Abstract
The global industrialization has brought profound lifestyle changes and environmental pollutions leading to higher risks of cardiovascular diseases. Such tremendous challenges outweigh the benefits of major advances in pharmacotherapies (such as statins, antihypertensive, antithrombotic drugs) and exacerbate the public healthcare burdens. One of the promising complementary non-pharmacologic therapies is the so-called intermittent hypoxia training (IHT) via activation of the human body's own natural defense through adaptation to intermittent hypoxia. This review article primarily focuses on the practical questions concerning the utilization of IHT as a non-pharmacologic therapy against cardiovascular diseases in humans. Evidence accumulated in the past five decades of research in healthy men and patients has suggested that short-term daily sessions consisting 3–4 bouts of 5–7 min exposures to 12–10% O2 alternating with normoxic durations for 2–3 weeks can result in remarkable beneficial effects in treatment of cardiovascular diseases such as hypertension, coronary heart disease, and heart failure. Special attentions are paid to the therapeutic effects of different IHT models, along with introduction of a variety of specialized facilities and equipment available for IHT, including hypobaric chambers, hypoxia gas mixture deliver equipment (rooms, tents, face masks), and portable rebreathing devices. Further clinical trials and thorough evaluations of the risks versus benefits of IHT are much needed to develop a series of standardized and practical guidelines for IHT. Taken together, we can envisage a bright future for IHT to play a more significant role in the preventive and complementary medicine against cardiovascular diseases.
Keywords: Intermittent hypoxia, cardioprotection, hypertension, coronary heart disease, heart failure, complementary therapy
Introduction
According to a recent systematic analysis from the Global Burden of Disease Study 2010, ischemic heart disease, stroke, chronic obstructive pulmonary disease, lower respiratory infections, lung cancer, and HIV/AIDS were the leading causes of death in 2010.1 Among these diseases, ischemic heart disease and stroke collectively killed 12.9 million people, or one in four deaths worldwide in 2010.1 Economic transitions for urbanization and industrialization around the globe have brought profound lifestyle changes and environmental pollutions leading to higher risks of cardiovascular diseases. Such tremendous challenges outweigh the benefits of several major advances in pharmacotherapies (such as statins and other antihypertensive and antithrombotic drugs) and exacerbate the burdens of public healthcare, financially as well as socially. Therefore, a continuing search for novel and effective therapies for prevention and treatment of cardiovascular diseases remains highly important. One of the promising complementary or alternative non-drug therapies is activation of the human body's own natural defense through adaptation to intermittent hypoxia.
In general, the biological responses to intermittent hypoxia may be either adaptive or maladaptive, depending on the severity, frequency, and duration of hypoxemia. These complex systemic, cellular, and molecular mechanisms underlying the effects of intermittent hypoxia have been thoroughly described and extensively discussed in several recent review articles2–6 as well as two monographs that we have previously published.7,8
The term of “intermittent hypoxia” in medical literature is often referred to pathological processes such as obstructive sleep apnea. The mechanisms of maladaptive responses to intermittent hypoxia were the focal topics in several recent reviews.3,9–14 Conversely, the use of the so-called intermittent hypoxia training (IHT) in athletic and military practices as well as medical applications for prevention and treatment of various diseases has gained increasing attention and interests during the past few decades.7,8 The drastically different detrimental versus beneficial effects of intermittent hypoxia may be dependent upon the different numbers of hypoxic episodes, severity, and total exposure duration of hypoxia, which may mobilize the body's adaptive mechanisms or provoke dangerous pathological processes in more severe or prolonged hypoxia events.
Whereas several IHT modalities have been commonly used by elite athletes to enhance their exercise performance, much less numbers of clinical studies were reported to date on the use of IHT for the treatment of human diseases, including cardiovascular disorders. Nevertheless, the potential beneficial effects of IHT via various approaches (e.g. adaptation to high altitude, exposure to hypobaric chamber, and inhalation of hypoxic gas mixtures) on human cardiovascular system have been either experimentally demonstrated or proposed (summarized in Table 1), including: (1) improvement of metabolic processes in the myocardium, (2) enhancement of the myocardial tolerance to ischemia–reperfusion injury (i.e. anti-ischemic effect), (3) reduction of free radical damage at cellular level, (4) improvement of endothelial function and microcirculation, (5) positive inotropic effect on cardiac function, (6) normalization of blood pressure, (7) reduction in activity of the sympathetic nervous system, and (8) limiting blood viscosity and platelet aggregation.
Table 1.
Chronological list of the representative published original studies reporting the therapeutic use of various forms of intermittent hypoxia training (IHT) in patients with cardiovascular diseases
| Authors, published year, reference # | No. and types of human subjects | Forms of IHT and equipment used | Protocol and duration of IHT | Beneficial effects | Country of researchers |
|---|---|---|---|---|---|
| Katiukhin et al. (1979)15,16 | Patients with Stages I or II hypertension | Hypobaric chamber | Blood pressure lowering effects in Stage I patients, but not Stage II patients, increase in stroke volume and right:left ventricle mass ratio, synergistic with the conventional pharmacotherapy | Russia | |
| Evgen'eva et al. (1989)17 | 44 pregnant women with Stages I–II hypertension and hypertensive neurocirculatory dystonia | Blood pressure lowering effects | Russia | ||
| Aleshin et al. (1993)65 | Patients with borderline hypertensive | Hypobaric chamber | 3500 m simulated altitude (30 min/day, 5 days/week for three weeks) | Blood pressure lowering effects | Russia |
| Potievskaia (1993)52 | Patients with hypertension | Blood pressure lowering effects; changes in salt and water metabolism may have contributing role | Russia | ||
| Vorob'ev et al. (1994)19 | 123 patients with Stages I and II essential hypertension | Normobaric IHT | 16–20 IHT sessions needed for Stage I hypertension and 26–30 sessions needed for Stage II patients | Blood pressure lowering effects | Russia |
| Agostoni et al. (2000)20 | 14 normal subjects and 38 patients with stable HF | Hypobaric chamber | Simulated altitude from 1000 to 3000 m | HF patients had good tolerance to IHT | Italy |
| Tin'kov and Aksenov (2002)21 | 46 male patients with coronary heart disease (including 36 had MI history, 16 had ischemic episodes) with abnormal blood profile of lipids | Hypobaric IHT in multipatient pressure chamber Ural-1 USSR | 22 daily 3 h sessions under 460 mmHg barometric pressure | 7–9% reduction in cholesterol, 11–13% reduction in LDL, 12% increase in HDL | Russia |
| Simonenko et al. (2003)22 | 30 hypertensive patients treated with antihypertensive medications combined with IHT and 32 drug-alone hypertensives | Normobaric IHT | 24 h blood pressure monitoring | Reduction in nocturnal arterial pressure and decreased number and duration of hypertensive episodes | Russia |
| Balykin et al. (2004)72 | Obese patients | Normobaric IHT combined with exercise | Combination of IHT and exercise increased cardiorespiratory functional reserves, physical performance, and aerobic capacity to greater extents | Russia | |
| Burtscher et al. (2004)24 | Healthy controls and patients with prior MI (50–70 years) | Normobaric IHT | 15 daily sessions of 3–5 hypoxic periods (14–10% FIO2), 3–5 min each followed by 3 min reoxygenation period | Increased hematocrit and hemoglobin; lowered heart rate, blood lactate accumulation during submaximum exercise; increase in oxygen consumption, workload, minute ventilation and arterial O2 content, and suppressed lactate accumulation during maximum exercise | Austria |
| del Pilar Valle et al. (2006)25 | Six normotensive male patients (age >53 years) with severe but stable CAD, all had CABG | Hypobaric chamber | 14 daily 4 h sessions of hypobaric IHT, progressively increasing to simulated altitude of 4200 m | Significant improvement in myocardial perfusion | Peru |
| Mukharliamov et al. (2006)23 | 56 patients with stages I–II hypertension | Normobaric IHT | 10 daily sessions of 10 cycles/day of 5 min hypoxia (10–14% O2) and 5 min normoxia | Enhanced efficacy of conventional antihypertensive medications on reduction of SBP and DBP, heart rate and peripheral resistance | Russia |
| Elizarov et al. (2007)26 | 30 patients with CAD | Normobaric IHT | 10 daily courses of IHT | Antianginal effect observed in 70% of patients, including reduced number of angina attacks per day and lowered daily doses of angina relief drugs—nitroglycerin and beta-blockers | Russia |
| Shatilo et al. (2008)27 | 45 elderly patients with stable angina and I and II functional classes | Normobaric IHT with “Hypotron” device | Each IHT session consisted of four cycles of 5 min hypoxic mixture breathing (12–14% oxygen) alternated with 5 min air breathing | Reduction in angina symptoms and daily cardiac ischemia duration. Normalization of lipid metabolism; increase in exercise tolerance | Ukraine |
| Ushakov et al. (2010)28 | Healthy subjects or patients | Hypobaric chamber with intermittent inhalation of hyperoxic gas mixtures | 10 daily 1 h sessions at simulated altitude 3000–5000 m. Each session comprised 7 min hypobaric hypoxia (breathing air) alternating with 3 min hyperoxia (breathing oxygen) | Improvement of the functional state and the resistance to extreme environmental conditions | Russia |
| Korkushko et al. (2010)29 | 29 elderly patients with Stage II hypertension | 10 days of IHT combined with the drug therapy of angiotensin-converting enzyme inhibitor (enalapril) | Reduced SBP at rest (5.8%) and during mild exercise (18.8%). Antihypertensive effects persisted for two months | Ukraine | |
| Tin'kov et al. (2011)18 | 46 postmenopausal women (age 54 ± 4 years) with arterial hypertension | Hypobaric IHT in multipatient pressure chamber Ural-1 USSR | 22 daily 3 h sessions under 460 mmHg barometric pressure | 13.9% reduction in SBP, 8.2% in DBP, 14.7% decrease in serum cholesterol, 21.3% lowered blood glucose, 19.3% increase in estradiol level | Russia |
| Minvaleev (2011)30 | Patients | Moderate IHT with hypobaric chamber | Decrease in total cholesterol and LDL, increase in HDL | Russia | |
| Lyamina et al. (2011)31 | Patients with Stage I arterial hypertension | Normobaric IHT | 20 daily sessions of 4–10 cycles of 3 min hypoxia (10% inspired O2) and 3 min room air breathing) | Reduction in blood pressure and increase in nitric oxide synthesis | Russia |
| Rachok et al. (2011)32 | 60 patients with ischemic cardiomyopathy and chronic HF LVEF <35% | Normobaric IHT with “Bio-Nova-204” one-patient hypoxicator | IHT prior to CABG | Reduction in ventricular arrhythmia and serum levels of endothelin-1, TNFα, and homocysteine; Better vascular endothelial function; less post-CABG perioperative MI, fewer ventricular fibrillation during resuscitation, lower demand for inotropic support | Russia |
| Saeed et al. (2012)33 | HF patients with LVEF <35% | Normobaric IHT with a “Hypoxico” sealed enclosure and air delivery unit | 10 daily 3–4 h sessions of normobaric IHT, equivalent to 1500–2700 m of altitude | Improvement in exercise time, 6 min walk distance, skeletal muscle strength, and quality of life scores, and LVEF. Sustained one month after IHT | USA |
CABG: coronary artery bypass graft surgery; CAD: coronary artery disease; DBP: diastolic blood pressure; HDL: high-density lipoprotein; HF: heart failure; LDL: low-density lipoprotein; LVEF: left ventricular ejection fraction; MI: myocardial infarction; SBP: systolic blood pressure.
In the overall context, this review article primarily focuses on the practical questions concerning the utilization of IHT as a non-pharmacologic therapy against cardiovascular diseases in humans. Special attentions are paid to the therapeutic effects of different IHT models, along with a brief introduction of a variety of specialized facilities and equipment that are currently available for the potential clinical applications of IHT.
Forms and types of therapeutic hypoxia
The concept of cardiac ischemic preconditioning has been well known for over 30 years in the cardiovascular research and clinical cardiology communities since the first report of this potent cardioprotective phenomenon in a canine model of myocardial infarction by Murray and colleagues in 1986.34 In the years around 2000, we and others have demonstrated in various animal species (mouse, rat, dog) that intermittent systemic hypoxia alone is sufficient to trigger adaptive responses that lead to the enhanced myocardial tolerance to ischemia–reperfusion injury.35–40 However, different models of systemic hypoxia may have specific effects on myocardial tolerance to ischemia: Whereas severe chronic hypoxia (long-term living at high altitude, chronic obstructive pulmonary disease and congenital heart disease, anemia, blood O2 carrying abnormalities, CO poisoning, chronically decreased tissue perfusion, etc.) invariably leads to depressed myocardial tolerance to ischemia, moderate chronic hypoxia may afford cardioprotective effects. In addition, it was reported that chronic hypoxia with daily repetitive short-term reoxygenation episodes is also cardioprotective.41 Indeed, there are substantial differences between effects of chronic intermittent and chronic constant hypoxia, which suggest that in addition to oxygen deprivation, the frequency of hypoxia–reoxygenation cycles is of importance in developing the adaptation mechanisms.4,42
High altitude sojourns: A natural form of hypoxia therapy for cardiovascular diseases?
The initial scientific observations of beneficial and detrimental effects of hypoxia on cardiovascular system were made among the high mountain resident or visitors. The human body's physiological responses to high altitude include hyperventilation, polycythemia, increased sympathetic tone, pulmonary vasoconstriction, elevated levels of heart rate, cardiac contractility and output, and increased capillary density in cardiac and skeletal muscles.43–45 Some of the adaptive responses may represent risk factors in patients with cardiovascular pathology, which include the further stimulation of the already activated sympathetic nervous system, unfavorable increase in heart rate, myocardial oxygen demand and ventricular afterload, as well as the enhanced interdependence between the right and left ventricle due to the increased pulmonary artery pressure.46 The consequences of stay at high altitude for patients with coronary artery disease, congestive heart failure, arterial hypertension, anomalies of the pulmonary circulation, arrhythmias, etc. appear to be detrimental.47,48
On the other hand, the curative effects of moderate high altitude on healthy humans and patients with cardiovascular disorders are also known for many decades.49,50 Lower incidence of myocardial infarction was reported in people living at high altitude.51 It was also shown that sojourn at moderate high altitude reduced arterial blood pressure, dopamine and epinephrine excretion, and plasma renin activity.52 Subsequently, it was observed that the hypertensive patients had reduced systolic and diastolic blood pressure during sojourn at moderate altitudes of 1285–2650 m.53 Interestingly, in 2009 Faeh et al. investigated the mortality rate from coronary heart disease and stroke at higher altitude regions in Switzerland and found that those who were born at high altitude had an additional and independent beneficial effect on coronary heart disease mortality.54 More recently, a new report from these researchers showed that in the model not adjusted for other environmental factors, the mortality ischemic heart diseases linearly decreased with increasing altitude resulting in a lower risk (hazard ratio 0.67 for those living > 1500 m (versus < 600 m).55 This association remained after adjustment for all other environmental factors 0.74.
In addition, Burtscher indicated that although humans show adaptive responses even to altitudes below 2000 m or corresponding normobaric hypoxia (FiO2: >16%), most of the subjects without severe pre-existing diseases well tolerate altitudes up to 3000 m (FiO2: 14.5%).56 Dehnert and Bartsch suggested that sojourn and exercise at high altitude of 3000–3500 m is generally safe for the patients with stable coronary heart disease and sufficient work capacity, although these patients should avoid travel to altitude above 4500 m.57 In addition, Sarybaev et al. investigated workers commuting between an elevation of 3700 and 4200 m (four-week work shift) and lowland (<500 m, four weeks of holiday) and they found that intermittent exposure to 4000 m for three years did not develop permanent pulmonary hypertension.58 Most recently, Vinnikov et al. studied 472 workers of a high-altitude mining company who used to have working shifts of two weeks at altitude of 4000 m, followed by two weeks of rest at lowland, cumulative exposure time—6 months and reported that one-year intermittent exposure to hypobaric hypoxia was not associated with significant increase in blood pressure.59
More recent evidence has emerged showing beneficial effects of high altitude sojourns or training on cardiovascular risk factors. Gutwenger et al. recently studied the effects of a two-week hiking vacation at moderate versus low altitude on adipokines and parameters of carbohydrate and lipid metabolism in patients with metabolic syndrome.60 They found that altitude training was more beneficial for normalizing lipid parameters than those trained at low altitude. However, another recent study reported that moderate-intensity activity with weekly hiking did not further reduce cardiovascular risk factors in elderly persons with a relatively normal cardiovascular risk profile.61
Nevertheless, the effects of high altitude exposure in older individuals and patients with coronary artery disease need further studies and it is recommended that a graded exercise test might be beneficial for elderly patients with cardiovascular diseases prior to high altitude in order to reduce the risk of possible adverse events.48
The periodic presence to the mountains for mining works at high altitude is independently associated with some health problems,62 such as exacerbated heart failure in the workers with pre-existing history of congestive heart failure.
Taken together, based on the published studies to date, the short stays at high altitude up to 3000–3500 m (corresponding to ∼14.5 to 13.5% inspired O2 at sea level) do not cause negative effects among the patients with subclinical coronary heart disease and hypertension. Actually in many cases the positive effects of high altitude sojourns on the patients' cardiovascular system were reported. However, high altitude sojourns require highly specialized medical and travel arrangements that usually involve higher financial costs and variabilities due to different mountain terrain and climate conditions. Therefore, it unlikely becomes a standardized mainstream medical therapy.
IHT in hypobaric chambers
Several decades ago, simulated altitude chambers were extensively used for training pilots, paratroopers, athletes, and spacemen in the former Soviet Union63 (Figure 1). This method has also been approved for prophylactic and rehabilitative medical application in the East European countries, including the use of intermittent hypobaric hypoxia for cardiovascular protection (Figure 2). These multipatient hypobaric chambers were usually used with daily treatment sessions at simulated altitudes of 1500–3500 m, which typically last from 30 min to 3 h/day for 10–30 days. Favorable effects on blood pressure were seen in approximately 60% of hypertensive patients completing the hypobaric training program.64
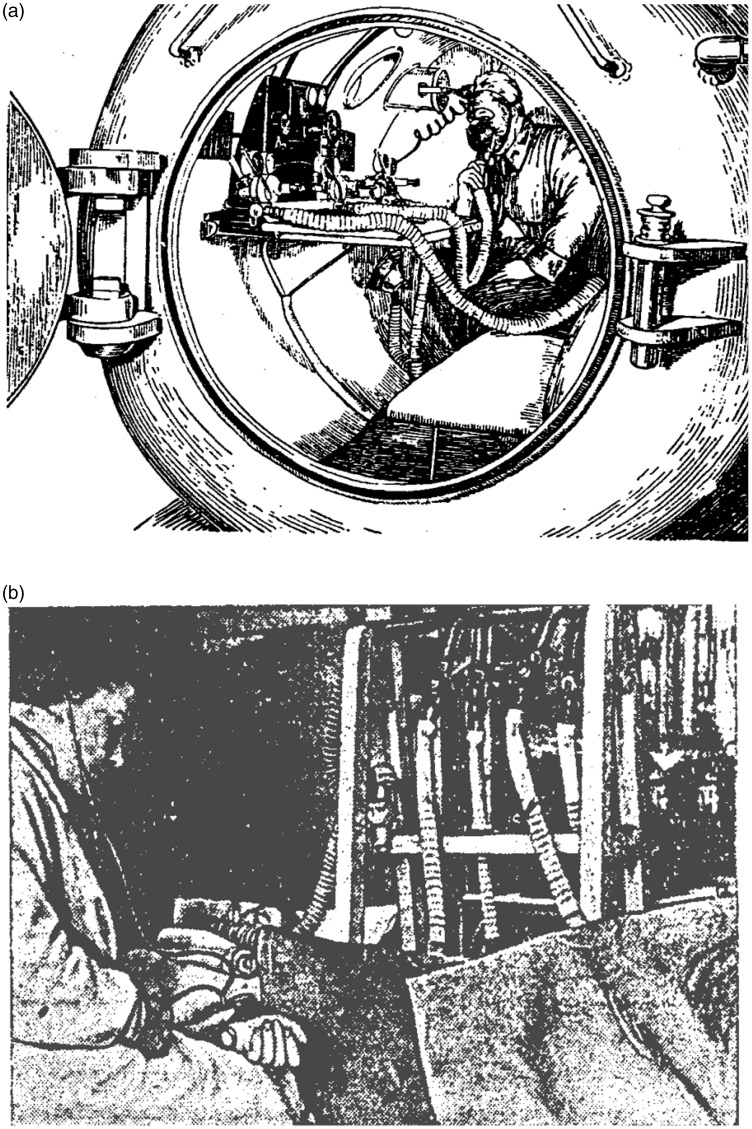
Figure 1.
Simulated altitude chambers that were extensively used for training pilots, paratroopers, athletes, and spacemen in the former Soviet Union. (a) Training barochamber in the Military Medical Academy, Leningrad, USSR (1930–1935). The picture is adopted from: Physiology and Hygiene of Altitude Flight (Edited by Krotkov FG), Narkomzdrav USSR, Moscow-Leningrad, 1938. (b) Experiment with hypoxic training. The image is adopted from Krotkov FG. Aviation Hygiene. In: Handbook in Military Hygiene. Narkomzdrav USSR, Moscow-Leningrad, 1939
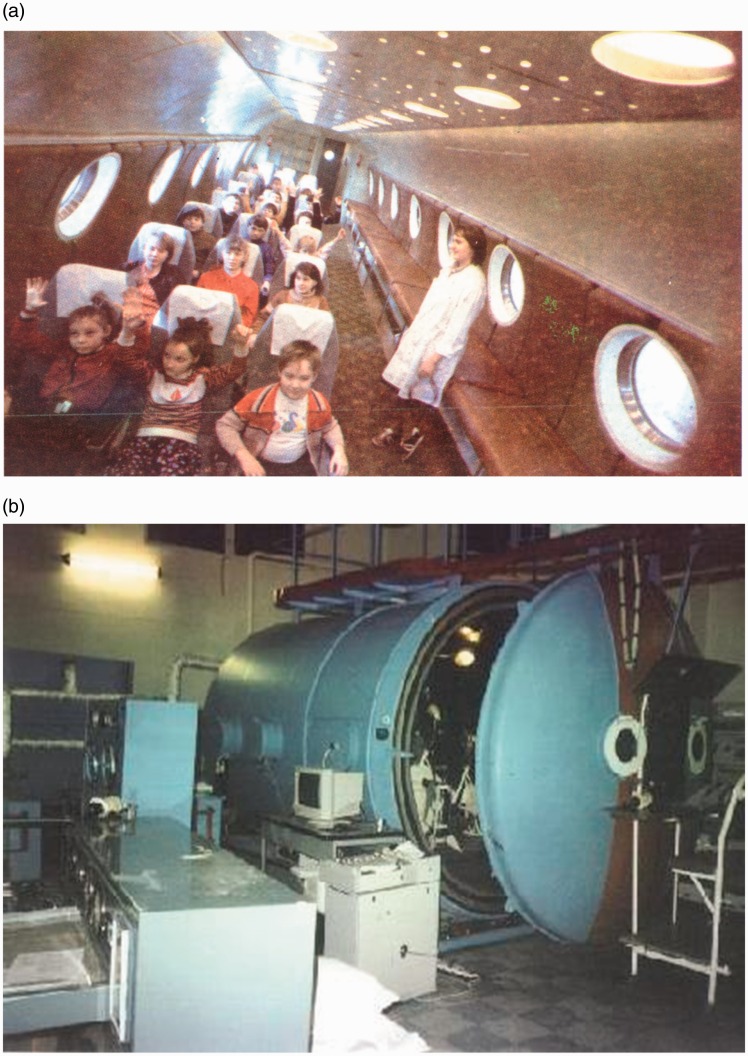
Figure 2.
(a) The barochamber Ural-1 USSR (Orenburg Medical University, 1970–1990). The picture is adopted from Meerson FZ, Tverdohlib VP, Boev VM, Frolov BA. Adaptation to Periodic Hypoxia in Therapy and Prophylaxis. Moscow-Orenburg, Nauka, 1989. (b) The training thermo-barochamber in Terskol, International Medico-Biological Scientific Centre, Russia-Ukraine (1970 to present). This large climatic test bench can reconstruct the atmospheric conditions equivalent to 9000 m altitude. The picture is adopted from http://www.terskol.com. (A color version of this figure is available in the online journal.)
As chronologically summarized in Table 1, these clinical research efforts have produced a number of publications particularly in the period of 1970s–1980s. For example, Meerson and co-workers reported a decrease in arterial pressure during adaptation to 3500 m simulated altitude (30 min/day, 5 days/week for three weeks) in borderline hypertensive patients.65 Similarly, Katiukhin et al. applied hypobaric IHT to patients with stages I or II hypertension. All patients reported feeling better, and most of them experienced appreciable reduction in arterial blood pressure.15,16 However, patients in stage II hypertension did experience a rise in blood pressure in the afternoon following each IHT session. An increased stroke volume without changes in heart rate and a decreased peripheral vascular resistance were observed by the end of the IHT program. IHT also produced electrocardiographic right axis shift and increased right:left ventricle mass ratio.15,16 These changes subsided within 2–3 weeks following the end of IHT sessions. Interestingly, the effectiveness of antihypertensive medications was increased after IHT, indicating a synergistic interaction between IHT and the conventional pharmacotherapy for hypertension.
In 2006, del Pilar Valle et al. studied six normotensive elderly male patients with severe but stable coronary artery disease who had coronary artery bypass surgery.25 They underwent 14 daily 4 h sessions of hypobaric IHT, progressively increasing to a maximal simulated altitude of 4200 m. Myocardial perfusion in these elderly patients was significantly improved after the IHT program.
A more recent study by Tin'kov et al. used the pressure chamber Ural-1 USSR for treatment of 46 postmenopausal women (mean age 54 ± 4 years) with arterial hypertension.18 Adaptation to hypobaric IHT (22 3 h daily sessions under 460 mmHg barometric pressure) reduced systolic pressure by 13.9% and diastolic pressure by 8.2%, along with improvement in cardiovascular risk factors (e.g. reduced serum cholesterol level by 14.7%, lowered glucose level by 21.3%, and increased estradiol level by 19.3%).18 In 2002, these researchers also published results on 46 male patients with coronary heart disease (including 36 had a history of myocardial infarction and 16 had ischemic episodes) with abnormal blood profile of lipids.21 At the end of the similar IHT protocol reduced blood levels of total cholesterol by 7% and this decrease maintained by 9% at three and six months after the IHT. High-density lipoprotein (HDL) levels increased by 12% at three months post-IHT follow-ups and remained significantly higher until six months. Conversely, low-density lipoprotein (LDL) levels declined on completion of IHT and were lower at three-month (13%) and six-month (11%) post-IHT period. Similar favorable changes were found in very LDL and triglycerides and the beneficial effects were more pronounced in the patients with higher baseline levels of serum lipids.21
In the medicated and stable hypertensive subjects, hypobaric IHT (2.5 h to four weeks at 1285–2650 m) reduced the resting systolic and diastolic blood pressure for 26 and 13 mmHg, respectively, whereas no significant effect of IHT on systolic and diastolic blood pressure was found in the healthy subjects.66
Another interesting combination protocol was used by Ushakov et al. to treat patients in a hypobaric chamber with intermittent inhalation of hyperoxic gas mixtures.28 Each patient received 10 1 h daily sessions at simulated altitude 3000–5000 m. Each session comprised a 7 min hypobaric hypoxia (breathing air) alternating with 3 min hyperoxia (breathing oxygen). This combination therapy was shown to be highly efficacious in improving the functional state of the organism and its resistance to extreme environmental conditions.28 In addition, Minvaleev and co-workers recently reported that the maximal rate of antiatherogenic changes of serum lipid profile (i.e. decreased total cholesterol and LDL, increased HDL) is characteristic for a combination of three conditions: (1) moderate IHT, (2) moderate physical activities, and (3) special exercises for increase of cold tolerance (a form of the Tibetan yoga).30
Nevertheless, as for any other therapies, the use of barochambers for treatment and prophylaxis of cardiovascular diseases in humans may carry its own risks and a third of the patients subjected to hypobaric IHT in simulated altitude chambers had side effects such as headache, stenocardia, and cardiac rhythm disturbances. Furthermore, barochambers for human use are often expensive to build and demand personnel with necessary medical specialty skills, which may not be practical in many healthcare settings. To determine and control the appropriate hypoxia dosage for each individual patient in the settings of hypobaric chamber is also a great challenge. It was suggested that intermittent hypobaric hypoxia may produce harmful biochemical changes, including decreased antioxidant capacity and increased lipid peroxidation, which may lead to suppression of vascular endothelial function and impairment of vascular hemodynamics.67
Intermittent hypoxia therapy with inhalation of hypoxic gas mixtures
The above-discussed limitations and disadvantages of hypobaric chambers prompted studies in recent decades on a different and more practical form of IHT—normobaric hypoxia training, which is carried out by breathing of hypoxic gas mixtures under normobaric environment. A variety of technical implementations for this treatment approach have been tested, including normobaric hypoxia rooms or suites and the so-called hypoxicators—a new class of biomedical devices that was first introduced by the former Soviet Union scientists for simulating altitude training in military personnel and sportsmen and for treatment of various human diseases.68,69 These equipment or devices typically contain polymer membranes, which can separate O2 and N2, are convenient for generating the hypoxic gas mixtures in open circuit devices and chambers.70 More technical details about the hypoxicators are described in our recent book.71 The following sections aim to provide an overview on the key clinical findings obtained from various types of normobaric IHT.
IHT in normobaric hypoxia rooms, tunnels, or tents
Only few published studies used the hypoxia rooms or tents for treating patients with cardiovascular disorders. For instance, Agostoni et al. studied 14 normal subjects and 38 patients with clinically stable heart failure at simulated altitude from 1000 to 3000 m.20 They demonstrated the safety of exposing stable heart failure patients to moderate altitude up to 3000 m. Another recent study by Saeed et al. on heart failure patients with left ventricular ejection fraction (LVEF) <35%,33 an “Hypoxico” sealed enclosure and air delivery unit was used for this study (Figure 3). Patients underwent and well tolerated the incremental normobaric IHT for 10 daily sessions (each 3–4 h). After completion of IHT, there were significant improvements in exercise time, 6 min walk distance, skeletal muscle strength, and quality of life scores and a trend toward improvement in LVEF, which were sustained after one month.
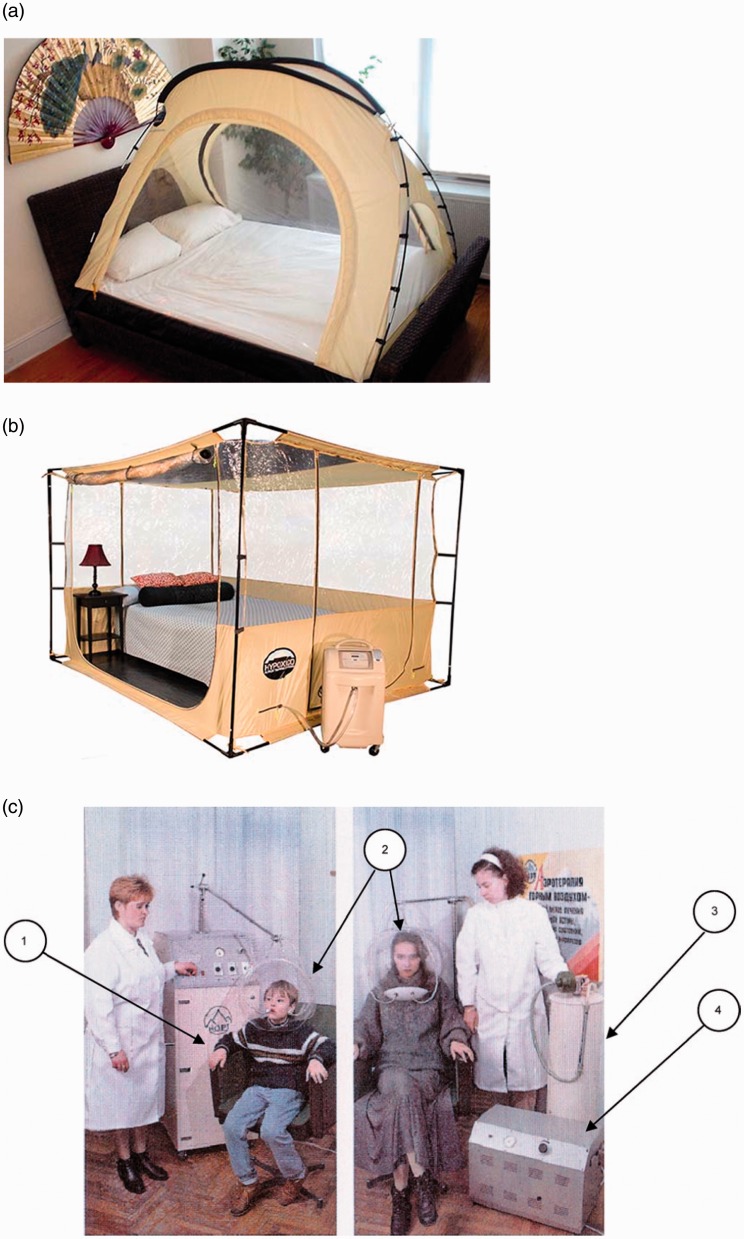
Figure 3.
(a) Hypoxico® portable altitude tent. The tent fits on the bed box spring or on the floor with a mattress inside. The image is adopted from: http://www.hypoxico.com/products/portable-altitude-tent/. (b) Hypoxico® at-home cubicle system. It offers the user a more comfortable and spacious atmosphere for in a more permanent setting. The picture is adopted from: http://www.hypoxico.com/products/at-home-cubicle/. (c) A portable device “Borei-5” (NORT Company, Ukraine). The arrows indicate: (1) control unit, (2) isolation helmet, (3) gas-separating column, and (4) compressor. The image is adopted from Orotherapy. Lectures of Academy of Hypoxia Problems. Edited by Berezovsky VA, Levashov MI, Kiev, Logos, 1998. (A color version of this figure is available in the online journal.)
Several companies have produced this line of IHT equipment that can be used in cardiovascular patients. For example, Hypoxico Inc. (New York, USA) produces altitude sleeping systems including working rooms or portable tents (Figure 3(a) and (b)). NORT Company (Ukraine) manufactures “Orothron” that allows IHT sessions for up to six patients simultaneously. A portable device “Borei-5” (NORT Company, Ukraine) consists of: (1) control unit, (2) isolation helmet, (3) gas-separating column, and (4) compressor (Figure 3(c)). Hypoxia-treatment complex “Edelweiss” (NVF METAKS Company, Russian Federation) uses membrane technologies and is equipped with a monitoring system of the internal environment and the patient's physiological parameters.
Benefits of normobaric IHT (face mask method) in patients with hypertension, coronary heart disease, and heart failure
Another more convenient, efficient, and low-cost means of normobaric IHT is the face mask method, which consists of a flow circuit containing face mask with valves for inspiration/expiration and a buffer container for hypoxic gas mixture in the line of inspiration (Figure 4). Special devices have been developed for this particular method.71 Using this method, the Russian and Ukrainian researchers have performed a number of studies concerning the effects of IHT on cardiovascular diseases, such as hypertension and coronary heart disease.
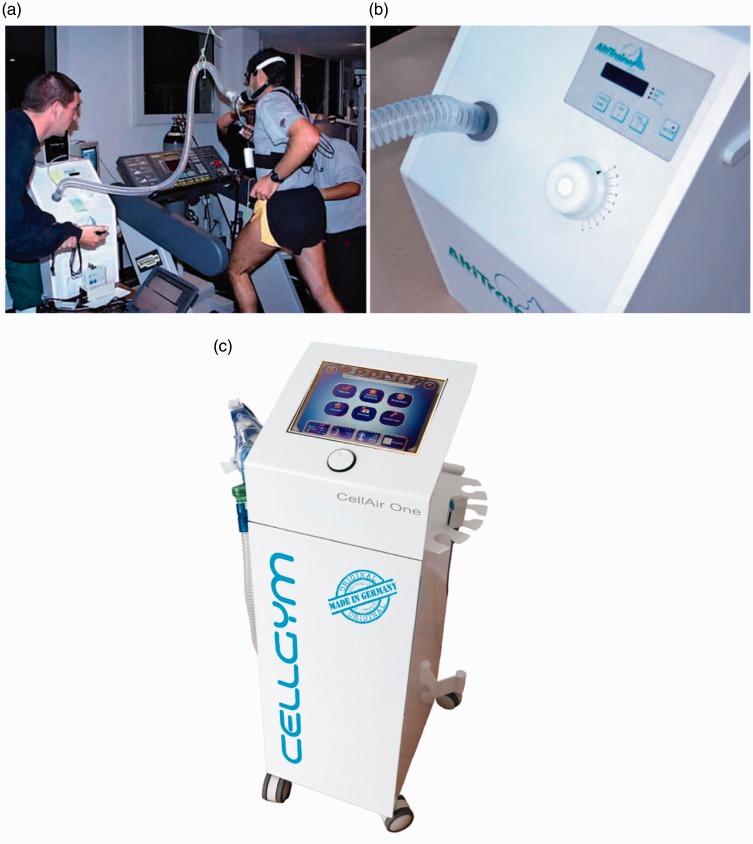
Figure 4.
The commercial image representing: (a) and (b): AltiTrainer200® (Switzerland). The picture is adopted from: http://www.smtec.net/en/products/altitrainer. (c) CellAir One® (Germany), a device for hypoxic–hyperoxic training. The picture is adopted from: http://cellgym.de/products/ihht-systems/?lang=en. (A color version of this figure is available in the online journal.)
For example, Vorob'ev et al. studied antihypertensive effects of IHT in 123 patients with stages I and II essential hypertension.19 The authors suggested 16–20 IHT sessions for patients with stage I hypertension and 26–30 sessions for stage II patients could provide optimal depressor effects. Similarly, Mukharliamov et al. conducted a 10-day IHT program of 10 cycles/day of 5 min hypoxia (10–14% O2) and 5 min normoxia in 56 patients with stages I–II hypertension.23 This IHT program enhanced the efficacy of conventional antihypertensive medications on reduction of systolic and diastolic blood pressure, heart rate, and peripheral resistance. In addition, Simonenko et al. studied 30 hypertensive patients receiving antihypertensive medications combined with normobaric IHT and 32 control hypertensive patients treated with drugs alone.22 Their results of 24 h blood pressure monitoring revealed that compared with the drugs alone controls, the combination therapy group (IHT + drugs) had more normalized 24 h arterial pressure profile, i.e. more pronounced reduction in nocturnal arterial pressure and decreased number and duration of hypertensive episodes.22
Lyamina et al. treated stage 1 hypertensive patients with 20-day IHT (4–10 cycles of 3 min hypoxia (10% inspired O2) and 3 min room air breathing).31 IHT increased nitric oxide synthesis and decreased blood pressure in the hypertensive patients. The IHT-induced enhancement of NO synthesis was especially robust in the patients with more than five years of hypertension history. The reduction in blood pressure persisted for more than three months. IHT also lowered blood pressure in pregnant women with hypertensive neurocirculatory dystonia and stages I–II hypertension.17 Potievskaia suggested that changes in salt and water metabolism may have contributed to these persistent hypotensive effects of hypoxia.52
Balykin et al. determined the changes in cardiorespiratory function in obese persons following various combinations of normobaric IHT and physical exercise and they reported that the combination of IHT and exercise increased cardiorespiratory functional reserves, physical performance, and aerobic capacity to greater extents than either of the modalities alone.72
Furthermore, clinical and functional effects of a 10-day course of normobaric IHT was evaluated in 30 patients with coronary heart disease in comparison with 30 patients without IHT.26 IHT course began with a pre-evaluation of hypoxia tolerance threshold for each patient based on electrocardiogram criteria and the occurrence of angina pectoris. Subsequent treatments were conducted with gradual reduction of oxygen content in the inhaled gas mixture for reaching hypoxia level up to 80% oxygen saturation. An antianginal effect was observed in 70% of patients following IHT, which also reduced the number of angina attacks per day and the average daily doses of nitroglycerin for rapid angina relief and beta-blockers—metoprolol. IHT also decreased myocardial oxygen consumption at rest as well as during standard exercise test, indicating an energy-saving effect of IHT on the myocardium of heart failure patients. An improvement of the quality of life score and self-reported well-being was also reported. Another application of the “Bio-Nova-204” one-patient hypoxicator in the settings of cardiothoracic surgery was reported by Rachok et al.32 The efficacy of IHT before coronary artery bypass grafting surgery (CABG) was tested in a cohort of 60 patients with ischemic cardiomyopathy and chronic heart failure (LVEF <35%). After a course of IHT, there were a shift in autonomic balance toward the prevalence of parasympathetic nervous system and a significant reduction in ventricular arrhythmia events. IHT also improved vascular endothelial function and decreased serum levels of endothelin-1, TNFα, and homocysteine. In addition, during the intra- and early postoperative period of CABG, the IHT-treated patients had less perioperative myocardial infarction and fewer ventricular fibrillation events during cardiac resuscitation as well as less demand for high dose inotropic support.
Normobaric IHT (face mask method) in elderly patients with cardiovascular diseases
The use of IHT in geriatric patients remains to be a matter for debate. Some researchers believed that elderly patients would neither tolerate nor benefit from IHT due to their increasing fragility of old age,73 including the age-dependent declines in gas exchange that maintain oxygenation, pulmonary vital capacity, and hypoxic ventilatory drive.74,75 It was demonstrated that IHT efficiency is decreased along with the aging process.76
On the other hand, there is considerable evidence that the elderly can readily acclimate to moderately high altitudes,75,77,78 and therefore should be able to tolerate the brief periods of moderate hypoxia during a typical IHT protocol. This concept is supported by the study results from Burtscher et al.24 in middle aged and elderly men (50–70 years) with and without prior myocardial infarction who completed 15 daily sessions of IHT. Each session consisted of 3–5 hypoxic periods (14–10% FIO2), 3–5 min each followed by a 3 min reoxygenation period. The IHT regimen slightly increased hematocrit and hemoglobin content, lowered heart rate, blood lactate accumulation and perceived exertion during submaximal exercise, and increased O2 consumption, workload, minute ventilation, and arterial O2 content during maximum exercise while again suppressing lactate accumulation. The authors concluded that such short-term IH exposures increase aerobic capacity and exercise tolerance not only in the healthy elderly persons but also the patients with coronary artery disease.
Subsequently, Korkushko et al. studied 29 elderly patients with stage II hypertension who completed 10 days of IHT combined with the drug therapy of angiotensin-converting enzyme inhibitor (enalapril). The completion of IHT program reduced systolic blood pressure at rest (by 5.8%) and during mild exercise (by 18.8%). The antihypertensive effects persisted for two months. Another study from this group was conducted in 45 elderly patients with stable angina and I and II functional classes.29 They used a “Hypoxytron” device as shown in Figure 5. Each IHT session consisted of four cycles of 5 min hypoxic mixture breathing (12–14% oxygen) alternated with 5 min air breathing. The IHT by “Hypoxytron” device was well tolerable and safe for vast majority of healthy elderly control subjects and elderly patients with ischemic heart disease. IHT led to the reduction in clinical symptoms of angina and of daily myocardial ischemia duration, normalization of lipid metabolism, and increased exercise tolerance. IHT also had positive effects on hemodynamics, microvascular endothelial function, and work capacity in healthy senior men.27
Figure 5.
A laboratory image representing the Rebreather Hypoxytron® (Ukraine) device, which is capable of buffer reservoir volume regulation for individual hypoxia dosage (Photo taken by T.V. Serebrovskaya). (A color version of this figure is available in the online journal.)
Collectively, these studies support the therapeutic application of normobaric IHT, alone or in combination with pharmacological remedies, to treat cardiovascular diseases in elderly patients. The described positive effects of IHT are generally opposite to the age-related characteristic changes of an elderly individual.
IHT with rebreathing technique
It is noteworthy that a special type of hypoxicator applies rebreathing principle in semi-closed flow circuit and was named as “autohypoxicator.”69 Its expiration line contains carbon dioxide absorber and its circuit has pneumatic connection to the atmosphere through buffer reservoir, either rigid or elastic.79 In such devices the process of hypoxic gas mixture formation depends upon three factors: (1) the patient's oxygen consumption, (2) binding of carbon dioxide, and (3) atmospheric air inflow into the circuit during inspiration. During a rebreathing session, the oxygen concentration gradually falls as the function of rebreathing time.
Some autohypoxicators80,81 have advanced features that are capable to regulate the buffer reservoir volume according to each patient's anthropometric parameters by fixing sylphon bellows in certain position or by spiral movement of the spring within the device71 (Figure 5). In brief, the initial inspired gas is atmospheric O2 (21%) and inspired O2 would fall to 12% after 60–90 s of rebreathing. Then O2 is added gradually to the device to maintain inspired O2 at 12% for the remaining 3.5–4 min with a final arterial O2 saturation typically 89–92%.
Although there is no report yet on the use of rebreathing techniques for treating cardiovascular diseases, we still consider this method as a promising modality for the future practice of IHT. This is because the rebreathing devices are easy and comfortable for the patients to use at home and are also much less expensive than the concentrators. However, a major restriction of the rebreathing device is the difficulty to disinfect, despite the presence of bacterial filter in its expiratory circuit. Therefore, each patient should have his/her own individual device to avoid cross-contamination of pathogens.
Optimal and individualized regimens of IHT for cardiovascular diseases
Substantial methodological variations exist among the published IHT studies up to date. The differences include: (1) intensity of hypoxia (ranging from 2 to 18% inspired oxygen); (2) duration of each of the hypoxic and normoxic episodes (ranging from 15 to 30 s to 12 h); (3) number of cycles per day (ranging from three to 25 sessions); and total training course (ranging from two to 90 days), which have apparently added complexity in comparing the results obtained from different studies. Therefore, seeking a standardization of the effective regimens for IHT has been a key demand in the field of intermittent hypoxia research.4,5,82
In addition, the proven influence of both hereditary and environmental parameters on physiological responses to hypoxia in humans underscores the importance of selection of individualized regimens for disease treatment. More than three decades ago, in a longitudinal twin investigation, we designed a nomogram to estimate individual non-specific reactivity and functional reserves for prognosis of the subject's adaptation to hypoxia.83 Various strategies were developed for customized IHT regimens according to the individually determined sensitivity to hypoxia.84 Also the heart rate changes during a steady decrease in arterial oxygen saturation (SaO2) were used by Russian scientists for predicting the prognosis of individual adaptation to hypoxia and for the selection of optimal treatment regimens. Estimation of individual patient sensitivity to hypoxia for selecting an optimal IHT regimen was also investigated.85
Furthermore, in 2009 Bassovich and Serebrovskaya proposed a novel approach to objectively quantify the dosage of hypoxia—the so-called Hypoxia Training Index (HTi).79 This parameter is calculated by analyzing the SaO2 curve during a hypoxic test and provides a more objective measure of the hypoxic stress delivered during the IHT session, as compared with the simple reliance on FiO2. HTi provides an index of dosage received by the individual at the end of the session. Knowledge of HTi can therefore be used to alter the training regimen for different individuals, compensating for individual variability, and to ensure that hypoxia exposure was correctly controlled for each subject.
An individual hypoxia dosing approach for elderly patients was also reported by Korkushko et al.86 In brief, before starting an IHT, the patients performed a standard hypoxic test with inhalation of 12% O2 gas mixture for 7 min. If the test was not interrupted during 7 min, because of either adverse effects or SaO2 drop to <80%, the subsequent IHT regimen started with 12% O2. Otherwise, the IHT session began with 14% O2 with a gradual reduction to 12% in the course of 3–5 sessions. Individual respiratory and cardiovascular functional status and blood sugar levels are among the confounding factors that need to be considered when designing the individual IHT protocols.
Contraindications of IHT
According to the guidelines published by the Russian and Ukrainian healthcare administrations, the general consensuses on the contraindications for IHT implementations are as follows: (1) acute stages of somatic diseases (myocardial infarction within the last three months, unstable angina, acute ischemic stroke within the last six months); (2) acute infectious diseases and conditions accompanied by fever and/or requiring intensive traditional therapy; (3) decompensated chronic renal failure requiring hemodialysis; (4) Hypertension Stage III with frequent hypertensive crisis; (5) significant extracranial blood flow disturbances; (6) congenital anomalies of the heart and great vessels; (7) thrombotic state and thromboembolic complications; (8) primary and secondary polycythemia; (9) individual intolerance to oxygen deficiency; and (10) intellectual or mental disorders.
Other modified protocols of IHT
During the past decade, a new mode of IHT has been explored, which combines periods of hypoxia (10–12% FiO2) and hyperoxia (30–35% FiO2).87–89 The researchers proposed that the cyclic generation of moderate levels of free radicals during the intermittent hypoxia–hyperoxia cycles may cause better induction of antioxidant enzymes than those triggered by the intermittent hypoxia–normoxia protocol. However, it remains to further prove that this new protocol would reduce the recovery time and in turn shorten the total duration of IHT sessions.
Notably, the hypoxia–hyperoxia IHT was used in treating patients with metabolic syndrome and such a modified IHT modality significantly reduced body weight of the obese patients.87 It was achieved mainly by reducing fat mass accompanied by the reduced blood levels of total cholesterol, LDL, and fasting glucose. Other improvements included normalized blood pressure, increased physical endurance, and improved mental status. Nevertheless, there is still no strong comparative evidence in humans to support that this method is much more efficient than the conventional hypoxia–normoxia mode.
Concluding remarks
Oxygen delivery and utilization are essential for human life. Adaptation to hypoxia is one of the best preserved survival mechanisms of the human body, including cardiovascular system. IHT represents a promising non-pharmacologic modality in prevention and treatment of cardiovascular diseases. The most notable features of IHT are its relatively non-invasive nature (i.e. brief hypoxic stimuli less than a half hour per day for only few weeks) that could provide long-lasting beneficial effects beyond several weeks. The proper choice of the hypoxia dosage depending on the individual reactivity to hypoxia, which should be titrated for each patient, in order to avoid negative effects of hypoxia while augmenting its favorable properties.
Evidence accumulated by more than five decades of extensive research in both healthy humans and patients with various diseases has clearly indicated that the short-term daily sessions consisting 3–4 bouts of 5–7 min exposures to 12–10% FiO2 alternating with equal durations of normoxia for 2–3 weeks can result in remarkable beneficial effects in treatment of major cardiovascular diseases such as hypertension and coronary heart disease, and chronic heart failure.
Careful monitoring of the vital parameters of cardiopulmonary function during IHT sessions is critical. Although 10% FiO2 or less was used in some cases of IHT in elite athletes, IHT in the patients with cardiovascular diseases and/or old age must be carried out under FiO2 no less than 12%. The duration and number of hypoxic episodes should also be individually selected.
Further evaluation of risk/benefit ratio is much needed to develop a series of standardized guidelines for IHT that can facilitate the potential clinical applications and resolve the confusions resulted from the complexity and differences in methods and dosages. Taken together, we can envisage a bright future for individualized IHT, which may play a more significant role in the preventive and complementary medicine against cardiovascular diseases.
Acknowledgements
This review work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' contributions
TVS and LX participated in designing and writing this review. The final version of manuscript was proof-read, edited, and approved by both authors.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin AA, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De LD, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dale EA, Ben MF, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology 2014; 29: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dempsey JA, Morgan BJ. Humans in hypoxia: a conspiracy of maladaptation?!. Physiology 2015; 30: 304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mateika JH, El-Chami M, Shaheen D, Ivers B. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. J Appl Physiol 2015; 118: 520–32. [DOI] [PubMed] [Google Scholar]
- 5.Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 2014; 307: R1181–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serebrovskaya TV, Xi L. Intermittent hypoxia in childhood: the harmful consequences versus potential benefits of therapeutic uses. Front Pediatr 2015; 3: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xi L, Serebrovskaya TV. Intermittent hypoxia and human diseases, 1st ed London: Springer, 2012. [Google Scholar]
- 8.Xi L, Serebrovskaya TV. Intermittent hypoxia: from molecular mechanisms to clinical applications, 1st ed New York: Nova Science Publishers, Inc, 2009. [Google Scholar]
- 9.Eisele HJ, Markart P, Schulz R. Obstructive sleep apnea, oxidative stress, and cardiovascular disease: evidence from human studies. Oxid Med Cell Longev 2015; 2015: 608438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birkbak J, Clark AJ, Rod NH. The effect of sleep disordered breathing on the outcome of stroke and transient ischemic attack: a systematic review. J Clin Sleep Med 2014; 10: 103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss JW, Tamisier R, Liu Y. Sympathoexcitation and arterial hypertension associated with obstructive sleep apnea and cyclic intermittent hypoxia. J Appl Physiol 2015; 119: 1449–54. [DOI] [PubMed] [Google Scholar]
- 12.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 2012; 92: 967–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baguet JP, Barone-Rochette G, Tamisier R, Levy P, Pepin JL. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol 2012; 9: 679–88. [DOI] [PubMed] [Google Scholar]
- 14.Levy P, Ryan S, Oldenburg O, Parati G. Sleep apnoea and the heart. Eur Respir Rev 2013; 22: 333–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katiukhin VN, Ochirova AT. [Change of sensitivity to hypotensive treatment under the effect of intermittent altitude hypoxia]. Vrach Delo 1979; 1: 32–5. [PubMed] [Google Scholar]
- 16.Katiukhin VN, Shliakhto EV, Shuiskaia GA. [Effect of discontinuous high-altitude barotherapy on the hemodynamics in arterial hypertension]. Kardiologiia 1979; 19: 107–8. [PubMed] [Google Scholar]
- 17.Evgen'eva IA, Karash I, Chizhov AI. [Preventive use of intermittent normobaric hypoxic hypoxia in pregnant women at high risk of developing late toxicosis]. Akush Ginekol (Mosk) 1989; 6: 50–3. [PubMed] [Google Scholar]
- 18.Tin'kov AN, Konstantinova OD, Kshniaseva SK. [Efficacy of hypobaric hypoxia in the treatment of arterial hypertension in postmenopausal women]. Ter Arkh 2011; 83: 16–9. [PubMed] [Google Scholar]
- 19.Vorob'ev LP, Chizhov AI, Potievskaia VI. [The possibilities of using intermittent normobaric hypoxia for treating hypertension patients]. Ter Arkh 1994; 66: 12–5. [PubMed] [Google Scholar]
- 20.Agostoni P, Cattadori G, Guazzi M, Bussotti M, Conca C, Lomanto M, Marenzi G, Guazzi MD. Effects of simulated altitude-induced hypoxia on exercise capacity in patients with chronic heart failure. Am J Med 2000; 109: 450–5. [DOI] [PubMed] [Google Scholar]
- 21.Tin'kov AN, Aksenov VA. Effects of intermittent hypobaric hypoxia on blood lipid concentrations in male coronary heart disease patients. High Alt Med Biol 2002; 3: 277–82. [DOI] [PubMed] [Google Scholar]
- 22.Simonenko VB, Ermolaev AL, Potievskaia VI, Stepaniants OS. [Hypoxic therapy of arterial hypertension in patients with different variability of arterial pressure]. Klin Med (Mosk) 2003; 81: 35–8. [PubMed] [Google Scholar]
- 23.Mukharliamov FI, Smirnova MI, Bedritskii SA, Liadov KV. [Interval hypoxic training in arterial hypertension]. Vopr Kurortol Fizioter Lech Fiz Kult 2006; 2: 5–6. [PubMed] [Google Scholar]
- 24.Burtscher M, Pachinger O, Ehrenbourg I, Mitterbauer G, Faulhaber M, Puhringer R, Tkatchouk E. Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int J Cardiol 2004; 96: 247–54. [DOI] [PubMed] [Google Scholar]
- 25.del Pilar Valle M, Garcia-Godos F, Woolcott OO, Marticorena JM, Rodriguez V, Gutierrez I, Fernandez-Davila L, Contreras A, Valdivia L, Robles J, Marticorena EA. Improvement of myocardial perfusion in coronary patients after intermittent hypobaric hypoxia. J Nucl Cardiol 2006; 13: 69–74. [DOI] [PubMed] [Google Scholar]
- 26.Elizarov A, Knyazev T, Badtieva V. Hypoxic adaptation method in the treatment of patients with ischemic heart disease. Kremlin Medicine 2007; 4: 32–5. [Google Scholar]
- 27.Shatilo VB, Korkushko OV, Ischuk VA, Downey HF, Serebrovskaya TV. Effects of intermittent hypoxia training on exercise performance, hemodynamics, and ventilation in healthy senior men. High Alt Med Biol 2008; 9: 43–52. [DOI] [PubMed] [Google Scholar]
- 28.Ushakov IB, Bukhtiiarov IV, Shishov AA, Olenev NI. [Hypobaric interval hypoxia as a tool for improving tolerance of harmful occupational factors]. Vestn Ross Akad Med Nauk 2010; 12: 3–7. [PubMed] [Google Scholar]
- 29.Korkushko OV, Shatilo VB, Ishchuk VA. [Effectiveness of intermittent normabaric hypoxic trainings in elderly patients with coronary artery disease]. Adv Gerontol 2010; 23: 476–82. [PubMed] [Google Scholar]
- 30.Minvaleev RS. [A comparison of rate human lipid profile changes at moderate altitude]. Fiziol Cheloveka 2011; 37: 103–8. [PubMed] [Google Scholar]
- 31.Lyamina NP, Lyamina SV, Senchiknin VN, Mallet RT, Downey HF, Manukhina EB. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J Hypertens 2011; 29: 2265–72. [DOI] [PubMed] [Google Scholar]
- 32.Rachok LV, Dubovik TA, Bulgak AG, Ostrovsky YP, Kolyadko MG, Belskaya MI, Zhujko EN, Russkikh II. The effects of using normobaric intermittent hypoxia training as a method of preoperative preparation for coronary bypass surgery of the ischemic cardiomyopathy patients. Cardiol Belarus 2011; 17: 28–45. [Google Scholar]
- 33.Saeed O, Bhatia V, Formica P, Browne A, Aldrich TK, Shin JJ, Maybaum S. Improved exercise performance and skeletal muscle strength after simulated altitude exposure: a novel approach for patients with chronic heart failure. J Card Fail 2012; 18: 387–91. [DOI] [PubMed] [Google Scholar]
- 34.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986; 74: 1124–36. [DOI] [PubMed] [Google Scholar]
- 35.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 2003; 108: 79–85. [DOI] [PubMed] [Google Scholar]
- 36.Xi L, Tekin D, Gursoy E, Salloum F, Levasseur JE, Kukreja RC. Evidence that NOS2 acts as a trigger and mediator of late preconditioning induced by acute systemic hypoxia. Am J Physiol Heart Circ Physiol 2002; 283: H5–12. [DOI] [PubMed] [Google Scholar]
- 37.Xie Y, Zhu Y, Zhu WZ, Chen L, Zhou ZN, Yuan WJ, Yang HT. Role of dual-site phospholamban phosphorylation in intermittent hypoxia-induced cardioprotection against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2005; 288: H2594–H2602. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Zhong N, Zhou ZN. Estradiol potentiates antiarrhythmic and antioxidative effects of intermittent hypoxic rat heart. Acta Pharmacol Sin 2000; 21: 609–12. [PubMed] [Google Scholar]
- 39.Zhu WZ, Dong JW, Ding HL, Yang HT, Zhou ZN. Postnatal development in intermittent hypoxia enhances resistance to myocardial ischemia/reperfusion in male rats. Eur J Appl Physiol 2004; 91: 716–22. [DOI] [PubMed] [Google Scholar]
- 40.Zong P, Setty S, Sun W, Martinez R, Tune JD, Ehrenburg IV, Tkatchouk EN, Mallet RT, Downey HF. Intermittent hypoxic training protects canine myocardium from infarction. Exp Biol Med 2004; 229: 806–12. [DOI] [PubMed] [Google Scholar]
- 41.Milano G, Corno AF, Lippa S, von Segesser LK, Samaja M. Chronic and intermittent hypoxia induce different degrees of myocardial tolerance to hypoxia-induced dysfunction. Exp Biol Med 2002; 227: 389–97. [DOI] [PubMed] [Google Scholar]
- 42.Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol 2014; 307: L129–L140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West JB. High-altitude medicine. Am J Respir Crit Care Med 2012; 186: 1229–37. [DOI] [PubMed] [Google Scholar]
- 44.Naeije R, Huez S, Lamotte M, Retailleau K, Neupane S, Abramowicz D, Faoro V. Pulmonary artery pressure limits exercise capacity at high altitude. Eur Respir J 2010; 36: 1049–55. [DOI] [PubMed] [Google Scholar]
- 45.Huez S, Faoro V, Guenard H, Martinot JB, Naeije R. Echocardiographic and tissue Doppler imaging of cardiac adaptation to high altitude in native highlanders versus acclimatized lowlanders. Am J Cardiol 2009; 103: 1605–9. [DOI] [PubMed] [Google Scholar]
- 46.Rimoldi SF, Sartori C, Seiler C, Delacretaz E, Mattle HP, Scherrer U, Allemann Y. High-altitude exposure in patients with cardiovascular disease: risk assessment and practical recommendations. Prog Cardiovasc Dis 2010; 52: 512–24. [DOI] [PubMed] [Google Scholar]
- 47.Bilo G, Villafuerte FC, Faini A, Anza-Ramirez C, Revera M, Giuliano A, Caravita S, Gregorini F, Lombardi C, Salvioni E, Macarlupu JL, Ossoli D, Landaveri L, Lang M, Agostoni P, Sosa JM, Mancia G, Parati G. Ambulatory blood pressure in untreated and treated hypertensive patients at high altitude: the High Altitude Cardiovascular Research-Andes study. Hypertension 2015; 65: 1266–72. [DOI] [PubMed] [Google Scholar]
- 48.Levine BD. Going high with heart disease: the effect of high altitude exposure in older individuals and patients with coronary artery disease. High Alt Med Biol 2015; 16: 89–96. [DOI] [PubMed] [Google Scholar]
- 49.Mortimer EA, Jr, Monson RR, MacMahon B. Reduction in mortality from coronary heart disease in men residing at high altitude. N Engl J Med 1977; 296: 581–5. [DOI] [PubMed] [Google Scholar]
- 50.Mirrakhimov MM. [The treatment of hypertension by adaptation to high-altitude hypoxia]. Kardiologiia 1992; 32: 5–10. [PubMed] [Google Scholar]
- 51.Hurtado A. Some clinical aspects of life at high altitudes. Ann Intern Med 1960; 53: 247–58. [DOI] [PubMed] [Google Scholar]
- 52.Potievskaia VI. [Mechanisms of therapeutic and preventive effects of adaptation to hypoxia in arterial hypertension]. Fiziol Zh 1993; 39: 94–107. [PubMed] [Google Scholar]
- 53.Greie S, Humpeler E, Gunga HC, Koralewski E, Klingler A, Mittermayr M, Fries D, Lechleitner M, Hoertnagl H, Hoffmann G, Strauss-Blasche G, Schobersberger W. Improvement of metabolic syndrome markers through altitude specific hiking vacations. J Endocrinol Invest 2006; 29: 497–504. [DOI] [PubMed] [Google Scholar]
- 54.Faeh D, Gutzwiller F, Bopp M. Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland. Circulation 2009; 120: 495–501. [DOI] [PubMed] [Google Scholar]
- 55.Faeh D, Moser A, Panczak R, Bopp M, Roosli M, Spoerri A. Independent at heart: persistent association of altitude with ischaemic heart disease mortality after consideration of climate, topography and built environment. J Epidemiol Community Health Epub ahead of print 20 Jan 2016. DOI: 10.1136/jech-2015-206210. [DOI] [PubMed] [Google Scholar]
- 56.Burtscher M. [Effects of acute altitude exposure: which altitude can be tolerated?]. Wien Med Wochenschr 2010; 160: 362–71. [DOI] [PubMed] [Google Scholar]
- 57.Dehnert C, Bartsch P. Can patients with coronary heart disease go to high altitude? High Alt Med Biol 2010; 11: 183–8. [DOI] [PubMed] [Google Scholar]
- 58.Sarybaev AS, Palasiewicz G, Usupbaeva DA, Plywaczewski R, Maripov AM, Sydykov AS, Mirrakhimov MM, Le RH, Kadyrov T, Zielinski J. Effects of intermittent exposure to high altitude on pulmonary hemodynamics: a prospective study. High Alt Med Biol 2003; 4: 455–63. [DOI] [PubMed] [Google Scholar]
- 59.Vinnikov D, Brimkulov N, Krasotski V. Chronic intermittent hypoxia and blood pressure: is there risk for hypertension in healthy individuals? High Alt Med Biol 2016; 17: 5–10. [DOI] [PubMed] [Google Scholar]
- 60.Gutwenger I, Hofer G, Gutwenger AK, Sandri M, Wiedermann CJ. Pilot study on the effects of a 2-week hiking vacation at moderate versus low altitude on plasma parameters of carbohydrate and lipid metabolism in patients with metabolic syndrome. BMC Res Notes 2015; 8: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gatterer H, Raab C, Pramsohler S, Faulhaber M, Burtscher M, Netzer N. Effect of weekly hiking on cardiovascular risk factors in the elderly. Z Gerontol Geriatr 2015; 48: 150–3. [DOI] [PubMed] [Google Scholar]
- 62.Vearrier D, Greenberg MI. Occupational health of miners at altitude: adverse health effects, toxic exposures, pre-placement screening, acclimatization, and worker surveillance. Clin Toxicol 2011; 49: 629–40. [DOI] [PubMed] [Google Scholar]
- 63.Gippenreiter E, West JB. High altitude medicine and physiology in the former Soviet Union. Aviat Space Environ Med 1996; 67: 576–84. [PubMed] [Google Scholar]
- 64.Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp Biol Med 2008; 233: 627–50. [DOI] [PubMed] [Google Scholar]
- 65.Aleshin IA, Kots I, Tverdokhlib VP, Galiautdinov GS, Vdovenko LG, Zabirov MR, Meerson FZ. [The nondrug treatment of hypertension patients by their adaptation to periodic hypoxia in a barochamber]. Ter Arkh 1993; 65: 23–9. [PubMed] [Google Scholar]
- 66.Wee J, Climstein M. Hypoxic training: clinical benefits on cardiometabolic risk factors. J Sci Med Sport 2015; 18: 56–61. [DOI] [PubMed] [Google Scholar]
- 67.Sanchis-Gomar F, Vina J, Lippi G. Intermittent hypobaric hypoxia applicability in myocardial infarction prevention and recovery. J Cell Mol Med 2012; 16: 1150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Starkova NT, Davydov AL, Budylina SM, Koroleva AV, Tkachuk EN, Tsvetkova AM, Erenburg IV. [Intersensory correlations in diabetic neuropathy during adaptation to intervalic normobaric hypoxia]. Zh Nevrol Psikhiatr Im S S Korsakova 1997; 97: 40–4. [PubMed] [Google Scholar]
- 69.Nemerovskii LI. [Principles of the design of the equipment for intermittent normobaric hypoxia]. Med Tekh 1992; 1: 3–8. [PubMed] [Google Scholar]
- 70.Rozhanchuk VN, Pukh NN, Samsonova IS, Osokina VK. [Membrane technology as a basis for creation of treatment-and-prophylactic equipment for inhalation therapy and normobaric hypoxia]. Fiziol Zh 1992; 38: 91–4. [PubMed] [Google Scholar]
- 71.Lopata VA, Serebrovskaya TV. Hypoxicators: review of the operating principles and constructions. In: Xi L, Serebrovskaya TV. (eds). Intermittent hypoxia and human diseases, 1st ed London: Springer, 2012, pp. 291–302. [Google Scholar]
- 72.Balykin MV, Vinogradov SN, Gening TP. [Effect of normobaric hypoxia and physical load on the functional indices of cardiorespiratory system in overweight people]. Vopr Kurortol Fizioter Lech Fiz Kult 2004; 1: 18–21. [PubMed] [Google Scholar]
- 73.Kolchinskaia AZ. [Medical use of stepwise adaptation to hypoxia]. Vestn Ross Akad Med Nauk 1997; 5: 12–9. [PubMed] [Google Scholar]
- 74.Levine BD, Zuckerman JH, deFilippi CR. Effect of high-altitude exposure in the elderly: the Tenth Mountain Division study. Circulation 1997; 96: 1224–32. [DOI] [PubMed] [Google Scholar]
- 75.Honigman B, Theis MK, Koziol-McLain J, Roach R, Yip R, Houston C, Moore LG, Pearce P. Acute mountain sickness in a general tourist population at moderate altitudes. Ann Intern Med 1993; 118: 587–92. [DOI] [PubMed] [Google Scholar]
- 76.Korkushko OV, Ivanov LA, Pisaruk AV, Chebotarev ND. [The respiratory function of blood in elderly and old age and the factors that determine it]. Fiziol Cheloveka 2009; 35: 40–6. [PubMed] [Google Scholar]
- 77.Serebrovskaya TV, Karaban IN, Kolesnikova EE, Mishunina TM, Swanson RJ, Beloshitsky PV, Ilyin VN, Krasuk AN, Safronova OS, Kuzminskaya LA. Geriatric men at altitude: hypoxic ventilatory sensitivity and blood dopamine changes. Respiration 2000; 67: 253–60. [DOI] [PubMed] [Google Scholar]
- 78.Roach RC, Houston CS, Honigman B, Nicholas RA, Yaron M, Grissom CK, Alexander JK, Hultgren HN. How well do older persons tolerate moderate altitude? West J Med 1995; 162: 32–6. [PMC free article] [PubMed] [Google Scholar]
- 79.Bassovich O, Serebrovskaya TV. Equipment and regimes for intermittent hypoxia therapy. In: Xi L, Serebrovskaya TV. (eds). Intermittent hypoxia: from molecular mechanisms to clinical applications, New York: Nova Science Publishers, 2009, pp. 561–72. [Google Scholar]
- 80.Serebrovskaya TV, Lopata VA, Roitman EM. Device for breathing with hypoxic mixtures “Hypoxytron.” Ukraine patent 44179. 25 September 2009.
- 81.Serebrovskaya TV, Roitman EM, Lopata VA, Osaulenko VL. Device for breathing with hypoxic mixtures “Hypoxydoz.” Ukraine patent 57257 À. 16 June 2003.
- 82.Serebrovskaya TV, Nosar VI, Bratus LV, Gavenauskas BL, Mankovska IM. Tissue oxygenation and mitochondrial respiration under different modes of intermittent hypoxia. High Alt Med Biol 2013; 14: 280–8. [DOI] [PubMed] [Google Scholar]
- 83.Serebrovskaia TV. [Evaluation of the degree of genetically determined reactions of the human cardiorespiratory system to hypoxia and hypercapnia]. Kosm Biol Aviakosm Med 1982; 16: 54–8. [PubMed] [Google Scholar]
- 84.Serebrovskaya TV, Xi L. Individualized intermittent hypoxia training: principles and practices. In: Xi L, Serebrovskaya TV. (eds). Intermittent hypoxia and human diseases, 1st ed London: Springer, 2012, pp. 281–9. [Google Scholar]
- 85.Berezovskii VA, Levashov MI. [The build-up of human reserve potential by exposure to intermittent normobaric hypoxia]. Aviakosm Ekolog Med 2000; 34: 39–43. [PubMed] [Google Scholar]
- 86.Korkushko OV, Shatilo VB, Ishchuk VA, Tourta MI. Use of intermittent normobaric hypoxia trainings in elderly people. In: Xi L, Serebrovskaya TV. (eds). Intermittent hypoxia: from molecular mechanisms to clinical applications, 1st ed New York: Nova Science Publishers, 2009, pp. 537–48. [Google Scholar]
- 87.Glazachev OS, Zvenigorodskaia LA, Dudnik EN, Iartseva LA, Mishchenkova TV, Platonenko AV, Spirina GK. [Interval hypoxic-hyperoxic training in the treatment of the metabolic syndrome]. Eksp Klin Gastroenterol 2010; 7: 51–6. [PubMed] [Google Scholar]
- 88.Arkhipenko YV, Sazontova TG, Zhukova AG. Adaptation to periodic hypoxia and hyperoxia improves resistance of membrane structures in heart, liver, and brain. Bull Exp Biol Med 2005; 140: 278–81. [DOI] [PubMed] [Google Scholar]
- 89.Gonchar O, Mankovska I. Moderate hypoxia/hyperoxia attenuates acute hypoxia-induced oxidative damage and improves antioxidant defense in lung mitochondria. Acta Physiol Hung 2012; 99: 436–46. [DOI] [PubMed] [Google Scholar]