Abstract
Background
This study is to explore the components and related mechanism responsible for the increase of work of breathing (WB) in chronic obstructive pulmonary disease (COPD) patients.
Methods
Eight COPD patients and eight healthy volunteers were recruited in the study. The rebreathing method was used to increase end-tidal CO2 partial pressure (PetCO2) and stimulate the increase in ventilation (VE). The increase in VE, WB, and changes in the compositions of WB were observed and analyzed. The WB and its components were calculated using the Campbell diagram.
Results
The inspiratory work (Wi) of breathing, a major component of total work of breathing (Wtot), in the COPD group was significantly higher than the control group during quiet breathing (P<0.05). As the minute VE increased, Wtot and Wi increased in a linear manner, and the slope of increase was significantly higher in the COPD group as compared to the normal group (P<0.05). The analyses of changes in overcoming airway resistance (Wrs) and lung/chest-wall elastance (Wel) indicated that the slope of increase (response to VE increase) of Wrs was not significantly different between the two groups (P>0.05) although the Wrs in the COPD group was always higher than the normal group (P<0.05). However, as the VE increased, the slope of the increase in Wel was significantly higher in the COPD group than the normal group. Work done to overcome the intrinsic PEEP (WPEEPi), a component of the Wel, was not observed in the control group. However, WPEEPi increased gradually as VE increased and accounted for 56% of Wel at the end of rebreathing trial in COPD group.
Conclusions
Airway resistance was the main cause for increased WB during quiet breathing. As the VE increased, an increase of WPEEPi became an important part of increased WB in COPD patients, so it is important to reduce dynamic hyperinflation in COPD patients.
Keywords: Work of breathing (WB), lung elastance, intrinsic PEEP (PEEPi), chronic obstructive pulmonary disease (COPD)
Introduction
Work of breathing (WB) refers to work or energy expenditure to overcome impedance of breathing motion and gas transportation during breathing, including the resistance of airway, elastance of chest wall and lungs as well as other impedance. In chronic obstructive pulmonary disease (COPD), there are many factors that may increase the WB, including increased airway resistance and intrinsic PEEP (PEEPi) due to dynamic hyperinflation. However, the contribution of different components of WB to the total work of breathing (Wtot) in COPD is not clear, especially in different conditions of ventilation (VE) demands. Rebreathing method can increase end-expiratory CO2 partial pressure (PetCO2) to stimulate the increase of respiratory central drive and VE. It is important to analyze the changes of WB and the related components responsible for the increase of WB during the dynamic increase of VE. So, this study is performed to investigate the contribution of different components of WB to the increased WB in different VE demands in COPD in order to elucidate the mechanisms responsible for increased WB. The results of the study will provide a clue for the management and prevention of increased WB in COPD.
Methods
Subjects
Eight males (65±8 years) with severe COPD in a stable condition without hypercapnia were recruited in the COPD Group for the study. Eight healthy non-smoker volunteers (7 males and 1 female, 34±4 years) were recruited as the control group (Table 1). For subjects from the COPD Group, the diagnosis of COPD was made on the findings of patient history, physical examinations, chest X-rays and pulmonary function tests, in consistence with the COPD diagnostic criteria (1). The exclusion criteria included cardiovascular, pleural, and chest wall disorders.
Table 1. Demographic data of COPD group and the control group.
| Groups | Case No. | Age (y) | FEV1 (L) | FVC (L) | FEV1 (%pred) (%) | Weight (kg) | Height (cm) | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|---|
| COPD group | 1 | 57 | 0.99 | 1.56 | 64 | 48 | 161 | 18.5 |
| 2 | 69 | 0.63 | 2.23 | 28 | 65 | 164 | 24.2 | |
| 3 | 51 | 0.73 | 1.22 | 60 | 64 | 170 | 22.1 | |
| 4 | 69 | 0.84 | 1.68 | 52 | 60 | 164 | 22.3 | |
| 5 | 64 | 1.36 | 2.16 | 65 | 45 | 165 | 16.5 | |
| 6 | 76 | 1.28 | 2.61 | 49 | 67 | 165 | 24.6 | |
| 7 | 64 | 0.89 | 2.69 | 42 | 62 | 174 | 20.5 | |
| 8 | 75 | 1.04 | 1.83 | 57 | 65 | 174 | 21.5 | |
| Mean ± SD | 65.6±8.6 | 1.0±0.3 | 2.0±0.5 | 52±12 | 59.5±8.3 | 167.1±4.9 | 21.3±2.7 | |
| Control group | 1 | 29 | 2.98 | 3.44 | 87 | 46 | 160 | 17.97 |
| 2 | 43 | 3.75 | 4.40 | 85 | 60 | 170 | 20.76 | |
| 3 | 32 | 3.00 | 3.41 | 88 | 56 | 164 | 20.82 | |
| 4 | 35 | 3.73 | 4.24 | 88 | 62 | 170 | 21.45 | |
| 5 | 36 | 3.79 | 4.29 | 88 | 70 | 175 | 22.86 | |
| 6 | 32 | 4.07 | 5.05 | 81 | 65 | 173 | 21.72 | |
| 7 | 36 | 4.00 | 4.57 | 87 | 59 | 173 | 19.71 | |
| 8 | 36 | 2.99 | 3.32 | 90 | 65 | 175 | 21.22 | |
| Mean ± SD | 34.9±4.2 | 3.5±0.5 | 4.1±0.6 | 87±3 | 60.4±7.2 | 170.0±5.4 | 20.8±1.5 |
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; BMI, body mass index; SD, standard deviations.
Measurement methods
Pressure transducers (Vigg-Spectramed Pte Ltd, US, model p23xl) were used for pressure test. Esophageal and gastric balloon catheters (2) were used to measure the esophageal and gastric pressures as surrogates for intrapleural pressure and intra-abdominal pressure. Airway pressure was measured with another pressure transducer simultaneously. Pneumotachograph (Fleish No2) and the differential pressure transducer (Validyne Co., US) were used to measure respiratory flow rate. Tidal volume was calculated by the integral of flow rate. NOVAMETRIC monitoring system (Novametric Co., US) was used to measure PetCO2.
All analog signals were connected to the analog-to-digital converter (12 bit 8-channel, Labview Co., US). Labview data acquisition program was used for data acquisition. Acquisition frequency was set at 3,000 Hz. After the end of the experiment, software package (Microcal Origin, US) was used for data analysis.
The measured and calculated parameters for the analysis
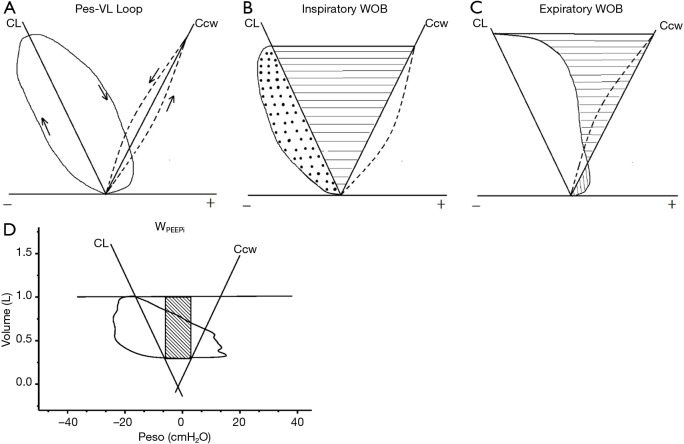
The measured and calculated parameters for the analysis were VE parameters that included flow rate (flow), tidal volume (VT), respiratory rate (RR), minute VE. The WB (2-5) was calculated with the integration of the changes in esophageal pressure over the change of lung volumes as shown on the Campbell diagram (Figure 1, calculation of area). Subjects were encouraged to take slow deep inspiration to establish the pulmonary static pressure-volume curve. The normal predicted curve of transthoracic pressure-volume was used as the chest wall static pressure-volume curve. For each level of VE (stratified by a stepwise increase of PetCO2 by 5 mmHg for each step), 6–8 breaths were taken to calculate the average WB, expressed as work done per liter of VE (Joule/liter, J/L).
Figure 1.
Campbell diagram and the work of breathing calculation. (A) Pressure and lung volume changes during breathing. CL represents for compliance of lung and Ccw for compliance of chest wall; (B) Wi = Wrs (dot area) + Wel (horizontal line area); (C) Wex was the vertical line area on the right of the Ccw; (D) the area inside the network was the work done to overcome intrinsic PEEP (WPEEPi). Wi, inspiratory work; Wrs, work to overcome airway resistance; Wel, work to overcome elestance; Wex, expiratory work; CL, compliance of lung; Ccw, compliance of chest wall.
Experimental procedure
Patient history was collected; physical examination, chest X-ray examination, and lung function tests were performed before the study. After a rest for at least 30 minutes, the patients underwent nasal topical anesthesia for placement of esophageal and gastric balloon catheters. Subjects maintained sitting position through the experimental study. Data acquisition started when the subjects reached a steady spontaneous breathing in a resting condition. Firstly, the static pressure-volume curve of the lung was measured with slow deep inspiration method by asking the subjects to take a slow and deep breath to reach total lung capacity (repeat 3 times at an interval of every 3 minutes). After a period of rest until the subject’s breathing became stable, the subjects started the rebreathing trial by breathing through a 6-liter bag filled with pure oxygen for 6–10 minutes. The end-tidal CO2 partial pressure (PetCO2), airway pressure and ventilator flow were monitored continuously throughout the study. The change of end-expiratory lung volume was monitored with inspiratory capacity (IC) method every minute by asking subjects to take a rapid deep inspiration to total lung capacity position. The trial was terminated once PetCO2 reached 70 mmHg or when the patient’s dyspnea became intolerable.
Statistical analysis
The SPSS10.0 software package was used for statistical analysis. All data were expressed as the mean ± standard deviations. A t-test was used to compare the differences between the two groups. Linear regression and non-linear regression tests were used to compare the differences of response to rebreathing between the COPD and control group. F test was used to compare the parameter differences between different levels of VE or PetCO2 in each group. P<0.05 was used as the cut-off for statistical significance.
Results
All of the healthy subjects and seven of the eight COPD patients were able to complete the rebreathing trial and reached the PetCO2 level of 70 mmHg. One COPD patient was unable to complete the study due to intolerance of dyspnea at PetCO2 level of 60 mmHg. During the test, all subjects complained of sweats, hot feeling, and a certain degree of dyspnea. No other adverse effects were observed.
The Wtot
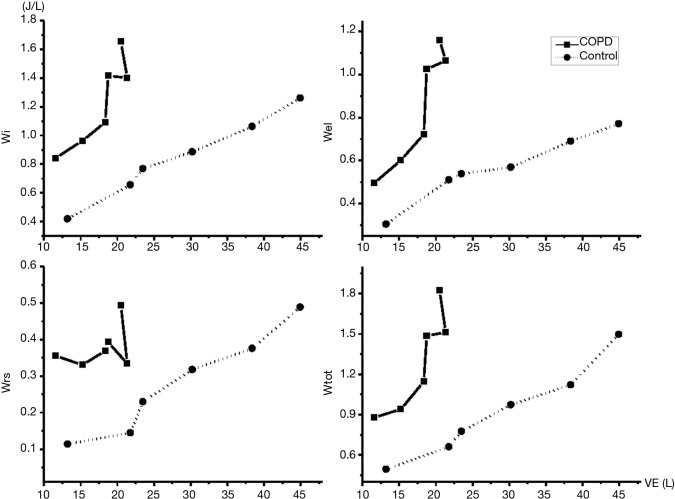
The changes of Wtot as the increase of PetCO2 in both groups were shown in Figure 2. It was noted that Wtot in the COPD group was significantly higher than the control group in the comparable levels of PetCO2 (P<0.05). The inspiratory work (Wi) in the COPD group (0.84±0.47 J/L) was significantly higher than that in the controlled group (0.42±0.22 J/L) during quiet breathing at rest (P<0.05) (Figure 3). The slope of increase of Wi was significantly higher in the COPD group than the control group (P<0.05). During quiet breathing at rest, the Wi/Wtot ratio was 95.5% in normal controls and 84% in COPD group. The ratio of Wi/Wtot remained similar as VE and PetCO2 increased throughout the rebreathing trial (P>0.05). So, Wi of breathing was the main component of WB even in the situation of increased ventilatory demand.
Figure 2.
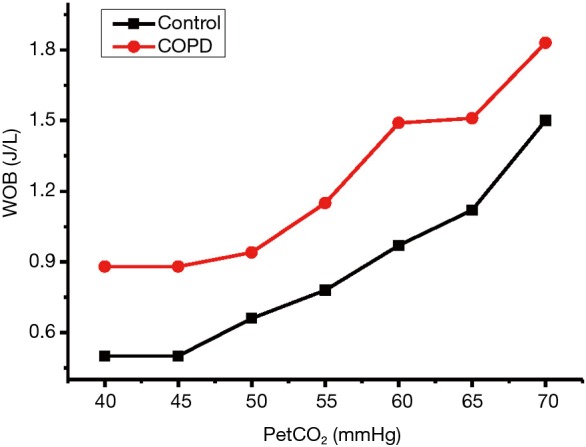
Wtot at different levels of PetCO2. Wtot, total work of breathing; PetCO2, end-tidal CO2 partial pressure.
Figure 3.
Work of breathing and its components in response to increased ventilation. VE, ventilation.
Dynamic changes of Wi of breathing and its components
The trend of change in overcoming airway resistance (Wrs): the Wrs increased as VE increased in both groups. At any level of VE, Wrs in the COPD group was significantly higher than the normal group (P<0.05). However, the slope of increase (response) was not significantly different (P>0.05) between the two groups.
The trend of change in overcoming lung/chest-wall elastance (Wel): as shown in Table 2, Wel in COPD group was not significantly different from the control group during quiet breathing. As PetCO2 increased to 70 mmHg, Wel in COPD Group was significantly higher than the control group (P<0.05). The slope of response in COPD group was significantly higher than control group (P<0.05).
Table 2. The different components of work of breathing at different PetCO2 levels.
| PetCO2 (mmHg) | Wi (J/L) | Wex (J/L) | Wtot (J/L) | Wrs (J/L) | Wel (J/L) | WPEEPi (J/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | COPD | Normal | COPD | Normal | COPD | Normal | COPD | Normal | COPD | Normal | COPD | ||||||
| 40 | 0.42±0.22* | 0.84±0.47 | 0.00±0.00* | 0.03±0.04 | 0.50±0.26 | 0.88±0.47 | 0.11±0.07** | 0.36±0.20 | 0.31±0.15 | 0.50±0.34 | 0 | 0.02±0.05 | |||||
| 50 | 0.66±0.33 | 0.96±0.34 | 0.00±0.01* | 0.11±0.11 | 0.66±0.34 | 0.94±0.32 | 0.14±0.14* | 0.33±0.16 | 0.51±0.21 | 0.60±0.25 | 0 | 0.13±0.15 | |||||
| 55 | 0.77±0.40 | 1.09±0.55 | 0.01±0.02* | 0.07±0.09 | 0.78±0.40* | 1.15±0.59 | 0.23±0.21 | 0.37±0.13 | 0.54±0.25 | 0.73±0.51 | 0 | 0.18±0.20 | |||||
| 60 | 0.89±0.46* | 1.42±0.48 | 0.09±0.15 | 0.07±0.11 | 0.97±0.47 | 1.49±0.55 | 0.32±0.24 | 0.39±0.16 | 0.57±0.28* | 1.02±0.47 | 0 | 0.51±0.27 | |||||
| 65 | 1.06±0.36 | 1.40±0.46 | 0.06±0.05 | 0.17±0.15 | 1.12±0.40 | 1.51±0.56 | 0.38±0.24 | 0.33±0.15 | 0.69±0.16 | 1.06±0.32 | 0 | 0.54±0.18 | |||||
| 70 | 1.26±0.45* | 1.65±0.63 | 0.24±0.28 | 0.17±0.21 | 1.50±0.71 | 1.83±0.73 | 0.49±0.45 | 0.49±0.27 | 0.77±0.15* | 1.16±0.40 | 0 | 0.65±0.26 | |||||
*, the P value of both groups was <0.05. **, the P value of both groups was <0.01. Wi, inspiratory work; Wex, expiratory work; Wtot, total work of breathing; Wrs, work to overcome airway resistance; Wel, work to overcome elestance; WPEEPi, work done to overcome intrinsic PEEP.
The trend of change in work done to overcome the intrinsic PEEP (WPEEPi): there was no WPEEPi in the control group even in the condition of increased ventilatory demand. However, WPEEPi in COPD group increased significantly as VE increased. During quiet breathing, the proportion of WPEEPi to Wel was only 4%. However, WPEEPi increased to 56% when PetCO2 increased to 70 mmHg, indicating that the increase of WPEEPi in COPD patients was an important component in the increased WB in condition of increased ventilatory demand.
Discussion
Expiratory flow limitation and elevated lung capacity are the major pathophysiological abnormalities in COPD patients, which lead to increased WB and reduction of inspiratory muscles strength. Dynamic hyperinflation often develops when VE increases (such as during exercises), lead to further increase of WB and reduction of inspiratory muscle strength. The imbalance between the WB and strength of inspiratory muscles is one of the important mechanisms related to dyspnea and respiratory failure. So it is necessary to dynamically monitor WB to understand the work of breathing in COPD patients in conditions of different VE demands and mechanisms related to the change of WB. At present, the main methods to induce the change of VE demand are exercise test and CO2 rebreathing test. The exercise test is mimic to the actual conditions in everyday life, which involves the activation of peripheral muscles, cardiac and ventilatory responses, as well as other confounding factors. CO2 rebreathing method stimulates the increase of VE by increasing the level of arterial CO2 (estimated through the monitoring of end-tidal CO2 levels), without a simultaneously involvement of peripheral muscles or cardiac functions. The CO2 rebreathing method is easy to perform and commonly used for respiratory physiology research. So in this study, we used CO2 rebreathing method to explore the change to WB and associated mechanisms in COPD patients as VE demand increased, in a hope that the results may improve our understanding of the mechanisms related to elevated WB and dyspnea and the potential intervention for improving these abnormalities in COPD.
WB can be calculated according to the changes in esophageal pressure and lung volume, or be calculated on the basis of the integration of airway pressure and lung volume in condition of positive airway pressure mechanical VE. Campbell diagram, the standard method for calculating WB and its components, combines the curve of esophageal pressure with the static pressure-volume curve of the lung and chest wall (Figure 1). In order to establish Campbell dagram, static pressure-volume curve of lung and chest wall must be established first. The slow deep breathing technique is the most commonly used method for the measurement of the static pressure-volume curve of the lungs as it is easy to be performed. However, the measurement of the static chest wall pressure-volume curve must be performed when all respiratory muscles are relaxed. In normal volunteers, static chest wall pressure-volume curve can be measured by asking the subjects to relax after deep breathing. The expiration flow is passed through a flow limitation tube to minimize the impact of expiratory flow on the pressure. In COPD subjects receiving intubation and mechanical VE under the condition of adequate sedation (no respiratory muscle activation), slow inspiration method can be used to measure the static chest wall pressure-volume curve by calculating the pressure difference between the airway pressure and transpulmonary pressure. This pressure difference against the lung volume change forms the chest wall pressure-volume curve. However, it is difficult to obtain the chest wall static pressure-volume curve when the patient is breathing spontaneously. Therefore, Estenne et al. (2) recommended the static chest wall pressure-volume curve from normal prediction to be used instead of measurement in each subject. Milic-Emili et al. (6) compared the chest wall pressure-volume curve of normal subjects with COPD patients. The results indicated that the pressure-volume curves were similar between healthy individuals and COPD patients. So static chest wall pressure-volume curve from the normal prediction was used in our study for data analyses.
In the calculation of WB, the determination of end-expiratory lung volume may have a significant impact on the results. In this study, inspiratory capacity (IC) method was used for determinant of the change of lung volume. IC method is easy to use in practice by asking subjects to take a deep inspiration intermittently to total lung capacity. After matching the total lung capacity position, the changes of end-expiratory lung volume can be calculated. It has been reported by Younes et al. (7) and Yan et al. (8) that IC method accurately determines the changes of expiratory lung volumes in normal volunteers and COPD patients. In our study, each subject practiced deep inspiration under the guidance of researchers before the study to ensure the appropriate use of IC method, with the variability of maximum esophageal pressure during IC measurement within 10% (the measured variability in all cases of our study was within 5.9–7.3%, with mean value at 6.28%).
In this study, we were aware of the age difference between the COPD group and the control group. However, we failed to recruit healthy volunteers with similar age as in COPD group. The main purpose of this study was to understand the mechanisms related to increased WB when VE level increased in COPD patients. The main comparison analysis was to compare the data at rest with those at increased VE level during CO2 rebreathing trial, but not the comparison between COPD and normal controls. So, the age difference between the COPD and control group should not have major impact on the main results of this study.
There have been reports in the literature on the WB in COPD patients with inconsistent results. Coussa et al. (4) and D’Angelo et al. (5) have reported that the work done to overcome resistance in COPD patients are twice as much as the normal individuals. Milic-Emili et al. (6) have reported that WB to overcome intrinsic PEEP (WPEEPi) accounted for 57% of the Wi of breathing. The disparity of these reports might be due to the difference of study subjects and subject’s status during the study. In this study with dynamic change of VE levels and WB, it was found that airway resistance was the main cause for increased WB during quiet breathing; WPEEPi increased gradually and became the main mechanism for increased WB at high level of VE status as the VE increased in COPD patients. The results of this study are similar to the study by Loring et al. (9). More attention should be paid to improve PEEPi to minimize the impact of increased WB in condition of increased VE demands (such as during acute exacerbation or exercise) in management of COPD patients.
To summarize, it is essential to reduce the WB and the respiratory muscle load in COPD in clinical practice. Airway resistance was the main cause for increased WB during quiet breathing. Optimizing medications treatment can improve lung capacity and prevent dynamic hyperinflation to decrease airway resistance, allowing for adequate respiratory muscle rests to improve dyspnea in COPD patients. For patients requiring mechanical VE support, we should appropriately prolong the expiratory time and use PEEP to prevent adversary effects of PEEPi (10).
Acknowledgements
None.
Ethical Statement: Informed consent was obtained in all the subjects involved in the study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.GOLD Executive Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (Revised 2011). Available online: www.goldcopd.com
- 2.Estenne M, Yernault JC, De Troyer A. Rib cage and diaphragm-abdomen compliance in humans: effects of age and posture. J Appl Physiol (1985) 1985;59:1842-8. [DOI] [PubMed] [Google Scholar]
- 3.Fleury B, Murciano D, Talamo C, et al. Work of breathing in patients with chronic obstructive pulmonary disease in acute respiratory failure. Am Rev Respir Dis 1985;131:822-7. [DOI] [PubMed] [Google Scholar]
- 4.Coussa ML, Guérin C, Eissa NT, et al. Partitioning of work of breathing in mechanically ventilated COPD patients. J Appl Physiol (1985) 1993;75:1711-9. [DOI] [PubMed] [Google Scholar]
- 5.D'Angelo E, Robatto FM, Calderini E, et al. Pulmonary and chest wall mechanics in anesthetized paralyzed humans. J Appl Physiol (1985) 1991;70:2602-10. [DOI] [PubMed] [Google Scholar]
- 6.Milic-Emili J. Work of breathing in COPD. In: Vincent JL. editor. Yearbook of Intensive Care and Emergency Medicine 1994. Heidelberg: Springer Berlin Heidelberg, 1994:561-71. [Google Scholar]
- 7.Younes M, Kivinen G. Respiratory mechanics and breathing pattern during and following maximal exercise. J Appl Physiol Respir Environ Exerc Physiol 1984;57:1773-82. [DOI] [PubMed] [Google Scholar]
- 8.Yan S, Kaminski D, Sliwinski P. Reliability of inspiratory capacity for estimating end-expiratory lung volume changes during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;156:55-9. 10.1164/ajrccm.156.1.9608113 [DOI] [PubMed] [Google Scholar]
- 9.Loring SH, Garcia-Jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol (1985) 2009;107:309-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koegelenberg CF, Slebos DJ, Shah PL, et al. Time for the Global Rollout of Endoscopic Lung Volume Reduction. Respiration 2015;90:430-40. 10.1159/000439311 [DOI] [PubMed] [Google Scholar]