Abstract
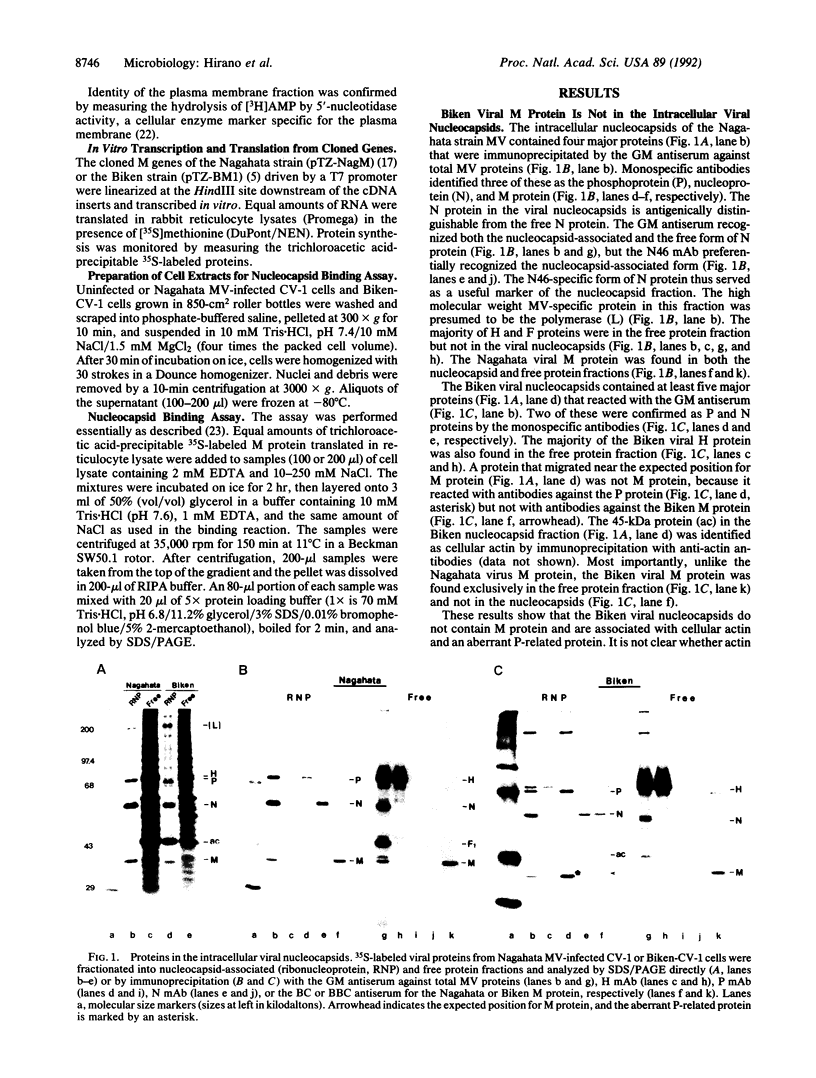
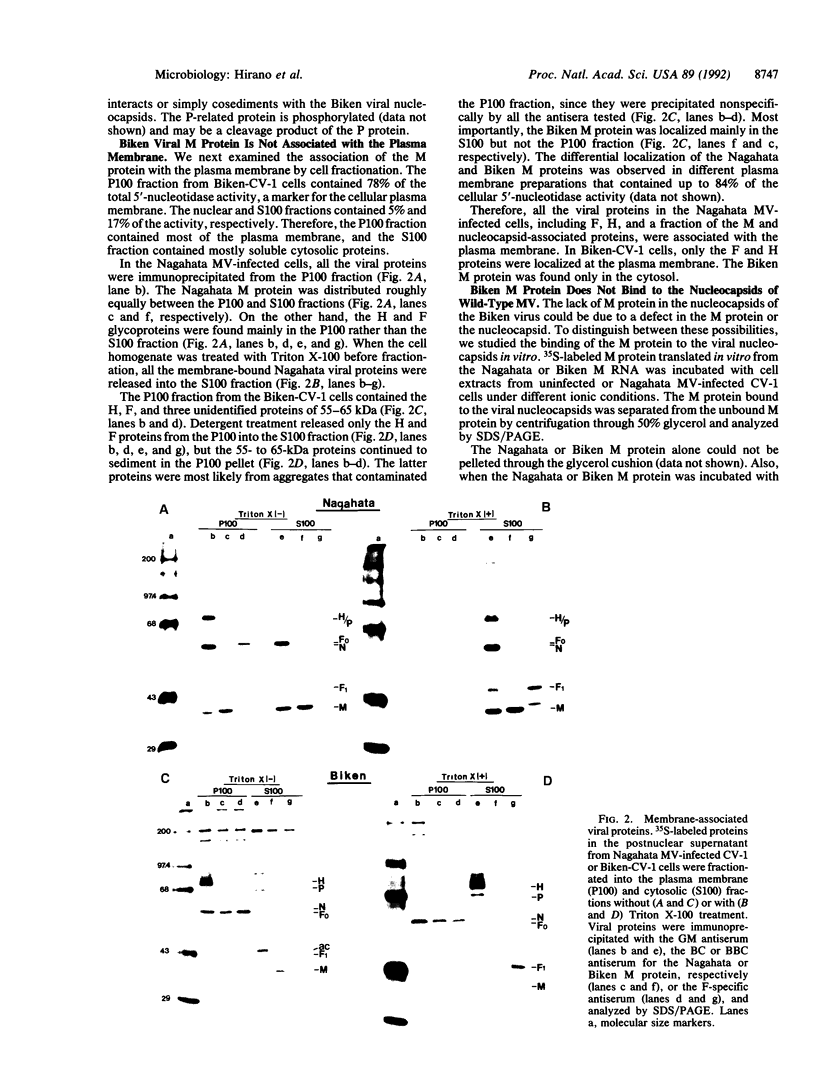
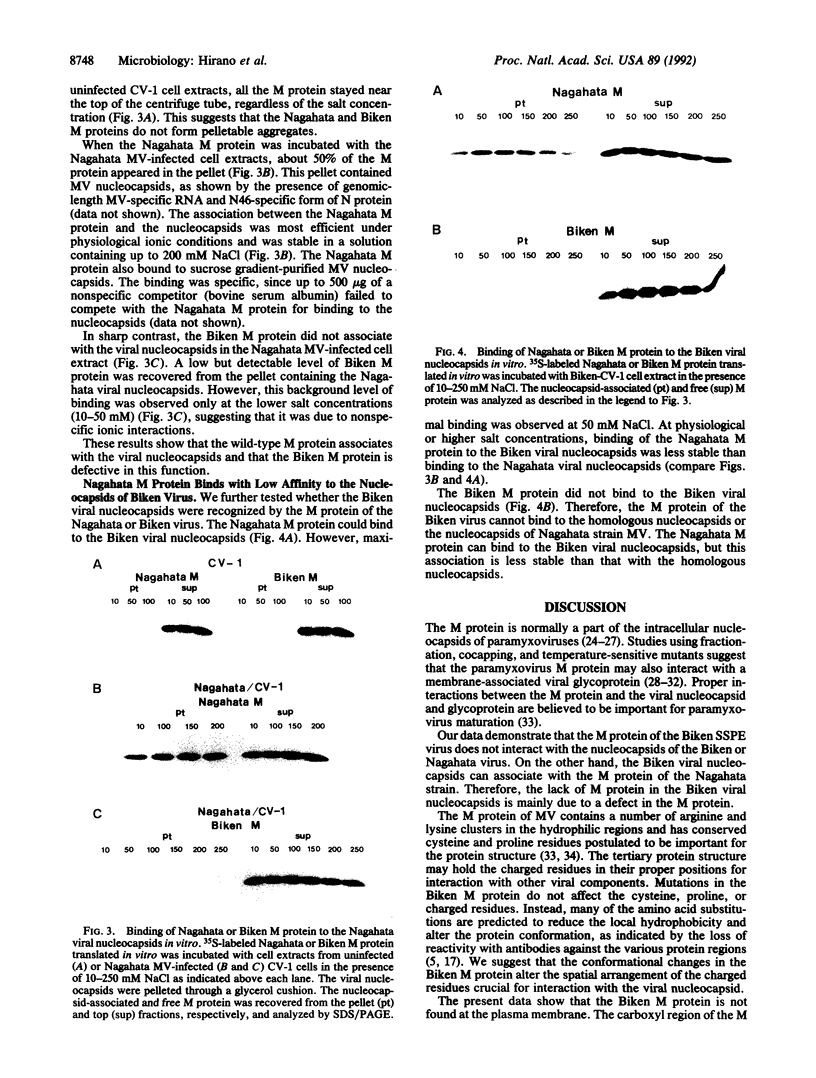
Persistence of measles virus in the brains of patients with subacute sclerosing panencephalitis (SSPE) is accompanied by changes in the viral matrix (M) protein. To understand the significance of these changes, cell culture and cell-free assays were developed to compare the functions of the M proteins of an SSPE virus Biken strain and its acute measles virus progenitor Nagahata strain. The Nagahata viral M protein is associated with the intracellular viral nucleocapsids and the plasma membrane, whereas the Biken viral M protein is localized mainly in the cytosol. The lack of M protein in the Biken viral nucleocapsids is due to a failure of the Biken M protein to bind to the viral nucleocapsids. The Biken M protein also fails to bind to the Nagahata viral nucleocapsids. Conversely, the Nagahata M protein can bind to the Biken viral nucleocapsids, although this association is not as stable at physiological salt concentration. These results offer concrete evidence that the M protein of an SSPE virus is functionally different from that of its progenitor acute measles virus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Ayata M., Hirano A., Wong T. C. Altered translation of the matrix genes in Niigata and Yamagata neurovirulent measles virus strains. Virology. 1991 Jan;180(1):166–174. doi: 10.1016/0042-6822(91)90020-c. [DOI] [PubMed] [Google Scholar]
- Ayata M., Hirano A., Wong T. C. Structural defect linked to nonrandom mutations in the matrix gene of biken strain subacute sclerosing panencephalitis virus defined by cDNA cloning and expression of chimeric genes. J Virol. 1989 Mar;63(3):1162–1173. doi: 10.1128/jvi.63.3.1162-1173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Billeter M., Cattaneo R., Budka H., ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986 Aug;59(2):472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Richardson C. D., Rozenblatt S., Lazzarini R. A. Matrix genes of measles virus and canine distemper virus: cloning, nucleotide sequences, and deduced amino acid sequences. J Virol. 1986 May;58(2):408–416. doi: 10.1128/jvi.58.2.408-416.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., ter Meulen V. Defective translation of measles virus matrix protein in a subacute sclerosing panencephalitis cell line. Nature. 1983 Sep 8;305(5930):153–155. doi: 10.1038/305153a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Rebmann G., Schmid A., Baczko K., ter Meulen V., Billeter M. A. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987 Mar;6(3):681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Billeter M. A., Sheppard R. D., Udem S. A. Multiple viral mutations rather than host factors cause defective measles virus gene expression in a subacute sclerosing panencephalitis cell line. J Virol. 1988 Apr;62(4):1388–1397. doi: 10.1128/jvi.62.4.1388-1397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Rebmann G., Baczko K., Ter Meulen V., Bellini W. J., Rozenblatt S., Billeter M. A. Accumulated measles virus mutations in a case of subacute sclerosing panencephalitis: interrupted matrix protein reading frame and transcription alteration. Virology. 1986 Oct 15;154(1):97–107. doi: 10.1016/0042-6822(86)90433-2. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Spielhofer P., Kaelin K., Baczko K., ter Meulen V., Pardowitz J., Flanagan S., Rima B. K., Udem S. A. Mutated and hypermutated genes of persistent measles viruses which caused lethal human brain diseases. Virology. 1989 Dec;173(2):415–425. doi: 10.1016/0042-6822(89)90554-0. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F., KATZ S. L., HOLLOWAY A. Development of attenuated measles-virus vaccines. A summary of recentinvestigation. Am J Dis Child. 1962 Mar;103:335–340. doi: 10.1001/archpedi.1962.02080020347030. [DOI] [PubMed] [Google Scholar]
- Giraudon P., Jacquier M. F., Wild T. F. Antigenic analysis of African measles virus field isolates: identification and localisation of one conserved and two variable epitope sites on the NP protein. Virus Res. 1988 May;10(2-3):137–152. doi: 10.1016/0168-1702(88)90011-1. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Scheid A., Choppin P. W. the relationship of conformational changes in the Sendai virus nucleocapsid to proteolytic cleavage of the NP polypeptide. Virology. 1981 Oct 30;114(2):555–562. doi: 10.1016/0042-6822(81)90235-x. [DOI] [PubMed] [Google Scholar]
- Hirano A. Subacute sclerosing panencephalitis virus dominantly interferes with replication of wild-type measles virus in a mixed infection: implication for viral persistence. J Virol. 1992 Apr;66(4):1891–1898. doi: 10.1128/jvi.66.4.1891-1898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W. Topography of phosphate residues in Sendai virus proteins. Virology. 1982 Jul 15;120(1):225–234. doi: 10.1016/0042-6822(82)90020-4. [DOI] [PubMed] [Google Scholar]
- Liebert U. G., Baczko K., Budka H., ter Meulen V. Restricted expression of measles virus proteins in brains from cases of subacute sclerosing panencephalitis. J Gen Virol. 1986 Nov;67(Pt 11):2435–2444. doi: 10.1099/0022-1317-67-11-2435. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980 Jan;33(1):152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. M., Re G. G., Kingsbury D. W. Complete sequence of the Sendai virus NP gene from a cloned insert. Virology. 1984 May;135(1):279–287. doi: 10.1016/0042-6822(84)90137-5. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Horikami S. M. Host cell proteins required for measles virus reproduction. J Gen Virol. 1990 Apr;71(Pt 4):775–783. doi: 10.1099/0022-1317-71-4-775. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Ogura H., Klenk H. Studies on the assembly of the envelope of Newcastle disease virus. Virology. 1976 Feb;69(2):523–538. doi: 10.1016/0042-6822(76)90482-7. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Yoshida T., Hamaguchi M., Iinuma M., Maeno K., Matsumoto T. Cross-linking of Newcastle disease virus (NDV) proteins. Arch Virol. 1978;58(1):15–28. doi: 10.1007/BF01315531. [DOI] [PubMed] [Google Scholar]
- Peeples M. E., Bratt M. A. Mutation in the matrix protein of Newcastle disease virus can result in decreased fusion glycoprotein incorporation into particles and decreased infectivity. J Virol. 1984 Jul;51(1):81–90. doi: 10.1128/jvi.51.1.81-90.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Murti K. G. Localization of P, NP, and M proteins on Sendai virus nucleocapsid using immunogold labeling. Virology. 1986 Apr 30;150(2):469–478. doi: 10.1016/0042-6822(86)90311-9. [DOI] [PubMed] [Google Scholar]
- Roux L., Beffy P., Portner A. Restriction of cell surface expression of Sendai virus hemagglutinin-neuraminidase glycoprotein correlates with its higher instability in persistently and standard plus defective interfering virus infected BHK-21 cells. Virology. 1984 Oct 15;138(1):118–128. doi: 10.1016/0042-6822(84)90152-1. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Koch T., Pinhasi O., Bratosin S. Infective substructures of measles virus from acutely and persistently infected cells. J Virol. 1979 Oct;32(1):329–333. doi: 10.1128/jvi.32.1.329-333.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K. W., Kingsbury D. W. Carboxyl-terminal region of Sendai virus P protein is required for binding to viral nucleocapsids. Virology. 1988 Nov;167(1):106–112. doi: 10.1016/0042-6822(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Sheppard R. D., Raine C. S., Bornstein M. B., Udem S. A. Rapid degradation restricts measles virus matrix protein expression in a subacute sclerosing panencephalitis cell line. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7913–7917. doi: 10.1073/pnas.83.20.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup K. C., Wechsler S. L., Fields B. N. Purification of measles virus and characterization of subviral components. J Virol. 1979 Apr;30(1):166–176. doi: 10.1128/jvi.30.1.166-176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Okuno Y., Hamamoto Y., Oya H. Subacute sclerosing panencephalitis (SSPE): isolation of a defective variant of measles virus from brain obtained at autopsy. Biken J. 1975 Jun;18(2):113–122. [PubMed] [Google Scholar]
- Ueda S., Takahashi M., Kurimura T., Minekawa Y., Suzuki N. Development of extremely attenuated live measles virus vaccine (CAM-EX). Biken J. 1972 Sep;15(3):173–177. [PubMed] [Google Scholar]
- Wechsler S. L., Meissner H. C. Measles and SSPE viruses: similarities and differences. Prog Med Virol. 1982;28:65–95. [PubMed] [Google Scholar]
- Wong T. C., Ayata M., Ueda S., Hirano A. Role of biased hypermutation in evolution of subacute sclerosing panencephalitis virus from progenitor acute measles virus. J Virol. 1991 May;65(5):2191–2199. doi: 10.1128/jvi.65.5.2191-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Wipf G., Hirano A. The measles virus matrix gene and gene product defined by in vitro and in vivo expression. Virology. 1987 Apr;157(2):497–508. doi: 10.1016/0042-6822(87)90292-3. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y., Maeno K., Iinuma M., Hamaguchi M., Matsumoto T., Nagayoshi S., Hoshino M. Studies on the role of M protein in virus assembly using a ts mutant of HVJ (Sendai virus). Virology. 1979 Jan 15;92(1):139–154. doi: 10.1016/0042-6822(79)90220-4. [DOI] [PubMed] [Google Scholar]