Abstract
Background
Tuberculous pleural effusion (TPE) exhibits different characteristics according to pleural fluid cellular predominance or whether the pleural fluid is free-flowing or loculated. However, its categorization based on either of these factors alone may be insufficient to properly reflect the heterogeneous manifestation of TPE. We evaluated the characteristics of the four TPE groups classified according to cellular predominance and whether the fluid is free-flowing or loculated.
Methods
A cohort of 375 patients with TPE was retrospectively reviewed. Clinical, radiological, and laboratory findings were compared between neutrophilic and lymphocytic TPE, and between free-flowing and loculated effusion for both neutrophilic and lymphocytic TPE.
Results
Lymphocytic TPE and neutrophilic TPE were observed in 336 (90%) and 39 (10%) patients, respectively. Pleural fluid loculation was present in 36% and 31% of the patients in the lymphocytic and neutrophilic groups, respectively. A few parameters of the laboratory findings between neutrophilic and lymphocytic TPE patients showed significant differences. However, these significant differences were prominently observed when comparing free-flowing and loculated subgroups of the respective neutrophilic and lymphocytic groups. Pleural fluid pH, lactate dehydrogenase, and adenosine deaminase levels were significantly different among the four subgroups. The neutrophilic loculated subgroup exhibited the most intense pleural inflammation and the highest mycobacterial yields when compared to the other subgroups. However, the percentage of neutrophils in the pleural fluid was not positively associated with the probability of culture-positive effusion.
Conclusions
The heterogeneous manifestation of TPE would be better characterized by using a classification system based on combined pleural fluid cellular predominance and loculation, with the neutrophilic loculated subgroup contributing to most of the clinically significant differences.
Keywords: Tuberculous pleural effusion (TPE), loculation, neutrophilic predominance
Introduction
Tuberculous pleural effusion (TPE) is the second most common type of extra-pulmonary tuberculosis (TB) (1,2) and is a common cause of pleural effusion in TB-endemic regions (3,4). Moreover, extra-pulmonary involvement tends to increase in frequency with an increasingly compromised immune system (5). TPE is generally characterized by lymphocytic exudates. However, around 10% of TPE cases are characterized by neutrophilic exudates (6,7). Neutrophilic predominance can be observed in the early stages of TPE and then shifts into lymphocytic predominance as time goes by (6,7). Neutrophilic TPE exhibits clinical and laboratory differences to lymphocytic TPE (6). In a retrospective study of 214 patients with TPE (6), those with neutrophilic predominance at the time of the first thoracentesis showed a higher yield of mycobacteria cultured from the pleural fluid on solid media, and higher pleural fluid adenosine deaminase (ADA) levels compared to lymphocytic TPE. Another study showed that the percentage of lymphocytes in the pleural fluid was negatively associated with the probability of culture-positive effusion (8).
Meanwhile, exudative pleural effusion often leads to the loculation of the pleural fluid, which reflects a more intense pleural inflammation in TPE as well as parapneumonic effusion. Recently, neutrophilic loculated TPE also showed a higher mycobacterial burden and higher ADA levels than neutrophilic free-flowing TPE (9). Failure to effectively contain mycobacteria in the pleural cavity may lead to a more complicated TPE, which can be associated with the development of loculation, a higher inflammatory response, and a higher frequency of positive mycobacterial cultures. Thus, a comparison between only pleural fluid cellular predominant types of TPE may be not enough. It may be necessary to determine whether TPE is free-flowing or loculated within the same cellular predominant type for better understanding heterogeneous manifestations of TPE.
Therefore, we hypothesized that the presence or absence of loculation is an additional factor that can be used to distinguish between heterogeneous manifestations of TPE within the same cellular predominant type. To test this hypothesis, we first compared the clinical, radiological, and laboratory findings between patients with neutrophilic and lymphocytic TPE. Next, we further classified patients with neutrophilic and lymphocytic TPE according to whether the pleural fluid was free-flowing or loculated, and compared the characteristics of these respective TPE groups.
Methods
Study population
We retrospectively reviewed all consecutive patients with TPE who underwent a diagnostic thoracentesis before receiving antituberculous therapy at the Kyungpook National University Hospital, South Korea, between January 2009 and May 2015. South Korea is an area with an intermediate prevalence of active TB (10). TPE was considered as confirmed when pleural fluid analysis was compatible with TPE and one of the following criteria was met: (I) positive culture for Mycobacterium tuberculosis (MTB) in the pleural fluid, pleural tissue, sputum, or bronchial aspirate; (II) pathologically chronic granulomatous inflammation with positive MTB polymerase chain reaction (PCR), positive acid-fast bacilli (AFB) smear, or caseous necrosis in pleural biopsy tissue; (III) chronic granulomatous inflammation alone in the pleural biopsy and pleural effusion that resolved with anti-TB treatment (8). A probable case of TPE was determined when the lymphocyte-predominant exudate at the first or subsequent thoracenteses contained ≥40 U/L of ADA levels in the pleural fluid, which is the most widely accepted value for the diagnosis of TPE, and clinical improvement was observed after anti-TB treatment (6). All patients with confirmed or probable TPE were included in the study and classified according to pleural fluid cellular predominance and whether the pleural fluid was free-flowing or loculated. Cases showing human immunodeficiency virus infection were excluded. In addition, cases with purulent pleural fluid showing gross pus at thoracentesis were also excluded.
Study design
Data regarding demographic, clinical, radiological, microbiological, and pleural fluid characteristics were collected from patients with TPE. Lymphocytic effusion was defined as effusion with >50% lymphocytes in the differential leukocyte count, while neutrophilic effusion was defined as effusion with ≥50% neutrophils. Differential leukocyte cell counts in the pleural fluid were manually performed using a Neubauer chamber (Marienfeld, Germany). Only the first pleural fluid cell counts and profiles were used for statistical analyses in patients who underwent repeated thoracentesis. AFB smears, MTB-PCR, and mycobacterial cultures on 3% Ogawa solid medium were performed as previously described (11). Pleural fluid ADA activity was measured in a routine clinical setting using an automated calorimetric assay kit (Runpia Liquid ADA, Kyokuto Pharmaceutical Industrial Co., Ltd., Japan) as described in the package insert.
Before diagnostic thoracentesis, all patients underwent chest radiographs (frontal, lateral, and both decubitus) and conventional chest CT with or without enhancement. These chest images were reviewed by a radiologist and a pulmonologist. They independently evaluated whether the pleural effusion was free-flowing or loculated and the following CT findings: (I) consolidative; (II) nodular; and (III) cavitary lesions (12). If a discrepancy was noted between interpretations, the images were further reviewed by another pulmonologist. The study protocols were reviewed and approved by the Institutional Review Board (IRB) of the Kyungpook National University Hospital (IRB No. 2016-01-006). Informed consent was waived by the IRB.
Statistical analysis
Statistical analyses were performed using the SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as the median [interquartile range (IQR)], and differences between groups were analyzed using the Mann-Whitney U test or Kruskal-Wallis test. Categorical variables were expressed as numbers and percentages, and were analyzed using the χ2 test or Fisher’s exact test. A P value <0.05 was considered statistically significant.
Results
The study population comprised 375 consecutive patients with TPE showing nonpurulent pleural fluid, of whom 336 (90%) had lymphocytic effusion and 39 (10%) had neutrophilic effusion according to the initial thoracentesis. Among the 336 TPE patients with lymphocytic effusion, 193 (57%) were confirmed by positive microbiological [pleural fluid (n=48), sputum (n=69), and bronchial aspirate (n=49)] and histologic (n=27) results; the remaining 143 (43%) were probable. All 39 TPE patients with neutrophilic effusion at the first thoracentesis were confirmed by positive microbiological [pleural fluid (n=8), sputum (n=18), and bronchial aspirate (n=9)] and histologic (n=4) results. They exhibited no evidence of bacterial infection in serial pleural fluid, sputum, or bronchial aspirates.
Clinical, radiological, and laboratory differences between neutrophilic and lymphocytic TPE patients
The median age and sex distribution were not significantly different between the two groups (Table 1). Symptom duration >14 days, chest pain, and fever were present in approximately 50% of the overall study population, and showed a similar frequency in both groups.
Table 1. Clinical, radiological, and laboratory differences between neutrophilic and lymphocytic TPE patients.
| Variable | Total (n=375) | Neutrophilic (n=39) | Lymphocytic (n=336) | P value |
|---|---|---|---|---|
| Demographic | ||||
| Age, [range] years | 65 [38–76] | 58 [36–75] | 66 [38–77] | 0.669 |
| Male, n [%] | 242 [65] | 30 [77] | 212 [63] | 0.088 |
| Clinical, n [%] | ||||
| Past TB history | 31 [8] | 5 [13] | 26 [8] | 0.351 |
| Symptoms duration >14 days | 203 [54] | 19 [49] | 184 [55] | 0.438 |
| Chest pain | 193 [51] | 19 [49] | 174 [52] | 0.676 |
| Fever | 179 [48] | 24 [62] | 155 [46] | 0.076 |
| Radiological, n [%] | ||||
| Any parenchymal lesion | 254 [68] | 30 [77] | 224 [67] | 0.195 |
| Consolidative | 107 [29] | 13 [33] | 94 [28] | 0.483 |
| Nodular | 199 [53] | 27 [69] | 172 [51] | 0.033 |
| Cavitary | 43 [11] | 10 [26] | 33 [10] | 0.007 |
| Loculated | 132 [35] | 12 [31] | 120 [36] | 0.540 |
| Pleural fluid | ||||
| Total cell counts, [range] cells/ìL | 1,600 [850–2,800] | 1,225 [300–4,500] | 1,663 [900–2,650] | 0.415 |
| Lymphocytes, [range] % | 95 [75–98] | 25 [5–40] | 95 [85–98] | <0.001 |
| pH, (range) | 7.42 (7.37–7.46) | 7.37 (7.29–7.47) | 7.42 (7.38–7.46) | 0.019 |
| Protein, (range) g/dL | 5.1 (4.6–5.6) | 4.9 (4.0–5.6) | 5.1 (4.6–5.6) | 0.073 |
| Glucose, [range] mg/dL | 98 [73–124] | 96 [66–145] | 98 [74–123] | 0.955 |
| Lactate dehydrogenase, [range] U/L | 880 [528–1,482] | 1,066 [441–2,045] | 860 [533–1,453] | 0.242 |
| ADA, [range] U/L | 83 [63–106] | 70 [45–111] | 84 [64–106] | 0.106 |
| AFB smear (+) | 2/371 [1] | 0/39 (0) | 2/332 [1] | 1.0 |
| TB-PCR (+) | 27/347 [8] | 7/37 [19] | 20/310 [7] | 0.016 |
| MTB culture (+) | 56/367 [15] | 8/38 [21] | 48/329 [15] | 0.294 |
Data are expressed as the median (IQR) or number (%). TB, tuberculosis; ADA, adenosine deaminase; AFB, acid fast bacilli; PCR, polymerase chain reaction; MTB, Mycobacterium tuberculosis.
Nodular and cavitary lesions on the chest CT were significantly more frequent in the neutrophilic group than in the lymphocytic group (P=0.033 and P=0.007, respectively). Loculation of pleural fluid on the initial chest images was present in 31% and 36% of the neutrophilic and lymphocytic groups, respectively, without a significant difference.
Pleural fluid pH was significantly lower in the neutrophilic group than in the lymphocytic group (P=0.019), while other biochemical markers, including ADA levels, were not significantly different between the two groups. The frequency of a positive TB-PCR result from the pleural fluid was significantly higher in the neutrophilic group than in the lymphocytic group (P=0.016). By contrast, the frequency of a positive AFB smear or MTB culture from the pleural fluid was not significantly different between the two groups.
Clinical, radiological, and laboratory differences between free-flowing and loculated effusion in the respective neutrophilic and lymphocytic TPE patients
Neutrophilic and lymphocytic TPE patients were sub-classified according to whether or not the pleural fluid was loculated on the initial chest images (Table 2). The frequency of patients with symptoms lasting >14 days was significantly greater in the loculated subgroup (75%) than in the free-flowing subgroup (37%) of the neutrophilic group (P=0.029). This significant difference was similar between the free-flowing and loculated subgroups of the lymphocytic group (P=0.018). In addition, chest pain and fever were more frequent in the loculated subgroup than in the free-flowing subgroup of the lymphocytic group (P=0.013 and P=0.028, respectively).
Table 2. Clinical, radiological, and laboratory differences between free-flowing and loculated effusion in the respective neutrophilic and lymphocytic TPE patients.
| Variable | Neutrophilic | Lymphocytic | |||||
|---|---|---|---|---|---|---|---|
| Free-flowing (n=27) | Loculated (n=12) | P value | Free-flowing (n=216) | Loculated (n=120) | P value | ||
| Demographic | |||||||
| Age, [range] years | 65 [38–77] | 50 [32–67] | 0.171 | 68 [41–77] | 63 [34–75] | 0.125 | |
| Male, n [%] | 20 [74] | 10 [83] | 0.693 | 132 [61] | 80 [67] | 0.312 | |
| Clinical, n [%] | |||||||
| Past TB history | 2 [7] | 3 [25] | 0.159 | 16 [8] | 10 [8] | 0.763 | |
| Symptoms duration >14 days | 10 [37] | 9 [75] | 0.029 | 108 [50] | 76 [63] | 0.018 | |
| Chest pain | 13 [48] | 6 [50] | 0.915 | 101 [47] | 73 [61] | 0.013 | |
| Fever | 19 [70] | 5 [42] | 0.153 | 90 [42] | 65 [55] | 0.028 | |
| Radiological, n [%] | |||||||
| Any parenchymal lesion | 20 [74] | 10 [83] | 0.693 | 144 [67] | 80 [67] | 1.0 | |
| Consolidative | 10 [37] | 3 [25] | 0.714 | 67 [31] | 27 [23] | 0.096 | |
| Nodular | 17 [63] | 10 [83] | 0.276 | 109 [51] | 63 [53] | 0.720 | |
| Cavitary | 4 [15] | 6 [50] | 0.043 | 22 [10] | 11 [9] | 0.764 | |
| Pleural fluid | |||||||
| Total cell counts, [range] cells/μL | 2,700 [842–5,600] | 163 [88–413] | <0.001 | 1,700 [925–2,950] | 1,475 [781–2,400] | 0.184 | |
| Lymphocytes, [range] % | 20 [5–40] | 25 [4–40] | 0.994 | 93 [80–98] | 98 [90–99] | <0.001 | |
| pH, (range) | 7.42 (7.35–7.50) | 7.27 (7.18–7.33) | <0.001 | 7.43 (7.40–7.46) | 7.41 (7.35–7.45) | <0.001 | |
| Protein, (range) g/dL | 5.0 (3.6–6.0) | 4.6 (4.2–5.2) | 0.494 | 5.1 (4.5–5.6) | 5.2 (4.8–5.6) | 0.192 | |
| Glucose, [range] mg/dL | 105 [71–146] | 74 [41–140] | 0.153 | 105 [85–126] | 88 [65–111] | <0.001 | |
| Lactate dehydrogenase, [range] U/L | 860 [422–1,572] | 1,504 [923–4,407] | 0.026 | 787 [474–1,254] | 1,140 [644–1,842] | <0.001 | |
| ADA, [range] U/L | 58 [27–83] | 114 [66–151] | 0.007 | 81 [59–103] | 89 [74–109] | 0.002 | |
| AFB smear (+) | 0/27 (0) | 0/12 (0) | 1.0 | 2/214 [1] | 0/118 (0) | 0.540 | |
| TB-PCR (+) | 1/26 [4] | 6/11 [55] | 0.001 | 16/201 [8] | 4/109 [4] | 0.142 | |
| MTB culture (+) | 3/26 [12] | 5/12 [42] | 0.034 | 27/213 [13] | 21/116 [18] | 0.183 | |
Data are expressed as the median (IQR) or number (%). TB, tuberculosis; ADA, adenosine deaminase; AFB, acid fast bacilli; PCR, polymerase chain reaction; MTB, Mycobacterium tuberculosis.
Regarding the parenchymal lung lesions on the chest CT, cavitary lesions were significantly greater in the loculated subgroup than in the free-flowing subgroup of the neutrophilic group (P=0.043). Other parenchymal lung lesions were not significantly different between the free-flowing and loculated subgroups in both groups.
Total leukocyte cell counts in the pleural fluid were significantly lower in the loculated subgroup than in the free-flowing subgroup of the neutrophilic group (P<0.001). The loculated subgroup showed a significantly lower pH and higher LDH and ADA levels than the free-flowing subgroup in both neutrophilic and lymphocytic groups (neutrophilic group: P<0.001, P=0.026, and P=0.007, respectively; lymphocytic group: P<0.001, P<0.001, and P=0.002, respectively). Additionally, pleural fluid glucose levels in the lymphocytic loculated subgroup were significantly lower than those in the lymphocytic free-flowing subgroup (P<0.001). Positive pleural fluid TB-PCR and MTB culture results were significantly higher in the neutrophilic loculated subgroup than in the neutrophilic free-flowing subgroup (P=0.001 and P=0.034, respectively), while there was no significant difference between the two lymphocytic subgroups.
Comparison of pleural fluid biochemistries and microbiological results among the four TPE subgroups according to pleural fluid cellular predominance and whether the pleural fluid is free-flowing or loculated
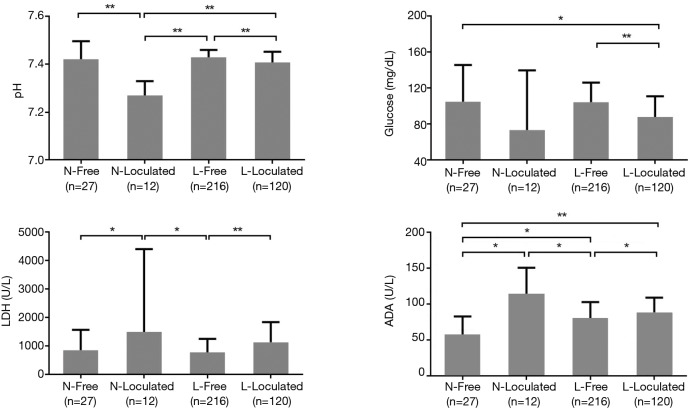
Figure 1 shows a comparison of pleural fluid biochemistry between the four subgroups. The pH of the pleural fluid was significantly lower in the neutrophilic loculated subgroup than in the two lymphocytic subgroups as well as in the neutrophilic free-flowing subgroup (P<0.001 for all groups). Pleural fluid glucose levels in the free-flowing subgroup of both the two groups were higher than in the lymphocytic loculated subgroup (P<0.05 or P<0.001). LDH levels in the loculated subgroup were significantly higher than in the free-flowing subgroup in both neutrophilic and lymphocytic groups (P<0.05 and P<0.001, respectively). In addition, LDH levels in the neutrophilic loculated subgroup were higher than in the lymphocytic free-flowing subgroup (P<0.05). ADA levels were the lowest in the neutrophilic free-flowing subgroup and were significantly different from those in the other three subgroups (P<0.05 or P<0.001), while ADA levels in the neutrophilic loculated subgroup were the highest among the four subgroups, and were significantly different from the levels in the two free-flowing subgroups (P<0.05).
Figure 1.
Comparison of pleural fluid biochemistries among the four tuberculous pleural effusion subgroups according to pleural fluid cellular predominance and whether the pleural fluid is free-flowing or loculated. N, neutrophilic; Free, free-flowing; L, lymphocytic; LDH, lactate dehydrogenase; ADA, adenosine deaminase. *, P<0.05; **, P<0.001.
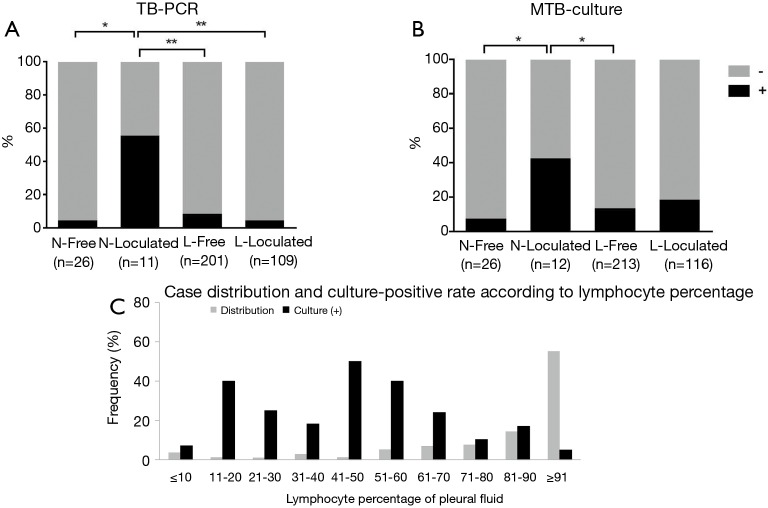
Pleural fluid from the neutrophilic loculated subgroup showed higher positive TB-PCR results than that from the other three subgroups (P<0.05 or P<0.001) (Figure 2A). This relationship was in line with the frequency of cavitary lesions between the four groups (P<0.05 between the neutrophilic loculated TPE subgroup and the other TPE subgroups in Table 2). A positive MTB culture from the pleural fluid was significantly higher in the neutrophilic loculated subgroup than in the two free-flowing subgroups (P<0.05) (Figure 2B).
Figure 2.
Comparison of pleural fluid microbiological results (A and B) among the four tuberculous pleural effusion subgroups according to pleural fluid cellular predominance and whether the pleural fluid is free-flowing or loculated, and case distribution and culture-positive rate according to the lymphocyte percentage of pleural fluid (C). TB-PCR, tuberculous-polymerase chain reaction; MTB, Mycobacterium tuberculosis; N, neutrophilic; Free, free-flowing; L, lymphocytic. *, P<0.05; **, P<0.001.
Figure 2C shows the case distribution and culture-positive rate according to the percentage of lymphocytes in the pleural fluid. The frequency of TPE cases positively correlated with the lymphocyte percentage (ρ=0.857, P=0.002). However, the culture-positive rate did not correlate with the lymphocyte percentage (ρ=‒0.359, P=0.309).
These findings in pleural fluid were similar to those for TPE subgroups that included only confirmed cases (Table S1).
Discussion
The main findings of the current study are as follows: (I) this study confirmed that neutrophilic TPE was present in approximately 10% of patients at the first thoracentesis and showed a few differences in laboratory findings compared to lymphocytic TPE; (II) these differences were markedly prominent between the free-flowing and loculated subgroups of the respective neutrophilic and lymphocytic TPE groups; (III) the neutrophilic loculated subgroup exhibited the most intense pleural inflammation and the highest mycobacterial yields from the pleural fluid than any of the other subgroups; and (IV) the percentage of lymphocytes in the pleural fluid positively correlated with the frequency of TPE cases, while it was not negatively associated with the probability of culture-positive effusion in the overall population. These results were consistently observed when comparing TPE subgroups that included only confirmed cases.
TPE may show neutrophilic predominance in the early phase (13). This is supported by experimental models of bacilli Calmette-Guerin (BCG) instilled into pleural spaces of rabbits (14). However, it appears unlikely that all cases of neutrophilic TPE, both free-flowing and loculated, present at the same phase. Our study showed that clinical symptoms were shorter in duration and pleural inflammation was lower in the free-flowing subgroup compared to the loculated subgroup. Loculated effusion generally reflects a more advanced or complicated stage in exudative effusion. If exudative free-flowing effusion proceeds unabated, it may lead to loculated effusion with a more intense inflammation, but it seems unlikely that the reverse will happen. Therefore, neutrophilic free-flowing TPE is more likely to be an earlier manifestation than neutrophilic loculated TPE. If these two neutrophilic subgroups are included in the same TPE group (as shown in Table 1 of the current study), the presence of the neutrophilic subgroups with different characteristics may go unnoticed (Table 2). Thus, differences in the relative proportion of these neutrophilic subgroups may show different results for neutrophilic TPE and explain some of the discrepancies between studies.
In our study, the case distribution according to lymphocyte percentage in the pleural fluid is in line with previous results (8). However, a culture-positive yield was not negatively associated with lymphocyte percentage, particularly in the neutrophilic group. It is likely that the probability of culture-positive effusion is associated with the mycobacterial burden of the pleural cavity. These mycobacterial burdens may vary in each patient with TPE. On the other hand, neutrophils initiate the inflammatory process in the pleural space in response to bacillary antigenic proteins or viable MTB regardless of mycobacterial burdens (15,16). In experimental animal models, the percentage of lymphocytes increased as serial thoracenteses were performed, while the number of BCG colonies cultured from pleural fluid decreased over time (14). The same BCG loads were initially introduced into the pleural space in this animal study. In clinical practice, if thoracenteses are serially performed on the same patients with TPE, we could expect that the lymphocyte percentage will inversely correlate with mycobacterial burden and the probability of culture-positive effusion will decrease, as shown in animal models (14). However, it is unclear whether lymphocyte percentage will uniformly increase and reflect the mycobacterial burden in individual TPE patients who present at different disease stages with initially different mycobacterial loads. Our results suggest that mycobacterial burdens differ among patients, even when the cellular proportion in the pleural fluid is similar.
TPE is often classified into free-flowing or loculated effusion, especially in the evaluation of residual pleural thickening after treatment (17,18). The different characteristics between lymphocytic and neutrophilic TPE, were also reported (6). In this dichotomous classification, the specificity of the neutrophilic loculated subgroup, which mainly accounts for the distinct characteristics compared to the counterpart group, may be obscured. The current study provides a better description of the heterogeneous characteristics of patients with TPE. Thus, this new classification system may be useful in clinical practice.
The main limitation of the current study is its retrospective nature, as a small number of microbiological data are missing. Another limitation is the small sample size of the neutrophilic group, particularly the neutrophilic loculated subgroup. This small sample size may influence the case distribution and culture-positive yield of the neutrophilic group. Further studies with a larger neutrophilic population are needed to verify our results.
In conclusion, our study highlighted the heterogeneous manifestation of TPE and specified the characteristics of the respective TPE status. The free-flowing and loculated subgroups of the neutrophilic group exhibited quite different characteristics. The neutrophilic loculated subgroup may contribute to most of the clinically significant differences when compared to the other groups. Although further validation is needed, our classification may be more helpful in improving our understanding of the heterogeneous manifestations of TPE.
Acknowledgements
This research was supported by Kyungpook National University Research Fund, 2015.
Table S1. Clinical, radiological, and laboratory differences between free-flowing and loculated effusion in the respective neutrophilic and lymphocytic TPE patients that included only the confirmed cases.
| Variable | Neutrophilic | Lymphocytic | |||||
|---|---|---|---|---|---|---|---|
| Free-flowing (n=27) | Loculated (n=12) | P value | Free-flowing (n=132) | Loculated (n=61) | P value | ||
| Demographic | |||||||
| Age, [range] years | 65 [38–77] | 50 [32–67] | 0.171 | 70 [46–79] | 62 [34–74] | 0.022 | |
| Male, n [%] | 20 [74] | 10 [83] | 0.693 | 90 [68] | 45 [74] | 0.431 | |
| Clinical, n [%] | |||||||
| Past TB history | 2 [7] | 3 [25] | 0.159 | 11 [8] | 7 [12] | 0.485 | |
| Symptoms duration >14 days | 10 [37] | 9 [75] | 0.029 | 73 [55] | 38 [62] | 0.361 | |
| Chest pain | 13 [48] | 6 [50] | 0.915 | 59 [45] | 34 [56] | 0.154 | |
| Fever | 19 [70] | 5 [42] | 0.153 | 61 [46] | 35 [57] | 0.149 | |
| Radiological, n [%] | |||||||
| Any parenchymal lesion | 20 [74] | 10 [83] | 0.693 | 104 [79] | 48 [79] | 0.987 | |
| Consolidative | 10 [37] | 3 [25] | 0.714 | 56 [42] | 23 [38] | 0.535 | |
| Nodular | 17 [63] | 10 [83] | 0.276 | 81 [61] | 44 [72] | 0.145 | |
| Cavitary | 4 [15] | 6 [50] | 0.043 | 20 [15] | 7 [12] | 0.494 | |
| Pleural fluid | |||||||
| Total cell counts, [range] cells/μL | 2,700 [842–5,600] | 163 [88–413] | <0.001 | 1675 [900–2,650] | 1,350 [588–2,350] | 0.195 | |
| Lymphocytes, [range] % | 20 [5–40] | 25 [4–40] | 0.994 | 93 [75–98] | 98 [90–100] | <0.001 | |
| pH, (range) | 7.42 (7.35–7.50)# | 7.27 (7.18–7.33)‡,§ | <0.001 | 7.44 (7.40–7.46) | 7.39 (7.31–7.43) | <0.001 | |
| Protein, (range) g/dL | 5.0 (3.6–6.0) | 4.6 (4.2–5.2) | 0.494 | 4.9 (4.4–5.5) | 5.1 (4.7–5.6) | 0.080 | |
| Glucose, [range] mg/dL | 105 [71–146]# | 74 [41–140] | 0.153 | 105 [81–127] | 86 [58–105] | <0.001 | |
| Lactate dehydrogenase, [range] U/L | 860 [422–1,572]# | 1,504 [923–4,407]‡ | 0.026 | 787 [488–1,254] | 1,263 [775–1,950] | <0.001 | |
| ADA, [range] U/L | 58 [27–83]#,* | 114 [66–151]† | 0.007 | 83 [53–106] | 98 [76–115] | 0.003 | |
| AFB smear (+) | 0/27 (0) | 0/12 (0) | 1.0 | 2/131 [2] | 0/60 (0) | 1.0 | |
| TB-PCR (+) | 1/26 [4] | 6/11 [55]†,§ | 0.001 | 11/122 [9] | 2/53 [4] | 0.349 | |
| MTB culture (+) | 3/26 [12]# | 5/12 [42] | 0.034 | 27/131 [21] | 21/58 [36] | 0.023 | |
Data are expressed as the median (IQR) or number (%). TB, tuberculosis; ADA, adenosine deaminase; AFB, acid fast bacilli; PCR, polymerase chain reaction; MTB, Mycobacterium tuberculosis. #, P<0.05, comparison between neutrophilic free-flowing and lymphocytic loculated subgroups; *, P<0.05, comparison between neutrophilic free-flowing and lymphocytic free-flowing subgroups; †, P<0.05, ‡, P<0.001, comparison between neutrophilic loculated and lymphocytic free-flowing subgroups; §, P<0.001, comparison between neutrophilic loculated and lymphocytic loculated subgroups.
Ethical Statement: The study protocols were reviewed and approved by the Institutional Review Board (IRB) of the Kyungpook National University Hospital (IRB No. 2016-01-006). Informed consent was waived by the IRB.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Peto HM, Pratt RH, Harrington TA, et al. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis 2009;49:1350-7. 10.1086/605559 [DOI] [PubMed] [Google Scholar]
- 2.Kruijshaar ME, Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999-2006. Thorax 2009;64:1090-5. 10.1136/thx.2009.118133 [DOI] [PubMed] [Google Scholar]
- 3.Porcel JM, Vives M. Etiology and pleural fluid characteristics of large and massive effusions. Chest 2003;124:978-83. 10.1378/chest.124.3.978 [DOI] [PubMed] [Google Scholar]
- 4.Maji A, Maikap MK, Jash D, et al. Role of common investigations in aetiological evaluation of exudative pleural effusions. J Clin Diagn Res 2013;7:2223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones BE, Young SM, Antoniskis D, et al. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis 1993;148:1292-7. 10.1164/ajrccm/148.5.1292 [DOI] [PubMed] [Google Scholar]
- 6.Bielsa S, Palma R, Pardina M, et al. Comparison of polymorphonuclear- and lymphocyte-rich tuberculous pleural effusions. Int J Tuberc Lung Dis 2013;17:85-9. 10.5588/ijtld.12.0236 [DOI] [PubMed] [Google Scholar]
- 7.Lin MT, Wang JY, Yu CJ, et al. Mycobacterium tuberculosis and polymorphonuclear pleural effusion: incidence and clinical pointers. Respir Med 2009;103:820-6. 10.1016/j.rmed.2008.12.023 [DOI] [PubMed] [Google Scholar]
- 8.Ruan SY, Chuang YC, Wang JY, et al. Revisiting tuberculous pleurisy: pleural fluid characteristics and diagnostic yield of mycobacterial culture in an endemic area. Thorax 2012;67:822-7. 10.1136/thoraxjnl-2011-201363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Lim JK, Lee SY, et al. Neutrophilic Loculated Tuberculous Pleural Effusion: Incidence, Characteristics and Differentiation From Complicated Parapneumonic Effusion. Am J Med Sci 2016;351:153-9. 10.1016/j.amjms.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Tuberculosis (TB). Available online: http://www.who.int/tb
- 11.Lee J, Lee SY, Yoo SS, et al. Clinical value of whole-blood interferon-gamma assay in patients with suspected pulmonary tuberculosis and AFB smear- and polymerase chain reaction-negative bronchial aspirates. Diagn Microbiol Infect Dis 2012;73:252-6. 10.1016/j.diagmicrobio.2012.03.019 [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Lee HJ, Kwon SY, et al. The prevalence of pulmonary parenchymal tuberculosis in patients with tuberculous pleuritis. Chest 2006;129:1253-8. 10.1378/chest.129.5.1253 [DOI] [PubMed] [Google Scholar]
- 13.Levine H, Szanto PB, Cugell DW. Tuberculous pleurisy. An acute illness. Arch Intern Med 1968;122:329-32. 10.1001/archinte.1968.00300090039009 [DOI] [PubMed] [Google Scholar]
- 14.Antony VB, Repine JE, Harada RN, et al. Inflammatory responses in experimental tuberculosis pleurisy. Acta Cytol 1983;27:355-61. [PubMed] [Google Scholar]
- 15.Antony VB, Sahn SA, Antony AC, et al. Bacillus Calmette-Guérin-stimulated neutrophils release chemotaxins for monocytes in rabbit pleural spaces and in vitro. J Clin Invest 1985;76:1514-21. 10.1172/JCI112131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowe DM, Redford PS, Wilkinson RJ, et al. Neutrophils in tuberculosis: friend or foe? Trends Immunol 2012;33:14-25. 10.1016/j.it.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 17.Cases Viedma E, Lorenzo Dus MJ, González-Molina A, et al. A study of loculated tuberculous pleural effusions treated with intrapleural urokinase. Respir Med 2006;100:2037-42. 10.1016/j.rmed.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 18.Cao GQ, Li L, Wang YB, et al. Treatment of free-flowing tuberculous pleurisy with intrapleural urokinase. Int J Tuberc Lung Dis 2015;19:1395-400. 10.5588/ijtld.15.0128 [DOI] [PubMed] [Google Scholar]