Abstract
Non-erythroid alpha spectrin (αIISp) is a structural protein which we have shown is present in the nucleus of human cells. It interacts with a number of nuclear proteins such as actin, lamin, emerin, chromatin remodeling factors, and DNA repair proteins. αIISp’s interaction with DNA repair proteins has been extensively studied. We have demonstrated that nuclear αIISp is critical in DNA interstrand cross-link (ICL) repair in S phase, in both genomic (non-telomeric) and telomeric DNA, and in maintenance of genomic stability following ICL damage to DNA. We have proposed that αIISp acts as a scaffold aiding to recruit repair proteins to sites of damage. This involvement of αIISp in ICL repair and telomere maintenance after ICL damage represents new and critical functions for αIISp. These studies have led to development of a model for the role of αIISp in DNA ICL repair. They have been aided by examination of cells from patients with Fanconi anemia (FA), a repair-deficient genetic disorder in which a deficiency in αIISp leads to defective ICL repair in genomic and telomeric DNA, telomere dysfunction, and chromosome instability following DNA ICL damage. We have shown that loss of αIISp in FA cells is due to increased breakdown by the protease, µ-calpain. Importantly, we have demonstrated that this deficiency can be corrected by knockdown of µ-calpain and restoring αIISp levels to normal. This corrects a number of the phenotypic deficiencies in FA after ICL damage. These studies suggest a new and unexplored direction for therapeutically restoring genomic stability in FA cells and for correcting numerous phenotypic deficiencies occurring after ICL damage. Developing a more in-depth understanding of the importance of the interaction of αIISp with other nuclear proteins could significantly enhance our knowledge of the consequences of loss of αIISp on critical nuclear processes.
Keywords: Non-erythroid alpha spectrin, DNA repair, DNA interstrand cross-links, telomeres, telomere dysfunction, chromosome stability, Fanconi anemia
Introduction
Spectrin is a structural protein, which is a major constituent of the cytoskeletal meshwork of proteins associated with the RBC membrane, and which has been long known for the essential role it plays in maintaining RBC membrane structure and flexibility.1–6 Spectrin is also present in non-erythroid cells and tissues where it is part of a cytoskeleton network that provides support for the plasma membrane and is important in maintaining cell shape.2–8 It is composed of heterodimers of α and β spectrin, which link together to form tetramers, and is present throughout the cytoplasm.2–6 Spectrin has been found to associate not only with the plasma membrane but also with organelle membranes, synaptic vesicle surfaces, and the nuclear envelope.6,9 In addition to maintaining cell architecture and plasma membrane stability, it has additional functions in the cell, which include trafficking of vesicles and organelles, synaptic transmission in neurons, adhesion of cells, progression of cells through the cell cycle, signal transduction, and cell growth and differentiation.2–5,8–12
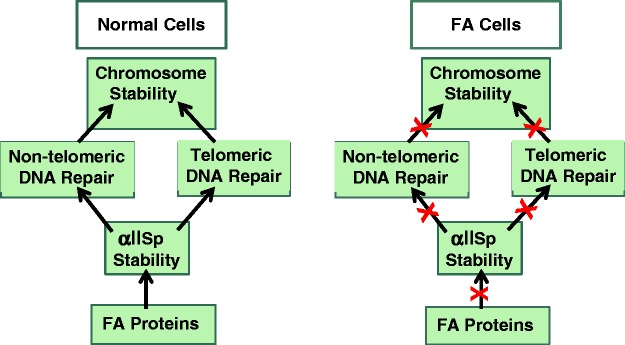
We have demonstrated that non-erythroid α-spectrin (αIISp) is also present in the nucleus of non-erythroid cells.13–15 Using sequence analysis, we have identified αIISp in the nuclei of normal human cells and showed that after DNA damage this protein was needed for DNA repair and chromosome stability.13–20 We have shown that αIISp is critical in DNA interstrand cross-links (ICL) repair and associates with proteins in the nucleus important in this repair process.15–22 We have proposed a model in which αIISp acts as a scaffold in the nucleus recruiting ICL repair proteins to sites of damage.15,16 We have shown that αIISp plays a role in repair of genomic (non-telomeric) and telomeric DNA, where it is needed for maintaining telomere function after ICL damage.15,20 Additionally, after DNA ICL damage, it is critical for maintaining chromosome stability.18–20 We propose that its involvement in repair of damage to both genomic (non-telomeric) and telomeric DNA is important in the critical role it plays in the maintenance of chromosomal stability after DNA ICL damage (Figure 1).15,18–20
Figure 1.
Chromosome stability in normal and Fanconi anemia (FA) cells. αIISp plays a critical role in maintenance of chromosomal stability in normal human cells. We propose that its involvement in repair of damage to both non-telomeric and telomeric DNA is essential for this process; FA proteins, in turn, are important in maintaining the stability of αIISp. We further propose that in FA cells deficiencies in FA proteins lead to reduced stability of αIISp in these cells, which in turn leads to decreased non-telomeric and telomeric DNA repair, which result in chromosome instability. (A color version of this figure is available in the online journal.)
The inherited bone marrow (BM) failure disorder, Fanconi anemia (FA), serves as an excellent model for elucidation of the effects of loss of αIISp in human cells. Classical manifestations of FA include chromosomal defects, congenital abnormalities, and a high predisposition to development of cancer.23–28 Cells from FA patients are strikingly hypersensitive to DNA ICL agents and are defective in ability to repair DNA ICLs.23–31 We have shown that in FA cells there is a deficiency in αIISp (decreased to 35–40% of normal levels) and that this deficiency correlates with diminished levels of repair of DNA ICLs in these cells (34–43% of normal).13,14,18 In FA cells, loss of αIISp is due to increased breakdown of this protein by µ-calpain, a protease which cleaves αIISp and whose levels are increased in FA cells.19 Of significance, we have shown that restoring αIISp levels in FA cells, by knockdown of µ-calpain, corrects a number of the phenotypic deficiencies observed in these cells after ICL damage, such as defective repair of genomic and telomeric DNA and chromosomal aberrations.19 This review will address the importance of αIISp in DNA repair in both non-telomeric and telomeric DNA and in maintaining genomic stability after DNA ICL damage, and demonstrate the deleterious effects that loss of αIISp can have on these processes as is seen in the DNA repair deficient genetic disorder, FA.
αIISp is critical for repair of DNA ICLs in genomic DNA
Repair of DNA ICLs involves a number of different steps and proteins. This process is especially critical at the time of DNA replication where ICLs, if left unrepaired, lead to stalled replication forks.32–35 Our finding that a significant decrease of αIISp in human cells leads to diminished cell survival after exposure to ICL agents and to decreased ability to repair DNA ICLs, specifically in S phase, points to an important role for αIISp in ICL repair.18–20 This involvement in ICL repair represents a new and critical function for αIISp in the cell.
αIISp binds directly to DNA-containing ICLs
Numerous lines of evidence show that αIISp has significant involvement in ICL repair. αIISp purified from bovine brain directly binds to a DNA substrate containing 4,5′,8-trimethylpsoralen (TMP) ICLs.16 αIISp from HeLa cell nuclei similarly binds to this cross-linked substrate.16 This binding is specific for TMP ICLs and not monoadducts and this is the first demonstration that αIISp interacts directly with DNA.16 Non-erythroid α spectrin consists of an array of triple α-helical repeat units.2–6 Examination of the crystal structure of spectrin shows that, in each of the repeat units, the α-helices contain significant numbers of polar residues.16 These residues are present on the surface of the helices (PDB entry 2SPC) indicating that α-spectrin could potentially bind to DNA.16 It is unlikely that spectrin interacts with DNA through the DNA backbone, since the numbers of positive and negative residues present in α-spectrin are similar (PDB entry 2SPC).16 α-spectrin, however, could interact with DNA via hydrogen bonding between its side chains and base atoms (N3 of purines and O2 of pyrimidines) in the minor groove of DNA.16 After ICL damage, the minor grove of DNA could open up, due to formation of the ICL, giving αIISp enhanced ability to interact with DNA after damage. This could thus account for the enhanced binding of αIISp to DNA after ICL damage.16
αIISp is critical for cell survival after DNA ICL damage
Identification of proteins important in ICL repair has been aided by determination of whether a deficiency in a specific protein leads to sensitivity of cells to ICL damage.36 αIISp is an essential protein in cells and, when it is completely depleted, this leads to cell death.11,18,37,38 We have shown, though, that levels of αIISp in normal cells can be knocked down by siRNA to those found in FA cells (35–40% of normal) and that these cells survive just as do FA cells.18 This loss of αIISp, however, results in increased sensitivity and decreased survival upon exposure to DNA ICL agents, providing strong evidence for its involvement in ICL repair.18,19
αIISp co-localizes with the ICL repair protein, XPF, in nuclear foci after DNA ICL damage
Another important indicator that αIISp functions in repair of ICLs is that it localizes to damage-induced nuclear foci after treatment of cells with a DNA ICL agent (psoralen plus UVA light or mitomycin C).17–19 Since αIISp binds directly to DNA-containing ICLs, this indicates that these foci represent localization of αIISp to sites of damage.16 In addition, αIISp co-localizes with the ICL repair protein, XPF, at sites of ICL damage (Figure 2).17,19,20 Formation of αIISp and XPF foci, after ICL damage, follows a similar time course.17 Foci first appear at 10 h after ICL damage with 8-methoxypsoralen (8-MOP) plus UVA light, peak at 16 h and are gone by 24 h after damage (Figure 2).17 Since XPF is involved in the incision, or unhooking, step in ICL repair,31,39,40 this indicates that endonucleolytic incisions are taking place during this period and that αIISp plays an important role in this step.
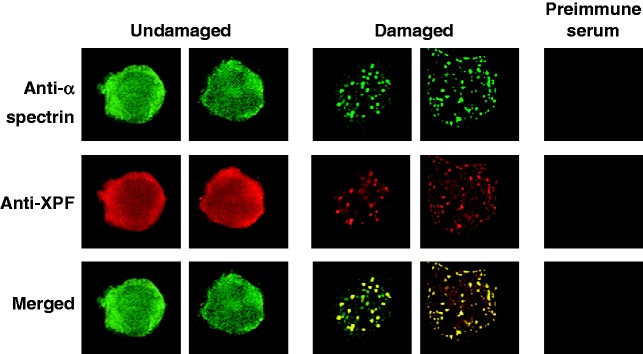
Figure 2.
αIISp and XPF co-localize to nuclear foci after treatment of normal cells with a DNA ICL agent. Normal human lymphoblastoid cells were either undamaged or treated with 8-MOP plus UVA light and the localization of αIISp and XPF foci in the nucleus examined 15 h after treatment. Dual staining was carried out using a monoclonal anti-α-spectrin antibody and a polyclonal anti-XPF antibody. When fluorescent signals for the αIISp and XPF were merged, the overlapping foci were yellow, indicating co-localization of these two proteins. Cells were also stained with the appropriate preimmune sera. (Reproduced from Sridharan et al.17 with permission from the Company of Biologists Ltd.)
αIISp is needed in recruitment of XPF to sites of ICLS
αIISp plays a critical role in the recruitment of XPF to sites of ICLs during the repair process as is demonstrated by studies which show that, after knockdown of αIISp, XPF fails to localize to these sites of damage.18,20 In addition, a monoclonal antibody (mAb) which specifically recognizes αIISp inhibits incisions produced by XPF in an in vitro system that contains a DNA substrate with a site-specific TMP ICL.16 In this in vitro system, purified αIISp has also been shown to enhance incisions produced by XPF.16 Based on these studies, we have proposed that αIISp acts as a scaffold in recruiting repair proteins, such as XPF, to sites of ICLs.16 In its absence, XPF is not recruited to these sites and incisions that it would otherwise produce do not take place.
Interaction of αIISp and FANCD2 in repair of DNA ICLs
In repair of DNA ICLs at stalled replication forks, monoubiquitination of the FA protein, FANCD2 (FANCD2-Ub), is a key event.29,41–43 Like αIISp, FANCD2-Ub has been shown to play a critical role in ICL repair where it is needed for recruiting XPF to damage sites.27–29 However, the relationship between αIISp and FANCD2 in the repair of ICLs, whether these two protein interact after production of ICLs, and whether αIISp is important in the monoubiquitination of FANCD2 and its localization to sites of damage and to chromatin are key questions which had not been addressed until our recent investigations.
Association of αIISp with FANCD2
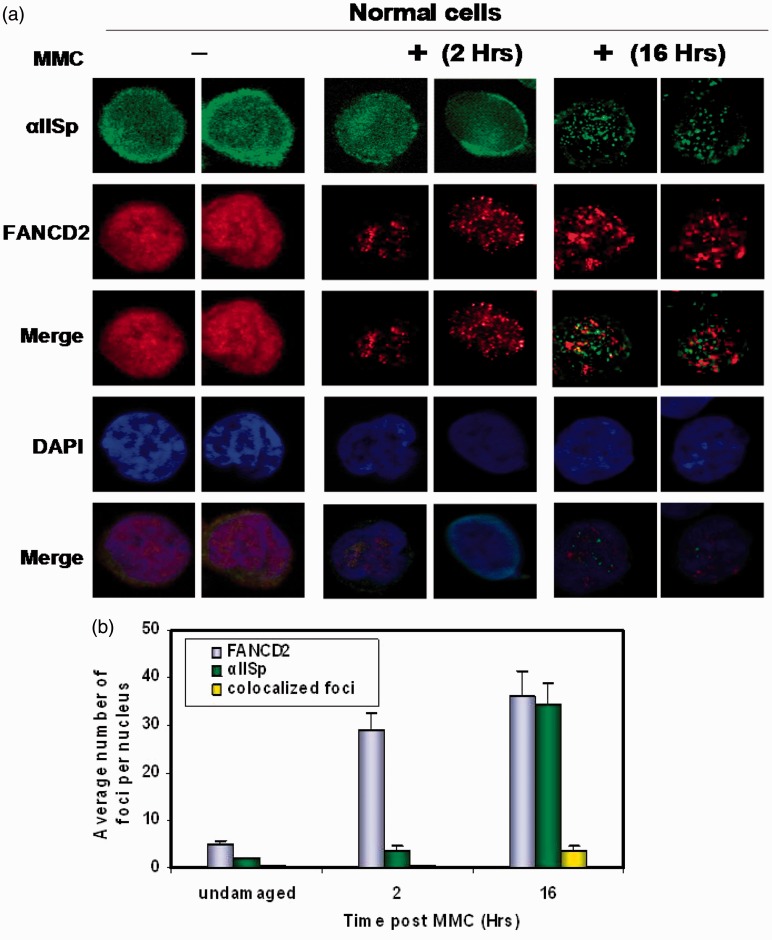
Our studies have demonstrated that FANCD2 associates with αIISp in normal human cells.44 However, after ICL damage, FANCD2 dissociates from αIISp and localizes to nuclear foci, which form before αIISp foci and do not co-localize with the αIISp foci (Figure 3).44 This indicates that after ICL damage FANCD2 localizes to different foci than does αIISp.44
Figure 3.
Localization of αIISp and FANCD2 to nuclear foci after DNA ICL damage. After DNA ICL damage, FANCD2 nuclear foci form in normal cells before formation of αIISp foci and do not co-localize with αIISp foci. Normal cells were untreated or treated with mitomycin C (MMC). (a) Formation of FANCF2 and αIISp foci was examined 2 and 16 h after treatment using indirect immunofluorescence and staining with anti-FANCD2 or anti-αIISp. Cells were also counter stained for the DNA-specific DAPI. The images were merged to examine co-localization of FANCD2 and αIISp foci. The images were also merged with the DAPI stained nuclei to show that these foci were present in the nucleus. (b) The average number of FANCD2 and αIISp foci per nucleus and the average number of FANCD2 and αIISp nuclear foci co-localizing were quantitated in cells 2 and 16 h after MMC treatment. Error bars represent SEM. (Reproduced from Zhang et al.44 with permission from Wiley Periodicals, Inc
Time course for formation of αIISp and FANCD2 foci after ICL damage
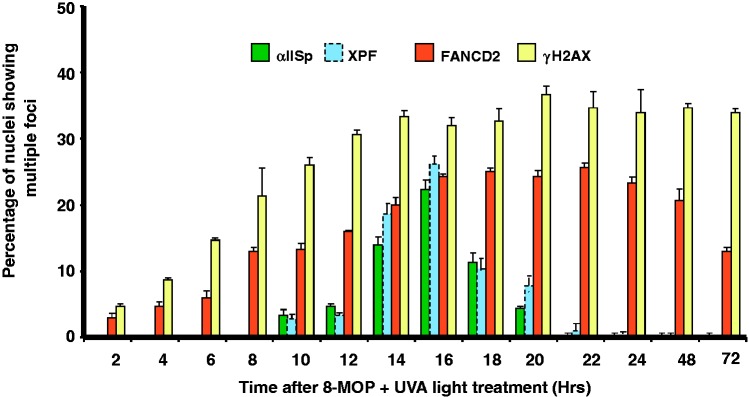
The view that αIISp and FANCD2 localize to different foci after ICL damage is further substantiated by the demonstration that formation of FANCD2 foci follows a different time course compared to that of αIISp and XPF foci, with FANCD2 foci forming before αIISp foci at 2 h after damage, compared to the 8–10 h for αIISp and XPF foci (Figure 4).17,44 These studies indicate that FANCD2 is recruited to sites of ICLs before αIISp and XPF and acts upstream of both of them.44 Similar results have been obtained using Xenopus egg extracts.40 These latter studies demonstrated that FANCD2 is recruited before XPF to sites of ICLs.40 This indicates that FANCD2 is loaded at these sites upstream of XPF.40 These results, combined with ours, indicate that both αIISp and XPF act downstream of FANCD2 after ICL damage.
Figure 4.
Time course for appearance of αIISp, XPF, FANCD2, and γ-H2AX nuclear foci after DNA ICL damage. Normal human cells were treated with 8-MOP plus UVA light and the percentage of nuclei showing multiple αIISp, XPF, FANCD2, and γ-H2AX foci determined at the indicated time post-treatment. Notation of XPF is from Sridharan et al.17 Nuclei containing four or more foci were counted as positive. Nuclear foci for 100 cells were counted for each time point after treatment. Error bars represent SEM. (Reproduced from Zhang et al.44 with permission from Wiley Periodicals, Inc.) (A color version of this figure is available in the online journal.)
FANCD2 foci formation plateaus at 16 h after ICL damage; foci start decreasing at 24 h but are still present at 72 h, unlike αIISp and XPF foci which peak at 16 h and are gone by 24 h (Figure 4).44 This strengthens the view that αIISp and XPF are involved in the incision steps in ICL repair and that FANCD2, in addition to a role in these steps, functions, as has been proposed, in subsequent steps in ICL repair.40
αIISp is not needed for monoubiquitination of FANCD2 or its localization to foci
When αIISp is knocked down, monoubiquitination of FANCD2 is not affected.44 After ICL damage, knockdown of αIISp also has no effect on localization of FANCD2 to chromatin or nuclear foci.44 These studies indicate that αIISp is not needed for the functioning of FANCD2-Ub, strengthening the view that it acts downstream of FANCD2-Ub in the repair process.44 Similarly, XPF, in a system utilizing Xenopus egg extracts, has been shown not to be required for the monoubiquitination of FANCD2 after ICL damage.40 Thus two proteins, αIISp and XPF, which play a role in ICL repair and are targeted to the same sites of damage, are not involved in monoubiquitination of FANCD2, which is targeted to a different site after ICL damage.
Model for the role of αIISp in DNA ICL repair
The studies described above thus demonstrate that αIISp and XPF co-localize to different sites of damage compared to those of FANCD2. They suggest that αIISp (1) acts downstream of FANCD2-Ub, like XPF, and is not needed for monoubiquitination of FANCD2 or for its localization to sites of ICLs; and (2) is critical for recruitment of XPF to damage sites and for incisions XPF produces at these sites.17,44
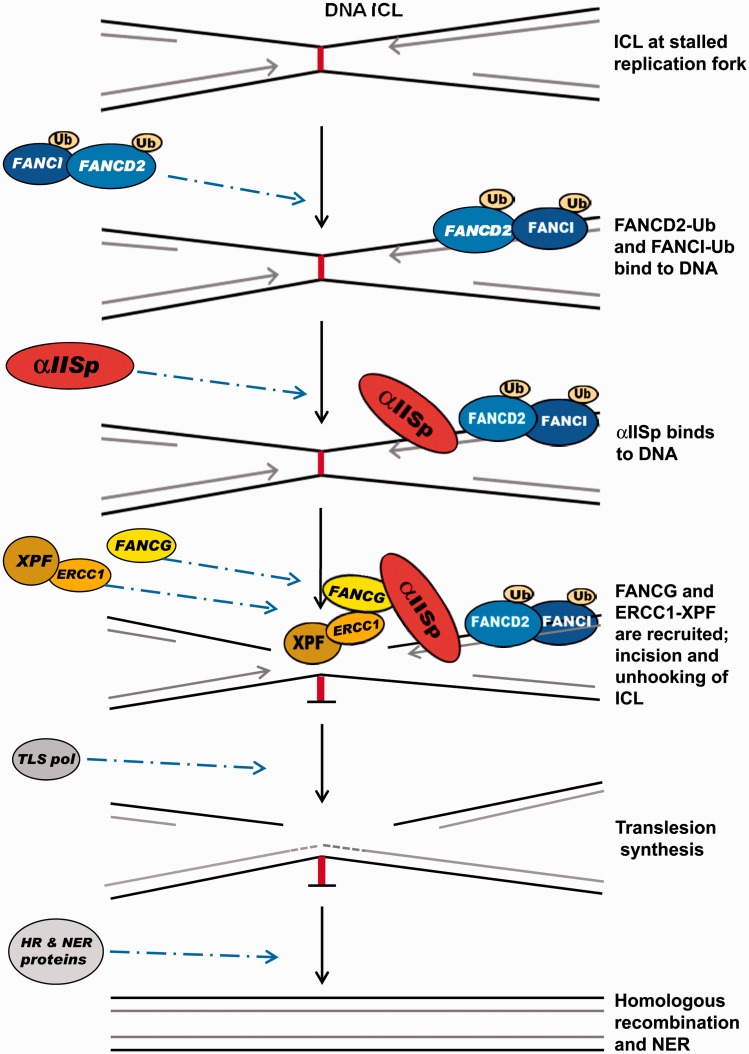
Based on this evidence, we now propose a model for the role of αIISp in repair of DNA ICLs (Figure 5). In this model: (1) When DNA replication is stalled at the site of an ICL, FANCD2 and FANCI are monoubiquitinated by the FA core complex and localize to the damaged DNA;26,29,41,42 (2) αIISp binds to DNA at sites of ICLs, downstream from FANCD2-Ub and in a different location than FANCD2-Ub.16 Whether FANCD2 is involved in recruitment of αIISp to sites of ICLs is as yet not known; (3) FANCG is recruited and binds to αIISp;45 (4) XPF-ERCC1 is then recruited and ERCC1 binds to FANCG;46 (5) XPF incises the DNA, unhooking the cross-link in conjunction with other nucleases such as SLX4;28,29,31,39,40,47 (6) translesion DNA synthesis occurs; and (7) the adducted base is removed by nucleotide excision repair and homologous recombination.28,29,34,35 This model thus links αIISp to the incision or unhooking step of the ICL repair process. Additional proteins that are involved in this process, such as SLX4 and other FA proteins, are not shown in this model since the emphasis is on the interaction of αIISp with XPF and the incision events occurring at the site of a DNA ICL.
Figure 5.
A model for a role for αIISp in the repair of DNA ICLs. After DNA replication is stalled at the site of an ICL, FANCD2 and FANCI are monoubiquitinated (FANCD2-Ub and FANCI-Ub) by the FA core complex and bind to the damaged DNA. αIISp binds to the DNA at the site of the ICL, downstream from FANCD2-Ub. FANCG is recruited and binds to αIISp. XPF-ERCC1 is then recruited and ERCC1 binds to FANCG. XPF, which is bound to ERCC1, incises the DNA unhooking the cross-linked DNA in conjunction with other nucleases, such as SLX4, which are not shown. Translesion DNA synthesis occurs by a translesion synthesis DNA polymerase (TLS pol) and the adducted base is removed and repair continues by a combination of nucleotide excision repair (NER) and homologous recombination (HR). This model does not show other proteins involved in this pathway, since it is emphasizing the role of αIISp in the ICL repair process
The link between αIISp and the unhooking step of DNA ICL repair
In the repair of DNA ICLs, as is indicated above, an important initial step is the unhooking of the ICL; XPF-ERCC1 and SLX4 are endonucleases which are proposed to be important in this unhooking process.31,39,40,47,48 αIISp has been shown to be critical in recruiting XPF to sites of ICLs in DNA and in incisions produced by it.17,19–21 An important question is what is the link between αIISp and incisions produced by XPF at sites of ICLs and by what mechanism does αIISp aid in this process?
The SH3 domain in αIISp
Non-erythroid α spectrin is composed of 20 triple-helical repeats.2–5 The ninth repeat contains a highly conserved Src-homology 3 (SH3) domain.2–5,49 SH3 domains are modular domains; they are important in protein–protein interactions and play a role in assembly of complex protein networks.50–53 These domains interact with proteins containing proline-rich motifs which have a minimal consensus sequence of PxxP.53–56 There are three major classes of protein ligands that bind to SH3 domains: class I ligands, class II ligands, and class I@ ligands, each of which is characterized by a different consensus sequence.53–58 The SH3 domain of αIISp preferentially binds to class I@ ligands.58 Examination of FA proteins has shown that a number of them have motifs that contain a consensus sequence that can bind to SH3 domains.45
FA proteins have consensus sequences recognizing SH3 domains
A number of FA proteins have motifs that contain either a class I, class II, or class I@ consensus sequence that can bind to SH3 domains; these include FANCA, FANCD1, FANCD2, FANCG, FANCI, FANCL, FANCM, FANCN, FANCP, and FANCQ.45 In FA proteins, these motifs may play a role in the interaction of FA proteins with cellular proteins involved in signal transduction and intracellular signaling. This constitutes another class of protein–protein interaction motifs present in some of the FA proteins.
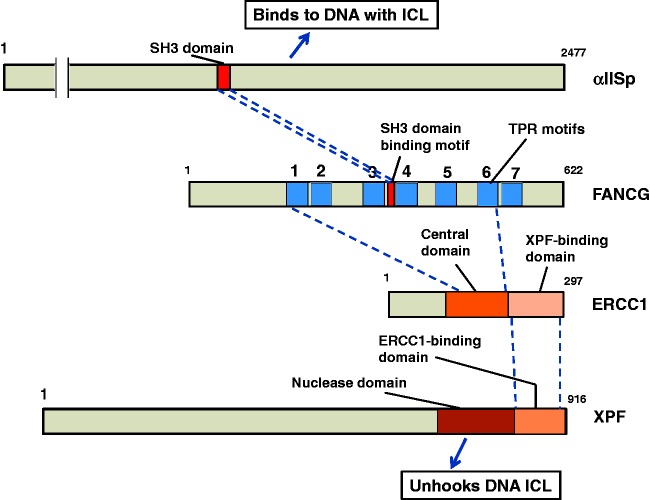
FANCG is of particular interest since it contains both a class I and a class I@ consensus sequence. The SH3 domain of αIISp preferentially binds to class 1@ ligands.58 We have shown, using site-directed mutagenesis and yeast two-hybrid analysis, that FANCG binds directly to the SH3 domain of αIISp by its class I@ consensus sequence together with its flanking C-terminal PxxP sequences (residues 380–394) (Figure 6).45 These flanking PxxP sequences may aid in the binding specificity of the 1@ motif in FANCG to the SH3 domain of αIISp.45 Flanking sequences, such as PxxP, have been shown to provide additional binding specificity for the -SH3 domain and could thus be of importance.49,55–57 These sequences can increase binding affinity significantly as well as selectivity of binding to the SH3 domain.49,55–57 The class 1 consensus sequence in FANCG does not bind to the SH3 domain of αIISp.45 Two of the FA proteins, FANCC and FANCF, which lack these motifs, do not bind to the SH3 domain of αIISp.45 Thus, FANCG has a motif with specificity for binding to SH3 domains and which does in fact bind to the SH3 domain of αIISp.
Figure 6.
Model for the mechanism of action of αIISp in the localization of XPF to sites of ICLs. αIISp binds to DNA at sites of ICLs; it also binds to FANCG via its SH3 domain. FANCG, in turn, binds to αIISp via a domain with a consensus sequence for SH3 domains; this could occur before or after αIISp binds to the DNA. ERCC1-XPF is recruited. ERCC1 interacts, through its central domain, with FANCG via the TPR motifs in FANCG. ERCC1 binds to XPF via a C-terminal XPF binding domain on ERCC1. XPF, in turn, has a binding domain for ERCC1 and it also has a nuclease domain, which aids in the ICL unhooking step
FANCG has a motif that binds to ERCC1-XPF
We have found that FANCG also has strong binding affinity for ERCC1 and moderate affinity for XPF.46 FANCG has seven tetratricopeptide repeat (TPR) motifs, which are motifs that mediate protein–protein interactions.59–62 The class 1@ consensus sequence of FANCG that binds to the SH3 domain of αIISp is located between TPR repeats 3 and 4 (Figure 6). Sites of interaction of FANCG with ERCC1 were mapped using site-directed mutagenesis and yeast two-hybrid analysis.46 These studies demonstrated that TPRs 1, 2, 3, and 6 are important for binding of FANCG to ERCC1 and that full length FANCG is need for this binding.46
ERCC1, in turn, binds to FANCG via its central domain (residues 120–220).46 ERCC1 binds to XPF via its C-terminal domain (residues 220–297) (Figure 6).63–65 XPF binds to ERCC1 at its (i.e. XPF’s) C-terminal domain, which is different from its nuclease domain which is involved in its incision activity (Figure 6).63–65 Thus αIISp, via its binding to FANCG, may interact with ERCC1-XPF and in this manner play a role in the unhooking step of the ICL repair process.
Model for the mechanism of action of αIISp in DNA ICL repair
Based on the results described above, as well as our studies on co-localization of αIISp and XPF at sites of DNA ICLs and on our co-immunoprecipitation data,17,19,45,46 we have proposed a mechanism by which αIISp is involved in the localization of XPF to sites of DNA ICLs and in the unhooking step in the repair process (Figure 6). According to this model: (1) αIISp binds to DNA at sites of ICLs at stalled replication forks; it also binds to FANCG via its (i.e. αIISp’s) SH3 domain;16,45 (2) FANCG, in turn, binds to αIISp via a motif with a consensus sequence for the SH3 domain present in αIISp; this could occur before or after αIISp binds to the DNA;45 (3) ERCC1-XPF is recruited. ERCC1 binds, through its central domain, to FANCG; specific TPR motifs in FANCG are critical for this binding;46 (4) ERCC1 binds to XPF via a C-terminal XPF binding domain on ERCC1;63–65 and (5) XPF, in turn, has a binding domain for ERCC1 and it also has a nuclease domain, which aids in the ICL unhooking step.63–65 We thus propose a mechanism of action for αIISp in DNA ICL repair which links it, via FANCG, to recruitment of XPF-ERCC1 to sites of ICLs and to an important step, production of incisions at these sites and unhooking of the ICL. We have shown that this interaction of αIISp with DNA and repair proteins at sites of damage is critical for repair of DNA ICLs.13–22
αIISp is critical for maintaining telomere function after DNA ICL damage
Chromosome stability is dependent not only on repair of genomic DNA after ICL damage but also on maintenance of telomeric DNA. Telomeres, which are located at the ends of chromosomes, are specialized nucleoprotein structures that are essential for preserving genomic integrity.66–68 They prevent chromosome ends from being considered as double-strand breaks (DSBs) by the cell and thereby prevent end-to-end fusions.66–68 A multiprotein complex, shelterin, specifically binds to telomeres helping to protect them and prevent telomere dysfunction, thus preserving chromosomal stability.66,68–70 Since telomere dysfunction can be an important factor leading to chromosome instability, insuring the integrity of telomeric DNA after DNA damage is essential. Because αIISp is critical for DNA ICL repair as well as chromosome stability, we addressed the important question of whether it is also critical for maintaining telomere function after DNA ICL damage.
Localization of αIISp to telomeres after ICL damage
Examination of telomeres using immunofluorescent staining of αIISp along with fluorescent in situ hybridization (immunoFISH) demonstrated that after DNA ICL damage a portion of αIISp in the nucleus localizes to telomeres and co-localizes with two telomere-specific proteins, TRF1 and TRF2, which are components of the shelterin complex.20 Since proteins important for telomere function are recruited by TRF1 and TRF2 to telomeres,66,68,71–74 this suggests that, after ICL damage, αIISp is also recruited by TRF1 and TFR2 to telomeres. Co-immunoprecipitation data support this view and show that, after ICL damage, binding of αIISp to TRF1 and TRF2 is markedly increased.20 Thus, after DNA ICL damage, αIISp is recruited to telomeres.
αIISp is critical for recruitment of XPF to telomeres after DNA ICL damage
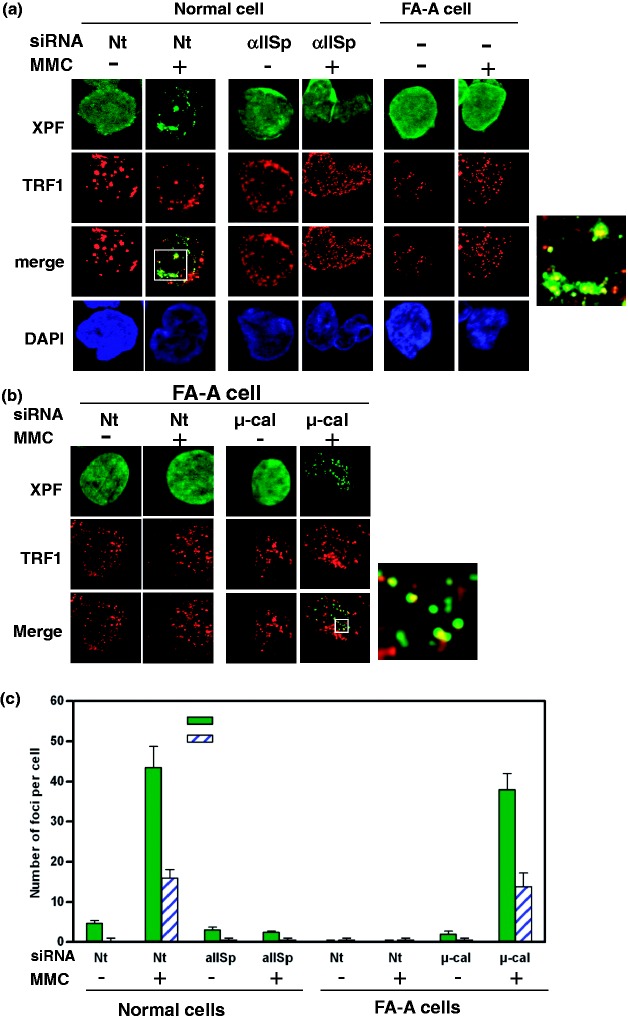
Our studies show that after DNA ICL damage αIISp is necessary for the localization of XPF to telomeres, indicating that αIISp is involved in repair of ICLs in telomeric DNA.20 XPF foci co-localize with αIISp foci at sites of ICL damage in telomeres.20 That αIISp is important in recruitment of XPF to telomeres after damage is further demonstrated by our studies which show that knocking down αIISp by siRNA results in failure of localization of XPF to telomeres20 (Figure 7). Since XPF has a key role in DNA ICL repair, these studies indicate that αIISp is critical for recruiting XPF to damage sites in telomeres which is an important step in the repair process and in maintenance of telomere stability after ICL damage.
Figure 7.
Knockdown of αIISp in normal cells leads to loss of localization of XPF to telomeres just as is observed in FA-A cells. (a) Normal cells, transfected with either Nt siRNA or αIISp siRNA, and FA-A cells were treated with MMC. Co-localization of XPF with TRF1 was examined 16 h after MMC treatment using immunoFISH and staining with anti-XPF (green) and anti-TRF1 (red) antibodies. Nuclear DNA was counterstained with DAPI (blue). Pictures were taken by z-stack. Only one optical slice is displayed. A magnified image of co-localization of XPF with TRF1 in MMC-treated Nt siRNA transfected normal cells is shown on the right. (b) Knocking down µ-calpain (µ-cal) in FA-A cells restores localization of XPF to telomeres after MMC treatment. FA-A cells were transfected with either Nt siRNA or µ-calpain siRNA and subsequently treated with MMC. Co-localization of XPF with TRF1 nuclear foci at telomeres was examined as above 16 h after MMC treatment. A magnified image of co-localization of XPF with TRF1 in MMC-treated µ-calpain siRNA transfected FA-A cells is shown on the right. (c) The number of XPF nuclear foci per cell and XPF nuclear foci that co-localized with TRF1 foci before and after MMC treatment in normal and FA-A cells was quantitated. Error bars represent SEM. (Reproduced from Zhang et al.20 with permission from Oxford University Press.)
αIISp localizes to telomeres in S phase after ICL damage
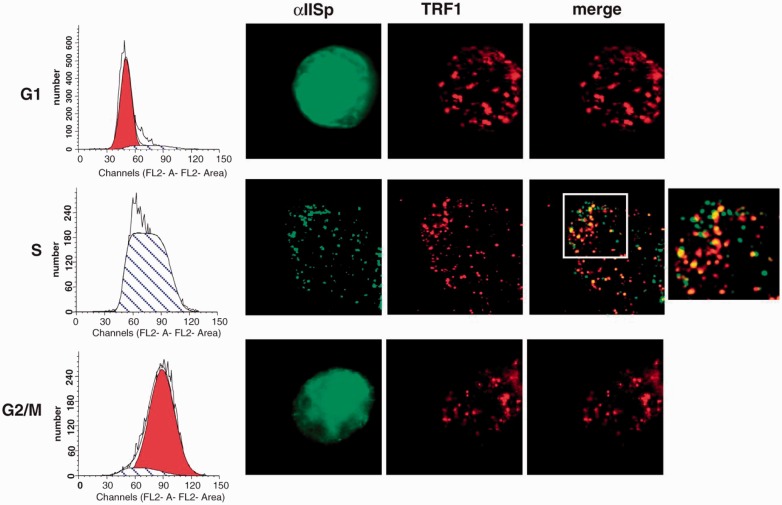
Telomeres undergo DNA replication in S phase.66–68,75 The presence of ICLs in telomeric DNA at the time of replication may lead to blocking of replication and to stalled replication forks. If these ICLs are not repaired, this could result in formation of aberrant telomeric structures and telomere dysfunction. Since, after DNA ICL damage, αIISp specifically localizes to telomeres in S phase (Figure 8), this indicates that it is important in the DNA damage response during replication of telomeric DNA, which in turn could aid in the re-initiation of the stalled replication fork.20
Figure 8.
αIISp specifically associates with telomeres (TRF1) after ICL damage in the S phase of the cell cycle. Normal cells were treated with MMC for 16 h and separated by centrifugal elutriation into G1, S, and G2/M phase of the cell cycle. Cell cycle distribution is shown on the left panel. Formation of αIISp (green) and TRF1 (red) foci and their co-localization was examined by immunoFISH (right panel). Pictures were taken by z-stack. A magnified image of αIISp and TRF1 co-localization is shown on the right. (Reproduced from Zhang et al.20 with permission from Oxford University Press.)
Loss of αIISp leads to telomere dysfunction after ICL damage
The role of αIISp in maintaining telomere function after DNA ICL damage has been further demonstrated by our studies which examined telomere dysfunction in normal human cells after knockdown of αIISp to levels that were 35% of normal.20 One indicator of telomere dysfunction in cells is the presence of telomere dysfunction-induced foci (TIF). This can be determined by examination of γ-H2AX foci which are markers for DNA DSBs and are used as an index of dysfunctional telomeres.76 DNA DSBs can arise when replication forks, stalled at sites of ICLs, fail to be efficiently restarted after damage.76 In normal cells, loss of αIISp results in a significantly increased numbers of TIF-positive cells after ICL damage.20 This is demonstrated by the increased levels of γ-H2AX foci that co-localize with telomeres.20 Another indicator of telomere dysfunction is an increase in chromosomal aberrations. In normal cells, knocking down αIISp results in a 10-fold increase in chromosome aberrations after ICL damage, the majority of these are sister chromatid end-to-end fusions.20 These studies thus show that αIISp is crucial for prevention of TIF formation and telomere dysfunction as well as chromosomal aberrations that arise after ICL damage.
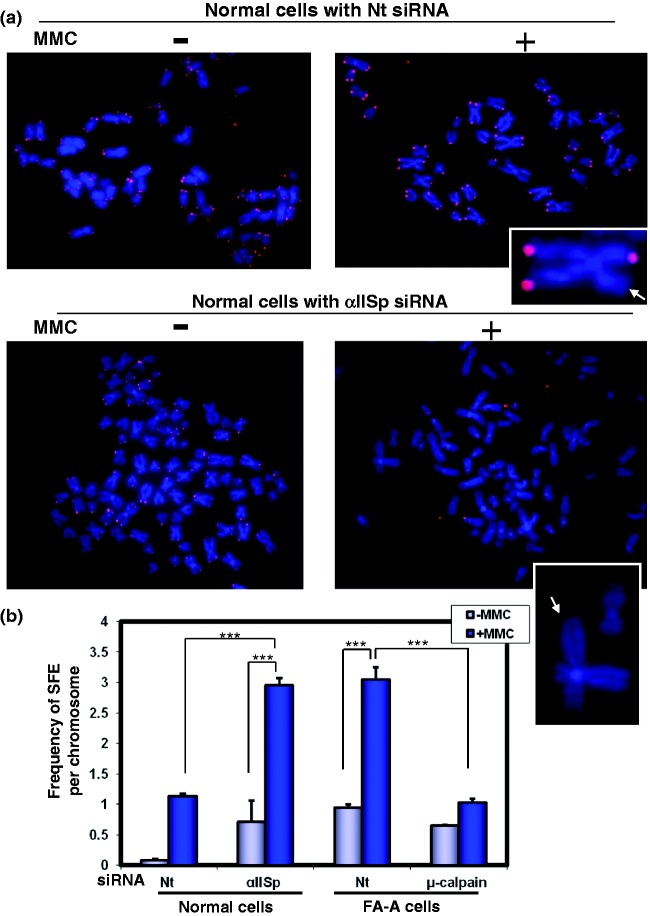
Another strong indication of telomere dysfunction is loss of telomeres. A proposed mechanism for this is that when telomeres are stalled at replication forks this results in collapse of the replication fork and formation of DSBs in DNA and in telomere breakage and loss.67,68 We have demonstrated that in normal human cells, following knockdown of αIISp, catastrophic loss of telomeres, leading to signal free ends (SFEs), occurs after DNA ICL damage (Figure 9).20 The number of SFEs per chromosome increases approximately threefold after damage.20 Based on these studies, we have hypothesized that when levels of αIISp are reduced, this prevents efficient repair of telomeric ICLs during S phase and that this results in replication fork stalling.20 This leads to incomplete telomere replication and formation of telomeric DSBs, which, in turn, promote a significant loss of telomeres.20 These studies provide further evidence for the critical role αIISp plays in maintaining telomere function after ICL damage.
Figure 9.
αIISp deficiency leads to enhanced loss of telomeres after ICL damage. (a) Normal cells were transfected with either Nt siRNA or αIISp siRNA and subsequently treated with MMC for 24 h. Metaphase spreads were prepared and chromosomes stained with DAPI (blue). Telomeric DNA was detected by FISH with a Cy3-labeled telomere-specific PNA probe (red). Inserted panels show magnified images of metaphase chromosomes. Arrowheads point to telomere signal free ends (SFEs). (b) Frequency of SFEs per chromosome for normal and FA-A cells was quantitated. Means are shown of five independent experiments in which 4600 chromosomes were counted per experiment. SEM *** P < 0.0001. (Reproduced from Zhang et al.20 with permission from Oxford University Press.)
Unlike αIISp foci, FANCD2 foci do not localize appreciably with telomeres after ICL damage even though they do localize to non-telomeric DNA.20 This suggests that FANCD2 does not play a role in ICL repair in telomeric DNA, even though it plays a role in repair of ICLs in genomic DNA. In the studies described above, human lymphoblastoid cells were used. These cells express telomerase. In human cells, during DNA replication telomere maintenance and extension of chromosome ends occur by either of two pathways. In one pathway, telomerase, a ribonucleoprotein enzyme complex, is expressed; the other pathway is telomerase independent.71,72,77 Cells which express telomerase include BM, peripheral blood cells, highly proliferating cells, stem cells, and 85–90% of cancer cells.78,79 Studies using HeLa cells, which express telomerase, have similarly shown that FANCD2 foci do not localize to telomeric DNA after DNA ICL damage but do associate with non-telomeric DNA in the nucleus.80 Additionally, these studies demonstrated in three other cell lines, which utilized a telomerase-independent pathway known as alternative lengthening of telomeres (ALT), that following ICL treatment FANCD2 foci co-localized at telomeres with TRF2 in addition to localizing with genomic DNA.80 These studies collectively indicate that, in telomerase-positive cells, FANCD2, unlike αIISp, does not play a role in ICL repair at telomeres. However, FANCD2 is involved in repair of telomeres in telomerase-negative ALT cells.80 It will be of interest to determine whether αIISp plays a role in repair of ICLs in telomeres in telomerase-negative cells and to further delineate the differences between αIISp and FANCD2 in these two pathways.
These studies demonstrate the important role of αIISp in maintenance of telomere function after DNA ICL damage. When there is loss of αIISp, three different telomeric phenotypes are observed associated with telomere dysfunction: (1) increased TIF formation, (2) increased formation of sister chromatid end-to-end fusions, and (3) dramatic loss of telomeres.20 These studies are the first demonstration of a role for αIISp in maintenance of telomeres after ICL damage. The link demonstrated above between αIISp and telomere function has not been previously explored but is one that is highly pertinent to the role αIISp plays in genomic stability.
Role of αIISp in FA and genomic stability
Examination of the physiological importance of αIISp in genomic stability after DNA ICL damage has been aided by studies using FA cells. FA is a genetic disorder characterized by progressive BM failure, diverse congenital abnormalities, chromosome instability, and an increased predisposition to develop cancer.23–28 Cells from FA patients have a marked hypersensitivity to DNA ICL agents and are defective in DNA ICL repair, which is considered an underlying basis for this disorder.23–31 In FA, 19 complementation groups have been identified; each group is characterized by mutations in a different FA gene.26,28,29,36,81 Analysis of αIISp from all FA groups examined (FA-A, -B, -C, -D1, -D2, -F, -G) has shown that αIISp levels are only 35–40% of normal.13,14,16,18 In these FA cells, this deficiency correlates with reduced levels of DNA ICL repair (34–43% of normal), as measured by determining levels of unscheduled (i.e. non-S-phase) DNA synthesis.20,30,31 FA thus serves as an excellent model for studying the effects of a deficiency in αIISp.
Reduced stability of αIISp in FA cells
The decreased αIISp in FA cells is not due to its reduced expression.82 It is due to reduced stability of αIISp, which we have shown results from increased breakdown by the protease, µ-calpain.19,20 In cells from a number of FA complementation groups (FA-A, -C, -D2, -F, -G) (i.e. all the FA groups we studied), the levels of µ-calpain activity are three to fourfold higher compared to those of normal cells, which can account for the increased breakdown of αIISp in FA cells.19 We have proposed that, in FA cells, this deficiency of αIISp is an important factor contributing to many of the phenotypic changes that characterize this disorder.
Loss of αIISp in FA leads to defective ICL repair and chromosome instability
Numerous lines of evidence demonstrate that in FA cells a deficiency in αIISp is a critical factor contributing to the defective ICL repair and chromosomal instability observed. In FA-A cells, the ICL repair protein, XPF, though present, does not localize to sites of damage in the nucleus after DNA ICL damage to cells.17,19,20 After knockdown of µ-calpain and restoration of αIISp levels to normal in FA-A cells, XPF localizes to nuclear foci after ICL damage and co-localizes with αIISp.19,20 This is accompanied by an increase in cell survival and DNA repair to levels similar to those in normal cells following ICL damage.17,19 Additionally, when levels of αIISp are restored in FA-A cells this additionally corrects a number of the phenotypic deficiencies. After ICL damage to FA-A cells there is a five to 10-fold increase in chromosomal aberrations (i.e. fusions/radials and breaks, interchromatid exchanges).19 However, following restoration of levels of αIISp to normal by knocking down µ-calpain, chromosomal aberrations are reduced after ICL damage and are similar to levels observed in ICL-treated normal cells.19
αIISp deficiency in FA leads to telomere dysfunction after DNA ICL damage
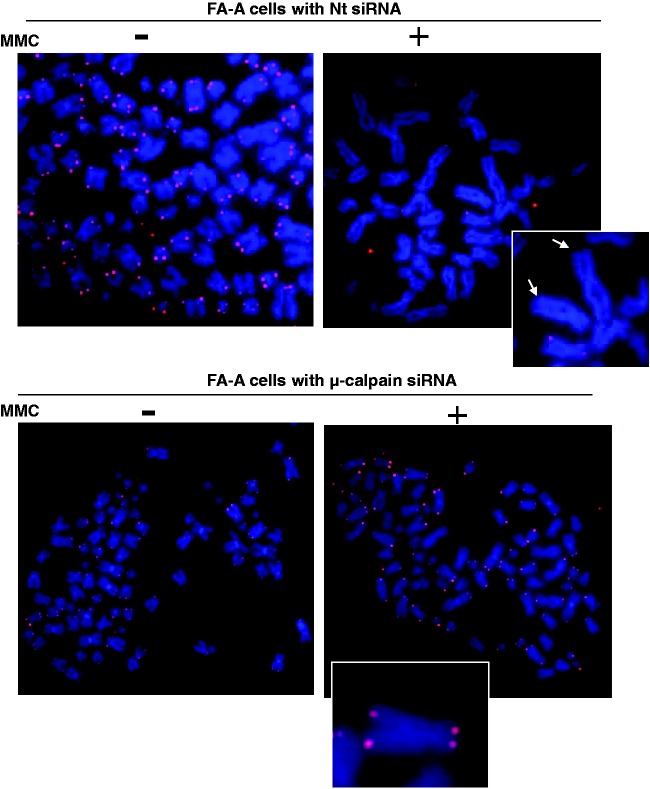
In FA-A cells there is a significant increase in telomere dysfunction after DNA ICL damage.20 Two major forms of evidence for this are that (1) the number of TIF positive cells increases approximately fourfold, and (2) there is, in addition, a significant loss of telomeres (i.e. levels of signal free chromosomes increase approximately threefold) after ICL damage (Figure 10).20 Importantly, restoration of levels of αIISp to normal by knocking down µ-calpain leads to a reduction in the number of TIF positive cells to levels found in normal as well as to a reduction in chromosomes with SFEs (Figure 10).20 Since αIISp localizes to telomeres in S phase, which is when telomeric DNA replicates, we have hypothesized that, after ICL damage, reduction of αIISp levels prevents efficient repair of telomeric DNA in S phase; this results in stalling of the replication fork and formation of telomeric DSBs.20 This, in turn, promotes TIF formation and a dramatic loss of telomeres.20 These studies thus demonstrate that in FA-A cells a deficiency in αIISp results in telomere dysfunction after ICL damage and show that αIISp is important in telomere maintenance following ICL damage.
Figure 10.
In FA-A cells, loss of telomeres is enhanced after damage with MMC and this loss is corrected after knockdown of µ-calpain. FA-A cells were transfected with (a) Nt siRNA or (b) µ-calpain siRNA and subsequently treated with MMC for 24 h. Metaphase spreads were prepared and chromosomes stained with DAPI (blue). Telomeric DNA was detected by FISH with a Cy3-labeled telomere-specific PNA probe (red). Inserted panels show magnified images of metaphase chromosomes. Arrowheads point to telomere SFEs. (Reproduced from Zhang et al.20 with permission from Oxford University Press.)
Our studies have also demonstrated that αIISp is critical for localization of XPF to sites of ICL damage on telomeric DNA, in addition to sites of ICLs on non-telomeric DNA.20 In FA-A cells, this increased co-localization of XPF with telomeres after ICL damage is not observed (Figure 7).20 Restoring αIISp levels to normal in FA-A cells by knockdown of µ-calpain reverses this and leads to association of normal levels of XPF with telomeres after ICL damage.20 Thus, loss of αIISp in FA cells is an important factor in defective repair of ICLs in telomeric DNA as well as genomic DNA, and in failure to recruit XPF-ERCC1 to sites of damage. Deficiencies in ICL repair in both telomeric and genomic DNA could contribute to genomic instability in these cells.
Role of αIISp in non-Ub FANCD2 function after DNA ICL damage
Our studies on whether αIISp plays a role in the functioning of non-ubiquitinated FANCD2 (non-Ub FANCD2) after DNA ICL damage have been aided by examination of FA-A cells. In FA-A cells, though FANCD2 is present it is not ubiquitinated and, following DNA damage, FANCD2 does not localize to nuclear foci.26,27,44,83 We have demonstrated, however, that in FA-A cells, after levels of αIISp are restored to normal by knockdown of µ-calpain, non-Ub FANCD2 localizes to nuclear foci at 80% of normal levels following ICL damage.44 Since in FA-A cells, FANCD2 is not monoubiquitinated after DNA ICL damage, as demonstrated by studies of ours44 as well as those of others,26,27,83 this indicates that FANCD2 present in these foci is not monoubiquitinated. It also indicates that non-Ub FANCD2, in addition to FANCD2-Ub, may play an important role in repair of ICLs.44 However, αIISp does not appreciably co-localize with FANCD2 foci, indicating that the role αIISp plays in FANCD2 foci formation may or may not be direct.44 We have additionally proposed that non-Ub FANCD2 and FANCD2-Ub localize to the same foci but that αIISp only plays a role in the localization of non-Ub FANCD2 to foci after ICl damage.44 The reason for this needs to be examined further.
Our studies showing that in FA-A cells, when αIISp is expressed, FANCD2 nuclear foci form after ICL damage, would appear to contradict studies carried out in FA-D2 cells, which express a ubiquitination resistant mutant of FANCD2 (K5612 mutation).42,83,84 These studies showed that in the FA-D2 cells, which expressed the mutant FANCD2, FANCD2 foci did not form after DNA ICL damage. These studies concluded that in order for FANCD2 to localize to nuclear foci after DNA ICL damage, it needs to be monoubiquitinated. However, what was not taken into account in these studies was that in the FA-D2 cell line used, we have shown that there is a deficiency in αIISp.13,14 We have, therefore, proposed an alternative interpretation for the studies investigating FA-D2 cells expressing the mutant non-Ub FANCD2.44 We propose that, since we have shown that αIISp is needed for formation of non-Ub FANCD2 foci after DNA ICL damage, non-Ub FANCD2 foci did not form in these FA-D2 cells because of the significantly reduced levels of αIISp present.44
There are numerous lines of evidence which support the viewpoint that non-Ub FANCD2 plays a role in repair of DNA ICLs and in replication fork recovery. It has been shown that non-Ub FANCD2 forms a complex with FANCD1/BRCA22, FANCG, and XRCC3.85–87 This complex of proteins, which includes non-Ub FANCD2, could be important in replication restart and promotion or modulation of the homologous recombination steps of DNA ICL repair, which occur when replication forks are blocked or break upon encountering a DNA ICL.85–88 This non-Ub FANCD2 complex would thus promote efficient restart of blocked or broken replication forks occurring at sites of DNA damage.85–88 It is thus possible that recruitment of non-Ub FANCD2 to sites of damage is an important factor in ICL repair and that αIISp plays a central role in this recruitment.44
Proposed role of FA proteins in maintenance of αIISp stability
Stability of αIISp in the nuclei of mammalian cells is of major importance in maintenance of genomic stability.18–20 This is evident in FA cells where loss of αIISp correlates with increased genomic instability after DNA ICL damage.19,20 We have demonstrated that in FA cells the reduction in αIISp levels is due to increased breakdown of αIISp rather than reduced expression of this protein.19,82 Specifically it is due to enhanced cleavage by the protease, µ-calpain, as evidenced by significantly increased µ-calpain activity (three to fourfold) in FA cells and increased levels of the µ-calpain 150 kDa breakdown product of αIISp.19 Since we have shown that in FA-A, FA-C, and FA-G cells, which have been corrected and express the appropriate FA protein, αIISp levels are increased to levels present in normal cells; this indicates that FA proteins play a role in maintenance of αIISp stability in these cells.14–19
FA proteins may regulate cleavage of αIISp by µ-calpain
We have proposed that FA proteins maintain αIISp stability by regulating the cleavage of αIISp by µ-calpain.19 Since there is a reduction in µ-calpain activity in corrected FA-A, FA-C, and FA-G cells, compared to this activity in normal cells, this indicates that the FANCA, FANCC, and FANCG proteins play a role in decreasing αIISp cleavage via their ability to inhibit µ-calpain activity.19 There are a number of ways in which this could be accomplished which are described below.
Model for maintenance of αIISp stability by FA proteins
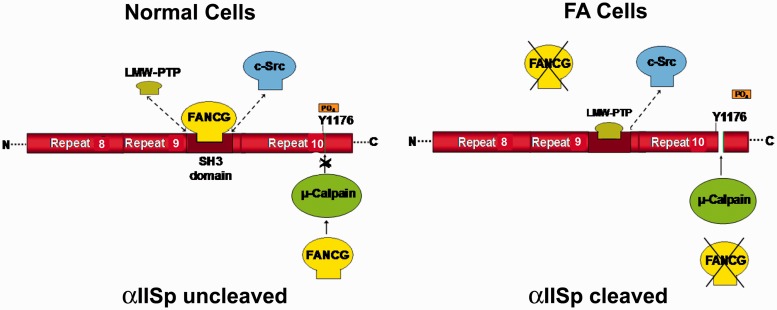
αIISp is cleaved by µ-calpain at Tyr1176 in repeat 10.89,90 Whether αIISp is cleaved by µ-calpain is controlled by phosphorylation of Tyr1176 by c-Src, a kinase that binds to the flanking SH3 domain of αIISp.89,90 When Tyr1176 is phosphorylated, αIISp becomes resistant to cleavage by µ-calpain.89,90 When low-molecular weight phosphotyrosine phosphatase (LMW-PTP) binds to the SH3 domain, Tyr1176 becomes dephosphorylated and µ-calpain can cleave αIISp.89,90 Based on our studies and those of other investigators, we have developed a model for the maintenance of αIISp stability in normal human cells and its breakdown in FA cells (Figure 11).19 We propose that in normal cells, a FA protein binds to the SH3 domain of αIISp, such as we have shown for FANCG.45 An equilibrium exists between the binding of a specific FA protein (i.e. FANCG), c-Src, and LMW-PTP for binding to the SH3 domain.19 Binding of the FA protein to the SH3 domain of αIISp, prevents LWM-PTP from binding to this domain, this inhibits dephosphorylation of Tyr1176 and prevents cleavage of αIISp by µ-calpain. A FA protein could also directly bind to µ-calpain and decrease or inhibit its activity. In support of this, we have shown, using yeast two-hybrid analysis, that FANCA and FANCG bind directly to µ-calpain.19 FA proteins could also potentially regulate αIISp stability by modulating binding of calmodulin to αIISp. When calmodulin binds to αIISp at its site adjacent to the µ-calpain cleavage site, µ-calpain cleavage of αIISp is enhanced.91 A FA protein could possibly inhibit binding of calmodulin to αIISp, which would decrease µ-calpain activity. Thus, in normal cells, FA proteins could maintain αIISp stability and inhibit its cleavage by µ-calpain in several different ways: (1) by binding to the SH3 domain of αIISp and inhibiting µ-calpain’s ability to cleave αIISp, (2) by binding to µ-calpain and inhibiting its activity and ability to cleave αIISp, and/or (3) by inhibiting the binding of calmodulin to αIISp and thus decreasing the ability of calmodulin to increase µ-calpain activity.
Figure 11.
Proposed model for involvement of FA proteins in cleavage of αIISp by µ-calpain. FANCG is used as an example in this figure. A portion of αIISp is shown containing repeats 8–10. In normal cells an equilibrium exists between LMW-PTP, FANCG, and c-Src for binding to the SH3 domain of αIISp. When c-Src binds to the SH3 domain of αIISp it phosphorylates Tyr1176 (Y1176) and prevents cleavage of αIISp by µ-calpain. It also prevents binding of LMW-PTP to the SH3 domain of αIISp. When FANCG binds to the SH3 domain (shown above), this prevents the binding of LMW-PTP to this site and the dephosphorylation of Tyr1176. This inhibits the ability of µ-calpain to cleave αIISp at this site, thus preventing the cleavage of αIISp.88,89 FANCG (or another FA protein) may also separately bind to µ-calpain and inhibit its ability to cleave αIISp, as shown. In FA cells (FA-G cells are used here as an example), there is absence of the FANCG protein and thus there is no binding of FANCG to the SH3 domain of αIISp. In the absence of FANCG, LMW-PTP can bind to the SH3 domain of αIISp and dephosphorylate Tyr1176, allowing µ-calpain to cleave αIISp at its cleavage site. There is also no FANCG to bind separately to µ-calpain and inhibit its ability to cleave αIISp. This results in µ-calpain breakdown of αIISp in FA-G cells. Similar events may occur in different FA complementation groups. (Reproduced from Zhang et al.19 with permission from the American Chemical Society.) (A color version of this figure is available in the online journal.)
Model for αIISp breakdown in FA cells
According to the model we have proposed, in FA cells a deficiency of a specific FA protein, such as FANCG, would lead to a defect in its ability to aid in the regulation of the activity of µ-calpain (Figure 11). This, in turn, would lead to an increase in µ-calpain activity and an increase in αIISp cleavage, as we have observed in cells from a number of FA complementation groups.19 Particularly interesting is the finding that there are at least 18 patient-derived mutations in FANCG which could result in FANCG proteins missing the motif that binds to the SH3 domain of αIISp, and which could affect their ability to bind to this domain in αIISp.45 This would enable LMW-PTP to bind to αIISp without interference from FANCG and to dephosphorylate Tyr1176, which would allow µ-calpain to cleave αIISp, leading to its increased breakdown.19 In addition, if a FA protein is inhibiting µ-calpain activity by binding to it, a deficiency in this FA protein could result in an increase in µ-calpain activity and in cleavage of αIISp. A deficiency in a FA protein could also potentially enhance µ-calpain activity and αIISp cleavage by inhibiting calmodulin binding to αIISp. Thus, in FA cells, increased breakdown of αIISp could result from loss of FA proteins critical for maintaining its stability and this could be a significant factor in the defective DNA repair and increased chromosomal instability observed.
A new role for FA proteins
We have proposed a critical and new role for FA proteins: maintaining αIISp stability in the cell.18–20 When this goal (i.e. maintenance of αIISp stability, and therefore normal levels of αIISp in the cell) is achieved by an alternate way in FA cells, such as by knockdown of µ-calpain, then these FA cells are able to perform functions that they otherwise cannot carry out (e.g. recruitment of DNA repair proteins to sites of ICLs, maintenance of telomeres, and chromosome stability after DNA ICL damage). In these FA cells in which αIISp levels are restored, one would expect that the presence or absence of a specific FA protein would be less critical. We have demonstrated this in studies in which restoration of αIISp levels to normal in FA-A cells, by knocking down µ-calpain, led to correction of the phenotypic defects normally observed in these cells even though there was still a deficiency in the FANCA protein in these cells.15,19,20 In further support of this view, we have shown that in normal cells, after knockdown of αIISp, the ICL repair protein, XPF, fails to localize to nuclear foci after ICL damage and there is defective DNA repair though FA proteins levels are normal.18
Thus, we propose that in FA cells increased breakdown of αIISp is a critical factor in the observed phenotype of this disorder, which includes defective DNA repair and increased chromosomal instability. We further propose that FA proteins are needed for maintaining αIISp stability and that this represents a critical role for these proteins in the cell.
Clinical importance of loss of αIISp
FA is not the only disorder in which a deficiency of αIISp has been demonstrated. There are studies which indicate there could be a link between spectrin and the pathogenesis of neoplastic BM disorders.92,93 Evidence also indicates that spectrin deficiencies could play a role in leukemogenesis, particularly acute myeloid leukemia (AML).92,93 In one study, 44% of the BMs examined from AML patients were found to have a loss of αIISp.92,93 Evidence that αIISp could potentially play a role in leukemogenesis is of particular interest since FA patients develop BM failure and have a strong predilection to develop AML.3,94 These studies combined with our studies, which demonstrate that FA cells are deficient in αIISp, indicate that in a number of BM disorders αIISp loss can be a factor important in the etiopathogenesis of these disorders. The role αIISp plays in DNA repair in both genomic and telomeric DNA may be an important factor in cellular responses to both endogenous and exogenous damage to DNA. Thus, a deficiency in αIISp could have a number of far-reaching consequences on various cellular functions which are particularly dependent on efficient repair of damaged DNA, and loss of which may lead to mutagenic and carcinogenic events.
Our studies in FA cells in which we were able to restore αIISp levels to those found in normal cells, by knockdown of µ-calpain, suggest a new and previously unexplored direction for therapeutic restoration of genomic stability in these cells.19,20 In these studies, a siRNA specific for the 80 kDa subunit of µ-calpain, which is the regulatory subunit and which is specific for µ-calpain,95 was used to knockdown µ-calpain in FA-A cells.19,20 This resulted in restoring αIISp levels to those found in normal cells and had little effect on cell viability.19 Levels of the FA or DNA repair proteins examined were also not effected. Studies in which a mouse model was used have shown that decreasing levels of µ-calpain by siRNA knockdown had no effect on development.95 It is thus possible that developing methods to target µ-calpain so as to reduce its activity in FA cells, such as by knocking it down, could be used effectively in prevention of the increased cleavage of αIISp observed in FA cells and aid in correcting a number of the phenotypic deficiencies occurring after ICL damage. This could be used, possibly along with other potential modalities, in therapeutic intervention in FA.
αII-Spectrin: Far-reaching importance
αII-Spectrin is an essential protein in cells and, if completely depleted, this results in cell death.11,18,37,38 In normal cells, when αIISp levels are knocked down to those found in FA cells (35–49% of normal), cells survive but after DNA ICL damage they show multiple phenotypic characteristics of FA cells, such as defective DNA repair, telomere dysfunction, and chromosomal aberrations after ICL damage.19,20 This poses the interesting question of what long-term effects a deficiency in this protein would have on various cellular processes, such as development. αIISp spectrin has been shown to be expressed throughout all developmental stages in mammalian cells and is critical in many developmental processes.12,96–98 It is possible that in the early developmental stages of an individual with FA, a deficiency of αIISp could lead to some of the congenital abnormalities observed.
Role of αIISp in cellular morphology and function
αIISp plays a role in the morphology of the cell. Loss of αIISp can result in changes in the shape of the cell, in the ability of the cell to proliferate, and in adhesion and spreading capabilities of the cell.8,11,12 For example, we have found that cultured normal human lymphoblastoid cells are very pleiomorphic and have numerous pseudopodia.18 After knockdown of αIISp, however, the cells decrease in size and become rounded with few pseudopodia, resembling FA-A lymphoblastoid cells in culture.18 Results similar to this have been reported in studies using a human melanoma cell line.11 In these studies, siRNA knockdown of αII-spectrin resulted in the cells becoming rounded and their size decreased.11 They showed defects in cell adhesion and spreading, cellular proliferation, as well as in organization of the actin skeleton. Studies suggest that αII-spectrin may be involved in regulating actin network formation.11 Of interest, macrophages in Fancc-/- mice have been reported to display decreased cell migration and adhesion, and reduced phagocytosis.99 The cells are rounded and lack the multiple protrusions found in wild-type macrophages.99 Filamentous actin (F-actin) rearrangements are dysregulated in these cells.99 Since we have found that there are reduced αIISp levels in cells from the BM of Fancc-/- mice (unpublished results), it is of interest to speculate that the morphological changes reported in Fancc-/- cells and on the rearrangements of actin observed in these cells are due to loss of αIISp. Collectively these studies suggest αIISp plays a role in the organization of the actin skeleton in the cytoplasm. As we and Wilson’s laboratory have shown, αIISp also interacts with actin in the nucleus of human cells22,100; this interaction could be of similar importance for the function of actin in the nucleus.
αIISp in cell cycle progression
α-spectrin is also important for cell cycle progression where it is required for proper cytokinesis and coordination of events involved in cell division such as organization of the spindle pole and cytokinesis.101 It plays a key role in linking the actin cytoskeleton to various cell components, such as microtubules, needed in cell division.101–103 A deficiency in α-spectrin in cells could significantly affect progression of the cell cycle.
Chimeric E2/E3 ubiquitin conjugating activity of αIISp
It is also possible that, in non-erythroid cells, α-spectrin has chimeric E2/E3 ubiquitin conjugating/ligating activity as it does in erythroid cells.6,104,105 Since α-spectrin is found both in the cytoplasm, associated with organelles and the plasma membrane,3,6,9 and in the nucleus,16–20,22 it would have a large number of potential target proteins for ubiquitination. This could have significant impact on cellular function and could influence αIISp’s actions in the nucleus.
Conclusions
There are thus a number of cellular functions in which αIISp has been demonstrated to play an important role. An involvement of αIISp in the nucleus in DNA repair and genomic stability is a unique and critical one. In all stages of development, cells can be damaged by endogenous as well as exogenous agents, and proper repair of damaged DNA is crucial for maintenance of cell function. A deficiency in a key repair protein, such as αIISp, could have far-reaching consequences, since it not only plays a role in DNA repair and interacts with DNA repair proteins, but it also interacts with structural proteins, such as actin, lamin, and emerin, and with chromatin remodeling proteins.22 αIISp could thus be essential for promoting interactions between the array of proteins with which it interacts in the nucleus. The nature of these interactions and their importance to nuclear function has been a relatively unexplored area. Developing a more in-depth understanding of the importance of αIISp, and its stability, for these interactions would significantly enhance our knowledge of the consequences loss of αIISp has on critical nuclear processes in the cell.
Acknowledgement
This work was supported by NHLBI, National Institutes of Health, Grant RO1 HL054860.
Declaration of Conflicting Interests
The author(s) declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Goodman SR, Shiffer K. The spectrin membrane skeleton of normal and abnormal human erythrocytes: a review. Am J Physiol 1983; 244: C121–41. [DOI] [PubMed] [Google Scholar]
- 2.Winkelmann JC, Forget BG. Erythroid and nonerythroid spectrins. Blood 1993; 81: 3173–85. [PubMed] [Google Scholar]
- 3.Goodman SR, Zimmer WE, Clark MB, Zagon IS, Barker JE, Bloom ML. Brain spectrin: of mice and men. Brain Res Bull 1995; 36: 593–606. [DOI] [PubMed] [Google Scholar]
- 4.de Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. Cell Sci 2000; 113: 2331–43. [DOI] [PubMed] [Google Scholar]
- 5.Bennet V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev 2001; 81: 353–92. [DOI] [PubMed] [Google Scholar]
- 6.Goodman SR, Chapa RP, Zimmer WE. Spectrin’s chimeric E2/E3 enzymatic activity. Exp Biol Med 2015; 240: 1039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman SR, Zagon IS, Kulikowski RR. Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci USA 1981; 78: 7570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machnicka B, Grochowalska R, Boguslawska DM, Sikorski AF, Lecomte MC. Spectrin-based skeleton as an actor in cell signaling. Cell Mol Life Sci 2012; 69: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zagon IS, Higbee R, Riederer BM, Goodman SR. Spectrin subtypes in mammalian brain: an immunoelectron microscopic study. J Neurosci 1986; 6: 2977–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gascard P, Mohandas N. New insights into functions of erythroid proteins in nonerythroid cells. Curr Opin Hematol 2000; 7: 123–9. [DOI] [PubMed] [Google Scholar]
- 11.Metral S, Machnicka B, Bigot S, Colin Y, Dhermy D, Lecomte M-C. αII-Spectrin is critical for cell adhesion and cell cycle. J Biol Chem 2009; 284: 2409–18. [DOI] [PubMed] [Google Scholar]
- 12.Stankewich MC, Cianci CD, Stabach PR, Ji L, Nath A, Morrow J. Cell organization, growth, and neural and cardiac development require αII-spectrin. J Cell Sci 2011; 124: 3956–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brois DW, McMahon LW, Ramos NI, Anglin LM, Walsh CE, Lambert MW. A deficiency in a 230 kDa DNA repair protein in Fanconi anemia complementation group A cells is corrected by the FANCA cDNA. Carcinogenesis 1999; 20: 1845–53. [DOI] [PubMed] [Google Scholar]
- 14.McMahon LW, Walsh CE, Lambert MW. Human α spectrin II and the Fanconi anemia proteins FANCA and FANCC interact to form a nuclear complex. J Biol Chem 1999; 274: 32904–8. [DOI] [PubMed] [Google Scholar]
- 15.Lambert MW. Functional significance of nuclear α spectrin. J Cell Biochem 2015; 116: 1816–30. [DOI] [PubMed] [Google Scholar]
- 16.McMahon LW, Sangerman J, Goodman SR, Kumaresan K, Lambert MW. Human alpha spectrin II and the FANCA, FANCC, and FANCG proteins bind to DNA containing psoralen interstrand cross-links. Biochemistry 2001; 40: 7025–34. [DOI] [PubMed] [Google Scholar]
- 17.Sridharan D, Brown M, Lambert WC, McMahon LW, Lambert MW. Nonerythroid alpha II spectrin is required for recruitment of FANCA and XPF to nuclear foci induced by DNA interstrand cross-links. J Cell Sci 2003; 116: 823–5. [DOI] [PubMed] [Google Scholar]
- 18.McMahon LW, Zhang P, Sridharan DM, Lefferts JA, Lambert MW. Knockdown of αII spectrin in normal human cells by siRNA leads to chromosomal instability and decreased DNA interstrand cross-link repair. Biochem Biophys Res Commun 2009; 381: 288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P, Sridharan D, Lambert MW. Knockdown of µ-calpain in Fanconi anemia, FA-A, cells by siRNA restores αII spectrin levels and corrects chromosomal instability and defective DNA interstrand cross-link repair. Biochemistry 2010; 49: 5570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang P, Herbig U, Coffman F, Lambert MW. Non-erythroid alpha spectrin prevents telomere dysfunction after DNA interstrand cross-link damage. Nucleic Acids Res 2013; 41: 5321–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumaresan K, Sridharan D, McMahon L, Lambert MW. Deficiency in incisions produced by XPF at the site of a DNA interstrand cross-link in Fanconi anemia cells. Biochemistry 2007; 46: 14359–68. [DOI] [PubMed] [Google Scholar]
- 22.Sridharan DM, McMahon LW, Lambert MW. αII-Spectrin interacts with five groups of functionally important proteins in the nucleus. Cell Biol Int 2006; 30: 866–78. [DOI] [PubMed] [Google Scholar]
- 23.Joenje H, Patel KJ. The emerging genetic and molecular basis of Fanconi anaemia. Nat Rev Genet 2001; 2: 446–57. [DOI] [PubMed] [Google Scholar]
- 24.Taniguchi T, D’Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood 2006; 107: 4223–33. [DOI] [PubMed] [Google Scholar]
- 25.Mathew CG. Fanconi anaemia genes and susceptibility to cancer. Oncogene 2006; 25: 5875–84. [DOI] [PubMed] [Google Scholar]
- 26.de Winter JP, Joenje H. The genetic and molecular basis of Fanconi anemia. Mutat Res Fundam Mol Mech Mutagen 2009; 668: 11–9. [DOI] [PubMed] [Google Scholar]
- 27.Moldovan G-L, D’Andrea A. How the Fanconi anemia pathway guards the genome. Rev Genet Ann 2009; 43: 223–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kottemann MC, Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature 2013; 493: 356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, D’Andrea AD. Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev 2012; 26: 1393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert MW, Tsongalis JT, Lambert WC, Parrish DD. Correction of the DNA repair defect in Fanconi anemia complementation groups A and D cells. Biochem Biophys Res Commun 1997; 230: 687–91. [DOI] [PubMed] [Google Scholar]
- 31.Kumaresan KR, Lambert MW. Fanconi anemia, complementation group A, cells are defective in ability to produce incisions at sites of psoralen interstrand cross-links. Carcinogenesis 2000; 21: 741–51. [DOI] [PubMed] [Google Scholar]
- 32.Akkari YM, Bateman R, Reifsteck CA, Olson SB, Grompe M. DNA replication is required to elicit cellular responses to psoralen-induced DNA interstrand cross-links. Mol Cell Biol 2000; 20: 8283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raschle M, Knipsheer P, Enoiu M, Angelov T, Sun J, Griffith JD, Ellenberger TE, Scharer OD, Walter JC. Mechanism of replication-coupled DNA interstrand crosslink repair. Cell 2008; 134: 969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knipscheer P, Raschle M, Smogorzewska A, Enoiu M, Ho TV, Scharer OD, Elledge SJ, Walter JC. The Fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science 2009; 326: 1698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legerski RJ. Repair of DNA interstrand cross-links during S phase of the mammalian cell cycle. Environ Mol Mutagen 2010; 51: 540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duxin PD, Walter JC. What is the DNA repair defect underlying Fanconi anemia? Curr Opin Cell Biol 2015; 37: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Coyne RS, Dubreuil RR, Goldstein LS, Branton D. Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J Cell Biol 1993; 123: 1797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norman KR, Moerman DG. αSpectrin is essential for morphogenesis and body wall muscle formation in Caenorhabditis elegans. J Cell Biol 2002; 157: 665–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumaresan KR, Hang B, Lambert MW. Human endonucleolytic incision of DNA 3′ and 5′ to a site-directed psoralen monoadduct and interstrand cross-link. J Biol Chem 1995; 270: 30709–16. [DOI] [PubMed] [Google Scholar]
- 40.Klein Douwel D, Boon RACM, Long DT, Szypowska AA, Raschle M, Walter JC, Knipscheer P. XPF-ERCC1 acts in unhooking DNA interstrand crosslinks in cooperation with FANCD2 and FANCP/SLX4. Mol Cell 2014; 54: 460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat Rev Genet 2007; 8: 735–48. [DOI] [PubMed] [Google Scholar]
- 42.Seki S, Ohzeki M, Uchiada A, Hirano S, Matsushita N, Kitao H, Oda T, Yamashita T, Kashihara N, Tsubahara A, Takata M, Ishiai M. A requirement of FancL and FancD2 monoubiquitination in DNA repair. Genes Cells 2007; 12: 299–310. [DOI] [PubMed] [Google Scholar]
- 43.Walden H, Deans AJ. The Fanconi anemia DNA repair pathway: structural and functional insights into a complex disorder. Ann Rev Biophys 2014; 43: 257–78. [DOI] [PubMed] [Google Scholar]
- 44.Zhang P, Sridharan D, Lambert MW. Nuclear α spectrin differentially affects monoubiquitinated versus non-ubiquitinated FANCD2 function after DNA interstrand cross-link damage. J Cell Biochem 2016; 117: 671–83. [DOI] [PubMed] [Google Scholar]
- 45.Lefferts JA, Wang C, Sridharan D, Baralt M, Lambert MW. The SH3 domain of αII spectrin is a target for the Fanconi anemia protein, FANCG. Biochemistry 2009; 48: 254–63. [DOI] [PubMed] [Google Scholar]
- 46.Wang C, Lambert MW. The Fanconi anemia protein, FANCG, binds to the ERCC1-XPF endonuclease via its tetratricopeptide repeats and the central domain of ERCC1. Biochemistry 2010; 49: 5560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Walter JC. Mechanism and regulation of incisions during DNA interstrand cross-link repair. DNA Repair 2014; 19: 135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhagwat N, Olsen AL, Wang AT, Hanada K, Stuckert P, Kanaar R, D'Andrea A, Niedernhofer LJ, McHugh PJ. XPF-ERCC1 participates in the Fanconi anemia pathway of cross-link repair. Mol Cell Biol 2009; 29: 6427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziemnicka-Kotula D, Xu J, Gui H, Potempska A, Kim KS, Jenkins EC, Trenkner E, Kotula L. Identification of a candidate human spectrin Src homology 3 domain-binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton. J Biol Chem 1998; 273: 13681–92. [DOI] [PubMed] [Google Scholar]
- 50.Kuriyan J, Cowburn D. Modular peptide recognition domains in eukaryotic signaling. Annu Rev Biophys Biomol Struct 1997; 26: 259–88. [DOI] [PubMed] [Google Scholar]
- 51.Stein R. SH2 and SH3 domains. Unraveling signaling networks with peptide antogonists. Methods Mol Biol 1998; 88: 187–95. [DOI] [PubMed] [Google Scholar]
- 52.McPherson PS. Regulatory role of SH3 domain-mediated protein-protein interactions in synaptic vesicle endocytosis. Cell Signal 1999; 11: 229–38. [DOI] [PubMed] [Google Scholar]
- 53.Mayer BJ. SH3 domains: complexity in moderation. J Cell Sci 2001; 114: 1253–63. [DOI] [PubMed] [Google Scholar]
- 54.Ren R, Mayer BJ, Cicchetti P, Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science 1993; 259: 1157–61. [DOI] [PubMed] [Google Scholar]
- 55.Fen S, Chen JK, Yu H, Simon JA, Schreiber SL. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science 1994; 266: 1241–7. [DOI] [PubMed] [Google Scholar]
- 56.Lim WA, Richards FM, Fox RO. Structural determinants of peptide-binding orientation and of sequence specificity in SH3 domains. Nature 1994; 372: 375–9. [DOI] [PubMed] [Google Scholar]
- 57.Kay BK, Williamson MP, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J 2000; 14: 231–41. [PubMed] [Google Scholar]
- 58.Cesareni G, Panni S, Nardelli G, Castagnoli L. Can we infer peptide recognition specificity mediated by SH3 domains? FEBS Lett 2002; 513: 38–44. [DOI] [PubMed] [Google Scholar]
- 59.Blatch GL, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein–protein interactions. Bioessays 1999; 21: 932–9. [DOI] [PubMed] [Google Scholar]
- 60.D’Andrea LD, Regan L. TPR proteins: the versatile helix. Trends Biochem Sci 2003; 28: 655–62. [DOI] [PubMed] [Google Scholar]
- 61.Blom E, van de Vrugt HJ, de Vries Y, de Winter JP, Arwert F, Joenje H. Multiple TPR motifs characterize the Fanconi anaemia FANCG protein. DNA Repair 2004; 3: 77–84. [DOI] [PubMed] [Google Scholar]
- 62.Hussain S, Wilson JB, Blom W, Thompson LH, Sung P, Gordon SM, Kupfer GM, Joenje H, Mathew CG, Jones NJ. Tetratricopeptide-motif-mediated interaction of FANCG with recombination proteins XRCC3 and BRCA2. DNA Repair 2006; 5: 629–40. [DOI] [PubMed] [Google Scholar]
- 63.de Laat WL, Sijbers AM, Odijk H, Jaspers NG, Hoeijmakers JH. Mapping of interaction domains between human repair proteins ERCC1 and XPF. Nucl Acids Res 1998; 26: 4146–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tripsianes K, Folkers GE, Ab E, Das D, Odijk H, Jaspers NGJ, Hoeijmakers JHJ, Kapten R, Boelens R. The structure of the human ERCC1/XPF interaction domains reveals a complementary role for the two proteins in nucleotide excision repair. Structure 2005; 13: 1849–58. [DOI] [PubMed] [Google Scholar]
- 65.Choi YJ, Ryu KS, Ko YM, Chae YK, Pleton JG, Wemmer DE, Choi BS. Biophysical characterization of the interaction domains and mapping of the contact residues in the XPF-ERCC1 complex. J Biol Chem 2005; 280: 28644–52. [DOI] [PubMed] [Google Scholar]
- 66.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005; 19: 2100–10. [DOI] [PubMed] [Google Scholar]
- 67.Murnane JP. Telomere dysfunction and chromosome instability. Mutat Res 2012; 730: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet 2008; 42: 301–34. [DOI] [PubMed] [Google Scholar]
- 69.O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol 2010; 11: 171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science 2012; 336: 593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem 2004; 73: 177–208. [DOI] [PubMed] [Google Scholar]
- 72.Blackburn EH. Telomeres and telomerase. FEBS Lett 2005; 579: 859–62. [DOI] [PubMed] [Google Scholar]
- 73.Kim H, Lee O-H, Xin H, Chen L-Y, Qin J, Chae HK, Lin S-Y, Safari A, Liu D, Songyang Z. TRF2 functions as a protein hub and regulates telomere maintenance by recognizing specific peptide motifs. Nat Struct Mol Biol 2009; 16: 372–9. [DOI] [PubMed] [Google Scholar]
- 74.Diotti R, Loayza D. Shelterin complex and associated factors at human telomeres. Nucleus 2011; 2: 119–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright WE, Tesmer VM, Liao ML, Shay JW. Normal human telomeres are not late replicating. Exp Cell Res 1999; 251: 492–9. [DOI] [PubMed] [Google Scholar]
- 76.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol 2003; 13: 1549–56. [DOI] [PubMed] [Google Scholar]
- 77.Cech TR. Beginning to understand the end of the chromosome. Cell 2004; 116: 272–9. [DOI] [PubMed] [Google Scholar]
- 78.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Cociello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science 1994; 266: 2011–5. [DOI] [PubMed] [Google Scholar]
- 79.Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci USA 1995; 92: 9082–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan Q, Zhang F, Barrett B, Ren K, Andreassen PR. A role for monoubiquitinated FANCD2 at telomeres in ALT cells. Nucleic Acids Res 2009; 37: 1740–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong H, Nebert DW, Bruford EA, Thompson DC, Joenje H, Vasiliou V. Update of the human and mouse Fanconi anemia genes. Hum Genet 2015;99:32–42. [DOI] [PMC free article] [PubMed]
- 82.Lefferts JA, Lambert MW. Fanconi anemia cell lines deficient in αII spectrin express normal levels of αII spectrin mRNA. Biochem Biophys Res Commun 2003; 307: 510–5. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, d’Andrea AD. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell 2001; 7: 249–62. [DOI] [PubMed] [Google Scholar]
- 84.Kachnic LA, Li L, Fournier L, Willers H. Fanconi anemia pathway revealed by cisplatin and oxaliplatin treatments. Cancer Lett 2010; 292: 73–9. [DOI] [PubMed] [Google Scholar]
- 85.Wilson JB, Yamamoto K, Marriott AS, Hussain S, Sung P, Hoatlin ME, Mathew CG, Takata M, Thompson LH, Kupfer GM, Jones NJ. FANCG promotes formation of a newly identified protein complex containing BRCA2, FANCD2 and XRCC3. Oncogene 2008; 27: 3641–52. [DOI] [PubMed] [Google Scholar]
- 86.Wilson JB, Blom E, Cunningham R, Xiao Y, Kupfer GM, Jones NJ. Several tetratricopeptide repeat (TPR) motifs of FANCG are required for assembly of the BRCA2/D1-D2-G-X3 complex, FANCD2 monoubiquitylation and phleomycin resistance. Mutat Res 2010; 689: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hussain S, Wilson JB, Blom W, Thompson LH, Sung P, Gordon SM, Kupfer GM, Joenje H, Mathew CG, Jones NJ. Tetratricopeptide-motif-mediated interaction of FANCG with recombination proteins XRCC3 and BRCA2. DNA Repair 2006; 5: 629–40. [DOI] [PubMed] [Google Scholar]
- 88.Raghunandan M, Chaudhury I, Kellich SL, Hanenberg H, Soebeck A. FANCD2, FANCJ and BRCA2 cooperate to promote replication fork recovery independently of the Fanconi anemia complex. Cell Cycle 2015; 143: 342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nicolas G, Fournier CM, Galand C, Malbert-Colas L, Bournier O, Kroviarski Y, Bourgeois M, Camonis JH, Dhermy D, Grandchamp B, Lecomte MC. Tyrosine phosphorylation regulates alpha II spectrin cleavage by calpain. Mol Cell Biol 2002; 22: 3527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nedrelow JH, Cianci CD, Morrow JS. C-Src binds αII spectrin’s Src homology 3 (SH3) domain and blocks calpain susceptibility by phosphorylating Tyr1176. J Biol Chem 2003; 278: 7725–41. [DOI] [PubMed] [Google Scholar]
- 91.Harris AS, Croall DE, Morrow JS. Calmodulin regulates fodrin susceptibility to cleavage by calcium-dependent protease I. J Biol Chem 1989; 264: 17401–8. [PubMed] [Google Scholar]
- 92.Gorman EB, Chen L, Albanese J, Ratech H. Pattern of spectrin expression in B-cell lymphomas: loss of spectrin isoforms is associated with nodule-forming and germinal center-related lymphomas. Mod Pathol 2007; 20: 1245–52. [DOI] [PubMed] [Google Scholar]
- 93.Wolqust LP, Cannizzarro LA, Rameshjk H, Xue X, Wang D, Bhattacharuya PK, Gong JZ, McMahon C, Albanese JM, Sunkara JL, Ratech H. Differential expression in normal hematopoiesis and alterations in neoplastic bone marrow disorders. Am J Clin Pathol 2011; 136: 300–8. [DOI] [PubMed] [Google Scholar]
- 94.Alter BP. Cancer in Fanconi anemia, 1927–2001. Cancer 2003; 97: 425–40. [DOI] [PubMed] [Google Scholar]
- 95.Goll DE, Thompson VF, Ki H, Wei W, Cong J. The calpain system. Physiol Rev 2003; 83: 731–801. [DOI] [PubMed] [Google Scholar]
- 96.Riederer BM, Zagon IS, Goodman SR. Brain spectrin(240/235) and brain spectrin(240/235E): differential expression during mouse brain development. J Neurosci 1987; 7: 864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zagon IS, Riderer BM, Goodman SR. Spectrin expression during mammalian brain ontogeny. Brain Res Bull 1987; 18: 799–807. [DOI] [PubMed] [Google Scholar]
- 98.Zimmer WE, Ma Y, Zagon IS, Goodman SR. Developmental expression of brain beta-spectrin isoform messenger RNAs. Brain Res 1992; 594: 75–83. [DOI] [PubMed] [Google Scholar]
- 99.Liu Y, Ballman K, Li D, Khan S, Derr-Yellin E, Shou W, Haneline LS. Impaired function of Fanconi anemia type C-deficient macrophages. J Leukocyte Biol 2012; 91: 333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holaska JM, Wilson KL. An emerin ‘proteome’: purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mRNA splicing, signaling, mechano-sensing and nuclear architecture. Biochem 2007; 46: 8897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Silverman-Gavrila RV, Solverman-Gavrila LB, Bilal KH, Bendeck MP. Spectrin alpha is important for rear polarization of the microtubule organizing center during migration and spindle pole assembly during division of neointimal smooth muscle cells. Cytoskeleton 2015; 72: 157–70. [DOI] [PubMed] [Google Scholar]
- 102.Kann M-L, Fouquet JP. Association of spectrin with manchette microtubules in mammalian spermatids. Biol Cell 1993; 7: 311–13. [DOI] [PubMed] [Google Scholar]
- 103.Rieder BM, Goodman SR. Association of brain spectrin isoforms with microtubules. FEBS Lett 2001;277:49–51. [DOI] [PubMed]
- 104.Chang TL, Cubillos FF, Kakhniashvili DG, Goodman SR. Ankyrin is a target of spectrin’s E2/E3 ubiquitin-conjugating/ligating activity. Cell Mol Biol 2004; 50: 59–66. [PubMed] [Google Scholar]
- 105.Hsu YJ, Zimmer WE, Goodman SR. Erythrocyte spectrin’s chimeric E2/E3 ubiquitin conjugating/ligating activity. Cell Mol Biol 2005; 51: 187–93. [PubMed] [Google Scholar]