Abstract
Peripheral arterial disease is a widely prevalent atherosclerotic occlusive disorder. Symptoms commence with exercise-induced pain in the lower extremities, known as claudication. Despite the fact that exercise has been shown to improve fibrinolytic profile some patients, the effect of exercise on coagulation and fibrinolysis cascades in claudicants has not been comprehensively defined. Literature search in English language yielded 13 studies of exercise on claudicants, including 420 patients. Claudicants tend to have a higher coagulation activity at rest compared to healthy individuals, a trend that persists even after exercise. Post-exercise coagulation activity of claudicants is increased when compared to their respective baseline levels, but it is so in a non-consistent manner. From the available data, it has been suggested that claudicants have a functional and effective fibrinolytic mechanism in place, operating continuously at a relatively higher activity level compared to healthy individuals. Fibrinolysis seems to be activated by exercise; a positive outcome with a prolonged effect as shown by a few of the studies. A final conclusion whether coagulation or fibrinolysis activity is affected mostly by exercise type and intensity in claudicants could not be answered. All conclusions regarding the effect of exercise on the coagulation and fibrinolysis mechanisms should be taken under cautious consideration, due to the limited number of studies, the small number of patients and the different exercise strategies employed in each study. Further randomized studies with similar exercise protocols could provide safer conclusions in the future.
Keywords: Arteries, claudicants, coagulation, exercise, fibrinolysis, peripheral arterial disease
Introduction
Peripheral arterial disease (PAD) is a prevalent atherosclerotic occlusive disease, which is characterized by increased morbidity and mortality, limitations in functional capacity and worsening of quality of life.1,2 Patients with PAD commonly experience exercise-induced pain in the lower extremities, known as claudication. The usual advice given to the claudicants is to “stop smoking and keep walking”.3 However, some of them do not comply and quit all exercise. As a result, these subjects are particularly susceptible to cardiovascular events owing to impairment in endogenous fibrinolysis favoring the formation of thrombus.4,5 Several studies have attempted to examine the relationship between the fibrinolytic activity and the daily physical activity in the PAD subjects. Suboptimal exercise training, such as gymnastics and cycling, has been shown to improve fibrinolytic profile by decreasing plasminogen activator inhibitor 1 (PAI-1) activity and increasing tissue polypeptide antigen (tPA) activity in some patients.6,7 On the other hand there is evidence that strenuous exercise, such as the marathon, may drive coagulation and fibrinolysis to a new equilibrium at a higher activity level.8 However given that the coagulation and fibrinolytic cascades are complex self-regulated mechanisms (Figure 1), the effect of different types and intensities of exercise on these two mechanisms in claudicants has not been adequately defined. As long as these two mechanisms maintain equilibrium the organism is protected from bleeding by coagulation and at the same time clot development stays within control of the thrombolytic mechanism. In PAD patients, who already present with an increased risk of thrombosis, the effect of exercise on this fine equilibrium of the two mechanisms can alter the status of these patients. The purpose of this review is to evaluate the effect of exercise on coagulation and fibrinolysis factors among patients with PAD.
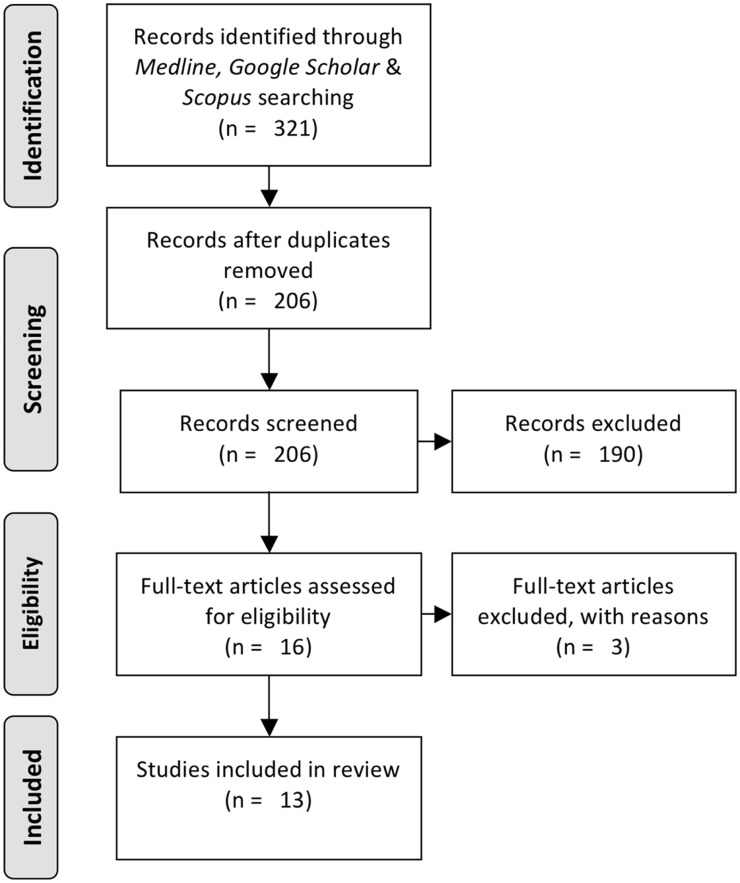
Figure 1.
PRISMA flowchart for studies selection
Methods and materials
An electronic search of medical literature in English was undertaken to identify all articles published until January 2016 and described the effect of exercise on coagulation/fibrinolysis in patients with PAD. Search terms included “peripheral arterial disease”, “peripheral arterial obstructive disease”, “claudicant”; “claudication”, “coagulation”, “thrombosis”, “clotting” or “fibrinolysis”; and “exercise” or “exertion”. Publications were retrieved via online indexing engines (Medline, Scopus, Google Scholar).
The literature search yielded 205 publications, after duplicates were removed. In order for a publication to be included in this review, the respective publication should report comparative data on fibrinolysis and coagulation between claudicants before and after a specific regime of exercise, and/or between claudicants and a control group after a specific regime of exercise. The exercise regime should be clearly stated in the publication. After an extensive short-listing based on the previously mentioned criteria, a total of 16 articles were considered relevant. In the subsequent evaluation three further publications were excluded due to the fact that they focus on platelet aggregation factors without reporting on coagulation and fibrinolysis factors. Exclusion criteria also included the following: systematic review or meta-analysis, case reports, and articles in languages other than English. Finally, 13 studies with total of 402 patients were included in this review (Table 1). The flowchart of the studies selection can be seen in Figure 1.
Table 1.
Publications included in the review process.
| Author | Year | Pts | Males | Age | Biomarkers studied | Exercise regimen | Walking distance | Pts characteristics | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Ηobbs et al.9 | 2007 | 34 | 27 | Mean 67 | TAT, PF1 + 2 | Rotation of exercise including treadmill 3 km/h at a 10% incline to absolute claudication distance or 1000 m 2 times a week | 20–500 m | IC; ABI < 0.9 | TAT levels are similar between the groups over the 6-month study period/Significant increase of PF1 + 2 in the group who receive cilostazol |
| Collins et al.2 | 2006 | 20 | 16 | 68 ± 8 | TAT, D-dimer | Treadmill to absolute claudication distance; 3.5 km/h, 5% incline | NA | IC; ABI < 0.8 | D-dimer rise similar to healthy individuals (P = .003); TAT rise 128% (P = .003) |
| Killewich et al.10 | 2004 | 21 | 21 | 68 ± 1 | tPA, PAI | Treadmill 3 times a week for six months | NA | IC; ABI < 0.9 | tPA rise by 28% (P = .003); PAI drop by 23% (P = 0.01) @6 m |
| Burns et al.11 | 2003 | 10 | 10 | Mean 61 (range 58–70) | TAT, PF1 + 2, D-dimer, FdDP | 3.5 km/h at a gradient of 12% until the absolute claudication distance | 25–300 m | ABI < 0.8; claudicant, as defined by Edinburgh Claudication Questionnaire | Rise in TAT, PF1 + 2, D-dimer, FDP (significant when compared to non-smoking controls) |
| Womack et al.12 | 2001 | 9 | 8 | 70 ± 6 | tPA, PAI | 65% of maximal intensity until 30 min | NA | Fontaine II | tPA levels rise by 180% (P = .017); PAI-1 and tPA antigen changed non-significantly; PAI-1 levels decreased by 43% (P = 0.017) |
| Gardner and Killewich13 | 2002 | 106 | NA | 70 ± 1 | tPA, PAI | Accelerometer during 48 h usual activity and then 6-min walking and treadmill exercise to ACD | NA | PAD and IC; Fontaine II | Impaired fibrinolysis in lower activity group; drop of tPA and rise of PAI |
| Constans et al.14 | 2000 | 20 | NA | 66 ± 10 | PF1 + 2, D-dimer, PAI, TFPI, sTM, vWF, sTM | 2 treadmill exercises in two weeks; 10% slope, 3.2 km/h | Mean 225 ± 180 | Fontaine II (ABI < 0.8) | At ACD P-selectin and sTM levels were significantly higher (P < .01); at recuperation period PAI-1 levels decreased (P < .01) while PF1 + 2 increased (P < .05) |
| Killewich et al.15 | 1998 | 80 | 80 | 69 | PAI-1 activity, tPA (Ag and activity) | 6-min walk during which pain-free walking time and distance were measured | NA | Mild claudicants (MC) with mean ABI 0.65 ± 0.04; severe claudicants (SC) with ABI 0.58 ± 0.03 | Increased PAI-1 activity in both MC and SC compared to healthy controls (P = .01 and P = .02 respectively); Increased tPA antigen levels (P = .001) and decreased tPA activity (P = .001) in SC group only |
| Mustonen et al.16 | 1998 | 15 | 10 | 59 ± 8 (42–70) | TAT, D-dimer, tPA:Ag, PAI-1:Ag, tPA ativity, PAP | Treadmill to max walking distance; at 3.2 km/h, flat for first 2 min; thereafter, the inclination angle was increased by 2° every 2 min | Median 567 m (140–1500 m) | Fontaine stage II | D-dimer significantly higher than control group (<.003); tPA/PAI-1 ratio almost constant; TAT rise (PT < .05) |
| Woodburn et al.17 | 1997 | 20 | 11 | 46–71 | tPA, PAI vWF | Treadmill to maximum walking distance or 200 m; 2 km/h, 10 incline | Median 94 m (76–116 m) | Symptomatic PAOD | Not significant alterations |
| Edwards et al.18 | 1994 | 11 | 7 | Mean 61 (range 56–71) | vWF | Treadmill to max walking distance; at 3 km/h, 10° inclination | NA | Stable intermittent claudication as a result of femoropopliteal disease; no clinical evidence of DM or ischemic heart disease | Significant vWF rise (P < 0.05) at 60 min post-exercise |
| Herren et al.19 | 1994 | 22 | 14 | Mean 65 (range 46–80) | PF1 + 2, TAT, D-dimer, fibrinogen | Treadmill until symptoms appeared; 10% inclination | 271.3 ± 149.4 | Fontaine II | No significant changes related to exercise; changes only related to severity and ABI, and ECG changes |
| De Buyzere et al.20 | 1993 | 34 | 26 | Mean 62 | PF1 + 2, TAT, D-dimer, fibrinogen | Treadmill at constant speed, inclination starting from 0% by 5% every 3 min to max 15% | 583 ± 40 | Fontaine II | Significant fibrinogen rise (P < 0.01); PF1 + 2, TAT, D-dimer higher in claudicants (P < 0.01 vs. control) but no significant rise after exercise |
FDP, fibrin degradation products; PAI, plasminogen activator inhibitor; PAP, plasmin-α2-antiplasmin; PF1 + 2, prothrombin factors 1 and 2; sTM, scanning tunneling microscope; TAT, thrombin–antithrombin complex; tPA, tissue polypeptide antigen; vWF, von Willebrand factor.
Results
In 2007, Hobbs et al. studied the effect of supervised exercised and/or cilostazol on the coagulation and fibrinolytic status of 34 claudicants with walking distance of 20–500 m.9 Exclusion criteria included major vascular pathology (myocardial infarction [MI], transient ischemic attack [TIA], cerebrovascular accident [CVA]) or percutaneous transluminal coronary angioplasty (PTCA) in the past three months; significant aorto-iliac disease; inability to walk the absolute claudication distance (ACD); renal impairment with GFR <20 mL/min; congestive heart failure; known predisposition for bleeding; or CYP3A4/CYP2C19 inhibitor use. Patients were randomized into four groups to receive best medical treatment (BMT), BMT plus cilostazol, BMT plus supervised exercise, or BMT plus cilostazol plus supervised exercise. No coagulation and fibrinolysis data were reported for the latter group. BMT was in accordance with the therapy strategy suggested by Burns et al.21 The exercise schedule comprised a three-month, twice-weekly, 1-h exercise program supervised by a physiotherapist. All patients were followed-up at three and six months. Cilostazol dosage was 100 mg twice daily, but upon intolerance the dose was halved. Coagulation status was studied by measuring the levels of thrombin–antithrombin complex (TAT) and prothrombin factors 1 and 2 (PF1 + 2). TAT is the product of thrombin inactivation by antithrombin III, whereas PF1 + 2 are the byproducts of prothrombin conversion to thrombin. TAT levels were found to remain at the same levels throughout the study in all groups (P = .123 for BMT, P = .65 for BMT + cilostazol and P = .929 for BMT + exercise). PF1 + 2 were increased in the BMT plus cilostazol group at six months (P = .002), the only significant change in all groups (P = .916 for BMT, P = .083 for BMT + exercise). Fibrinolysis was assessed by measuring the levels of PAI-1 antigen levels, tissue plasminogen activator (t-PA) antigen, and t-PA activity levels. t-PA antigen levels did not vary significantly within six months in any group (P = .779 for BMT, P = .717 for BMT + cilostazol, P = .084 for BMT + exercise), as well as t-PA activity (P = .889 for BMT, P = .55 for BMT + cilostazol, P = .553 for BMT + exercise). Both the fibrinolysis and the coagulation markers did not related to ankle-brachial pressure index (ABPI), intermittent claudication distance (ICD) or absolute claudication distance.
Collins et al. compared the effect of exercise on the hemostasis, renal function, and inflammation in 20 claudicants who received statin and acetylosalicilic acid (ASA) with 20 healthy individuals.2 Patients on clopidogrel, anti-inflamatory medication, cilostazol or praxilene were excluded from the study. Patients who were considered unable to exercise due to co-morbidities and diabetics were also excluded. All patients were subjected to treadmill exercise to their ACD. The controls walked on the treadmill for 3 minutes 20 seconds, which corresponded to the average patient exercise duration. TAT and fibrin D-dimer levels were measured using enzyme-linked immunosorbent assay (ELISA) and immunoassay, respectively. No difference in baseline TAT levels existed between the claudicants and the controls. TAT levels in claudicants increased immediately after exercise at a median percentage of 128% (P = .003) and remained high during the first hour (P = .009 to baseline). Baseline D-dimer levels were significantly higher in claudicants compared to controls (P = .003). Following exercise, D-dimer levels increased in both groups at a median percentage of 7% (P = .035) and 9% (P = .008) for controls and claudicants, respectively. Changes in both groups were comparable, but the fibrin D-dimer levels remained elevated at 1 h in the claudicants group.
In the most recent study by Killewich et al. 21 claudicants were submitted to regular (3 times a week) treadmill exercise for six months while their t-PA blood level and PAI-1 activity were measured and compared to a similar group of non-exercising claudicants and a group of healthy individuals.10 t-PA activity was lower at baseline in the two claudicant groups compared to the controls, though not statistically significant. After six months of exercise, the t-PA activity level in the exercising claudicant group increased by 28% (P = .003). The non-exercising claudicant group did not demonstrate any change compared to baseline (P = .55). PAI-1 activity was significantly increased in the two claudicant groups compared to the control group before exercise. After six months, PAI-1 activity decreased by 23% in the exercising group (P = .001). The non-exercising claudicant group did not demonstrate any change in the PAI-1 activity. Claudicants who followed the supervised exercise therapy who had the highest pre-exercise PAI-1 activity levels experienced the greatest benefit from exercise, as manifested by the highest decreases in PAI-1 activity. On the contrary, claudicants with the lowest or near normal PAI-1 activity levels experienced the least benefit from exercise, as manifested by the smallest decreases in PAI-1 activity (P = .001).
Burns et al. studied the effect of one-time exertion on the thrombin generation and the fibrin turnover in 10 claudicants and compared these results with 10 healthy age-matched individuals, 5 of whom were smokers.11 Claudicants underwent a treadmill exercise to the ACD, while the controls exercised for 3 min. Exclusion criteria comprised of DM, administration of warfarin, current viral infection, and hemoglobin lower than 11 g/dL. At baseline claudicants had a fibrinogen concentration that was significantly higher than the smoking controls (P = .019), but when compared to non-smoking controls fibrinogen levels were insignificantly increased (P = .181). These differences persisted even after exertion. At baseline, TAT and PF1 + 2 concentrations were similar between the claudicants and the smoking controls, but upon exertion the claudicants group demonstrated significantly higher levels of both TAT and PF1 + 2 (P = .011 and P = .001 vs. smoking and non-smoking controls for TAT; P = .129 and P = .005, respectively, for PF1 + 2). At baseline and post-exertion, d-Dimer and fibrin degradation products (FDP) concentrations were similar between claudicants and the smoking controls. When compared to non-smoking controls, the d-Dimer and FDP levels of claudicants were significantly higher.
In the largest patients’ series to date, Gardner and Killewich studied the effect of exercise on claudicants after the latter who were divided into low, moderate and high daily activity groups depending on the physical activity recorded for two consecutive weekdays using an accelerometer.13 Subjects performed a progressive, graded treadmill protocol at a speed of 2 mph, with no inclination and with 2% increase every 2 min until ACD was reached. Moderate and high activity groups demonstrated a significant increase in tPA levels compared to the low activity group (P < .05 for both). The high activity group also demonstrated a higher tPA increase when compared to moderate activity group (P < .05). The claudicants were also subjected to 6-min supervised walking and results were similar to the above described results after the treadmill exercise (P < .05 for all). In a similar fashion, PAI-1 blood levels were decreased after both 6-min walking and the treadmill exercise in moderate and high activity groups compared to low activity group (P < .05).
In 2001, Womack et al. studied the levels of tPA and PAI-1 in Fontaine class II claudicants after exercising for 30 min at 65% of maximal exertion achieved on a previous graded exercise test.12 The cumulative 30 min exercise time was reached by all subjects with a mean of 2 ± 1 breaks (range 0–4). Claudicants were subjected to 2 miles/h supervised walking. Blood samples were drawn before, immediately after, 30 min, and 60 min after exertion. tPA and PAI-1 antigen levels did not significantly change at any time interval compared to the baseline. In the post-exertion period, tPA blood levels increased significantly by 180% and PAI-1 levels decreased significantly by 43% (P < .017 for both).
Constans et al. studied a number of factors in claudicants after treadmill exercise tests performed twice in two weeks at 10% slope and speed of 3.2 km/h.14 Blood samples were drawn before the exertion, at ACD, and after a period of recuperation. Blood analysis was performed using ELISA for all factors. PF1 + 2 levels did not change at ACD, but was significantly increased during the recuperation period (P < .05). PAI-1 levels were significantly lower in the recuperation period (P < .01) but not changed at ACD. At ACD, scanning tunneling microscope (sTM) levels were significantly higher (P < .01), but later decreased to levels not significantly different to the pre-exertion levels. D-dimer and von Willebrand factor (vWF) levels did not change significantly at any point compared to the pre-exertion baseline.
In 1998, Killewich et al. studied the effect of pain-free supervised walking on the levels of PAI-1 activity, tPA antigen, and tPA activity in mild and severe claudicants (MC and SC, respectively).15 The two claudicant groups had significantly reduced baseline ABIs compared to the healthy controls, but no significant difference inbetween them. Both PAD groups demonstrated an increase in PAI-1 activity compared to healthy individuals (P = .01 and P = .02 for MC and SC, respectively). tPA antigen levels between the healthy controls and the MC group were similar, but the SC group demonstrated a significantly increased tPA antigen level (P = .001). The MC group showed a similar tPA activity to that of healthy controls, but the SC group had a significant decrease in tPA activity (P < .01). Linear regression analysis showed decreasing tPA antigen levels analog to time period of pain-free walking.
Mustonen et al. studied the effect of exercise on PAI-1 antigen and activity, tPA antigen and activity, and concentrations of vWF, TAT, plasmin-α2-antiplasmin (PAP), and D-dimer in Fontaine class II claudicants who have undertaken physical exercise.16 The method comprised 15-min walk followed by a baseline blood test, then a treadmill exercise at a constant speed of 3.2 km/h. In the beginning, the treadmill remained flat for 2 min and the inclination was increased by 2° every 2 min. Then a second blood sample and an ABI were obtained, the subjects rested lying down for 30 min and another blood sample was obtained. Claudicants’ TAT levels increased significantly immediately after exercise compared both to healthy controls after exercise and to the baseline TAT levels of claudicants (P < .01 and P < .05, respectively). tPA baseline levels in claudicants were significantly higher compared to healthy controls’ baseline levels (P < .05). This difference in tPA levels between the two groups persisted immediately after the exercise (P < .01) and after recuperation (P < .05). Both the control and the claudicants’ groups had their tPA levels significantly increased compared to their respective baseline levels (P < .001 for both groups). PAI-1 antigen levels were significantly higher in the healthy control at baseline (P < .01), immediately after exercise (P < .001) and after recuperation (P < .05). A significant decrease in PAI-1 antigen levels was demonstrated in claudicants after rest compared to the port-exertion levels (P < .01).
Woodburn et al. studied PAI-1 activity, tPA, and vWF levels in claudicants after been subjected to a treadmill exercise at a speed of 2 km/h with an incline of 10° until 200 m or the ACD is reached.17 Blood samples were drawn prior to and 2 min after the exercise. None of the factors studied changed significantly with exercise.
In 1994, Edwards et al. subjected claudicants to a 5-min treadmill exercise to ACD at 3 km/h with a 10° incline.18 Patients with DM and ischemic heart disease were excluded from this study. Blood samples were drawn prior to, immediately after and at 5, 15, 30 and 60 min after exercise. vWF levels were measured using ELISA. Baseline vWF levels in claudicants was significantly higher compared to healthy controls (P < .001); a difference that persisted throughout all phases of the study. In claudicants, the vWF raised significantly at 60 min post-exertion compared to baseline (P < .05).
Herren et al. studied the fibrin formation and degradation in claudicants using PF1 + 2, TAT, D-dimers, and fibrinopeptide a (FPA).19 The blood levels of these factors were measured prior to and immediately after symptom limited treadmill exercise with an incline of 10°. All claudicants underwent the treadmill test under ECG surveillance. FPA levels were similar between the PAD patients and the healthy controls both at baseline and after exercise. The blood levels of the rest of the studied factors were higher in the PAD group compared to healthy controls both prior to and after the exercise (P < .05 for all factors before and after exercise). TAT and PF1 + 2 levels did not largely change with exercise and D-dimers decreased after exercise; statistical significance was not available for these data. After exercise, TAT and PF1 + 2 levels increased significantly only in those patients with ST-segment depression in the stress-test ECG compared to their baseline (P < .01 and P < .001, respectively).
De Buyzere et al. studied the effect of exercise on the blood levels of TAT, PF1 + 2, D-dimers, and fibrinogen in claudicants.20 Subjects underwent a standardized multistage exercise test on a treadmill at an inclination of 0% for the first 3 min, and then the inclination increased every 3 min by 5% up to 15%. Speed was maintained constant for each subject. Blood samples were drawn before and immediately after the exercise and blood levels of all factors were measured using ELISA. Baseline fibrinogen levels were similar between the healthy controls and the PAD patients, but the latter saw a significant increase after exertion (P < .05). Baseline TAT, D-dimers, and PF1 + 2 levels were significantly higher in the claudicants group compared to healthy controls ( < .01), but exercise did not impact these levels in the PAD group significantly.
Discussion
In healthy individuals, the mechanisms of coagulation and fibrinolysis are in dynamic balance. A number of studies have demonstrated the benefit in healthy individuals of any age, especially those who otherwise follow a sedentary life-style.7,22,23 Specific patients’ subgroups also find benefit in exercise, such as those who suffered an MI and diabetics.6,7,24
Thrombin, or Factor IIa, is formed from prothrombin under the enzymic action of Factor Xa. It belongs to the proteases of mixed nucleophile, superfamily A (PA clan) and has a molecular weight of 36,000 Da. It activates coagulation factors XI, VIII, V, XIII to their respective active forms. It also catalyzes the conversion of fibrinogen to fibrin and promotes platelet activation and aggregation via activation of protease-activated receptors on the cell membrane of the platelet. Thrombin also has a negative feedback effect on coagulation by binding to thrombomodulin and activating protein C, and by inactivating factors V and VIII. Thrombin blood levels could theoretically demonstrate the activity of the coagulation mechanism, but thrombin blood levels cannot be directly measured as thrombin is quickly inactivated by antithrombin. Levels of thrombin cleavage products (prothrombin fragments 1 and 2; PF1 + 2) and levels of TAT complexes can be directly measured and therefore act as indicators of coagulation activity.
Approximately half of the previously mentioned studies reported the effect that exercise has on TAT levels in claudicants.2,11,16,19,20,25 Three studies found that no significant difference exist between the pre-exercise baseline TAT levels of control groups and claudicants,2,11,16 while two studies reported that claudicants presented with a higher TAT level at baseline compared to healthy controls.19,20 These two contradictory reports are supported by similar number of age-matched subjects, therefore no safe conclusion can be drawn from these studies regarding the baseline TAT level in claudicants. On the contrary the majority of studies supports that TAT levels rise significantly immediately after and up to 1 h after exertion when compared to controls but this increase is not significant when compared to baseline.2,11,16,19,20 According to one of these studies, this post-exercise TAT increase can persist even after 30 min of rest.16 This rise in post-exertion TAT levels is disputed only by Hobbs et al., who report that TAT levels do not rise significantly even up to three months post-exertion (to a total of 6 months from commencing exercise), but the study method is unique and has not been reproduced—even in a similar fashion—in any other of the series.9
The other markers of coagulation activity, PF1 + 2, are reported by two studies to be at a higher baseline level compared to controls and this difference between claudicants and healthy control persists in the early post-exertion period.19,20 Only one study did not report any difference between the PF1 + 2 pre-exertion levels of claudicants and smoking controls, but a significant increase was reported when compared to non-smoking controls.11 Constans et al. take a different approach and compares the immediate post-exertion and the post-resting levels of PF1 + 2 to the respective baseline, reporting that immediately after exercise there is no significant increase to baseline, but after a period of rest PF1 + 2 levels rise significantly.14 Once more, the work of Hobbs et al. reports totally different results: a lower pre-exertion level of PF1 + 2 in claudicants receiving cilostazol compared to all other groups9 and in a similar comparison at three and six months, PF1 + 2 levels of the cilostazol group were significantly higher.
From the above, it is apparent that even at rest claudicants tend to have a higher level of coagulation activity, a trend that persists even after exercise. Compared to the claudicants baseline, coagulation activity does not clearly increase after exercise. These two conclusions regarding the coagulation activity levels should be taken under consideration with caution, as it is clear that the limited number of studies and the total number of subjects cannot statistically support the results.
In regard to fibrinolysis, most publications report tPA and PAI-1 antigen (Ag) and activity levels, D-dimer concentration, and less frequently include PAP levels, and tPA Ag/PAI-1 Ag ratio in their results. Fibrinolytic activity can be estimated by measuring the blood levels of PAI-1 antigen, tPA antigen, tPA activity, and D-dimer levels. tPA is single-chain serine proteinase with molecular weight of approximately 70,000 Da, consisting of 527 amino acids with Ser as the N-terminal amino acid.26 tPA converts plasminogen into plasmin, which is the main enzyme of fibrin degradation and therefore of clot dissolving. High tPA activity levels and low tPA antigen levels result in increased fibrinolysis, when low tPA activity levels and high tPA antigen levels show a hypofibrinolytic state along increased PAI-1 activity levels. PAI-1 or serpin E1 is a protein encoded by the SERPINE1 gene.27 PAI-1 is a serine protease inhibitor (serpin) that inhibits the effect of tPA and urokinase, both activators of fibrinolysis. It is produced by the endothelium and increased PAI-1 levels lead to an increased thrombotic risk and atherosclerosis. D-dimer is a FDP created by the consecutive action of thrombin, factor XIIIa, and plasmin. D-dimer consists of two cross-linked D fragments of fibrin proteins. Increased D-dimer blood level indicates a significant degradation of fibrin, alas higher fibrinolysis activity.
Baseline tPA activity does not significantly differ from the activity in healthy controls10,16,17 or in claudicants with low physical activity.15 Post-exertion tPA activity is significantly increased when compared to the baseline value.10,12,13,16 This increase persists up to 1 h after the exercise,12 something that can explain the continuous and persisting tPA activity increase after frequent bouts of exercise reaching a six-month period.10 tPA Ag levels showed either to be stable throughout the exercise period or to decrease.12,15
Baseline PAI-1 activity in claudicants is reported to be significantly higher than in healthy controls.10,12,15,16 This difference in PAI-1 activity does not depend on the severity of the PAD symptoms and it is significant even in patients with MC.15 Post-exercise PAI-1 activity is lower compared to both the baseline value12–14 and the lower-intensity exercise PAD group.15 This decrease in PAI-1 activity is prolonged up to 2 h post-exertion including a period of rest.12,13 Patients with PAI-1 levels higher than other claudicants were demonstrated a bigger decrease in post-exertion PAI-1 levels, thus they benefited more from exercise.10 The intensity of the exercise also correlates inversely to the degree of PAI-1 activity drop.12
In most studies, baseline D-dimer levels are reported to be higher in claudicants compared to healthy controls.2,16,19,20 D-dimer levels significantly increase after exercise compared to the respective levels of healthy controls,2,16,19,20 and it appears to persist up to 1 h post-exercise.2 This increase is not significant when compared to the baseline values. It is interesting that other FDP appear to have higher baseline and post-exertion concentrations in claudicants when compared to non-smoking control, but there is no significant difference between claudicants and smoking controls.11
Post-exertion fibrinolysis in claudicants appears to be activated by both higher levels of tPA activity and lower antagonizing effect of PAI-1 activity. These two changes in the fibrinolytic mechanism are reported to persist even well after cessation of exercise. It is not clear if tPA or PAI-1 activity overwhelms the other, but to a degree a balance between the two antagonizing effects is reached.16 Ratios between the tPA and PAI-1 activity and antigen levels would be useful to explore the state of balance, but unfortunately these are not used but in one of the already mentioned studies.16
If we consider the few available data on post-exertion antigen valid, post-exertion tPA Ag concentration appears to decrease and PAI-1 Ag concentration appears to remain stable,12,15 meaning a degree of clotting degradation. Patients’ post-exertion D-dimer blood levels are higher compared to healthy controls, but not higher compared to baseline. This could indicate that claudicants have a functional fibrinolytic mechanism in place, but it continuously operates at a higher level. Despite these post-exertion changes in fibrinolysis, exercise does not impact clot strength and clot formation time in claudicants.28
As mentioned above, the small number of studies, the small number of subjects within studies, and the different modalities and regime of exercise present some of the limitations of this review. It is rather impossible to categorize the mentioned studies into groups with same exercise regimes and reach safe conclusions on how exercise of given intensity and type affects fibrinolysis and coagulation. Despite their potential role, some factors (e.g. fibrinogen, vWF, P-selectin) have not been studied thoroughly in the included literature, something that might mislead conclusions.29 One other important limitation is that patients in most of the above mentioned studies have not been randomized, leading to potential bias and statistical errors.
Exercise has been proved to improve the quality of life, increase walking distance, and extend life expectancy of patients with PAD.30–34 The modality, the intensity, and the regime of exercise influence the outcome for patients.12,35 To date, data is insufficient to draw safe conclusions on how physical activity alters the coagulation and the fibrinolytic state of PAD patients. Larger randomized controlled studies, all employing a similar and comparable exercise regime, are necessary to examine the results of exercise in claudicants. After clarifying the mechanism involved and the expected results from exercise in claudicants, a further step would be to optimize the exercise regime in such a way that enhances the fibrinolytic effect while suppresses the coagulation mechanism in the long term.
Authors’ contribution
All authors participated in drafting the manuscript, reviewing of the manuscript and approving the version to be published; NP and GK collected the data; GNK assisted in data analysis and interpretation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
No funding was received from any source.
References
- 1.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001; 286: 1317–24. [DOI] [PubMed] [Google Scholar]
- 2.Collins P, Ford I, Croal B, Ball D, Greaves M, Macaulay E, Brittenden J. Haemostasis, inflammation and renal function following exercise in patients with intermittent claudication on statin and aspirin therapy. Thromb J 2006; 4: 9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes JR, Solomon LJ, Naud S, Fingar JR, Helzer JE, Callas PW. Natural history of attempts to stop smoking. Nicotine Tob Res 2014; 16: 1190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindgren A, Lindoff C, Norrving B, Astedt B, Johansson BB. Tissue plasminogen activator and plasminogen activator inhibitor-1 in stroke patients. Stroke 1996; 27: 1066–71. [DOI] [PubMed] [Google Scholar]
- 5.Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med 1995; 332: 635–41. [DOI] [PubMed] [Google Scholar]
- 6.Estelles A, Aznar J, Tormo G, Sapena P, Tormo V, Espana F. Influence of a rehabilitation sports programme on the fibrinolytic activity of patients after myocardial infarction. Thromb Res 1989; 55: 203–12. [DOI] [PubMed] [Google Scholar]
- 7.Stratton JR, Chandler WL, Schwartz RS, Cerqueira MD, Levy WC, Kahn SE, Larson VG, Cain KC, Beard JC, Abrass IB. Effects of physical conditioning on fibrinolytic variables and fibrinogen in young and old healthy adults. Circulation 1991; 83: 1692–7. [DOI] [PubMed] [Google Scholar]
- 8.Prisco D, Paniccia R, Bandinelli B, Fedi S, Cellai AP, Liotta AA, Gatteschi L, Giusti B, Colella A, Abbate R, Gensini GF. Evaluation of clotting and fibrinolytic activation after protracted physical exercise. Thromb Res 1998; 89: 73–8. [DOI] [PubMed] [Google Scholar]
- 9.Hobbs SD, Marshall T, Fegan C, Adam DJ, Bradbury AW. The effect of supervised exercise and cilostazol on coagulation and fibrinolysis in intermittent claudication: a randomized controlled trial. J Vasc Surg 2007; 45: 65–70. discussion. [DOI] [PubMed] [Google Scholar]
- 10.Killewich LA, Macko RF, Montgomery PS, Wiley LA, Gardner AW. Exercise training enhances endogenous fibrinolysis in peripheral arterial disease. J Vasc Surg 2004; 40: 741–5. [DOI] [PubMed] [Google Scholar]
- 11.Burns P, Wilmink T, Fegan C, Bradbury AW. Exercise in claudicants is accompanied by excessive thrombin generation. Eur J Vasc Endovasc Surg 2003; 26: 150–5. [DOI] [PubMed] [Google Scholar]
- 12.Womack CJ, Ivey FM, Gardner AW, Macko RF. Fibrinolytic response to acute exercise in patients with peripheral arterial disease. Med Sci Sports Exerc 2001; 33: 214–9. [DOI] [PubMed] [Google Scholar]
- 13.Gardner AW, Killewich LA. Association between physical activity and endogenous fibrinolysis in peripheral arterial disease: a cross-sectional study. Angiology 2002; 53: 367–74. [DOI] [PubMed] [Google Scholar]
- 14.Constans J, Seigneur M, Blann AD, Lestage B, Resplandy F, Renard M, Chaudet B, Amiral J, Guérin V, Boisseau MR, Conri C. Endothelial function, platelet activation and coagulation in lower limb occlusive arterial disease during treadmill exercise: correlations with transcutaneous oxygen pressure. Thromb Res 2000; 99: 557–61. [DOI] [PubMed] [Google Scholar]
- 15.Killewich LA, Gardner AW, Macko RF, Hanna DJ, Goldberg AP, Cox DK, Flinn WR. Progressive intermittent claudication is associated with impaired fibrinolysis. J Vasc Surg 1998; 27: 645–50. [DOI] [PubMed] [Google Scholar]
- 16.Mustonen P, Lepantalo M, Lassila R. Physical exertion induces thrombin formation and fibrin degradation in patients with peripheral atherosclerosis. Arterioscler Thromb Vasc Biol 1998; 18: 244–9. [DOI] [PubMed] [Google Scholar]
- 17.Woodburn KR, Rumley A, Murtagh A, Lowe GD. Acute exercise and markers of endothelial injury in peripheral arterial disease. Eur J Vasc Endovasc Surg 1997; 14: 140–2. [DOI] [PubMed] [Google Scholar]
- 18.Edwards AT, Blann AD, Suarez-Mendez VJ, Lardi AM, McCollum CN. Systemic responses in patients with intermittent claudication after treadmill exercise. Br J Surg 1994; 81: 1738–41. [DOI] [PubMed] [Google Scholar]
- 19.Herren T, Stricker H, Haeberli A, Do DD, Straub PW. Fibrin formation and degradation in patients with arteriosclerotic disease. Circulation 1994; 90: 2679–86. [DOI] [PubMed] [Google Scholar]
- 20.De Buyzere M, Philippe J, Duprez D, Baele G, Clement DL. Coagulation system activation and increase of D-dimer levels in peripheral arterial occlusive disease. Am J Hematol 1993; 43: 91–4. [DOI] [PubMed] [Google Scholar]
- 21.Burns P, Gough S, Bradbury AW. Management of peripheral arterial disease in primary care. BMJ 2003; 326: 584–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahraman S, Bediz CS, Piskin O, Aksu I, Topcu A, Yuksel F, Demirkan F. The effect of the acute submaximal exercise on thrombin activatable fibrinolysis inhibitor levels in young sedentary males. Clin Appl Thromb Hemost 2011; 17: 414–20. [DOI] [PubMed] [Google Scholar]
- 23.Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol 2008; 59: 119–32. [PubMed] [Google Scholar]
- 24.Khalil O, Sherif MM, ME G, Fahmy D, Fawzy M. Effect of aerobic exercises on blood coagulation and fibrinolytic system in type 2 diabetic patients. Int J Adv Res 2015; 3: 64–70. [Google Scholar]
- 25.Hobbs SD, Marshall T, Fegan C, Adam DJ, Bradbury AW. The constitutive procoagulant and hypofibrinolytic state in patients with intermittent claudication due to infrainguinal disease significantly improves with percutaneous transluminal balloon angioplasty. J Vasc Surg 2006; 43: 40–6. [DOI] [PubMed] [Google Scholar]
- 26.Pennica D, Holmes WE, Kohr WJ, Harkins RN, Vehar GA, Ward CA, Bennett WF, Yelverton E, Seeburg PH, Heyneker HL, Goeddel DV, Collen D. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature 1983; 301: 214–21. [DOI] [PubMed] [Google Scholar]
- 27.Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost 2005; 3: 1879–83. [DOI] [PubMed] [Google Scholar]
- 28.Mauer K, Exaire JE, Stoner JA, Saucedo JF, Montgomery PS, Gardner AW. Effect of exercise training on clot strength in patients with peripheral artery disease and intermittent claudication: an ancillary study. SAGE Open Med 2015; 3: 2050312115575938–2050312115575938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paraskevas KI, Baker DM, Vrentzos GE, Mikhailidis DP. The role of fibrinogen and fibrinolysis in peripheral arterial disease. Thromb Res 2008; 122: 1–12. [DOI] [PubMed] [Google Scholar]
- 30.Vemulapalli S. Revascularisation plus supervised exercise is superior to supervised exercise alone for the treatment of intermittent claudication. Evid Based Med 2016;21:91. [DOI] [PubMed]
- 31.Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014; 7: CD000990–CD000990. [DOI] [PubMed] [Google Scholar]
- 32.Parmenter BJ, Raymond J, Fiatarone Singh MA. The effect of exercise on fitness and performance-based tests of function in intermittent claudication: a systematic review. Sports Med 2013; 43: 513–24. [DOI] [PubMed] [Google Scholar]
- 33.Parmenter BJ, Raymond J, Dinnen P, Singh MA. A systematic review of randomized controlled trials: walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis 2011; 218: 1–12. [DOI] [PubMed] [Google Scholar]
- 34.Delaney CL, Miller MD, Chataway TK, Spark JI. A randomised controlled trial of supervised exercise regimens and their impact on walking performance, skeletal muscle mass and calpain activity in patients with intermittent claudication. Eur J Vasc Endovasc Surg 2014; 47: 304–10. [DOI] [PubMed] [Google Scholar]
- 35.Lauret GJ, Fakhry F, Fokkenrood HJ, Hunink MG, Teijink JA, Spronk S. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev 2014; 7: CD009638–CD009638. [DOI] [PubMed] [Google Scholar]