Abstract
Due to the limitations of research using human embryos and the lack of a biological model of human liver development, the roles of the various markers associated with liver stem or progenitor cell potential in humans are largely speculative, and based on studies utilizing animal models and certain patient tissues. Human pluripotent stem cell-based in vitro multistage hepatic differentiation systems may serve as good surrogate models for mimicking normal human liver development, pathogenesis and injury/regeneration studies. Here, we describe the implications of various liver stem or progenitor cell markers and their bipotency (i.e. hepatocytic- and biliary-epithelial cell differentiation), based on the pluripotent stem cell-derived model of human liver development. Future studies using the human cellular model(s) of liver and biliary development will provide more human relevant biological and/or pathological roles of distinct markers expressed in heterogeneous liver stem/progenitor cell populations.
Keywords: Liver stem cell, liver progenitor, hepatoblast, human liver development, pluripotent stem cells, hepatic differentiation, biliary differentiation, bipotency
Introduction
For years, embryonic and liver development has been studied thoroughly based on animal models, resulting in progress in the fields of developmental biology as well as regenerative medicine. These studies laid the groundwork for directed differentiation of human pluripotent stem cells (PSC) into hepatocytes, which in turn have augmented our understanding of the signaling processes governing the various stages of liver development and the differentiation stage specific markers.1–10 While the phenotypic markers for undifferentiated pluripotent stage, endoderm stage, and the mature liver stage are better established, it has been unclear which markers represent multipotent hepatic stem cells or hepatoblast-like bipotent liver progenitor cells. Here, we describe the hepatocytic and biliary commitment of human liver progenitor cells, and the implications of various liver stem/progenitor markers with an emphasis on human stem cell based model of liver development.
Liver development and implicated signaling
Germ-line specification occurs during gastrulation, forming the three germ layers; which give rise to various organs. The liver develops from the anterior definitive endoderm, driven by fibroblast growth factor (FGF) signaling11 from the adjacent cardiac mesoderm12,13 and bone morphogenetic protein (BMP) signaling from the septum transversum mesenchyme cells (STM) in the 2–4 somite stage of the embryo.14 STM cells highly express GATA4, a zinc finger transcription factor, which is critical for the growth of the liver bud.15,16 The secretion of BMP4, which is also critical for the expansion of the liver and hepatic specification, is regulated by GATA4.17 In addition, WNT signaling plays a complex role in hepatic development. At the early somite stage in the posterior endoderm, WNT signaling represses the expression of hhex (hematopoietically expressed homeobox protein), a vital regulator of hepatic specification in mouse.18 WNT antagonists in the anterior endoderm are required to relieve WNT signaling, and thus the hhex repression to facilitate the endodermal commitment to a hepatic fate.19 However, in multiple model systems WNT signaling appears to promote hepatogenesis,19–21 but does not seem to be critical for the process. Forkhead box A1 (FOXA1) and Forkhead box A2 (FOXA2) seem to be especially critical for FGF signaling driven early hepatic specification,22 however, the later stages of hepatocyte differentiation following the specification of liver progenitors are independent of FOXA1/2.23 Since a majority of these reports are based on non-human organism based research studies, knowledge of human liver development and the associated signaling mechanisms is limited.
Identification of human liver stem cells and hepatoblasts
Hepatic stem cells in the human liver are multipotent cells, located in the ductal plates in fetal and neonatal livers, and in the Canals of Hering in pediatric and adult liver.24 Human hepatic stem cells are reported to express epithelial cell adhesion molecule (EpCAM), CD133, SOX9, cytokeratins (CK) 8/18/19, neural cell adhesion molecule (NCAM), and also markers associated with endoderm such as CXCR4, SOX17, and FOXA2. They do not express alpha-fetoprotein (AFP), intercellular adhesion molecule (ICAM) 1, cytochrome P450s, and only show weak or negligible expression of albumin (ALB).25,26 These hepatic stem cells have been isolated from donor livers of all ages by dual immunoselection for EpCAM+/NCAM+ cells. In adult human livers, with their inherently scarce population of hepatoblast-like cells, selection for EpCAM+ cells results in isolation of hepatic stem cell population.25,26 In contrast, immunoselection for EpCAM+ cells from fetal livers results in predominantly hepatoblast population isolation with only a small percentage of hepatic stem cells.25,26 These isolated hepatic stem cells are capable of self-renewal and differentiate both in vitro and in vivo into hepatocytes and cholangiocytes, the epithelial cells of bile-duct.26,27
The hepatoblast cells within the aforementioned fetal liver bud express AFP and are bipotent, capable of generating hepatocytes and cholangiocytes.28 These bipotent hepatoblasts have been isolated from human fetal liver (18–20 gestational age) by dual immuno-selection for EpCAM+/ICAM+ cells.29 In human adult livers, AFP+ hepatocytes have been reported to increase with disease or acute injury.28,30 Human hepatoblasts and hepatic stem cells share an overlap in their phenotypic markers. They both express EpCAM and both do not express hematopoietic markers (CD45 and CD34) or mesenchymal markers (CD146 and KDR). They are discernable from each other in that hepatoblasts express ICAM1, CK7, AFP and early P450s, while hepatic stem cells express Neural cell adhesion molecule (NCAM) and claudin 3.24,25,31
Hepatocytic and biliary commitment of hepatoblast-like bipotent liver progenitors
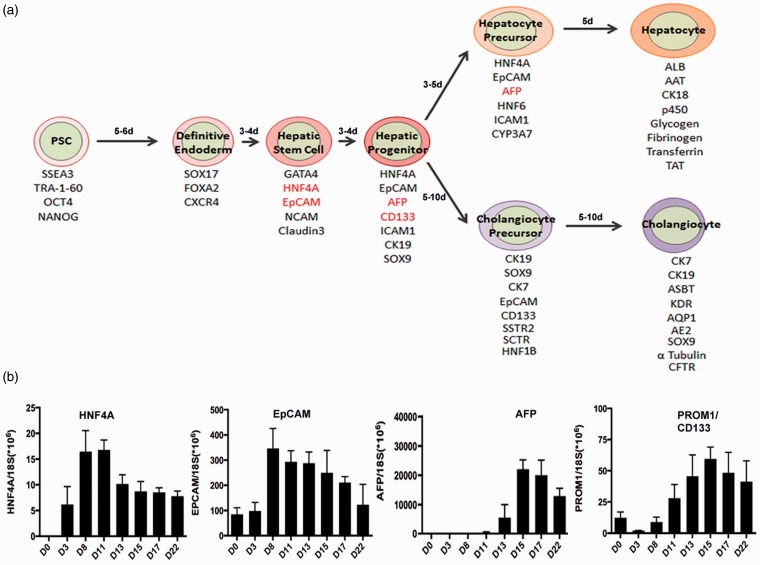
A delicate balance between several signaling pathways such as the transforming growth factor β(TGF-β), WNT, FGF, and BMP is required for the development of liver.19,32 In animal liver buds, developing hepatoblasts are exposed to multiple growth signals from various cell sources33–35 promoting development into hepatocytes and cholangiocytes; the hepatoblasts near the portal vein differentiate and become committed to the cholangiocyte lineage, whereas the hepatoblasts exposed to Oncostatin M differentiate and commit to the hepatocyte fate.36 Hepatocytes from human PSC-derived hepatoblast-like hepatic progenitors have been generated by others and us (Figure 1) harnessing the above cues,3,8,37–40 with significantly higher efficiencies than those generated from other cell sources such as primary cells,40,41 cell lines,42–44 and mesenchymal stem cells.45,46 We have also shown both the in vitro and the in vivo functionalities of human stem cell-derived multistage hepatic cells by demonstrating their potential in disease modeling, drug screening as well as liver engraftment and regeneration.1,2,7,41
Figure 1.
Human iPSC-based in vitro model of liver development. (a) Human iPSC-based model of hepatic and bile ductal development depicting the stages passed through during endodermal commitment, liver stem cell and hepatoblast-like liver progenitor formation, hepatocyte- and cholangiocyte- precursor formation and terminal differentiation into mature hepatocytes and cholangiocytes (biliary epithelial cells). Stage-specific markers and time lines are denoted at each stage. (b) Representative expression kinetics of HNF4A, EpCAM, AFP, and CD133 with regards to the differentiation day of an in vitro human iPSC-based model of liver development. (A color version of this figure is available in the online journal.)
Cholangiocytes are epithelial cells that line the intra- and extra-hepatic bile-ducts. In humans, around the 8th gestational week, hepatocytes near the portal mesenchyme form the ductal plate, a ring of cells from which cholangiocytes develop.47 NOTCH pathway is one of the most important pathways driving biliary commitment, by inducing SOX9 expression, which controls bile duct morphogenesis and is thus considered to be the earliest and most-specific marker of biliary cells in the developing liver.47 SALL4 also plays a key role in biliary commitment of hepatoblasts, by inhibiting their differentiation toward hepatocytes and instead driving cholangiocyte fate.48 Hepatoblast-like progenitor cells derived from human induced pluripotent stem cell (iPSC), embryonic stem cell (ESC), and HepaRG cells have been differentiated into cholangiocytes by employing different combinations of epidermal growth factor (EGF), interleukin-6, growth hormone (which regulates the insulin-like growth factor pathway),49 and sodium taurocholate hydrate.50–52 Others and we have recently demonstrated that these cholangiocytes can develop into functional cysts and biliary ducts in 3D culture conditions.50,53,54 Recent advances have demonstrated the in vivo ability of these human hepatic progenitor cells derived from primary,40,41,55,56 cell line42 or hepatic stem cell57 sources to engraft into animal model livers and differentiate into cholangiocytes that are usually located near or in bile ducts.
Markers for human liver stem and progenitor cells
To identify liver stem cells, hepatoblast-like bipotent progenitors and committed precursors for hepatocytes and cholangiocytes, studies have generated considerable information about the markers that specify early hepatic development, as summarized in the Table 1. Terminology for these reported markers is not very clear and many have used the terms liver stem cells, progenitors, and hepatoblasts without a clear distinction, some have also used the progenitors and precursors interchangeably, as if they have the same capacity. By definition, 1) the term “liver stem cells” should ideally be used for the multipotent and self-renewing liver stem cells which can generate hepatocytes, ductal epithelial cells (cholangiocytes), and other cell lineages in liver, 2) “liver progenitor cells” should be designated for the bipotent, hepatoblast-like cells which can give rise to both hepatocytes and cholangiocytes, 3) the term “hepatoblasts” should be used for fetal (prenatal) bipotent liver progenitor cells, and 4) the term “liver precursor cells” should be used for the unipotent hepatocyte- or cholangiocyte- precursors which have already committed to only one direction in further differentiation potential.
Table 1.
Hepatic stem or progenitor cell markers.
| Marker | Reported function in liver | Studied species | References |
|---|---|---|---|
| HNF4A | Early hepatic endoderm, hepatic stem or progenitor marker. Transcription factor controls the expression of various hepatocytic genes, and maintain adult liver function. | Mouse, human | 72–74,78,79,82,83 |
| EpCAM | Early hepatic stem or progenitor marker. Roles in cell adhesion, proliferation, differentiation, migration, cell cycle progression and regeneration following ductular reaction in liver. | Mouse, rat, human | 26,87,88,89,92,94,95 |
| CK19 | Hepatoblast, hepatic progenitor, or cholangiocyte marker. Roles in liver development and regeneration. | Rat, human | 118–120 |
| CK7 | Ductal precursor, cholangiocytes, or hepatic progenitor marker. Roles in liver development and regeneration. | Rat, human | 118–121 |
| AFP | Hepatoblast or fetal hepatocyte marker. Increase in acute liver failure and hepatocellular carcinoma. | Mouse, human | 28,99,103,104,105,107 |
| CD133 (PROM1) | Possible hepatic stem or progenitor marker. Roles in fibrosis and cancer stem cell identity maintenance. | Mouse, human | 112–115 |
| SOX9 | Hepatic progenitor or cholangiocyte marker. Role in regeneration following ductular reaction in liver. | Mouse, human | 123–126 |
| NCAM | Immature biliary cells and hepatic stem cell marker Roles in intrahepatic duct development, ductular reactions and liver regeneration | Rat, human | 26,135,139,140,141 |
| ICAM1 | Hepatoblast marker Role in leukocyte recruitment at inflammatory sites and in liver regeneration | Rat, human | 26,150,149,143 |
The cells that give rise to hepatic endoderm express key phenotypic markers denoting a hepatocytic cell fate such as ALB, AFP, transthyretin (TTR), retinol binding protein (RBP), and hepatocyte nuclear factor 4A (HNF4a) between the 7 and 11 somite stage of the mouse embryo.58 Studies have further implicated the role of other transcriptional regulators in later hepatic development, namely Onecut-1 and Onecut-2 that are vital for hepatoblast migration,59 and Prospero Homeobox 1 (PROX1) which promotes hepatoblast proliferation and also has a role in their migration.60 The critical regulators of hepatoblast differentiation such as HNF4A and CCAAT/enhancer binding protein (c/EBPa) are highly expressed by migrating hepatoblasts, while the expression of cholangiocyte fate regulators hepatocyte nuclear factors (HNF) such as HNF6 and HNF1β is very low. Oval cells, that are thought to arise from the canals of Hering61,62 and are bipotent,63–65 express TROP2 in mice.66 In mice, Foxl1 has also been proposed as a marker of bipotent liver progenitors.67 In normal human liver, cholangiocytes express osteopontin,68 and its expression in liver is increased in response to acute inflammation69,70 and liver fibrogenesis.71 Thus, there are a wide variety of suggested markers for liver stem or progenitor cells. Below, we discuss the most relevant and speculated markers for liver stem cells and progenitor cells.
HNF4A has been implicated as a marker for liver stem or progenitor cells in humans72 and animals,73,74 and for early hepatic endoderm.75–77 HNF4A is a nuclear hormone receptor transcription factor and has a critical role in controlling the expression of various hepatocytic genes.78,79 It is considered to be at the pinnacle of all transcription factors that power hepatocytic differentiation80 and is vital for hepatocyte differentiation in murine fetal liver,74,81 and also plays a role in maintenance of liver function in adult.82,83 HNF4A, along with HNF1A and HNF1b is also implicated in development and functioning of the pancreas.84,85 Thus, HNF4A is important for the development and functionality of hepatocytes and beta cells. Based on our human iPSC-based model of liver development (Figure 1), HNF4A is highly expressed during the very early hepatic specification stage (i.e., day 8 to day 11 of hepatic differentiation) following definitive endoderm stage and decreases when AFP starts to increase, suggesting that HNF4A might be a marker for early liver stem cells rather than a hepatoblast-like hepatic progenitor.
EpCAM, a transmembrane glycoprotein, has diverse roles in cellular processes such as cell adhesion,86 proliferation,87,88 differentiation,89 migration,90 and cell cycle progression.91 In addition, EpCAM has been reported as a marker for human liver stem or progenitor cells.26,92,93 In humans, hepatoblasts are tethered to the canals of Hering near the portal triads, which are the sites of ductular reaction during regeneration, by exclusively membranous EpCAM expression.94 Regeneration responses following liver necrosis involve the proliferation of human EpCAM+ hepatic stem and/or progenitor cells.94 EpCAM+ human fetal stem/progenitor cells are located in ductal plate in situ, and on isolation are capable of self-renewal and differentiation into both hepatocytes and cholangiocytes, and further on transplantation are capable of engraftment in the livers of immunodeficient mice.26,95 In human embryonic liver, bulk of the hepatocytes express EpCAM; however in adult liver, hepatocytes do not express EpCAM,93 while the bile duct epithelium does.96 EpCAM is highly expressed in murine and human embryonic stem cells and is down-regulated during spontaneous differentiation (on LIF withdrawal or embryoid body differentiation).89,97 Concurrently, EpCAM also has an important role in enhancing pluripotency reprogramming and iPSC generation through OCT4 upregulation and a possible suppression of p53-p21 pathway.98 In line with these findings, our human iPSC-based model of liver development demonstrates that EpCAM which is already expressed at a low level in undifferentiated iPSCs, sharply increases in early hepatic stem/progenitor stage cells and gradually decreases with hepatocytic maturation (Figure 1).
AFP expression is high in fetal hepatocytes, and reduces sharply as they mature to an adult phenotype in mouse liver.99 Zhx2 is a key transcriptional regulator, which induces the repression of AFP, H19, and GPC3100,101; while ZBTB20 has a key role in suppression of AFP during the switch from fetal to adult hepatocyte phenotype.102 In murine and human liver, AFP has been known as a fetal hepatoblast or bipotent liver progenitor marker.28,103–105 While AFP expression is high in fetal liver, it is still expressed in adult human liver106,107 and normal range of detectable AFP in human circulation is <20 ng/mL.107 In liver cancer, it increases to >400 ng/mL.108 In our human iPSC-based model of hepatic development, AFP was expressed from hepatic progenitor stage cells to early hepatocyte stage cells and decreased in more mature hepatocytes2,3,7,8 (Figure 1). Our findings are consistent with previously established data suggesting AFP as a hepatoblast-like progenitor or early hepatocyte marker in human tissue findings described above.
Prominin 1 (CD133) has long been regarded as a primitive hematopoietic and neural stem cell marker,109 however recent evidence suggests it may also be a cancer stem cell marker in solid cancers such as brain tumors,110 renal tumors,111 liver cancer,112 and colon113 and prostate carcinomas.114 Recent evidence suggests that CD133 is also a marker for the oval cells in adult murine liver, which have the gene expression profile and function of bipotent, primitive liver stem cells,115 CD133, thus has been considered as a liver progenitor marker. However, CD133 is also suggested as fibrosis marker, since hepatic stellate cells express CD133 and are involved in liver fibrosis,116 especially in biliary atresia-associated liver fibrosis.117 Therefore, CD133 in human liver may be a marker for a more multipotent progenitor, which produces not only hepatocytes and biliary cells but also hepatic stellate cells. Based on our human iPSC-based model of liver development, CD133 (PROM 1) is expressed in both hepatic stem and progenitor stage cells, and its expression decreased upon further hepatocyte differentiation and maturation (Figure 1).
CK19 and CK7 have been considered to be indicative markers of biliary differentiation118 and liver progenitors in both rat and human tissue studies.119,120 In a human tissue study, low expression of CK19 is seen in hepatoblasts which rises steadily in differentiating cholangiocytes; while CK7 expression is only detectable when cholangiocytes have already committed to their fate.118 On the other hand, there have been many reports suggesting that these two cytokeratins are indicative of liver progenitors, regenerating hepatocytes in adult human liver119–121 or ductal precursors.118,122 The roles for these markers in liver development or regeneration are seemingly overlapping, and it is not clear if these markers are restricted to ductal precursors or whether they are expressed in subsets of bipotent liver progenitors within the human liver.
SOX9 (Sex Determining Region Y-Box 9) is also regarded as a murine liver progenitor marker.123 SOX9 expression at E11.5 may be an indication of hepatoblast commitment to a biliary fate, as SOX9 expression is first detected at E11.5 in liver epithelial cells (hepatoblasts) which are located near the portal vein branches, where biliary cells differentiate; while at the later developmental stages SOX9 expression is restricted to the biliary cells in mice.47 In mice, the reactive ductular cells and many hepatocytic cells near the periportal tracts, where the ductular reaction occurs, are positive for SOX9.124 In humans with severe nonalcoholic fatty liver disease (NAFLD), the ductular reaction observed in response to the disease is characterized by increased expression of Hedgehog target genes such as SOX9, SPP1, and Jagged-1.125,126 SOX9 thus seems to have a key role in induction of liver regeneration. Along with ductular progenitors, hepatic stellate cells also express SOX9 in human and rodent liver,127,128 however, this is a highly debated issue, as some groups have not been able to confirm this. Murine lineage tracing experiments have shown that embryonic SOX9+ cholangiocytes can also give rise to hepatocytes.123,129 Abnormal expression of SOX9 in fetal human hepatocytes results in ectopic expression of genes encoding components of the extracellular matrix. Also during liver damage, hepatic stellate cells regulated by a SOX9-dependent process, proliferate into myofibroblasts that migrate to the adjoining parenchymal tissue and secrete extracellular matrix components. Activated hepatic stellate cells in turn lead to SOX9 expression and Collagen 1 production which is mediated by TGFβ signaling.130 SOX9 is also a critical regulator of Osteopontin, and extracellular matrix component, which serves as a biomarker for detecting the severity of liver fibrosis.128 Overexpression of SOX9 has been noted in hepatocellular carcinoma and is associated with poor clinical prognosis.131 However, it is not clear how SOX9 is regulated in normal human liver development. It is therefore of vital interest to identify the roles of SOX9 in human liver development and pathogenesis.
NCAM has been known to have roles in morphogenesis, migration and remodeling via cell–cell and cell–matrix interactions in several organs.132–134 In adult (human and rat) liver, the immature biliary cells present in the reactive bile ductules express NCAM.120,135,136 These ductules occur in various liver diseases and contribute to an atypical ductular reaction,137,138 which is modulated by NCAM.139 In humans, NCAM may have a role in intrahepatic duct development since its patchy expression is observed in the duplicated ductal plates and merging bile ducts from gestational 16th to 40th week. In contrast, ductal plate malformations such as extra-hepatic bile duct atresia and congenital hepatic fibrosis are associated with an overexpression of NCAM.140 Activated portal fibroblasts observed in the regenerating livers of rats following partial hepatectomy also express NCAM, further supporting the morphogenesis roles of NCAM.141 Hepatic stem cells have also been known to express NCAM, in addition to EpCAM and Claudin 3.26
ICAM-1, a member of the immunoglobulin superfamily,142 is expressed on the surface of various cells at inflammatory and immune reactive sites,143 and is induced by pro-inflammatory cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α.144 ICAM1 expression has been observed in various inflammatory liver disorders such as human liver allograft rejection,145 autoimmune liver diseases146,147 and hepatitis B.147,148 In mice, ICAM1 also plays a role in liver regeneration through recruitment of leukocytes and triggers the proliferation of hepatocytes via kupffer cell-dependent release of cytokines TNF α and IL-6.149 Studies have shown that the hepatoblasts isolated from E13 rats were ICAM1+.150 Human hepatoblasts differentiated from isolated ICAM-fetal hepatic stem cells, have been shown to be express ICAM1, CK19, CD133, EpCAM, and AFP.26
Conclusions and perspectives
Due to the limitations of research using developing human embryo or liver tissue and lack of a biological model of human liver development, the roles of the various markers associated with liver stem or progenitor potential and cell fate determination in human liver are currently highly speculative. Based on our human iPSC-based model of liver development, expression of some liver stem markers such as HNF4A and EpCAM increases in early hepatic differentiation stage cells right after the definitive endoderm stage, and decreases with hepatocytic maturation, while the hepatoblast marker AFP is expressed later during the hepatic specification stage and decreases in mature hepatocytes (Figure 1). Therefore, we hypothesize that HNF4A and EpCAM might be markers for more early stage hepatic stem cells, and AFP may be a marker for a later stage or less potent, hepatoblast-like liver progenitors in the human liver development. However, considering the current uncertainty of roles of these known markers for liver stem or progenitor cells (Table 1), and potential heterogeneity of these primitive liver cell subsets, further research warrants extensive biological studies for determining the roles of each marker for identifying liver stem, progenitor or committed precursor cell populations in a human relevant setting. Human iPSC-based in vitro hepatocytic and ductal differentiation systems will serve as good surrogate models for mimicking human liver development and for liver disease studies, and may provide a highly human relevant research tool for determining biological functions of many liver stem/progenitor markers; and potentially for discovery of new more reliable stage-specific markers in human liver development and the cell fate determination process associated with liver disease pathogenesis.
Methods
Human iPSC culture, directed hepatic, hepatocytic, and ductal differentiation and quantification of target gene mRNA was performed as previously described.2,3,7,8,37,54
Acknowledgements
This work was supported in part by grants from Maryland Stem Cell Research Funds (2010-MSCRFII-0101 and 2013-MSCRFII-0170) and by NIH (R43 ES023514, R21AA020020, and P30DK089502).
Author Contributions
PC and YYJ wrote the manuscript. PC, YYJ, and LT collected the data, and performed data analysis. AD helped in writing the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Choi SM, Kim Y, Liu H, Chaudhari P, Ye Z, Jang YY. Liver engraftment potential of hepatic cells derived from patient-specific induced pluripotent stem cells. Cell Cycle 2011; 10: 2423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi SM, Kim Y, Shim JS, Park JT, Wang RH, Leach SD, Liu JO, Deng C, Ye Z, Jang YY. Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology 2013; 57: 2458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi SM, Liu H, Chaudhari P, Kim Y, Cheng L, Feng J, Sharkis S, Ye Z, Jang YY. Reprogramming of EBV-immortalized B-lymphocyte cell lines into induced pluripotent stem cells. Blood 2011; 118: 1801–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun YS, Chaudhari P, Jang YY. Applications of patient-specific induced pluripotent stem cells; focused on disease modeling, drug screening and therapeutic potentials for liver disease. Int J Biol Sci 2010; 6: 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan J, Robert C, Jang YY, Liu H, Sharkis S, Baylin SB, Rassool FV. Human induced pluripotent cells resemble embryonic stem cells demonstrating enhanced levels of DNA repair and efficacy of nonhomologous end-joining. Mutat Res 2011; 713: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang YY, Ye Z, Cheng L. Molecular imaging and stem cell research. Mol Imaging 2011; 10: 111–22. [PubMed] [Google Scholar]
- 7.Liu H, Kim Y, Sharkis S, Marchionni L, Jang YY. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med 2011; 3: 82ra39–82ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Ye Z, Kim Y, Sharkis S, Jang YY. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology 2010; 51: 1810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharkis SJ, Jones RJ, Civin C, Jang YY. Pluripotent stem cell-based cancer therapy: promise and challenges. Sci Transl Med 2012; 4: 127ps9–127ps9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith C, Abalde-Atristain L, He C, Brodsky BR, Braunstein EM, Chaudhari P, Jang YY, Cheng L, Ye Z. Efficient and allele-specific genome editing of disease loci in human iPSCs. Mol Ther 2015; 23: 570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science 1999; 284: 1998–2003. [DOI] [PubMed] [Google Scholar]
- 12.Douarin NM. An experimental analysis of liver development. Med Biol 1975; 53: 427–55. [PubMed] [Google Scholar]
- 13.Fukuda-Taira S. Hepatic induction in the avian embryo: specificity of reactive endoderm and inductive mesoderm. J Embryol Exp Morphol 1981; 63: 111–25. [PubMed] [Google Scholar]
- 14.Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev 2001; 15: 1998–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci USA 2004; 101: 12573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watt AJ, Zhao R, Li J, Duncan SA. Development of the mammalian liver and ventral pancreas is dependent on GATA4. BMC Dev Biol 2007; 7: 37–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemer G, Nemer M. Transcriptional activation of BMP-4 and regulation of mammalian organogenesis by GATA-4 and -6. Dev Biol 2003; 254: 131–48. [DOI] [PubMed] [Google Scholar]
- 18.Bort R, Martinez-Barbera JP, Beddington RS, Zaret KS. Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development 2004; 131: 797–806. [DOI] [PubMed] [Google Scholar]
- 19.McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development 2007; 134: 2207–17. [DOI] [PubMed] [Google Scholar]
- 20.Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, Bourque C, Strijbosch R, Haramis AP, Puder M, Clevers H, Moon RT, Zon LI. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol 2008; 320: 161–74. [DOI] [PubMed] [Google Scholar]
- 21.Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature 2006; 442: 688–91. [DOI] [PubMed] [Google Scholar]
- 22.Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature 2005; 435: 944–7. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, White P, Tuteja G, Rubins N, Sackett S, Kaestner KH. Foxa1 and Foxa2 regulate bile duct development in mice. J Clin Invest 2009; 119: 1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, Mendel G, Wauthier E, Barbier C, Alvaro D, Reid LM. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011; 53: 1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells 2006; 24: 1852–8. [DOI] [PubMed] [Google Scholar]
- 26.Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, Kulik M, Sherwood S, Tallheden T, Cheng N, Furth ME, Reid LM. Human hepatic stem cells from fetal and postnatal donors. J Exp Med 2007; 204: 1973–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, Costello MJ, Cardinale V, Wauthier E, Barbier C, Gerber DA, Alvaro D, Reid LM. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology 2011; 53: 293–305. [DOI] [PubMed] [Google Scholar]
- 28.Saxena R, Theise N. Canals of Hering: recent insights and current knowledge. Semin Liver Dis 2004; 24: 43–8. [DOI] [PubMed] [Google Scholar]
- 29.Turner WS, Schmelzer E, McClelland R, Wauthier E, Chen W, Reid LM. Human hepatoblast phenotype maintained by hyaluronan hydrogels. J Biomed Mater Res B Appl Biomater 2007; 82: 156–68. [DOI] [PubMed] [Google Scholar]
- 30.Abelev GI. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res 1971; 14: 295–358. [DOI] [PubMed] [Google Scholar]
- 31.Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. Identification of bipotential progenitor cells in human liver development. Hepatology 1996; 23: 476–81. [DOI] [PubMed] [Google Scholar]
- 32.Dessimoz J, Opoka R, Kordich JJ, Grapin-Botton A, Wells JM. FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev 2006; 123: 42–55. [DOI] [PubMed] [Google Scholar]
- 33.Berg T, Rountree CB, Lee L, Estrada J, Sala FG, Choe A, Veltmaat JM, De Langhe S, Lee R, Tsukamoto H, Crooks GM, Bellusci S, Wang KS. Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta-catenin activation. Hepatology 2007; 46: 1187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto K, Miki R, Nakayama M, Tatsumi N, Yokouchi Y. Wnt9a secreted from the walls of hepatic sinusoids is essential for morphogenesis, proliferation, and glycogen accumulation of chick hepatic epithelium. Dev Biol 2008; 319: 234–47. [DOI] [PubMed] [Google Scholar]
- 35.Onitsuka I, Tanaka M, Miyajima A. Characterization and functional analyses of hepatic mesothelial cells in mouse liver development. Gastroenterology 2010; 138: 1525–35, 35 e1–6. [DOI] [PubMed] [Google Scholar]
- 36.Kamiya A, Kinoshita T, Ito Y, Matsui T, Morikawa Y, Senba E, Nakashima K, Taga T, Yoshida K, Kishimoto T, Miyajima A. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J 1999; 18: 2127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhari P, Prasad N, Tian L, Jang YY. Determination of functional activity of human iPSC-derived hepatocytes by measurement of CYP metabolism. Methods Mol Biol 2016; 1357: 383–94. [DOI] [PubMed] [Google Scholar]
- 38.Takayama K, Inamura M, Kawabata K, Katayama K, Higuchi M, Tashiro K, Nonaka A, Sakurai F, Hayakawa T, Furue MK, Mizuguchi H. Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4alpha transduction. Mol Ther 2012; 20: 127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shan J, Schwartz RE, Ross NT, Logan DJ, Thomas D, Duncan SA, North TE, Goessling W, Carpenter AE, Bhatia SN. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat Chem Biol 2013; 9: 514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, Cantafora A, Wauthier E, Furth ME, Inverardi L, Dominguez-Bendala J, Ricordi C, Gerber D, Gaudio E, Alvaro D, Reid L. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 2011; 54: 2159–72. [DOI] [PubMed] [Google Scholar]
- 41.Nowak G, Ericzon BG, Nava S, Jaksch M, Westgren M, Sumitran-Holgersson S. Identification of expandable human hepatic progenitors which differentiate into mature hepatic cells in vivo. Gut 2005; 54: 972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higuchi Y, Kawai K, Yamazaki H, Nakamura M, Bree F, Guguen-Guillouzo C, Suemizu H. The human hepatic cell line HepaRG as a possible cell source for the generation of humanized liver TK-NOG mice. Xenobiotica 2014; 44: 146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deurholt T, van Til NP, Chhatta AA, ten Bloemendaal L, Schwartlander R, Payne C, Plevris JN, Sauer IM, Chamuleau RA, Elferink RP, Seppen J, Hoekstra R. Novel immortalized human fetal liver cell line, cBAL111, has the potential to differentiate into functional hepatocytes. BMC Biotechnol 2009; 9: 89–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerec V, Glaise D, Garnier D, Morosan S, Turlin B, Drenou B, Gripon P, Kremsdorf D, Guguen-Guillouzo C, Corlu A. Transdifferentiation of hepatocyte-like cells from the human hepatoma HepaRG cell line through bipotent progenitor. Hepatology 2007; 45: 957–67. [DOI] [PubMed] [Google Scholar]
- 45.Snykers S, De Kock J, Tamara V, Rogiers V. Hepatic differentiation of mesenchymal stem cells: in vitro strategies. Methods Mol Biol 2011; 698: 305–14. [DOI] [PubMed] [Google Scholar]
- 46.Wu XB, Tao R. Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat Dis Int 2012; 11: 360–71. [DOI] [PubMed] [Google Scholar]
- 47.Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, Jacquemin P, Pierreux CE, Clotman F, Lemaigre FP. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology 2009; 136: 2325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oikawa T, Kamiya A, Zeniya M, Chikada H, Hyuck AD, Yamazaki Y, Wauthier E, Tajiri H, Miller LD, Wang XW, Reid LM, Nakauchi H. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology 2013; 57: 1469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takayama K, Nagamoto Y, Mimura N, Tashiro K, Sakurai F, Tachibana M, Hayakawa T, Kawabata K, Mizuguchi H. Long-term self-renewal of human ES/iPS-derived hepatoblast-like cells on human laminin 111-coated dishes. Stem Cell Rep 2013; 1: 322–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dianat N, Dubois-Pot-Schneider H, Steichen C, Desterke C, Leclerc P, Raveux A, Combettes L, Weber A, Corlu A, Dubart-Kupperschmitt A. Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology 2014; 60: 700–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogawa M, Ogawa S, Bear CE, Ahmadi S, Chin S, Li B, Grompe M, Keller G, Kamath BM, Ghanekar A. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat Biotechnol 2015; 33: 853–61. [DOI] [PubMed] [Google Scholar]
- 52.Sampaziotis F, Cardoso de Brito M, Madrigal P, Bertero A, Saeb-Parsy K, Soares FA, Schrumpf E, Melum E, Karlsen TH, Bradley JA, Gelson WT, Davies S, Baker A, Kaser A, Alexander GJ, Hannan NR, Vallier L. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol 2015; 33: 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao D, Chen S, Cai J, Guo Y, Song Z, Che J, Liu C, Wu C, Ding M, Deng H. Derivation and characterization of hepatic progenitor cells from human embryonic stem cells. PLoS One 2009; 4: e6468–e6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian L, Deshmukh A, Ye Z, Jang YY. Efficient and controlled generation of 2D and 3D bile duct tissue from human pluripotent stem cell-derived spheroids. Stem Cell Rev Rep 2016. DOI:10.1007/s12015-016-9657–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brilliant KE, Mills DR, Callanan HM, Hixson DC. Engraftment of syngeneic and allogeneic endothelial cells, hepatocytes and cholangiocytes into partially hepatectomized rats previously treated with mitomycin C. Transplantation 2009; 88: 486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krause P, Rave-Frank M, Wolff HA, Becker H, Christiansen H, Koenig S. Liver sinusoidal endothelial and biliary cell repopulation following irradiation and partial hepatectomy. World J Gastroenterol 2010; 16: 3928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu B, He ZY, You P, Han QW, Xiang D, Chen F, Wang MJ, Liu CC, Lin XW, Borjigin U, Zi XY, Li JX, Zhu HY, Li WL, Han CS, Wangensteen KJ, Shi Y, Hui LJ, Wang X, Hu YP. Reprogramming fibroblasts into bipotential hepatic stem cells by defined factors. Cell Stem Cell 2013; 13: 328–40. [DOI] [PubMed] [Google Scholar]
- 58.Bort R, Signore M, Tremblay K, Martinez Barbera JP, Zaret KS. Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev Biol 2006; 290: 44–56. [DOI] [PubMed] [Google Scholar]
- 59.Margagliotti S, Clotman F, Pierreux CE, Beaudry JB, Jacquemin P, Rousseau GG, Lemaigre FP. The Onecut transcription factors HNF-6/OC-1 and OC-2 regulate early liver expansion by controlling hepatoblast migration. Dev Biol 2007; 311: 579–89. [DOI] [PubMed] [Google Scholar]
- 60.Sosa-Pineda B, Wigle JT, Oliver G. Hepatocyte migration during liver development requires Prox1. Nat Genet 2000; 25: 254–5. [DOI] [PubMed] [Google Scholar]
- 61.Dorrell C, Grompe M. Liver repair by intra- and extrahepatic progenitors. Stem Cell Rev 2005; 1: 61–4. [DOI] [PubMed] [Google Scholar]
- 62.Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev 2003; 120: 117–30. [DOI] [PubMed] [Google Scholar]
- 63.Lemire JM, Shiojiri N, Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol 1991; 139: 535–52. [PMC free article] [PubMed] [Google Scholar]
- 64.Tee LB, Kirilak Y, Huang WH, Morgan RH, Yeoh GC. Differentiation of oval cells into duct-like cells in preneoplastic liver of rats placed on a choline-deficient diet supplemented with ethionine. Carcinogenesis 1994; 15: 2747–56. [DOI] [PubMed] [Google Scholar]
- 65.Tian YW, Smith PG, Yeoh GC. The oval-shaped cell as a candidate for a liver stem cell in embryonic, neonatal and precancerous liver: identification based on morphology and immunohistochemical staining for albumin and pyruvate kinase isoenzyme expression. Histochem Cell Biol 1997; 107: 243–50. [DOI] [PubMed] [Google Scholar]
- 66.Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, Tsujimura T, Nakamura K, Miyajima A. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development 2009; 136: 1951–60. [DOI] [PubMed] [Google Scholar]
- 67.Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, Kaestner KH, Greenbaum LE. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology 2009; 49: 920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown LF, Berse B, Van de Water L, Papadopoulos-Sergiou A, Perruzzi CA, Manseau EJ, Dvorak HF, Senger DR. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell 1992; 3: 1169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawashima R, Mochida S, Matsui A, YouLuTu ZY, Ishikawa K, Toshima K, Yamanobe F, Inao M, Ikeda H, Ohno A, Nagoshi S, Uede T, Fujiwara K. Expression of osteopontin in Kupffer cells and hepatic macrophages and Stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochem Biophys Res Commun 1999; 256: 527–31. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Mochida S, Kawashima R, Inao M, Matsui A, YouLuTu ZY, Nagoshi S, Uede T, Fujiwara K. Increased expression of osteopontin in activated Kupffer cells and hepatic macrophages during macrophage migration in Propionibacterium acnes-treated rat liver. Journal of gastroenterology 2000; 35: 696–701. [DOI] [PubMed] [Google Scholar]
- 71.Lorena D, Darby IA, Gadeau AP, Leen LL, Rittling S, Porto LC, Rosenbaum J, Desmouliere A. Osteopontin expression in normal and fibrotic liver. altered liver healing in osteopontin-deficient mice. J Hepatol 2006; 44: 383–90. [DOI] [PubMed] [Google Scholar]
- 72.DeLaForest A, Nagaoka M, Si-Tayeb K, Noto FK, Konopka G, Battle MA, Duncan SA. HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells. Development 2011; 138: 4143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torres-Padilla ME, Fougere-Deschatrette C, Weiss MC. Expression of HNF4alpha isoforms in mouse liver development is regulated by sequential promoter usage and constitutive 3′ end splicing. Mech Dev 2001; 109: 183–93. [DOI] [PubMed] [Google Scholar]
- 74.Li J, Ning G, Duncan SA. Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha. Genes Dev 2000; 14: 464–74. [PMC free article] [PubMed] [Google Scholar]
- 75.Chen WS, Manova K, Weinstein DC, Duncan SA, Plump AS, Prezioso VR, Bachvarova RF, Darnell JE., Jr Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev 1994; 8: 2466–77. [DOI] [PubMed] [Google Scholar]
- 76.Duncan SA, Manova K, Chen WS, Hoodless P, Weinstein DC, Bachvarova RF, Darnell JE., Jr Expression of transcription factor HNF-4 in the extraembryonic endoderm, gut, and nephrogenic tissue of the developing mouse embryo: HNF-4 is a marker for primary endoderm in the implanting blastocyst. Proc Natl Acad Sci USA 1994; 91: 7598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li K, Zhang H, Wang Y, Wang Y, Feng M. Differential expression of HNF4alpha isoforms in liver stem cells and hepatocytes. J Cell Biochem 2006; 99: 558–64. [DOI] [PubMed] [Google Scholar]
- 78.Battle MA, Konopka G, Parviz F, Gaggl AL, Yang C, Sladek FM, Duncan SA. Hepatocyte nuclear factor 4alpha orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc Natl Acad Sci USA 2006; 103: 8419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bolotin E, Liao H, Ta TC, Yang C, Hwang-Verslues W, Evans JR, Jiang T, Sladek FM. Integrated approach for the identification of human hepatocyte nuclear factor 4alpha target genes using protein binding microarrays. Hepatology 2010; 51: 642–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuo CJ, Conley PB, Chen L, Sladek FM, Darnell JE, Jr, Crabtree GR. A transcriptional hierarchy involved in mammalian cell-type specification. Nature 1992; 355: 457–61. [DOI] [PubMed] [Google Scholar]
- 81.Kyrmizi I, Hatzis P, Katrakili N, Tronche F, Gonzalez FJ, Talianidis I. Plasticity and expanding complexity of the hepatic transcription factor network during liver development. Genes Dev 2006; 20: 2293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gonzalez FJ. Regulation of hepatocyte nuclear factor 4 alpha-mediated transcription. Drug Metab Pharmacokinet 2008; 23: 2–7. [DOI] [PubMed] [Google Scholar]
- 83.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol 2001; 21: 1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maestro MA, Cardalda C, Boj SF, Luco RF, Servitja JM, Ferrer J. Distinct roles of HNF1beta, HNF1alpha, and HNF4alpha in regulating pancreas development, beta-cell function and growth. Endocr Dev 2007; 12: 33–45. [DOI] [PubMed] [Google Scholar]
- 85.Shahjalal HM, Shiraki N, Sakano D, Kikawa K, Ogaki S, Baba H, Kume K, Kume S. Generation of insulin-producing beta-like cells from human iPS cells in a defined and completely xeno-free culture system. J Mol Cell Biol 2014; 6: 394–408. [DOI] [PubMed] [Google Scholar]
- 86.Litvinov SV, Bakker HA, Gourevitch MM, Velders MP, Warnaar SO. Evidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epithelial cell-cell adhesion. Cell Adhes Commun 1994; 2: 417–28. [DOI] [PubMed] [Google Scholar]
- 87.Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M, Gires O. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol 2009; 11: 162–71. [DOI] [PubMed] [Google Scholar]
- 88.Munz M, Kieu C, Mack B, Schmitt B, Zeidler R, Gires O. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene 2004; 23: 5748–58. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez B, Denzel S, Mack B, Conrad M, Gires O. EpCAM is involved in maintenance of the murine embryonic stem cell phenotype. Stem Cells 2009; 27: 1782–91. [DOI] [PubMed] [Google Scholar]
- 90.Sankpal NV, Willman MW, Fleming TP, Mayfield JD, Gillanders WE. Transcriptional repression of epithelial cell adhesion molecule contributes to p53 control of breast cancer invasion. Cancer Res 2009; 69: 753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaves-Perez A, Mack B, Maetzel D, Kremling H, Eggert C, Harreus U, Gires O. EpCAM regulates cell cycle progression via control of cyclin D1 expression. Oncogene 2013; 32: 641–50. [DOI] [PubMed] [Google Scholar]
- 92.Yoon SM, Gerasimidou D, Kuwahara R, Hytiroglou P, Yoo JE, Park YN, Theise ND. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology 2011; 53: 964–73. [DOI] [PubMed] [Google Scholar]
- 93.Gires O. EpCAM in hepatocytes and their progenitors. J Hepatol 2012; 56: 490–2. [DOI] [PubMed] [Google Scholar]
- 94.Zhang L, Theise N, Chua M, Reid LM. The stem cell niche of human livers: symmetry between development and regeneration. Hepatology 2008; 48: 1598–607. [DOI] [PubMed] [Google Scholar]
- 95.Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology 2008; 47: 636–47. [DOI] [PubMed] [Google Scholar]
- 96.de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol 1999; 188: 201–6. [DOI] [PubMed] [Google Scholar]
- 97.Ng VY, Ang SN, Chan JX, Choo AB. Characterization of epithelial cell adhesion molecule as a surface marker on undifferentiated human embryonic stem cells. Stem Cells 2010; 28: 29–35. [DOI] [PubMed] [Google Scholar]
- 98.Huang HP, Chen PH, Yu CY, Chuang CY, Stone L, Hsiao WC, Li CL, Tsai SC, Chen KY, Chen HF, Ho HN, Kuo HC. Epithelial cell adhesion molecule (EpCAM) complex proteins promote transcription factor-mediated pluripotency reprogramming. J Biol Chem 2011; 286: 33520–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spear BT, Jin L, Ramasamy S, Dobierzewska A. Transcriptional control in the mammalian liver: liver development, perinatal repression, and zonal gene regulation. Cell Mol Life Sci 2006; 63: 2922–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morford LA, Davis C, Jin L, Dobierzewska A, Peterson ML, Spear BT. The oncofetal gene glypican 3 is regulated in the postnatal liver by zinc fingers and homeoboxes 2 and in the regenerating liver by alpha-fetoprotein regulator 2. Hepatology 2007; 46: 1541–7. [DOI] [PubMed] [Google Scholar]
- 101.Perincheri S, Dingle RW, Peterson ML, Spear BT. Hereditary persistence of alpha-fetoprotein and H19 expression in liver of BALB/cJ mice is due to a retrovirus insertion in the Zhx2 gene. Proc Natl Acad Sci USA 2005; 102: 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie Z, Zhang H, Tsai W, Zhang Y, Du Y, Zhong J, Szpirer C, Zhu M, Cao X, Barton MC, Grusby MJ, Zhang WJ. Zinc finger protein ZBTB20 is a key repressor of alpha-fetoprotein gene transcription in liver. Proc Natl Acad Sci USA 2008; 105: 10859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shiojiri N. Enzymo- and immunocytochemical analyses of the differentiation of liver cells in the prenatal mouse. J Embryol Exp Morphol 1981; 62: 139–52. [PubMed] [Google Scholar]
- 104.Hua M, Zhang W, Li W, Li X, Liu B, Lu X, Zhang H. Molecular mechanisms regulating the establishment of hepatocyte polarity during human hepatic progenitor cell differentiation into a functional hepatocyte-like phenotype. J Cell Sci 2012; 125: 5800–10. [DOI] [PubMed] [Google Scholar]
- 105.Wang P, Zhang H, Li W, Zhao Y, An W. Promoter-defined isolation and identification of hepatic progenitor cells from the human fetal liver. Histochem Cell Biol 2008; 130: 375–85. [DOI] [PubMed] [Google Scholar]
- 106.Arrieta O, Cacho B, Morales-Espinosa D, Ruelas-Villavicencio A, Flores-Estrada D, Hernandez-Pedro N. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer 2007; 7: 28–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnu L, Zoli M, Borzio F, Bernardi M, Trevisani F. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006; 101: 524–32. [DOI] [PubMed] [Google Scholar]
- 108.Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–36. [DOI] [PubMed] [Google Scholar]
- 109.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997; 90: 5002–12. [PubMed] [Google Scholar]
- 110.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63: 5821–8. [PubMed] [Google Scholar]
- 111.Bruno S, Bussolati B, Grange C, Collino F, Graziano ME, Ferrando U, Camussi G. CD133+ renal progenitor cells contribute to tumor angiogenesis. Am J Pathol 2006; 169: 2223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rountree CB, Ding W, He L, Stiles B. Expansion of CD133-expressing liver cancer stem cells in liver-specific phosphatase and tensin homolog deleted on chromosome 10-deleted mice. Stem Cells 2009; 27: 290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007; 445: 106–10. [DOI] [PubMed] [Google Scholar]
- 114.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005; 65: 10946–51. [DOI] [PubMed] [Google Scholar]
- 115.Rountree CB, Barsky L, Ge S, Zhu J, Senadheera S, Crooks GM. A CD133-expressing murine liver oval cell population with bilineage potential. Stem Cells 2007; 25: 2419–29. [DOI] [PubMed] [Google Scholar]
- 116.Kordes C, Sawitza I, Muller-Marbach A, Ale-Agha N, Keitel V, Klonowski-Stumpe H, Haussinger D. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun 2007; 352: 410–7. [DOI] [PubMed] [Google Scholar]
- 117.Mavila N, James D, Shivakumar P, Nguyen MV, Utley S, Mak K, Wu A, Zhou S, Wang L, Vendyres C, Groff M, Asahina K, Wang KS. Expansion of prominin-1-expressing cells in association with fibrosis of biliary atresia. Hepatology 2014; 60: 941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Van Eyken P, Sciot R, Callea F, Van der Steen K, Moerman P, Desmet VJ. The development of the intrahepatic bile ducts in man: a keratin-immunohistochemical study. Hepatology 1988; 8: 1586–95. [DOI] [PubMed] [Google Scholar]
- 119.del Castillo G, Alvarez-Barrientos A, Carmona-Cuenca I, Fernandez M, Sanchez A, Fabregat I. Isolation and characterization of a putative liver progenitor population after treatment of fetal rat hepatocytes with TGF-beta. J Cell Physiol 2008; 215: 846–55. [DOI] [PubMed] [Google Scholar]
- 120.Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, Desmet V. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. J Hepatol 1998; 29: 455–63. [DOI] [PubMed] [Google Scholar]
- 121.Matsukuma S, Takeo H, Kono T, Nagata Y, Sato K. Aberrant cytokeratin 7 expression of centrilobular hepatocytes: a clinicopathological study. Histopathology 2012; 61: 857–62. [DOI] [PubMed] [Google Scholar]
- 122.Van Eyken P, Sciot R, Desmet VJ. A cytokeratin immunohistochemical study of alcoholic liver disease: evidence that hepatocytes can express ‘bile duct-type’ cytokeratins. Histopathology 1988; 13: 605–17. [DOI] [PubMed] [Google Scholar]
- 123.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 2011; 43: 34–41. [DOI] [PubMed] [Google Scholar]
- 124.Michelotti GA, Tucker A, Swiderska-Syn M, Machado MV, Choi SS, Kruger L, Soderblom E, Thompson JW, Mayer-Salman M, Himburg HA, Moylan CA, Guy CD, Garman KS, Premont RT, Chute JP, Diehl AM. Pleiotrophin regulates the ductular reaction by controlling the migration of cells in liver progenitor niches. Gut 2016; 65: 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moylan CA, Pang H, Dellinger A, Suzuki A, Garrett ME, Guy CD, Murphy SK, Ashley-Koch AE, Choi SS, Michelotti GA, Hampton DD, Chen Y, Tillmann HL, Hauser MA, Abdelmalek MF, Diehl AM. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology 2014; 59: 471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Swiderska-Syn M, Suzuki A, Guy CD, Schwimmer JB, Abdelmalek MF, Lavine JE, Diehl AM. Hedgehog pathway and pediatric nonalcoholic fatty liver disease. Hepatology 2013; 57: 1814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Michelotti GA, Xie G, Swiderska M, Choi SS, Karaca G, Kruger L, Premont R, Yang L, Syn WK, Metzger D, Diehl AM. Smoothened is a master regulator of adult liver repair. J Clin Invest 2013; 123: 2380–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pritchett J, Harvey E, Athwal V, Berry A, Rowe C, Oakley F, Moles A, Mann DA, Bobola N, Sharrocks AD, Thomson BJ, Zaitoun AM, Irving WL, Guha IN, Hanley NA, Hanley KP. Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology 2012; 56: 1108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carpentier R, Suner RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, Leclercq IA, Sander M, Lemaigre FP. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology 2011; 141: 1432–8, 8 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hanley KP, Oakley F, Sugden S, Wilson DI, Mann DA, Hanley NA. Ectopic SOX9 mediates extracellular matrix deposition characteristic of organ fibrosis. J Biol Chem 2008; 283: 14063–71. [DOI] [PubMed] [Google Scholar]
- 131.Guo X, Xiong L, Sun T, Peng R, Zou L, Zhu H, Zhang J, Li H, Zhao J. Expression features of SOX9 associate with tumor progression and poor prognosis of hepatocellular carcinoma. Diagn Pathol 2012; 7: 44–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Muller-Rover S, Peters EJ, Botchkarev VA, Panteleyev A, Paus R. Distinct patterns of NCAM expression are associated with defined stages of murine hair follicle morphogenesis and regression. J Histochem Cytochem 1998; 46: 1401–10. [DOI] [PubMed] [Google Scholar]
- 133.Ronn LC, Berezin V, Bock E. The neural cell adhesion molecule in synaptic plasticity and ageing. Int J Dev Neurosci 2000; 18: 193–9. [DOI] [PubMed] [Google Scholar]
- 134.Ronn LC, Hartz BP, Bock E. The neural cell adhesion molecule (NCAM) in development and plasticity of the nervous system. Exp Gerontol 1998; 33: 853–64. [DOI] [PubMed] [Google Scholar]
- 135.Fabris L, Strazzabosco M, Crosby HA, Ballardini G, Hubscher SG, Kelly DA, Neuberger JM, Strain AJ, Joplin R. Characterization and isolation of ductular cells coexpressing neural cell adhesion molecule and Bcl-2 from primary cholangiopathies and ductal plate malformations. Am J Pathol 2000; 156: 1599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Roskams T, van den Oord JJ, De Vos R, Desmet VJ. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol 1990; 137: 1019–25. [PMC free article] [PubMed] [Google Scholar]
- 137.Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology 1992; 16: 1069–83. [DOI] [PubMed] [Google Scholar]
- 138.Sell S. Comparison of liver progenitor cells in human atypical ductular reactions with those seen in experimental models of liver injury. Hepatology 1998; 27: 317–31. [DOI] [PubMed] [Google Scholar]
- 139.Tsuchiya A, Lu WY, Weinhold B, Boulter L, Stutchfield BM, Williams MJ, Guest RV, Minnis-Lyons SE, MacKinnon AC, Schwarzer D, Ichida T, Nomoto M, Aoyagi Y, Gerardy-Schahn R, Forbes SJ. Polysialic acid/neural cell adhesion molecule modulates the formation of ductular reactions in liver injury. Hepatology 2014; 60: 1727–40. [DOI] [PubMed] [Google Scholar]
- 140.Libbrecht L, Cassiman D, Desmet V, Roskams T. Expression of neural cell adhesion molecule in human liver development and in congenital and acquired liver diseases. Histochem Cell Biol 2001; 116: 233–9. [DOI] [PubMed] [Google Scholar]
- 141.Nakatani K, Tanaka H, Ikeda K, Sakabe M, Kadoya H, Seki S, Kaneda K, Nakajima Y. Expression of NCAM in activated portal fibroblasts during regeneration of the rat liver after partial hepatectomy. Arch Histol Cytol 2006; 69: 61–72. [DOI] [PubMed] [Google Scholar]
- 142.Staunton DE, Marlin SD, Stratowa C, Dustin ML, Springer TA. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell 1988; 52: 925–33. [DOI] [PubMed] [Google Scholar]
- 143.Springer TA. Adhesion receptors of the immune system. Nature 1990; 346: 425–34. [DOI] [PubMed] [Google Scholar]
- 144.Menger MD, Richter S, Yamauchi J, Vollmar B. Role of microcirculation in hepatic ischemia/reperfusion injury. Hepato-gastroenterology 1999; 46(Suppl 2): 1452–7. [PubMed] [Google Scholar]
- 145.Adams DH, Hubscher SG, Shaw J, Rothlein R, Neuberger JM. Intercellular adhesion molecule 1 on liver allografts during rejection. Lancet 1989; 2: 1122–5. [DOI] [PubMed] [Google Scholar]
- 146.Adams DH, Hubscher SG, Shaw J, Johnson GD, Babbs C, Rothlein R, Neuberger JM. Increased expression of intercellular adhesion molecule 1 on bile ducts in primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology 1991; 14: 426–31. [PubMed] [Google Scholar]
- 147.Volpes R, van den Oord JJ, Desmet VJ. Immunohistochemical study of adhesion molecules in liver inflammation. Hepatology 1990; 12: 59–65. [DOI] [PubMed] [Google Scholar]
- 148.Volpes R, van den Oord JJ, Desmet VJ. Hepatic expression of intercellular adhesion molecule-1 (ICAM-1) in viral hepatitis B. Hepatology 1990; 12: 148–54. [DOI] [PubMed] [Google Scholar]
- 149.Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology 2003; 124: 692–700. [DOI] [PubMed] [Google Scholar]
- 150.Kubota H, Reid LM. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc Natl Acad Sci USA 2000; 97: 12132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]