRDA patients and 15 pairwise‐matched controls (Table 1) received structural and functional MRI on a 3 Tesla Tim Trio MR scanner (Siemens Medical Systems, Erlangen, Germany). For the fMRI scan, T2*‐weighted images parallel to the AC/PC‐line were obtained using an echo‐planar imaging sequence (EPI) with the following parameters: TR = 4000 msec, TE = 30 msec, FoV = 200 mm, 64 × 64 matrix, 36 slices and slice thickness = 3.12 mm. Images were acquired within the first 2080 msec of the TR, leaving 1920 msec of no data acquisition, in which the subject could generate words.26 High‐resolution T1‐weighted images were acquired using a magnetization‐prepared rapid gradient‐echo sequence (TR = 2300 msec, TE = 2.98 msec, TI = 900 msec, FoV = 250 mm, 240 × 256 matrix, 176 sagittal slices, slice thickness = 1 mm). DTI was performed using a spin‐echo echo‐planar sequence (TR = 8800 msec TE = 84 msec, FoV = 224 mm, 112 × 112 matrix, 2 mm slice thickness) including seven b0‐images without diffusion gradients and 48 with diffusion weightings along predefined gradient directions with b = 1000 sec/mm2.
Verbal fluency paradigm
Using an fMRI block‐design with a paced–EPI technique26, we applied four different tasks (phonological and semantic fluency, overt and covert speech) with alternating resting blocks (fixation cross). In the phonemic task, subjects were instructed to generate words starting with the phonemes S, P, M and K. During the semantic blocks subjects had to name words belonging to the categories ‘animals’, ‘fruits’, ‘furniture’ and ‘clothes’. Overt and covert speech tasks were administered as control conditions to adjust for articulation and retrieval of words (motor controlling). Here, participants were requested to count aloud (overt speech) and silently (covert speech) from one to ten. In total, the stimulus design consisted of 16 task blocks (four for each condition) presented in a randomized order, and 16 intervening rest‐intervals. Before each block started, a written instruction was presented. All blocks included ten trials each one lasting 4s. Subjects were asked to generate only one word (or count) per trial in the delay time of the TR. The fMRI data were acquired in the first 2.08 sec of each trial using a bunched‐early sequence.26 After acquisition of one whole‐brain EPI volume, a speech bubble appeared for the remaining 1.92 sec within the time frame of no fMRI data recording, indicating the subjects to utter the word during this silent period. By using this procedure of paced word‐generation, the subjects’ verbal responses did not interfere with scanner noise and speech‐induced motion artifacts were reduced since subjects only spoke when no fMRI was recorded.26 Before scanning, subjects familiarized with the task using different phonemes and categories in order to avoid practice effects (i.e., B, T, H, D; male, female names).
Analysis of functional MRI data
Image processing and statistical analyses were performed using Statistical Parametric Mapping software (SPM8, www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 7.14 (MathWorks Inc., Natick, MA, USA). To allow magnetic field saturation the first three scans were discarded. All remaining functional images were realigned to the mean EPI volume, which was co‐registered with the anatomical image of each subject. Images were then normalized to the MNI (Montreal Neurological Institute) space using the SPM8 EPI standard template, and smoothed with an 8 mm full‐width‐at‐half‐maximum (FWHM) isotropic Gaussian kernel. On the first level, the hemodynamic response function was convolved with the onset of every block, and realignment parameters were entered as regressors of no interest to account for motion artifacts. Contrast images were generated for the task conditions versus baseline and against the control condition (i.e., phonemic > overt speech, semantic > overt speech, overt > covert speech) to test for group differences on the second level after including age and gender as covariates. Note that first level contrast images were also used to calculate individual laterality indices (LI) for the left‐handed subjects (3 patients, 3 controls; Table 1) using the LI‐toolbox.27 In none of these subjects LI indicated right‐hemispheric cortical dominance for verbal fluency and were in accordance with previous research reporting that a greater portion of left‐handers show typical left‐hemispheric language dominance.28 Activation differences between patients and controls were assessed on the second level using t‐contrasts. Based on functional imaging literature on verbal fluency activation and speech networks10, 12, 29, we prespecified anatomical region‐of‐interest (ROI), including Brodmann area (BA) 44, BA45, insula, anterior cingulate cortex (ACC), primary motor cortex (M1, 4a/4p), and cerebellar lobules VI, VIIa (Crus I, II) and VIIb. For ROI analyses, significance was accepted at a statistical threshold of family‐wise error (FWE) corrected P ≤ 0.05 (cluster extent threshold k E ≥ 20 contiguous voxels). Outside our ROI, results are reported after FWE cluster‐level correction with P ≤ 0.05 (uncorrected at the voxel‐level with P ≤ 0.001) across the whole brain.
In addition to functional activity alterations, we further investigated differences in functional cerebello‐cortical connectivity between groups, using the CONN‐toolbox.30 First‐level processing was performed as reported above and after component‐based noise reduction in BOLD effects from WM and cerebrospinal fluid, realignment and artifact detection parameters, and main condition effects. Bivariate regression coefficients were estimated representing the linear temporal association between ROI. On the second‐level, group comparisons were performed to detect task‐related differences in functional connectivity between source and target ROI. Since projections between cerebral and cerebellar cortices are widely contralateral and language functions are linked with the right posterior cerebellum, we assessed the co‐activation profile between left cortical and right cerebellar regions. ROI‐to‐ROI functional connectivity was tested after including age and gender as covariates and using a false discovery rate (FDR) corrected P ≤ 0.05 (across all source‐target pairs).
Analysis of macrostructure: voxel‐based morphometry
Regional gray (GM) and white (WM) matter alterations were assessed by means of VBM in 14 patients and 14 controls. Due to motion artifacts in one patient, the anatomical data of this subject and the healthy counterpart were discarded. T1‐weighted images were processed according to the VBM8 protocol (http://dbm.neuro.uni-jena.de/vbm) implemented in SPM8. Images were spatially normalized to the MNI space and segmented into GM, WM and CSF. To correct for individual brain sizes and allow comparing the absolute amount of tissue volume31, voxel values were multiplied (modulated) by the nonlinear component of the Jacobian determinant derived from the spatial normalization. Modulated images were then smoothed with a Gaussian kernel of 8 mm FWHM. Differences between groups were tested using two‐sample t‐tests and by including age and gender as covariates. Regression analyses were performed to assess associations between regional volumes and verbal fluency performance in the neuropsychological test‐battery, separately for patients and controls controlled for age and gender. Results were thresholded at P ≤ 0.05 (FWE‐corrected) across the whole brain and within ROI. For ROI analysis within GM we used the masks defined above. For WM, alteration in the superior cerebellar peduncle (SCP), which contains ascending efferent fibers of the dentate nuclei, is a promising biomarker in FRDA and has been shown to correlate with clinical progression.3, 32, 33, 34, 35
Analysis of microstructure: diffusion tensor imaging
DTI data were processed using FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS)36 and DTI‐ToolKit (DTI‐TK).37 First, raw diffusion‐weighted MR images were corrected for eddy‐current distortions and subject movement, skull‐stripped, and voxel‐wise fitted to a diffusion tensor model using FSL.36 Successively, diffusion tensor images from all subjects were utilized to create a population‐based template with DTI‐TK, which was mapped to a standard ICBM template. Tensor volumes in their native spaces were realigned to the template using the DTI‐TK in three successive steps including rigid, affine, and diffeomorphic transformations. After realignment, fractional anisotropy (FA) measures were extracted from each tensor volume of each subject and merged into 4D files. The mean FA image and its skeletonized version, which delineate the centers of all fiber tracts, were created. FA data of each subject were then projected onto this skeleton and a voxel‐wise statistical analysis of tract‐based spatial statistics (TBSS)38 was performed. Permutation‐based testing with 10.000 iterations and the threshold‐free cluster enhancement (TFCE) option was applied to assess significant differences between FRDA and controls at P ≤ 0.05 (adjusted for age and gender). Similar to VBM analysis as described above, voxel‐wise regression analyses were performed for each group separately to assess significant associations between verbal fluency performance and WM integrity in the SCP.
Correlation analyses between imaging modalities
To investigate the association between the functional profile in the verbal fluency paradigm and cerebellar degeneration, we conducted post hoc correlation analyses. For this, we extracted functional parameter estimates for each significant ROI per subject, as well as mean GM, WM and FA values in respective areas. Partial correlations (adjusted for age and gender) were computed between functional activity and GM volumes, and between cerebellar functional connectivity and WM/FA in the SCP. Due to the exploratory nature of this approach, we did not correct for multiple testing but set a more stringent threshold of P ≤ 0.01 to reduce the number of false positives.
Results
Neuropsychological test performance
FRDA patients did not significantly differ from controls in both the screening instrument for general cognition (MoCA), nor estimated premorbid intelligence (MWT‐B; Table 2). There were also no differences between groups in verbal learning and digit‐span. In the Stroop test, patients performed worse than controls in the three task conditions (t 42 ≥ 4.31, P < 0.001). However, Stroop performances were correlated with patients’ SARA‐speech disturbances score (r ≥ 0.47, P ≤ 0.026) and PATA‐repeats of the SCAFI (r ≥ −0.64, P ≤ 0.001). In contrast, when comparing the ‘speed‐corrected’ nomination and selectivity indices, there were no significant differences between groups (Table 2). Patients further performed worse compared to controls in the PASAT and in both phonemic and semantic verbal fluency tasks, with the highest effect size observed for phonemic fluency (Table 2). Impaired phonemic fluency performance was associated with longer disease duration (r = −0.551, P = 0.01). We did not find significant correlations between verbal fluency performances and SARA‐speech scores or PATA‐repeats in patients, and neither showed a significant effect as a covariate in subsequent ANCOVA. Additionally, there were no significant differences in verbal fluency or PASAT scores when comparing the 11 patients with mild speech problems with the 11 more severely affected patients, indicating that articulatory deficits had no significant impact on verbal fluency or PASAT. Finally, patients had significantly lower scores in the Faux‐Pas test, especially in the appraisal of intentions and for empathy, while there were no significant differences in neutral stories or control questions (Table 2).
Table 2.
Neuropsychological test performance of FRDA patients and controls
| All subjects | MRI subgroup | |||||||
|---|---|---|---|---|---|---|---|---|
| FRDA patients n = 22 Mean (SD) | Controls n = 22 Mean (SD) | Effect size d | P | FRDA patients n = 15 Mean (SD) | Controls n = 15 Mean (SD) | Effect size d | P | |
| Screening | ||||||||
| MoCA | ||||||||
| Dementia screening | 26.64 (3.06) | 27.82 (1.56) | 0.49 | 0.114 | 27.20 (2.24) | 28.33 (1.54) | 0.59 | 0.118 |
| MWT‐B | ||||||||
| Estimated premorbid IQ | 105.27 (14.05) | 110.86 (12.19) | 0.43 | 0.166 | 105.47 (12.21) | 109.80 (12.82) | 0.35 | 0.351 |
| Memory and learning | ||||||||
| CVLT | ||||||||
| Total immediate recall | 29.77 (3.59) | 30.36 (3.44) | 0.17 | 0.580 | 30.73 (2.92) | 31.27 (1.87) | 0.22 | 0.556 |
| Total delayed recall | 15.91 (2.60) | 16.41 (2.20) | 0.21 | 0.494 | 16.13 (2.50) | 17.20 (1.01) | 0.56 | 0.143 |
| Recognitiona | 8.64 (0.73) | 8.77 (0.53) | 0.21 | 0.628 | 8.73 (0.59) | 8.93 (0.26) | 0.44 | 0.276 |
| Attention and working memory | ||||||||
| Digit‐span | ||||||||
| Forward | 7.45 (1.77) | 7.45 (1.54) | 0.00 | 1.000 | 7.87 (1.64) | 7.67 (1.63) | 0.12 | 0.740 |
| Backward | 5.82 (1.97) | 6.82 (1.62) | 0.55 | 0.073 | 6.00 (2.33) | 6.87 (1.89) | 0.41 | 0.272 |
| PASAT | ||||||||
| Correct responses (%) | 76.15 (17.53)b | 89.24 (9.06) | 0.94 | 0.006 | 78.07 (18.43) | 87.73 (10.08) | 0.65 | 0.089 |
| Executive functions | ||||||||
| Stroop | ||||||||
| Nomination index | 57.23 (6.00) | 56.73 (11.74) | 0.05 | 0.860 | 56.33 (5.77) | 54.60 (11.75) | 0.19 | 0.614 |
| Interference index | 53.95 (8.12) | 53.05 (8.65) | 0.11 | 0.721 | 54.40 (8.22) | 54.20 (8.04) | 0.02 | 0.947 |
| Verbal fluency | ||||||||
| Phonemic (A, F) | 28.10 (9.56)c | 37.05 (7.63) | 1.03 | 0.002 | 30.14 (7.45)c | 36.67 (8.48) | 0.82 | 0.037 |
| Semantic (Food) | 35.09 (10.68) | 42.14 (8.67) | 0.72 | 0.021 | 36.73 (9.22) | 41.93 (9.94) | 0.54 | 0.149 |
| Social cognition | ||||||||
| Faux pas testa | ||||||||
| Faux pas stories | 24.18 (4.93) | 27.09 (5.04) | 0.58 | 0.012 | 24.93 (3.41) | 28.33 (3.29) | 1.01 | 0.004 |
| Intentions | 12.77 (2.33) | 14.00 (2.25) | 0.54 | 0.045 d | 12.87 (1.92) | 14.47 (1.46) | 0.94 | 0.024 d |
| Beliefs | 8.00 (2.05) | 8.73 (2.07) | 0.35 | 0.369d | 8.40 (1.30) | 9.33 (0.98) | 0.81 | 0.135d |
| Empathy | 3.36 (1.33) | 4.41 (1.01) | 0.89 | 0.015 d | 3.60 (1.12) | 4.60 (0.91) | 0.98 | 0.021 d |
| Neutral stories | 9.45 (1.54) | 9.55 (0.86) | 0.07 | 0.552 | 9.87 (0.52) | 9.33 (0.97) | 0.68 | 0.073 |
| Control questions | 19.77 (0.61) | 19.86 (0.47) | 0.17 | 0.624 | 19.73 (0.70) | 19.80 (0.56) | 0.10 | 0.944 |
SD, standard deviation; FRDA, Friedreich's ataxia; MoCA, Montreal cognitive assessment; MWT‐B, Multiple choice vocabulary test; CVLT, California verbal learning test – short version: PASAT, Paced Auditory Serial Addition Test. In bold: significant group differences at P < 0.05.
Group differences tested with Mann–Whitney U‐tests (remaining group comparisons assessed with two‐sample t‐tests).
n = 20.
n = 21 (14 in MRI group).
Bonferroni‐adjusted P‐values.
Overall, similar results were obtained when considering only subjects undergoing MRI, except that the group differences for PASAT and semantic verbal fluency did not reach significance (Table 2).
Functional imaging results
Verbal fluency performance in the paced fMRI task did not significantly differ between groups (phonemic: t 28 = 0.57, P = 0.576; semantic: t 28 = −0.79, P = 0.437). Differences in BOLD‐response between FRDA patients and controls were found particularly for the phonemic condition (Table 3, Fig. 1). Compared to baseline, patients exhibited higher activity than controls in left BA44 and anterior insula, and outside of ROI in the left precentral gyrus, premotor cortex and supplementary motor area, anterior mid‐cingulate cortex, and middle occipital gyrus. Decreased functional activity was observed in the ventral posterior cingulate cortex with patients deactivating this area in contrast to controls. To control for motor demand, we contrasted phonemic activity against overt speech and found increased activity in patients compared to controls in the left anterior insula, right cerebellar lobule VI and Crus I bilaterally, though differences in right Crus I were more pronounced (Table 3, Fig. 1B). Note that cerebellar activation differences were also significant across the whole brain with cluster‐level FWE correction at P < 0.05. Here, patients also showed higher activity in the left precentral and middle frontal gyrus, right inferior parietal lobe, and bilateral occipital regions (Table 3, Fig. 1B). For the semantic condition, we only found reduced activity in patients versus controls in the anterior cerebellum (I–IV). For overt versus covert speech, patients exhibited increased activity compared to controls in M1 (Table 3).
Table 3.
Significant differences in BOLD‐response between FRDA patients and controls during verbal fluency tasks and overt speech
| Anatomical region | MNI co‐ordinates | MNI co‐ordinates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | T | Anatomical region | x | y | z | T | |||
| Phonemic verbal fluency | Phonemic verbal fluency | ||||||||||
| Patients < controls (phonemic vs. baseline) | Patients > controls (phonemic vs. overt speech) | ||||||||||
| Posterior cingulate cortex | L/R | 2 | −38 | 10 | 4.40 | Anterior insula | L | −34 | 24 | 6 | 5.22 |
| Cerebellum, VI | R | 34 | −70 | −24 | 4.96 | ||||||
| Patients > controls (phonemic vs. baseline) | Cerebellum, VIIa Crus I | R | 18 | −78 | −22 | 4.54 | |||||
| L | −20 | −84 | −22 | 4.12 | |||||||
| Broca's area (BA 44) | L | −60 | 6 | 18 | 4.27 | Precentral gyrus, middle frontal gyrus | L | −32 | −6 | 48 | 5.00 |
| Anterior insula | L | −32 | 22 | 6 | 4.74 | Inferior parietal lobe | R | 42 | −74 | 34 | 4.62 |
| Precentral gyrus | L | −42 | 0 | 36 | 5.01 | Middle temporal gyrus | R | 50 | −66 | 22 | 4.49 |
| Premotor cortex (BA 6) | L | −36 | −8 | 46 | 6.10 | Superior occipital gyrus | L | −20 | −96 | 18 | 4.35 |
| Supplementary motor area (BA 6) | L | −8 | 10 | 46 | 4.73 | Middle occipital gyrus | L | −40 | −90 | 6 | 4.66 |
| R | 2 | −4 | 66 | 4.64 | R | 36 | −86 | 22 | 5.60 | ||
| Anterior mid‐cingulate cortex | L | −10 | 18 | 30 | 4.27 | Inferior occipital gyrus | L | −30 | −90 | −10 | 3.91 |
| Middle occipital gyrus | L | −22 | −90 | −2 | 4.25 | R | 32 | −84 | −4 | 4.51 | |
| Semantic verbal fluency | Overt speech | ||||||||||
| Patients < controls (semantic vs. baseline) | Patients > controls (overt vs. covert speech) | ||||||||||
| Cerebellum I–IV | L/R | 4 | −50 | 2 | 4.03 | Motor cortex (M1, 4a) | L | −14 | −34 | 72 | 4.46 |
Results are FWE corrected at P < 0.05 within ROI (BA44, Insula, cerebellum VI/VIIa, M1) or cluster‐level FWE corrected at P < 0.05 across the whole brain (uncorrected at the voxel‐level with P < 0.001).
T, maximum T‐value for the anatomical area; BA, Brodmann area.
Figure 1.
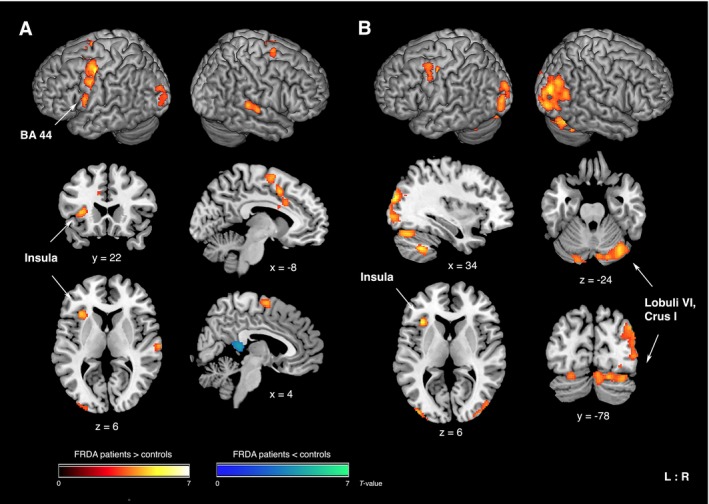
Significant differences in BOLD‐response between FRDA patients and controls during phonemic verbal fluency (A) versus baseline and (B) versus overt speech. Higher functional activity in patients compared to controls is displayed in red‐yellow, lower activity in blue‐green (for visualization purposes only: significant ROI [BA44, insula, cerebellar lobules] are displayed at an uncorrected voxel‐level threshold of P < 0.001 within ROI; results outside of ROI are cluster‐level FWE corrected at P ≤ 0.05 across the whole brain). L/R: left/right; coordinates in MNI space; color bars represent T‐values.
ROI‐to‐ROI functional connectivity analysis showed reduced connectivity in patients compared to controls between right cerebellar lobule VI and left BA44/insula during phonemic verbal fluency (Table 4, Fig. 2A). While patients showed no correlations between right lobule VI and left BA44 (post hoc one sample t‐test: t 14 = −0.375, P = 0.713), we found anticorrelations in patients for the connectivity between right lobule VI and left insula (t 14 = −2.19, P = 0.046) that was also related to impaired phonemic fluency performance (r = 0.565, P = 0.044; Fig. 2B). In contrast, patients showed higher positive functional connectivity between cortical ROI, that is, BA44‐M1/ACC, BA45‐M1 (Table 4, Fig. 2B). For semantic verbal fluency, we also observed this pattern of higher functional co‐activation between cortical ROI (BA45‐ACC, BA45‐Insula), and additionally between cerebellar ROI (VIIb‐Crus I, VIIb‐Crus II). Similar results were found for overt speech (BA44‐M1, VIIb‐Crus II; Table 4, Fig. 2B).
Table 4.
Functional connectivity differences between FRDA patients and controls during verbal fluency tasks and overt speech
| ROI‐to‐ROI connectivity | T | p FDR | ROI‐to‐ROI connectivity | T | p FDR | ||
|---|---|---|---|---|---|---|---|
| Phonemic verbal fluency | Phonemic verbal fluency | ||||||
| Patients < controls | Patients > controls | ||||||
| R Cerebellum VI – L BA 44 | 3.48 | 0.0347 | L BA 44 – L Motor cortex (M1, 4p) | 3.54 | 0.0347 | ||
| R Cerebellum VI – L Insula | 3.26 | 0.0347 | L BA 44 – L Anterior cingulate cortex | 3.06 | 0.0460 | ||
| L BA 45 – L Motor cortex (M1, 4p) | 3.34 | 0.0347 | |||||
| Semantic verbal fluency | Overt speech | ||||||
| Patients > controls | Patients > controls | ||||||
| L BA 45 – L Anterior cingulate cortex | 3.32 | 0.0350 | L BA 44 – L Motor cortex (M1, 4a) | 3.32 | 0.0396 | ||
| L BA 45 – L Insula | 3.26 | 0.0350 | L BA 44 – L Motor cortex (M1, 4p) | 3.65 | 0.0257 | ||
| R Cerebellum VIIb – R VIIa, Crus II | 4.57 | 0.0047 | R Cerebellum VIIb – R VIIa, Crus II | 4.91 | 0.0019 | ||
| R Cerebellum VIIb – R VIIa, Crus I | 4.31 | 0.0047 | |||||
Results are FDR corrected at P < 0.05 across all ROI‐to‐ROI connections (analysis‐level).
T, T‐value for the ROI‐to‐ROI functional connectivity. R, right; L, left; BA, Brodmann area.
Figure 2.
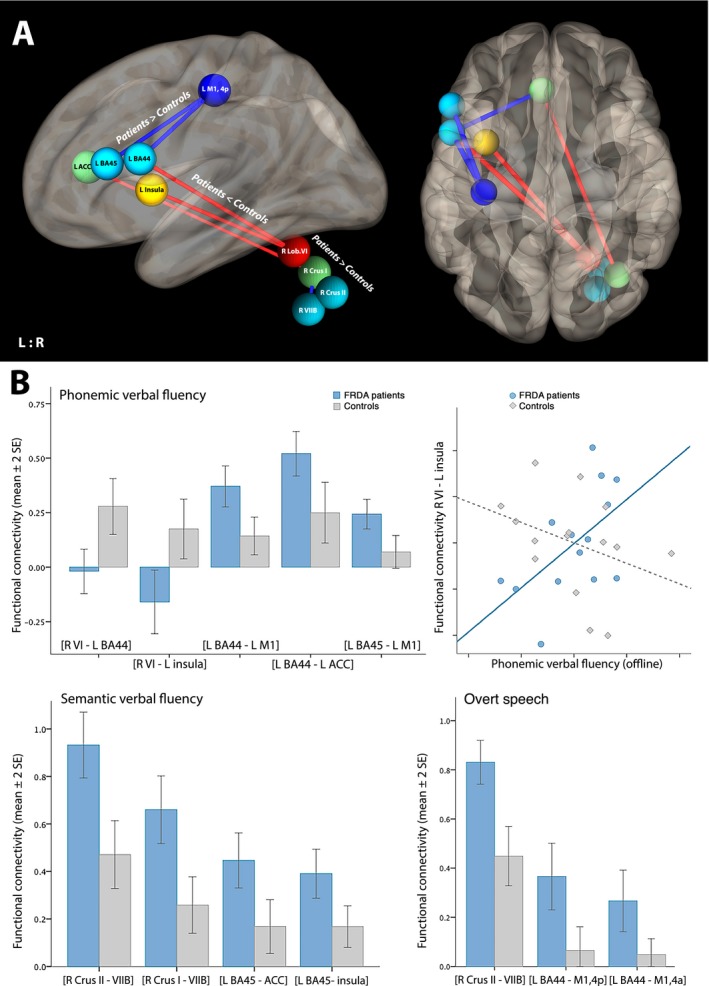
(A) Schematic overview of ROI‐to‐ROI functional connectivity differences between FRDA patients and controls during verbal fluency tasks and overt speech. (B) Beta values showing functional connectivity differences for each significant ROI‐to‐ROI pair and each task condition. Scatter plot demonstrates the correlation between lobule VI – insular connectivity and phonemic verbal fluency performance (continuous line: significant correlation at P < 0.05, dotted line: not significant). L/R: left/right, BA: Brodmann area, M1: primary motor cortex, ACC: anterior cingulate cortex.
Macroanatomical alterations (VBM)
Whole‐brain VBM analysis revealed cerebellar GM atrophy in patients compared controls in the bilateral lobule VI (left cluster maxima [MNI coordinates x/y/z]: −4/−69/−12, t = 6.77, k E = 26; right: 4/−67/−12, t = 6.37, k E = 78; Fig. 3A). ROI analyses showed GM reductions in FRDA patients versus controls in all posterior cerebellar lobules bilaterally (VI, Crus I/II, VIIb) and in left M1 (4p; Fig. 3A). VBM analysis for WM degeneration yielded two large clusters, one located in the medulla extending bilaterally to the inferior cerebellar peduncle (ICP; 4/−49/−63, t = 12.08, k E = 1,216; Fig. 3B), the second cluster was found in the bilateral SCP, adjacent to the dentate nucleus area (−4/−40/−26, t = 9.57, k E = 1,142; Fig. 3B). Another smaller cluster showed atrophy in the midbrain (2/−22/−18, t = 12.08, k E = 80). There were no significant increases in GM or WM in patients compared to controls.
Figure 3.
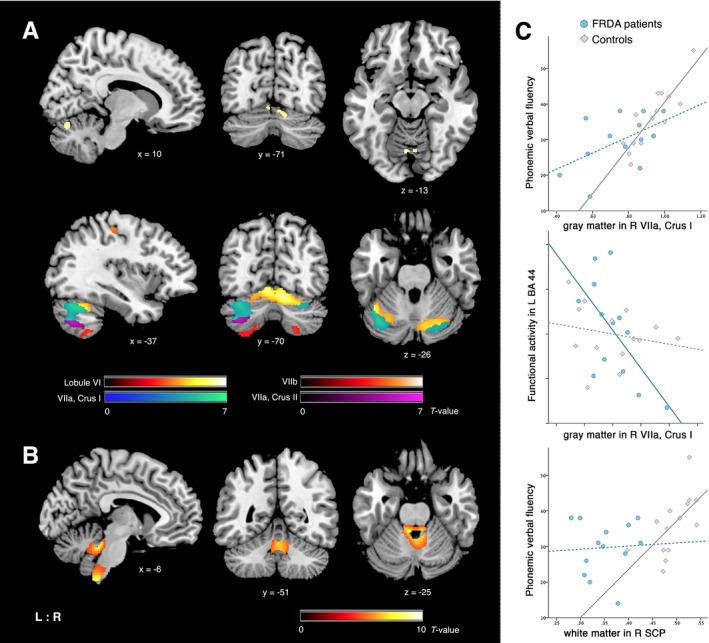
VBM results showing significant volume reductions in patients compared to controls (A) for gray matter in the bilateral cerebellar lobule VI after FWE correction at P ≤ 0.05 across the whole‐brain (top row), and in posterior lobules VI, VIIa and VIIb FWE corrected at P ≤ 0.05 within ROI (second row). (B) for white matter after FWE correction at P ≤ 0.05 across the whole‐brain. (C) Scatter plots demonstrating the association between gray and white matter volumes with phonemic verbal fluency performance, and functional activity in left BA44 (continuous lines indicate significant correlation, dotted lines non‐significant). Coordinates in MNI space; color bars represent T‐values. L/R: left/right, BA: Brodmann area, SCP: superior cerebellar peduncle.
Regression analysis did not indicate associations between GM reductions and verbal fluency performance in patients. However, in controls higher phonemic fluency correlated with cerebellar volumes in right Crus I (50/−66/−45, t = 7.49, k E = 49, 36; Fig. 3C) and left VIIb (−36/−57/−56, t = 5.39, k E = 27). Post hoc correlations of extracted GM volumes within this right cerebellar area revealed significant negative associations with functional activity in the left BA44 and left insula for phonemic fluency execution in patients (r = −0.778, P = 0.005; r = −0.836, P = 0.001, respectively; Fig. 3C). Lower GM volumes in M1 (4p) were associated with higher functional connectivity between left BA45‐M1 in patients (r = −0.735, P = 0.006).
Regression analyses with WM did not yield significant correlations with verbal fluency performance, though post hoc correlations with extracted values showed a positive association between right SCP and phonemic fluency in controls (r = 0.658, P = 0.014; Fig. 3C). In controls, the right SCP also correlated negatively with functional connectivity within the cerebellum (r = −0.72, P = 0.008), whereas this association tended to be positive in patients (r = 0.696, P = 0.012).
Microstructural alterations (DTI)
Patients showed widespread decreases in FA compared to controls, mainly in bilateral SCP, ICP, corticospinal tract, cerebral peduncles, fornix, posterior thalamic radiation, corpus callosum (splenium, body and genu), internal capsule, corona radiata (mainly posterior), forceps major, inferior longitudinal and fronto‐occipital fasciculus, as well as the left hippocampal cingulum (Fig. 4). Correlation analyses of SCP alterations with verbal fluency performance or cerebellar functional connectivity were not significant.
Figure 4.
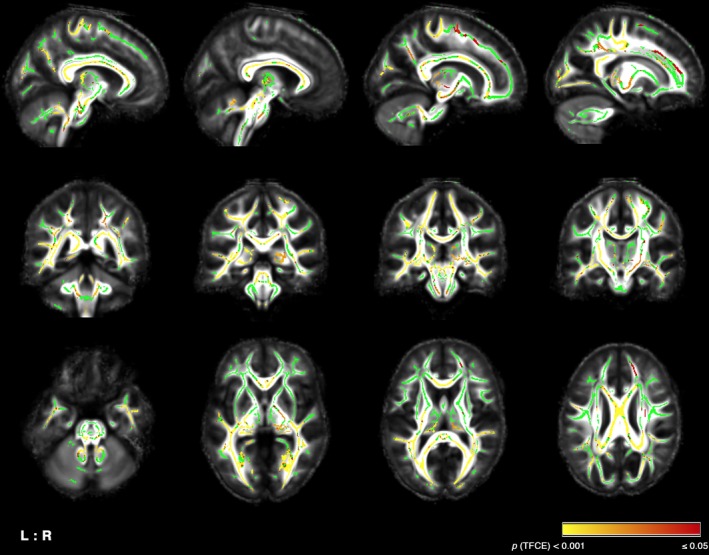
Diffusion tensor imaging: Statistical map showing reduced fractional anisotropy (FA) in FRDA patients compared to controls corrected for multiple comparisons at P ≤ 0.05 using threshold free‐cluster enhancement (TFCE; displayed in red‐yellow). Results are superimposed on the skeleton mask (green) and mean FA template. L/R: left/right.
Discussion
This study provides evidence of neurocognitive deficits and cortico‐cerebellar alterations in FRDA. During executive processing, patients exhibited increased neural response in cerebellar and cortical regions that was accompanied by impaired cortico‐cerebellar functional coupling and related to cerebellar volume reductions.
Neuropsychological testing of 22 patients showed poorer performances in verbal fluency, working memory and social cognition. Global cognitive abilities, memory and learning, digit‐span and selective attention were relatively preserved. Generally, these finding are in line with previous studies on cognition in FRDA.4, 6 Importantly, impairment in verbal fluency was not merely explainable by articulatory deficits, according with previous data.4, 5, 7, 39, 40 As expected, differences in semantic fluency were less pronounced than for phonemic search, and did not reach significance in the MRI subgroup. A selective impairment for phonemic fluency in FRDA compared to semantic word‐retrieval has been suggested before5, 39 and is consistent with the common observation that cerebellar damage affects phonemic fluency more than semantic search.9, 16 This is explained by the higher cognitive demand of letter fluency tasks requiring less automated search strategies with greater executive/prefrontal control.11, 16 While patients did not differ from controls in the digit‐span tasks4, 7, they performed worse in the PASAT. The PASAT may therefore be a more sensitive tool to assess working memory deficits in FRDA, for which we did not find an impact of slowed speech. In contrast, Stroop‐subtests were associated with speech impairment, while speed‐corrected nomination or interference indices did not show selective attention deficits, as reported by others.4, 7 Verbal learning and memory measured by the CLVT were also preserved in FRDA, confirming previous findings.4, 7, 39 A novel finding in our cohort is that we found social cognition deficits in the Faux‐Pas test. Given that cerebellar activity is observed in social cognition tasks41 and patients with cerebellar damage do exhibit social cognition deficits42, impairment in this domain is reasonable in FRDA and may have important implications for patients’ quality of life and caregivers.
Functional activity differences between patients and controls were found predominantly for phonemic fluency. Patients exhibited increased neural response in task‐related cortical regions (including left prefrontal areas, BA44, anterior insula) and the posterior‐lateral cerebellum (VI, Crus I) that was accompanied by impaired cortico–cerebellar functional coupling. In contrast, during semantic search patients showed reduced activity in the anterior ‘motor’ cerebellum, and increased activity in M1 for overt speech. Both the left BA44 and anterior insula are involved in phonological processing.12, 26, 43 Increased activity may hence indicate that individuals with FRDA engage these areas to a greater extent than controls, possibly to compensate for deficits in this domain. Within the cerebellum, language processing is particularly localized in right lobules VI and VIIa (i.e., contralateral to the dominant cerebral hemisphere), and these lobules are also involved in executive functions.10 Importantly, enhanced activity in lobules VI and Crus I emerged after controlling for motor demand, indicating that the cerebellum contributes to cognitive processing independent of motor‐related task‐requirements. Bilateral activation in Crus I further supports a compensatory recruitment of the posterior cerebellum in patients, as suggested previously for motor functioning in FRDA.44
Functional connectivity analysis showed increased intracortical coupling in patients between BA45 and insula/ACC for semantic fluency, while for phonemic fluency effects were rather shown for BA44. This indicates a task‐specific enhancement in neural coupling, since BA44 and BA45 were shown to contribute differentially to phonologically and semantically cued word‐retrieval, respectively.26, 43 Specific to the phonemic condition, we also observed reduced interregional connectivity between cerebellar lobule VI and BA44/insula. As suggested previously4, 5, 6, our data thus provide evidence that cognitive deficits in FRDA are associated with disruptions in cerebro‐cerebellar circuits, or in case phonological processing can be related to impaired functional coupling between the cerebellum and frontoinsular cortex. Based on effective connectivity analyses of imaging data from different word‐generation tasks, Eickhoff and colleagues29 postulated a speech system, in which the cerebellum receives input from BA44 via the left anterior insula and projects to the premotor cortex, from where it is forwarded to M1. In accordance with this, controls displayed positive co‐activations between lobule VI and BA44/insula during phonemic fluency, whereas neural coupling between the cerebellum and BA44 was absent in patients, and even anticorrelated with the insula. Although the physiological process underlying negative co‐activation is not entirely understood, this inverse coupling may indicate dysfunctional competing network activity, which in patients was associated with poorer task performance. Evidence of a mixed pattern in functional activation has further been shown in other fMRI studies examining motor dysfunctions in FRDA.40, 44, 45, 46 This heterogeneity in neural response seems to reflect both cerebellar and cortical dysfunctions presumably related to regional cerebellar damage with compensatory significance.44, 45
WM degeneration as shown by VBM and DTI was much more pronounced and widespread than GM atrophy in FRDA, which is in line with previous work.1, 35, 47, 48 We found GM degeneration mainly affecting cerebellar lobule VI, and reduced GM in posterior lobules VI–VII and M1 in the more sensitive ROI‐approach. In controls, higher volumes in right Crus I were associated with better phonemic fluency performance, confirming the posterior cerebellum's role in phonological processing. In patients, reduced GM in Crus I correlated with the increased contralateral cortical activity (left BA44, insula). Hence, cerebellar volume reductions might lead to a disinhibition of the dentate nucleus resulting in enhanced cortical excitability. Lower volumes in M1 were related to the increased coupling between BA45 and M1, which is indicative of diminished cortical inhibition. Both observations support a putative compensatory enhancement of functional activity due to disturbed cerebellar input, as well as a reduced intranetwork distinctiveness due to regional degeneration.49
WM differences were observed in the cerebellum and brainstem, and loss of microstructural integrity was even more widespread including cerebral regions.1, 3, 34, 35, 48, 50 Higher SCP volumes were associated with better phonemic fluency performance in controls. Again, this emphasizes the significance of cerebellar input for cognitive functioning, since the SCP in particular contain ascending efferent fibers of the dentate nuclei. Degeneration in this area would therefore likely lead to disruptions of cerebello‐thalamic‐cortical projections. This is supported by a recent DTI study demonstrating that cognitive deficits in FRDA correlate with degeneration in the dentato‐rubral tract.13 In controls we further found that higher SCP volumes were related to less (dys)functional coupling between cerebellar lobules. In FRDA, however, greater WM loss seemed to be associated with reduced intracerebellar co‐activation. Although such coherence between reduced structural and functional connectivity might be consequential, it does however not explain the increased intranetwork functional connectivity in FRDA. This suggests a differential structure‐function relationship for WM degeneration than for GM reductions, which was related to an increase in BOLD response. Pronounced WM deterioration may therefore lead to reduced cerebellar innervation of cortical areas resulting in impaired cortico‐cerebellar coupling. Incipient regional GM loss on the other hand seems more predictive to explain the increase in functional activity and intranetwork connectivity either in terms of a compensatory mechanism to maintain function or due to a reduced functional differentiation. This may be caused by primary regional tissue damage or as secondary abnormal cortico‐cortical disinhibition, which in turn would account for the anticorrelated co‐activation between the insula and cerebellum (e.g., via cortico‐ponto‐cerebellar projections). As we cannot infer any causality and none of the fMRI studies in FRDA have directly assessed structural changes or interdependencies with function, the specific effects of structural degeneration on cortico‐cerebellar dysfunctions need to be further evaluated. Nevertheless, our multi‐modal MRI approach clearly indicates both cerebellar and cortical upregulation during executive processing in FRDA and provides evidence that the abnormal pattern of neural response seems to be related to structural cerebellar damage affecting cortico‐cerebellar loops.
One limitation of our study is the small sample of imaging data. As this is inherently an issue when investigating rare diseases, accumulating evidence from the recently growing number of studies in FRDA will further elucidate the neural correlates of functional decline in this disease. We tried to overcome this drawback by using a more sensitive ROI‐approach for those areas known to be involved in verbal fluency processing and particularly affected by the disease pathology, while controlling for false positives where appropriate. Another potential caveat regarding our FRDA sample is that we also included patients with late‐onset FRDA, who seem to differ with respect to the rate of clinical decline and cognitive profile from individuals with typical FRDA.8, 51 On the other hand, the inclusion of a heterogeneous clinical sample peculiar to the disease itself increases the variance in parameters of interest, whereby interdependencies between different disease markers can be outlined. Larger samples allowing subgroup stratification are needed to investigate differential aspects of functional reorganisation related to the age of disease onset. Another limitation concerns our connectivity‐analysis. Functional ‘co‐activation’ reveals interregional relationships based on temporal fluctuations in BOLD response, which are not necessarily structurally interconnected. We complemented this approach by using TBSS‐based structural data to investigate whether functional connectivity alterations are related to microstructure deterioration. Further methods (e.g. ‘effective connectivity’ analyses, fiber‐tracking) may elaborate pathway disruptions in FRDA. Finally, in FRDA cerebellar degeneration is mainly found in the deep dentate nuclei2, which are difficult to map with MRI due to their small size and high iron content. Other imaging‐techniques (e.g., susceptibility‐weighted or ultra‐high‐field imaging) allow the quantification of volumes and activity at the level of cerebellar nuclei.46, 52
In conclusion, we demonstrated that individuals with FRDA have neurocognitive deficits, in particular pertaining to executive functioning. Using a multi‐modal MRI approach, our results indicate differential activation profiles in both cerebellar and cortical areas accompanied by dysfunctional cortico‐cerebellar coupling underlying phonological processing. The observed functional alterations seem to be mediated by structural cerebellar damage interfering with cortico‐cerebellar loops, and reflect functional reorganisation in FRDA that exceed beyond spinocerebellar deficits. Our findings emphasize the need for multimodal investigations into brain structure–function relationships to unravel the complex neuronal interactions in this multisystem disorder. In addition to structural MRI, functional imaging data hold valuable information for a more comprehensive understanding of neural dynamics in FRDA.
Author Contributions
Conception and design of the study: ET, ID, ASC, CJW, SH, KF, SS, DT, IAG, TK, JBS, KR. Acquisition or analysis of data: ET, ID, SR, SM, ASC, KF, KR. Drafting or revision of the manuscript: ET, ID, SR, SM, ASC, CJW, SH, KF, SS, DT, IAG, TK, JBS, KR.
Conflicts of Interest
The authors report no conflicts of interest that could have influenced the results of the study.
Acknowledgements
The authors thank the EFACTS study participants and their families. We also thank Laura Hausmann and Franziska Wilhelm for help with database and patient assessment. Most authors are part of the European Friedreich's Ataxia Consortium for Translational Studies (EFACTS), which was funded by an FP7 Grant from the European Commission (HEALTH‐F2‐2010‐242193). KR and SR positions are funded partly by the Excellence Initiative of the German federal and state governments, and the Federal Ministry of Education and Research (BMBF 01GQ1402 to KR). The funders had no role in the present study. This work was supported by the Brain Imaging Facility of the Interdisciplinary Center for Clinical Research within the Faculty of Medicine at the RWTH Aachen University.
References
- 1. Della Nave R, Ginestroni A, Giannelli M, et al. Brain structural damage in Friedreich's ataxia. J Neurol Neurosurg Psychiatry 2008;79:82–85. [DOI] [PubMed] [Google Scholar]
- 2. Koeppen AH, Michael SC, Knutson MD, et al. The dentate nucleus in Friedreich's ataxia: the role of iron‐responsive proteins. Acta Neuropathol 2007;114:163–173. [DOI] [PubMed] [Google Scholar]
- 3. Della Nave R, Ginestroni A, Diciotti S, et al. Axial diffusivity is increased in the degenerating superior cerebellar peduncles of Friedreich's ataxia. Neuroradiology 2011;53:367–372. [DOI] [PubMed] [Google Scholar]
- 4. Nieto A, Correia R, de Nobrega E, et al. Cognition in Friedreich ataxia. Cerebellum 2012;11:834–844. [DOI] [PubMed] [Google Scholar]
- 5. de Nobrega E, Nieto A, Barroso J, Monton F. Differential impairment in semantic, phonemic, and action fluency performance in Friedreich's ataxia: possible evidence of prefrontal dysfunction. J Int Neuropsychol Soc 2007;13:944–952. [DOI] [PubMed] [Google Scholar]
- 6. Corben LA, Georgiou‐Karistianis N, Fahey MC, et al. Towards an understanding of cognitive function in Friedreich ataxia. Brain Res Bull 2006;70:197–202. [DOI] [PubMed] [Google Scholar]
- 7. Nachbauer W, Bodner T, Boesch S, et al. Friedreich ataxia: executive control is related to disease onset and GAA repeat length. Cerebellum 2014;13:9–16. [DOI] [PubMed] [Google Scholar]
- 8. Reetz K, Dogan I, Costa AS, et al. Biological and clinical characteristics of the European Friedreich's Ataxia Consortium for Translational Studies (EFACTS) cohort: a cross‐sectional analysis of baseline data. Lancet Neurol 2015;14:174–182. [DOI] [PubMed] [Google Scholar]
- 9. Stoodley CJ, Schmahmann JD. The cerebellum and language: evidence from patients with cerebellar degeneration. Brain Lang 2009;110:149–153. [DOI] [PubMed] [Google Scholar]
- 10. Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta‐analysis of neuroimaging studies. NeuroImage 2009;44:489–501. [DOI] [PubMed] [Google Scholar]
- 11. Argyropoulos GP. The cerebellum, internal models and prediction in ‘non‐motor’ aspects of language: a critical review. Brain Lang 2015. [DOI] [PubMed] [Google Scholar]
- 12. Wagner S, Sebastian A, Lieb K, et al. A coordinate‐based ALE functional MRI meta‐analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neurosci 2014;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akhlaghi H, Yu J, Corben L, et al. Cognitive deficits in Friedreich ataxia correlate with micro‐structural changes in dentatorubral tract. Cerebellum 2014;13:187–198. [DOI] [PubMed] [Google Scholar]
- 14. Georgiou‐Karistianis N, Akhlaghi H, Corben LA, et al. Decreased functional brain activation in Friedreich ataxia using the Simon effect task. Brain Cogn 2012;79:200–208. [DOI] [PubMed] [Google Scholar]
- 15. Harding IH, Corben LA, Storey E, et al. Fronto‐cerebellar dysfunction and dysconnectivity underlying cognition in friedreich ataxia: the IMAGE‐FRDA study. Hum Brain Mapp 2016;37:338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leggio MG, Silveri MC, Petrosini L, Molinari M. Phonological grouping is specifically affected in cerebellar patients: a verbal fluency study. J Neurol Neurosurg Psychiatry 2000;69:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burk K, Malzig U, Wolf S, et al. Comparison of three clinical rating scales in Friedreich ataxia (FRDA). Mov.Disord. 2009;24:1779–1784. [DOI] [PubMed] [Google Scholar]
- 18. Jacobi H, Rakowicz M, Rola R, et al. Inventory of Non‐Ataxia Signs (INAS): validation of a new clinical assessment instrument. Cerebellum 2013;12:418–428. [DOI] [PubMed] [Google Scholar]
- 19. Schmitz‐Hubsch T, Giunti P, Stephenson DA, et al. SCA Functional Index: a useful compound performance measure for spinocerebellar ataxia. Neurology 2008;71:486–492. [DOI] [PubMed] [Google Scholar]
- 20. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 21. Lehrl S. Mehrfachwahl‐Wortschatz‐Intelligenztest MWT‐B. Balingen: Spitta Verlag, 2005. [Google Scholar]
- 22. Niemann H, Sturm W, Thöne‐Otto AIT, Willmes K. California Verbal Learning Test ‐ German adaptation. Frankfurt: Pearson Assessment, 2008. [Google Scholar]
- 23. Gronwall DM. Paced auditory serial‐addition task: a measure of recovery from concussion. Percept Mot Skills 1977;44:367–373. [DOI] [PubMed] [Google Scholar]
- 24. Bäumler G. Farbe‐Wort‐Interferenztest nach J.R. Stroop (FWIT). Göttingen: Hogrefe Verlag, 1985. [Google Scholar]
- 25. Stone VE, Baron‐Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci 1998;10:640–656. [DOI] [PubMed] [Google Scholar]
- 26. Heim S, Eickhoff SB, Amunts K. Specialisation in Broca's region for semantic, phonological, and syntactic fluency? NeuroImage 2008;40:1362–1368. [DOI] [PubMed] [Google Scholar]
- 27. Wilke M, Lidzba K. LI‐tool: a new toolbox to assess lateralization in functional MR‐data. J Neurosci Methods 2007;163:128–136. [DOI] [PubMed] [Google Scholar]
- 28. Knecht S, Drager B, Deppe M, et al. Handedness and hemispheric language dominance in healthy humans. Brain 2000;123(Pt 12):2512–2518. [DOI] [PubMed] [Google Scholar]
- 29. Eickhoff SB, Heim S, Zilles K, Amunts K. A systems perspective on the effective connectivity of overt speech production. Philos Trans A Math Phys Eng Sci 2009;367:2399–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whitfield‐Gabrieli S, Nieto‐Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. [DOI] [PubMed] [Google Scholar]
- 31. Good CD, Johnsrude IS, Ashburner J, et al. A voxel‐based morphometric study of ageing in 465 normal adult human brains. NeuroImage 2001;14:21–36. [DOI] [PubMed] [Google Scholar]
- 32. Clemm von Hohenberg C, Schocke MF, Wigand MC, et al. Radial diffusivity in the cerebellar peduncles correlates with clinical severity in Friedreich ataxia. Neurol Sci 2013;34:1459–1462. [DOI] [PubMed] [Google Scholar]
- 33. Akhlaghi H, Corben L, Georgiou‐Karistianis N, et al. Superior cerebellar peduncle atrophy in Friedreich's ataxia correlates with disease symptoms. Cerebellum 2011;10:81–87. [DOI] [PubMed] [Google Scholar]
- 34. Rizzo G, Tonon C, Valentino ML, et al. Brain diffusion‐weighted imaging in Friedreich's ataxia. Mov Disord 2011;26:705–712. [DOI] [PubMed] [Google Scholar]
- 35. Della Nave R, Ginestroni A, Tessa C, et al. Brain white matter tracts degeneration in Friedreich ataxia. An in vivo MRI study using tract‐based spatial statistics and voxel‐based morphometry. NeuroImage 2008;40:19–25. [DOI] [PubMed] [Google Scholar]
- 36. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004;23(Suppl. 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 37. Zhang H, Yushkevich PA, Alexander DC, Gee JC. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal 2006;10:764–785. [DOI] [PubMed] [Google Scholar]
- 38. Smith SM, Jenkinson M, Johansen‐Berg H, et al. Tract‐based spatial statistics: voxelwise analysis of multi‐subject diffusion data. NeuroImage 2006;31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 39. Wollmann T, Barroso J, Monton F, Nieto A. Neuropsychological test performance of patients with Friedreich's ataxia. J Clin Exp Neuropsychol 2002;24:677–686. [DOI] [PubMed] [Google Scholar]
- 40. Mantovan MC, Martinuzzi A, Squarzanti F, et al. Exploring mental status in Friedreich's ataxia: a combined neuropsychological, behavioral and neuroimaging study. Eur J Neurol 2006;13:827–835. [DOI] [PubMed] [Google Scholar]
- 41. Vollm BA, Taylor AN, Richardson P, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage 2006;29:90–98. [DOI] [PubMed] [Google Scholar]
- 42. Hoche F, Guell X, Sherman JC, et al. Cerebellar contribution to social cognition. Cerebellum 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Costafreda SG, Fu CH, Lee L, et al. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp 2006;27:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Akhlaghi H, Corben L, Georgiou‐Karistianis N, et al. A functional MRI study of motor dysfunction in Friedreich's ataxia. Brain Res 2012;1471:138–154. [DOI] [PubMed] [Google Scholar]
- 45. Ginestroni A, Diciotti S, Cecchi P, et al. Neurodegeneration in friedreich's ataxia is associated with a mixed activation pattern of the brain. A fMRI study. Hum Brain Mapp 2012;33:1780–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stefanescu MR, Dohnalek M, Maderwald S, et al. Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich's ataxia. Brain 2015;138:1182–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Franca MC Jr, D'Abreu A, Yasuda CL, et al. A combined voxel‐based morphometry and 1H‐MRS study in patients with Friedreich's ataxia. J Neurol 2009;256:1114–1120. [DOI] [PubMed] [Google Scholar]
- 48. Rezende TJ, Silva CB, Yassuda CL, et al. Longitudinal magnetic resonance imaging study shows progressive pyramidal and callosal damage in Friedreich's ataxia. Movement 2016;31:70–78. [DOI] [PubMed] [Google Scholar]
- 49. Werner CJ, Dogan I, Sass C, et al. Altered resting‐state connectivity in Huntington's disease. Hum Brain Mapp 2014;35:2582–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vieira Karuta SC, Raskin S, Carvalho Neto A, et al. Diffusion tensor imaging and tract‐based spatial statistics analysis in Friedreich's ataxia patients. Parkinsonism Relat Disord 2015;21:504–508. [DOI] [PubMed] [Google Scholar]
- 51. Nieto A, Correia R, de Nobrega E, et al. Cognition in late‐onset Friedreich ataxia. Cerebellum 2013;12:504–512. [DOI] [PubMed] [Google Scholar]
- 52. Diedrichsen J, Maderwald S, Kuper M, et al. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. NeuroImage 2011;54:1786–1794. [DOI] [PubMed] [Google Scholar]


