Introduction
Primary tracheobronchial neoplasms represent approximately 0.1% of all pulmonary tumors (1). They have been a topic of interest for many decades due to the challenge in their diagnosis and management. Many of tracheobronchial tumors are found incidentally. They are sometimes misdiagnosed as asthma or chronic obstructive pulmonary disease. The past few decades have seen an increase in the diagnosis of these tumors due to the rising popularity of flexible bronchoscopy and advancement in related techniques. We present a review of rare airway tumors (RATs) that have been presented in the literature as case reports or case series and discuss pertinent aspects of the different airway tumors with focus on epidemiology, pathology and genetics, clinical presentation, diagnosis, treatment and outcomes. The Cochrane Library and Medline from 1990 to 2015 was searched to identify the rare airway tumors and to provide a comprehensive review for tumors of the tracheobronchial tree that are considered rare. Following search terms were used: rare airway tumor, tracheal tumors, and rare bronchial tumors. We have focused on rare neoplasms based on incidence and atypical presentation that arise in the tracheobronchial tree from the subglottic to the segmental bronchioles. These tumors were classified based on cell type as well as metastatic potential (Table 1) (1).
Table 1. List of rare airway tumors classified based on cell type and malignant potential.
| Tissue type | Name of tumor |
|---|---|
| Salivary gland | |
| Benign | Oncocytoma |
| Mucus gland adenoma | |
| Malignant | Adenoid cystic carcinoma (ACC) |
| Mucoepidermoid carcinoma (MEC) | |
| Myoepithelial carcinoma | |
| Epithelial cells | |
| Benign | Papilloma |
| Malignant | Carcinoid tumor (CT) |
| Mesenchymal cells | |
| Benign | Leiomyoma |
| Schwannoma | |
| Hamartoma | |
| Hemangioma | |
| Lipoma | |
| Chondroma | |
| Glomus tumor (GT) | |
| Granular cell tumor (GCT) | |
| Malignant | Non-Hodgkin’s lymphoma |
| Fibrosarcoma | |
| Chondrosarcoma | |
| Others | |
| Benign | Inflammatory myofibroblastic tumor (IMT) |
| Extramedullary plasmacytoma (EMP) |
Epidemiology
It is difficult to determine the actual prevalence, geographical distribution, gender predominance, age predominance and associated risk factors of these rare tumors (1-4). Furthermore, there is no epidemiological data due to the lack of large prospective or retrospective clinical studies. Through individual review of most up-to-date case reports and reviews of each individual tumor, we present the prevalence of each tumor as percentage of primary pulmonary tumors (1-15) or as number of reported cases as well as gender predominance, age predominance and associated risk factors, Table 2 (16-28).
Table 2. Epidemiological criteria of rare airway tumors including prevalence, gender predominance, age predominance and associated risk factors.
| Rare airway tumor | Prevalence (percent or number of cases) | Gender predominance (male:female) | Age predominance (years of age) | Associated risk factors |
|---|---|---|---|---|
| Oncocytoma | 10 cases | M>F | − | Smoking |
| Mucus gland adenoma | − | F>M | − | − |
| Adenoid cystic carcinoma | 0.04–0.2% | 1:1 | 46 | − |
| Mucoepidermoid carcinoma | 0.1–0.2% | 1:1 | <30 | − |
| Myoepithelial carcinoma | 20 cases | 1:1 | − | − |
| Papilloma | 50 cases | |||
| Squamous | 6:1 | 54 | HPV | |
| Glandular | 1.2:1 | 68 | − | |
| Mixed | 5:1 | 58 | − | |
| Carcinoid tumor | 1–2% | − | <35 | − |
| Leiomyoma | 2% | M>F | 35–40 | − |
| Schwannoma | 51 cases | 2:3 | Adults | − |
| Hamartoma | 43 cases | 6.2:1 | 62 | − |
| Hemangioma | 10 cases | − | Infants | − |
| Lipoma | 0.1–0.5% | M>F | 50–60 | Smoking, obesity |
| Chondroma | 0.2% | 3:2 | 46 | − |
| Glomus tumor | 31 cases | 2:1 | 52 | − |
| Granular cell tumor | 31 cases | − | Adults | − |
| Non-Hodgkin’s Lymphoma | 0.23% | F>M | 40–60 | Smoking |
| Fibrosarcoma | 3.6% | M>F | Children and young adults | Radiation |
| Chondrosarcoma | − | 1.3:1 | 30–60 | − |
| Inflammatory myofibroblastic tumor | 0.04–0.07% | − | <16 | − |
| Solitary extramedullary plasmacytoma | 5 cases | 4:1 | 56 | − |
Pathology, genetics and immunohistochemistry
There have been very limited reports on the genetics, pathology and associations of RATs. Some of that information has been an extrapolation based on known pathogenesis of these tumors that occurs in other organs. In this paper, we discuss available information on some of the unique genetic and pathological criteria as well as the associations of some of these rare tracheobronchial tumors.
Pulmonary salivary gland-type tumors account for <1% of pulmonary tumors but are distinct group (29). Mucoepidermoid carcinoma (MEC) is the most common type (Figure 1) followed by adenoid cystic carcinoma (ACC) (29). These tumors are indistinguishable histologically from their salivary gland counterparts. They originate from the submucosal glands of the tracheobronchial tree and have structural homology to the exocrine glands. They can be distinguished by cell type as MEC is composed of mucoid and epidermoid cells that are squamous cells while ACC is composed of cribriform, tubular, or solid growth patterns with monomorphic tumor cells. A recent review on the salivary gland tumor reports the difference in term of immunohistochemistry (IHC) and molecular features between these tumors (30). Pulmonary MEC was reported to have positive immune sensitivity for p63, p40, cytokeratin (CK)7 and CK5/6 and negative IHC for CK20, thyroid transcription factor-1 (TTF-1), and napsin A (6,31). This immunohistochemical distinction is important because adenocarcinoma and adenosquamous carcinoma of the lung are difficult to differentiate from MEC by cytomorphology. On the other hand, ACC was determined to be immunoreactive to pan Cytokeratin, CD117 and proto-oncogene MYB (30). In term of molecular features, around 40% to 75% of MEC of the head and neck region have shown a characteristic association with translocation, t(11;19)(q21;p13). In one study 24 cases of pulmonary MEC showed a mastermind-like 2 (MAML2) on chromosome 11q21 rearrangement by fluorescence in situ hybridization thus demonstrating an importance of this test in diagnostic work up (31). Recent review also showed that around 50% of studied ACC demonstrate tumor-specific t(6;9)(q22-23;p23-24) translocation generating a MYB (v-myb avian viral oncogene homolog) proto-oncogene fusion with the transcription factor gene NFIB (nuclear factor IB) (30).
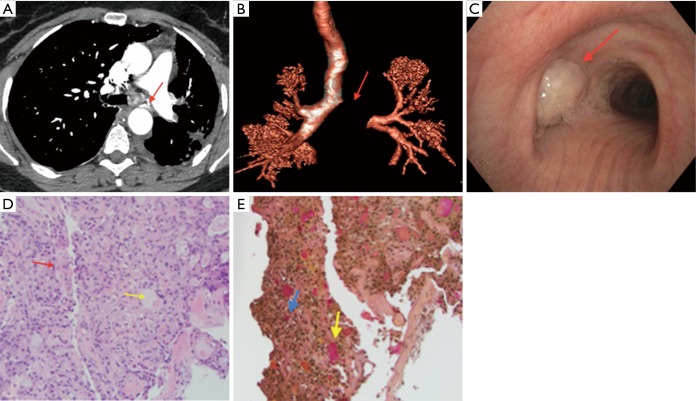
Figure 1.
Mucoepidermoid carcinoma. (A) axial chest CT image showing the complete obstruction of the left main stem bronchus. The obstructive lesion is highly vascularized with a bronchial artery branch originating from the descending aorta (arrow); (B) chest CT three-dimensional reconstruction showing the complete obstruction of the left main stem (arrow); (C) bronchoscopic image showing the complete obstruction of the left main stem by a large tumor (arrow); (D) microscopic image of biopsy showing mucoepidermoid carcinoma. The image shows neoplastic tissue composed of round to oval epithelioid cells and occasional goblet cells (red arrow) punctuated by mucin containing cystic spaces (yellow arrow). Hematoxylin and Eosin stain (200×); (E) microscopic image showing mucicarmine staining of mucoepidermoid carcinoma. The image shows intracellular (blue arrow) and extracellular mucin (yellow arrow). Mucicarmine stain (200×).
Another interesting rare malignant airway tumor is the epithelial-myopeithelial carcinoma. P53 gene and adenomatous polyposis coli (APC) gene has been implicated in the pathogenesis of epithelial-myoepithelial carcinoma (32-34). Although involvement was shown in a case report, more information is needed to show direct association. The immunohistochemical stain for Ki-67 (a cellular marker for proliferation) revealed increased mitotic activity (32).
Papilloma is another unusual tumor due to its various cell types and association with human papilloma virus (HPV) (Figure 2). Papilloma is classified into 3 categories: squamous, glandular and mixed. Squamous cell papillomas have been associated with tobacco smoking as well as HPV (8). A study by Inamura et al. reported 78% of HPV identification in squamous papilloma while none in mixed and glandular cases suggesting that sub types of papilloma’s differ in etiology (8). Katial et al. found HPV DNA in squamous papilloma (reference). The study by Shibuya et al. and Lang et al. showed that HPV serotype 6 and 11 is the most common type associated with papilloma with low risk of malignant transformation (35,36). Go et al. showed that p53 status is not a molecular marker for malignant transformation in papillomas and its expression is variable (37).
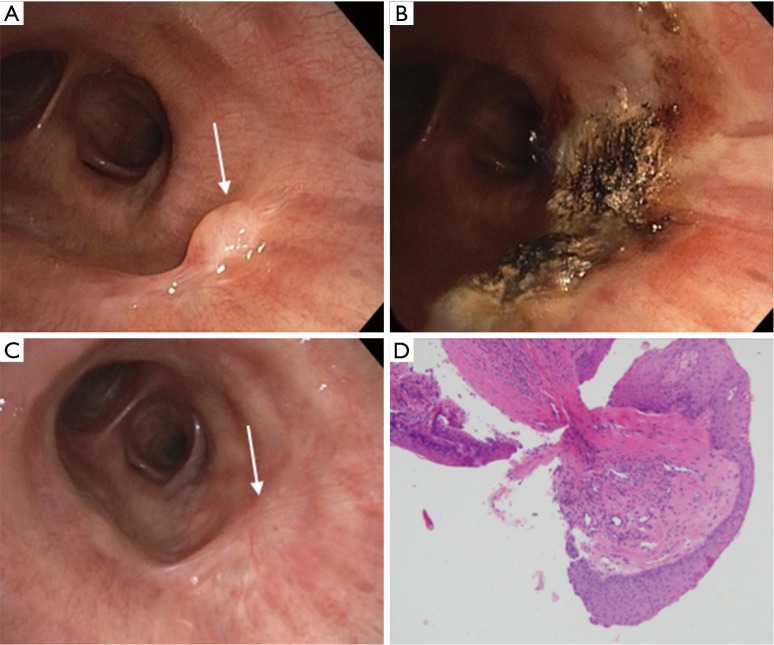
Figure 2.
Endobronchial papilloma. (A) bronchoscopic view showing a small a polypoid endobronchial lesion (arrow) in the lateral wall of the bronchus intermedius; (B) ablation using argon plasma coagulation of the endobronchial lesion in the bronchus intermedius; (C) a six-month follow up showing endobronchial minimal scaring and no evidence of recurrence; (D) microscopic image of a papilloma. The image shows tissue fragments composed of fibrovascular core lined by benign squamous epithelium. Hematoxylin and Eosin stain (100×).
The World Health Organization (WHO) have classified neuroendocrine tumors of the lung into three main entities: carcinoid tumors (typical/atypical), large cell neuroendocrine carcinomas (LCNEC), and small cell carcinomas (SCC) (9). Classification of typical and atypical carcinoid tumors, LCNEC and SCC are based on the histologic appearance, and presence of necrosis and mitoses (9). This review focused on typical carcinoid (TC) and atypical carcinoid (AC) tumors. The WHO diagnostic criteria for TC are: a tumor with neuroendocrine morphology and <2 mitoses/2 mm2 (10 high power fileds (HPF), lacking necrosis and tumor 0.5 cm or larger. AC are tumors with neuroendocrine morphology with 2 to 10 mitoses/2 mm2 and/or necrosis (often punctate). About 75–90% of bronchopulmonary carcinoids are centrally located while only 10–25% are peripheral. TCs represent 90% of pulmonary carcinoid tumors. They tend to occur in younger patients and metastasize to lymph node in 5–15% and to distant sites in 3% at the time of presentation (38). On the contrary, AC are rare (0.1–0.2%) lung tumors; however, metastasis to lymph node (40–50%) or distant sites (20%) are common at presentation. Furthermore, neuroendocrine tumors have also been associated with multiple endocrine neoplasia type I (MEN1), an autosomal dominant disorder associated with the gene locus on 11q13. In one series around 5% of patient with MEN1 were associated with pulmonary carcinoid, while higher incidence of 31% was reported in another study (39). Inactivation of the MEN1 gene by mutation is evident in TC (47%), AC (70%), LCNEC (52%), and SCLC (41%) (40-42). Onuki et al. noted that 11q13 gene mutation was more common in LCNEC (71%) and SCLC (67%). Abnormal expression or loss of heterogenosity and point mutations of the p53 locus have been detected less commonly in TC (4%) than AC (29%) (43,44). Expression of p53 protein in AC is correlated with a higher apoptotic index. An older study reports 10q and 13q sequences and allow further cytogenetic differences between AC and TC (45).
Leiomyoma is a benign tumor, which arises from smooth muscle fibers surrounding the airway or in the blood vessels. Multiple cases of leiomyoma have been reported in patients with HIV/AIDS and cellular immunity deficiency (46). Along with HIV virus, leiomyoma have been reported in immunocompromised patients with Epstein-Barr Virus (EBV) infection. The exact pathophysiology is not yet clear and many cases of leiomyoma have been reported in immunocompetent patients suggesting the possibility of multiple etiologies (47,48).
Inflammatory myofibroblastic tumors (IMT) are distinctive tumors composed of myofibroblastic spindle cell population as well as inflammatory infiltrate of plasma cells, lymphocytes and eosinophils. The etiology for this tumor is not well understood but many believe it is initiated due to an inflammatory process like trauma or surgery and even infection (49). One study suggests COX2 and VEGF, as mediators of angiogenesis, which may play a role in the pathogenesis and growth of IMT (50). In the past couple of years, IMT have got recognition as true tumors than inflammatory process due to reported cases showing presence of clonal chromosomal abnormalities of 2p23 and rearrangement of the anaplastic lymphoma kinase (ALK) receptor tyrosine-kinase gene locus or fusion of ALK with the clathrin heavy chain gene localized on 17q23 (51,52).
Diagnosis
Clinical presentation
Many of the RATs are diagnosed incidentally on computed tomogram (CT) imaging or bronchoscopy. Although they tend to have similar clinical presentation and course, RATs may present with different degree of symptomatic severity, depending on the location, size, degree of obstruction, growth rate, and other characteristics of these neoplasms (3-26). However, when symptomatic, they all share common constellation of signs and symptoms attributed to tracheobronchial obstruction (Figure 3). Most RATs present with cough and dyspnea, and sometimes with hemoptysis, wheezing and stridor. Other less common symptoms include pleuritic chest pain, hoarseness and dysphagia (1). Associated signs include hypoxia, wheezing and stridor. In some cases, RATs may be misdiagnosed as asthma or chronic obstructive pulmonary disease especially when the onset of symptoms is gradual.
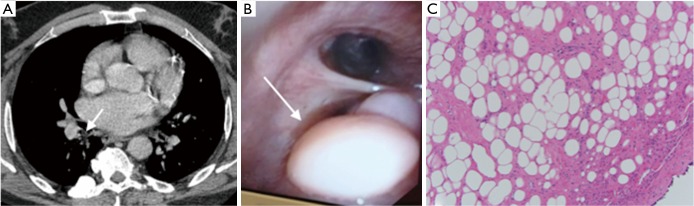
Figure 3.
Endobronchial lipoma. (A) axial chest CT showing an endobronchial abnormality in the superior segment of the right lower lobe; (B) bronchoscopic image of the large endobronchial soft polypoid lesion originating from the superior segment of the right lower lobe; (C) microscopic image of an airway lipoma. The image shows airway epithelium on the right lower corner and submucosal tissue replaced by benign adipocytes. Hematoxylin and Eosin stain (200×).
Diagnostic radiology and bronchoscopy
Conventional chest radiographs are not usually diagnostic, yet are commonly obtained as the initial radiological test. Chest CT is the most useful radiologic method to assess the airway tumors and to delineate the extraluminal extent of the tumor (5). MRI provides no clear advantage over CT in the assessment of airway tumors, except for those with salivary gland-type tumors (53). In those cases, MRI T1-weighted imaging provides better visualization of the glands hyper-intense fatty tissue and delineates the border of the tumor. Pulmonary function testing can be sometimes helpful if there is presence of obstructive pattern or abnormal flow-volume loops that have characteristic flattening as seen in dynamic extrathoracic/intrathoracic obstruction or fixed airway obstruction (1). Bronchoscopy is the diagnostic modality of choice for airway tumors as it allows for direct visualization for localization, tissue sampling and staging (5,54) The extent of the airway tumor and its relation to the surrounding structure can be evaluated using the radial probe endobronchial ultrasound (RP-EBUS) (55,56). Besides, the curvilinear EBUS can be used to evaluate the mediastinal lymph nodes for metastasis (57).
The diagnostic approach for most the rare airway tumors discussed in this review follow the above approach (1-15). There are subtle differences in the diagnostic work-up for these tumors depending on the extent of the disease. General summary of the diagnostic tool and associated findings of each tumor is presented below (Table 3) (16-26,30-33,36,38,48,58,59) .
Table 3. Radiological, endoscopic and histochemical diagnostic findings of rare airway tumors based on literature reports.
| Tumor | Radiological imaging | Bronchoscopy | Histopathology & immunohistochemical analysis |
|---|---|---|---|
| Oncocytoma | Undetectable on X-ray or CT | Solitary polypoid nodules ranging from 1.0–3.5 cm in diameter | Uniform large polygonal cells with eosinophilic cytoplasm and small round nuclei forming sheets, trabeculae, acinar structures |
| Mucus gland adenoma | Undetectable on X-ray or CT | Solitary polypoid nodules | Mucous gland adenoma cells appear as cystic mucus-filled glands on microscope |
| Adenoid cystic carcinoma | Detectable on chest X-ray and CT scan; Positive PET-CT scans (6–10% of cases) | Nodular or vascular lesions; ice-berg structure with wide base and narrow intraluminal projection of nodular or lobulated | Monomorphic uniform cells with scant cytoplasm. Three histological subtypes including tubular, cribriform and solid (most aggressive type) Tissue stains positive with, keratin, CK7, CD117S-100, and SMA |
| Mucoepidermoid | Sharply marginated, ovoid or lobulated polypoid nodules on chest X-ray described as “pneumonic consolidation” and “punctuate calcifications”; CT findings similar to those of the bronchial carcinoid tumors but less enhancement due to less vasculature | Smooth, well-circumsized glossy lesion with no visible vessels | Tissue analysis consistent with variable proportions of mucus-secreting cells, squamous cells, and so-called intermediate cells that show no particular differentiating characteristics |
| Myoepithelial carcinoma | Opaque shadow with well-circumcised borders on radiological chest X-ray and CT scan | Smooth, well-circumsized endobronchial mass with clear borders, spontaneous bleeding to touch | Glandular differentiation with a dual population of epithelial and myoepithelial cells; Tissue stains positive for p53 and c-Kit (CD117);focal atypia and increased mitotic activity can be present |
| Papilloma | Abnormal shadows or infiltrates on chest X-ray; Focal bronchiectasis is a common radiographic feature | Polypoid or pedunculated friable tan to red, glistering, no visible vessels, no spontaneous bleeding | Tissue analysis consistent with intracellular mucin which is positive for MUC5AC (expressed in goblet cells); columnar cells which are diffusely positive for CAM5.2 and CK19; CEA and CA19-9 are focally positive |
| Carcinoid tumor | Well-defined spherical or ovoid nodule with a slightly lobulated border on CT scan; Vascular enhancement on CT scanVariable uptake on FDG and PET-CT compared to other malignant tumors depending on the mitotic figures | Large fleshy polypoidal growth with well-defined narrow stalk arising from the luminal wall | Tissue stains positive for chromogranin and synaptophysin and negative for smooth-muscle actin antibodies; Intracytoplasmic dense-core granules seen under electron microscope |
| Leiomyoma | Detectable lesions on X-ray, specially lateral view; CT scan helps confirm the size and extent of the tumor | Smoothly contoured, polypoid mass pink in color with broad base, rare spontaneous bleeding | Tissue analysis consistent with pseudostratified columnar epithelium; bundles and whorls of spindle shaped cells with monomorphous fusiform nuclei and acidophilic cytoplasm; Tissue stains positive for desmin and caldesmon |
| Schwannoma | Spherical and slightly heterogeneous lesions on CT or MRI | Smooth, round, whitish color mass with no visible vessels, no spontaneous bleeding | Tissue analysis consistent with presence of a capsule, presence of compacted bipolar cells with nuclei arranged in a palisade form and/or loosely arranged spindle cells within myxoid matrix and positive staining for S-100 |
| Hamartoma | Variable findings on CT scan such as internal fat or “popcorn” calcifications; Little to no uptake on FDG PET-CT | Round, pink mass with no visible vessels, not spontaneous bleeding | Tissue analysis consistent cartilage, fat, fibrous tissue, and epithelial components |
| Hemangioma | X-ray can detect lesions depending on size and location | Polypoid lesion with visible vessels, usually appear hemorrhagic | Tissue analysis consistent with benign lobular capillary hemangioma |
| Lipoma | Enlarged hilar shadow, or atelectatic pattern on chest X-ray | Soft, poorly vascular, white, or yellowish glistening mass | Definitive diagnosis in fewer than 50% of biopsies due to existence of a fibrous, firm sheath around the tumor which may prevent adequate tissue sampling |
| Chondroma | Lobar consolidation or atelectasis on chest X-ray | Pedunculated, vascularized, pink lesions | Biopsies shows characteristic chondromatous tissue |
| Glomus tumor | Distinguished from other tumors on CT scan based on smooth borders and marked contrast enhancement due to their rich vascular supply | A mass with hyperemic, meaty or flesh solid surface on gross bronchoscopic visualization | Tissue analysis consistent with medium-sized cells with round nuclei and eosinophilic cytoplasm; arranged in sheets that form collars around capillary sized vessels; Tissue stains positive for smooth muscle actin and vimentin |
| Granular cell tumor | Detectable airway masses on CT scan | Pedunculated polypoid endobronchial lesion partially obstructing the bronchial lumen | Tissue analysis consistent with granular cells that stain positive for S-100 but negative for smooth muscle, calretinin and inhibin |
| Endobronchial T-cell lymphoma | Chest CT imaging reveal an endobronchial mass. PET avid endobronchial lesion | Obstructing bronchial lesion | Positive for CD3, CD4, and CD5 and negative for CD8 and CD20 |
| Fibrosarcoma | Well-marginated, smooth or lobular nodules or masses on X-ray or CT; They can also manifest as atelectasis or postobstructive pneumonitis | Multi-nodular mass, usually manifesting as endobronchial atelectasis or post-obstructive pneumonitis | Closely packed spindle cells in herringbone pattern |
| Chondrosarcoma | Large chest wall masses with bone destruction and soft-tissue involvement on CT; Scattered areas of calcification in the chondroid matrix detected with CT. Intermediate signal intensity on T1-weighted MR images and are heterogeneous on T2-weighted images, typically with scattered areas of high signal intensity | Polypoid endotracheal or endobronchial masses on bronchoscopy | Tissue analysis consistent with moderately hypercellular cartilaginous and binuclear cells with large nuclei and open chromatin; mitotic figures were absent |
| Inflammatory myofibroblastic tumors | Non-specific obstructive lesions on chest X-ray and CT scan | Smooth-surfaced, lobulated mass lesion with visible vessels, spontaneous bleeding | Tissue stains positive for vimentin, muscle-specific actin, SMA and ALK-1 (up to 60% cases) |
| SEPs | Solitary nodule, lobar consolidation or as diffuse pulmonary infiltrate on chest X-ray or CT scan | Smooth-surface mass 1–2 cm in size | Tissue analysis consistent with kappa chain-type and lambda chain-type tumors; Diagnosis is based on 5 criteria including single extramedullary mass of clonal plasma cells, histologically normal bone marrow, absence of anemia, normal skeletal survey and lack or decrease in serum or urinary level of monoclonal immunoglobulin |
Treatment
The treatment of airway tumors requires a multi-disciplinary approach. The interventional pulmonologist, surgeon, anesthesiologist and pathologist all play a crucial role in the management of these tumors. While the treatment of these tumors is complex, good short and long-term outcomes that are possible with a well-planned and well-executed treatment paradigm justify the effort involved.
The first step in the treatment includes a diagnostic biopsy. Easy as that seems, bronchoscopic biopsy requires astute clinical judgment and comfort level and experience with rigid bronchoscopy and multiple hemostatic techniques utilized to control airway hemorrhage that may ensue from a biopsy. The goals of bronchoscopy are:
To assess the precise location and extent of the disease along with the depth of invasion: this may require the use of regular adult bronchoscopes along with low profile, ultra-slim bronchoscopes to bypass the tumor and assess the distal airway. Assessment of a patent distal airway confers a certain degree of safety should an endoscopic destruction be considered. It also provides invaluable guidance for surgical planning, something a virtual bronchoscopy may not be able to provide;
To obtain a histologic diagnosis: the histology of the disease process guides treatment. Most pathology identified in the Table 1 is curable with complete resection. However, some pathology may be better dealt with by chemotherapy or radiation. For example, it is only in the rare situation that resection, surgical or endoscopic would be recommended for metastatic melanoma of the airway. The need for a histologic diagnosis must be balanced against the risk of bleeding. For example, if a lesion looks like a classic carcinoid in an eminently resectable location in a fit patient, it may be entirely reasonable to go for surgery without histologic diagnosis. However, as a general rule, it is useful to have histology before treatment. The risk of bleeding can be often assessed by pre-operative scanning with IV contrast (useful for identifying hemangiomas) or with the use of curvilinear or radial endobronchial ultrasound;
To render a blocked airway patent: there are two situations in which this becomes particularly important. First, in the case of a patient with metastatic disease where the stage of the disease precludes curative resection, a palliative endobronchial destruction to improve the quality of life is the optimal course. Second, in the case of a patient with a completely obstructing endobronchial tumor with distal collapse or infection, opening up the airway helps with assessment of resectability as well as to obtain time to optimize the patient for surgery. In addition, this may be necessary for anesthetic management. The classic example of this situation is a carinal tumor that near-obstructs the left mainstem bronchus. As the surgical approach would be through a right thoracotomy, intubation of the left mainstem to obtain lung isolation is critical. In this situation, the optimal approach would be to open up the left mainstem with multimodality bronchoscopic techniques, place an endotracheal tube in the left mainstem bronchus to obtain good lung isolation and then approach the resection via right thoracotomy.
Bronchoscopic destruction of endobronchial tumors can be accomplished in several ways. These include electrocautery, cryotherapy, argon plasma coagulation (APC), and laser (light amplification by stimulated emission of radiation) using Neodymium-Yttrium-Aluminum-Garnet (Nd:YAG), Neodymium-Yttrium-Aluminum-Perovskite (Nd:YAP), carbon dioxide lasers and others (60). Electrocautery involves tissue destruction using an electric current. It is best suited for small intraluminal tumors in early stages with curative intent (61). It has risk of intraluminal bleeding and perforation. APC, on the other hand, is a mode of noncontact electro-coagulation that involves forming plasma at the tip of a probe which produces coagulative necrosis in the targeted tissue (61). APC is useful in targeting lesions at sharp angles and provides excellent hemostasis. Laser therapy is useful for tumor debulking and among other used laser types, Nd:YAG is the most commonly used (61). Nd:YAG Laser has an invisible beam with wavelength that lies in the infrared region with depth up to 10 mm (compared to 1–5 mm for APC). With the exception of the carbon dioxide laser that requires rigid bronchoscopy, most other laser types can be used through the flexible bronchoscope. In contrast, cryotherapy relies on the repeated freeze-thaw cycles using extremely cold temperatures to destroy tumor tissue (61). Cryotherapy results in both physical and vascular damage of the tumor. It has very low risk of bleeding and perforation. Airway dilation using sequential balloon or rigid bronchoscopy techniques is another approach that is usually used in combination with other bronchoscopic modalities or as a palliative measure to maintain airway patency (61).
The factors that impact the successful bronchoscopic intervention are the extension of the tumor to the distally beyond the central airways, complex tumors with wider mucosal extension (more than three bronchial rings), bronchial wall invasion toward other mediastinal structures, and determining cartilage invasion with radial probe EBUS (54). Endoscopic approach is preferred over surgical approach in patients with multiple co-morbidities and high surgical risk. Other treatment such as chemotherapy, radiotherapy and anti-inflammatory drugs may be used in conjunction with surgical or endoscopic management or as palliation for patient who have tumors that are candidates for endoscopic or surgical management (3,4,6,7,9,13-23). A summary of most employed treatment modalities for rare airway tumors is provided in Table 4 (26,32,36,47,49,58-60,62-67).
Table 4. Treatment modalities for rare airway tumors.
| Tumor | Treatment modality |
|---|---|
| Oncocytoma | Surgical resection |
| Multimodality bronchoscopic resection | |
| Mucus gland adenoma | Multimodality bronchoscopic resection |
| Surgical resection | |
| Adenoid cystic carcinoma | (I) Surgical resection |
| (II) Multimodality bronchoscopic resection | |
| (III) Pneumonectomy if there is extensive bronchial involvement | |
| (IV) Radiotherapy with radical doses of 60 Gy for palliative therapy | |
| Mucoepidermoid carcinoma | Multimodality bronchoscopic resection |
| Surgical resection | |
| Myoepithelial carcinoma | Surgical resection |
| Papilloma | Multimodality bronchoscopic resection |
| Carcinoid tumor | (I) Surgical resection if tumor is localized (if metastatic there is little to offer) |
| (II) Bronchoscopic ablation | |
| Leiomyoma | (I) Surgical resection: |
| • tracheal sleeve resection | |
| • segmental tracheal resection (anastomotic failure due to dehiscence and stenosis) | |
| • carinal resection | |
| • endoscopic resection | |
| (II) Multimodality bronchoscopic resection | |
| (III) Segmentectomy or lobectomy for tumors located at the lobar bronchus or more distal locations | |
| Schwannoma | (I) Surgical resection |
| (II) Endoscopic excision for more localized tumors and patients with poor pulmonary function | |
| Hamartoma | (I) Multimodality bronchoscopic resection |
| (II) Surgical resection | |
| Hemangioma | (I) Multimodality bronchoscopic resection |
| (II) Selective bronchial artery embolization by interventional radiology | |
| (III) High-dose corticosteroids in children and young adults | |
| Lipoma | (I) Multimodality bronchoscopic resection |
| (II) Surgical resection if there is atypical features | |
| (III) Lobectomy or pneumonectomy if there is parenchymal involvement | |
| Chondroma | Endoscopic excision |
| Glomus tumor | (I) Sleeve resection with primary reconstruction of the trachea |
| (II) Multimodality bronchoscopic resection if tumor is confined to the airway lumen without extension into the wall or urgent situations to maintain airway patency | |
| Granular cell tumor | (I) Surgical resection |
| (II) Multimodality bronchoscopic resection | |
| Endobronchial T-cell lymphoma | (I) Systemic chemotherapy: pirarubicin, cyclophosphamide, vincristine, and steroids |
| (II) Surgical resection (after chemotherapy) | |
| Fibrosarcoma | Multimodality bronchoscopic resection |
| Chondrosarcoma | (I) Surgical resection |
| (II) Adjuvant chemotherapy and/or radiation therapy for extensive tumors | |
| Inflammatory myofibroblastic tumor | (I) Surgical resection (segmental tracheal resection) |
| (II) Multimodality bronchoscopic resection | |
| (III) Wide local excision along with adjuvant radiation therapy | |
| (IV) NSAIDs, steroids and ALK-inhibitors such as crizotinib in localized, unresectable tumors | |
| Solitary extramedullary plasmacytoma | (I) Argon plasma coagulation via rigid bronchoscopy for narrow-base tumors |
| (II) Surgical resection followed by radiotherapy for wide-base tumors |
Surgical treatment is aimed at obtaining negative surgical margins while sparing as much lung parenchyma as possible. In tumors distal to the carina, sleeve resection is preferred over pneumonectomy whenever feasible. In lesions at or proximal to the carina, often, complete sparing of all lung parenchyma is possible. While it is beyond the scope of this article to discuss the details of all possible combinations of resection, it is important to note that the resections should be carefully planned by an experienced team that includes the interventional pulmonologist, surgeon and anesthesiologist. All patients should be planned on being extubated on the table. The interventional pulmonologist’s involvement continues into the post-operative period in order to handle post-resection stenosis, even though this should be rare (<5%).
Outcomes
The overall survival of rare airway tumors depends on multiple factors including tumor malignant potential, patient’s co-morbidities, location, and risks of treatment modality. Benign tumors are usually localized and amendable to resection with no or minimal risk of recurrence (1). Benign tumors are usually treated with surgical resection with very low recurrence rate. Tumors that are treated with endoscopic excision have varying degrees of recurrence, but re-excision is usually feasible (1,54,62). Imaging and bronchoscopy are usually used to survey for possible recurrence (5,54). The outcome of malignant tumors is variable and depends on location, lymph node involvement and mediastinal invasion of vital organs (1,26).
Acknowledgements
None.
Footnotes
Conflicts of Interest: KH is a consultant for cook medical. The other authors have no conflicts of interest to declare.
References
- 1.Macchiarini P. Primary tracheal tumours. Lancet Oncol 2006;7:83-91. 10.1016/S1470-2045(05)70541-6 [DOI] [PubMed] [Google Scholar]
- 2.Kim TS, Lee KS, Han J, et al. Sialadenoid tumors of the respiratory tract: radiologic-pathologic correlation. AJR Am J Roentgenol 2001;177:1145-50. 10.2214/ajr.177.5.1771145 [DOI] [PubMed] [Google Scholar]
- 3.Hsu AA, Tan EH, Takano AM. Lower Respiratory Tract Adenoid Cystic Carcinoma: Its Management in the Past Decades. Clin Oncol (R Coll Radiol) 2015;27:732-40. 10.1016/j.clon.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 4.Hu MM, Hu Y, He JB, et al. Primary adenoid cystic carcinoma of the lung: Clinicopathological features, treatment and results. Oncol Lett 2015;9:1475-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park CM, Goo JM, Lee HJ, et al. Tumors in the tracheobronchial tree: CT and FDG PET features. Radiographics 2009;29:55-71. 10.1148/rg.291085126 [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Adams AL. Mucoepidermoid carcinoma of the bronchus: a review. Arch Pathol Lab Med 2007;131:1400-4. [DOI] [PubMed] [Google Scholar]
- 7.Paganin F, Prevot M, Noel JB, et al. A solitary bronchial papilloma with unusual endoscopic presentation: case study and literature review. BMC Pulm Med 2009;9:40. 10.1186/1471-2466-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inamura K, Kumasaka T, Furuta R, et al. Mixed squamous cell and glandular papilloma of the lung: a case study and literature review. Pathol Int 2011;61:252-8. 10.1111/j.1440-1827.2011.02659.x [DOI] [PubMed] [Google Scholar]
- 9.Hadda V, Madan K, Mohan A, et al. Successful flexible bronchoscopic management of dynamic central airway obstruction by a large tracheal carcinoid tumor. Case Rep Pulmonol 2014;2014:349707. [DOI] [PMC free article] [PubMed]
- 10.Park JS, Lee M, Kim HK, et al. Primary leiomyoma of the trachea, bronchus, and pulmonary parenchyma--a single-institutional experience. Eur J Cardiothorac Surg 2012;41:41-5. 10.1016/j.ejcts.2011.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swarnakar R, Sinha S. Endobronchial leiomyoma: A rare and innocent tumour of the bronchial tree. Lung India 2013;30:57-60. 10.4103/0970-2113.106175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumoulin E, Gui X, Stather DR, et al. Endobronchial schwannoma. J Bronchology Interv Pulmonol 2012;19:75-7. 10.1097/LBR.0b013e318241e5aa [DOI] [PubMed] [Google Scholar]
- 13.Sim JK, Choi JH, Oh JY, et al. Two Cases of Diagnosis and Removal of Endobronchial Hamartoma by Cryotherapy via Flexible Bronchoscopy. Tuberc Respir Dis (Seoul) 2014;76:141-5. 10.4046/trd.2014.76.3.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings S, Tharion J, Jones P, et al. Bronchial haemangioma: exceptionally rare cause of haemoptysis. Heart Lung Circ 2013;22:1030-2. 10.1016/j.hlc.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Triviño A, Mora-Cabezas M, Vallejo-Benitez A, et al. Endobronchial lipoma: a rare cause of bronchial occlusion. Arch Bronconeumol 2013;49:494-6. [Article in English, Spanish]. 10.1016/j.arbr.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Bussy S, Labarca G, Descalzi F, et al. Endobronchial chondromas. Respir Care 2014;59:e193-6. 10.4187/respcare.02673 [DOI] [PubMed] [Google Scholar]
- 17.Choi IH, Song DH, Kim J, et al. Two cases of glomus tumor arising in large airway: well organized radiologic, macroscopic and microscopic findings. Tuberc Respir Dis (Seoul) 2014;76:34-7. 10.4046/trd.2014.76.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakr L, Palaniappan R, Payan MJ, et al. Tracheal glomus tumor: a multidisciplinary approach to management. Respir Care 2011;56:342-6. 10.4187/respcare.00761 [DOI] [PubMed] [Google Scholar]
- 19.Jivan V, Litwin D, Kaini N. Submucosal Granular Cell Tumor-A Rare Case Of Lower Airway Involvement. Am J Respir Crit Care Med. 2013;187:A6136. [Google Scholar]
- 20.Matsumoto S, Fujimoto N, Fuchimoto Y, et al. Endobronchial T-cell lymphoma in a patient with chronic pyothorax. Respirol Case Rep 2015;3:44-7. 10.1002/rcr2.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding J, Chen Z, Shi M. Tracheal stenting for primary tracheal mucosa-associated lymphoid tissue lymphoma. Eur J Med Res 2013;18:8. 10.1186/2047-783X-18-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomonov A, Zuckerman T, Goralnik L, et al. Non-Hodgkin's lymphoma presenting as an endobronchial tumor: report of eight cases and literature review. Am J Hematol 2008;83:416-9. 10.1002/ajh.21112 [DOI] [PubMed] [Google Scholar]
- 23.Gladish GW, Sabloff BM, Munden RF, et al. Primary thoracic sarcomas. Radiographics 2002;22:621-37. 10.1148/radiographics.22.3.g02ma17621 [DOI] [PubMed] [Google Scholar]
- 24.Massey C, Laver N, Bedi H, et al. Primary fibrosarcoma of the trachea presenting with acute airway loss. Am J Otolaryngol 2015;36:287-9. 10.1016/j.amjoto.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 25.Özgül MA, Toru Ü, Acat M, et al. A rare tumor of trachea: Inflammatory myofibroblastic tumor diagnosis and endoscopic treatment. Respir Med Case Rep 2014;13:57-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo Park C, Kim W, Jae Oh I, et al. Solitary extramedullary plasmacytoma presenting as an endobronchial mass. Intern Med 2013;52:2113-6. 10.2169/internalmedicine.52.0572 [DOI] [PubMed] [Google Scholar]
- 27.Yang FF, Gao R, Miao Y, et al. Primary tracheobronchial non-Hodgkin lymphoma causing life-threatening airway obstruction: a case report. J Thorac Dis 2015;7:E667-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joung MK, Lee YJ, Chung CU, et al. A case of granular cell tumor of the trachea. Korean J Intern Med 2007;22:101-5. 10.3904/kjim.2007.22.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer 2007;110:2253-9. 10.1002/cncr.23048 [DOI] [PubMed] [Google Scholar]
- 30.Falk N, Weissferdt A, Kalhor N, et al. Primary Pulmonary Salivary Gland-type Tumors: A Review and Update. Adv Anat Pathol 2016;23:13-23. 10.1097/PAP.0000000000000099 [DOI] [PubMed] [Google Scholar]
- 31.Roden AC, Garcia JJ, Wehrs RN, et al. Histopathologic, immunophenotypic and cytogenetic features of pulmonary mucoepidermoid carcinoma. Mod Pathol 2014;27:1479-88. 10.1038/modpathol.2014.72 [DOI] [PubMed] [Google Scholar]
- 32.Ru K, Srivastava A, Tischler AS. Bronchial epithelial-myoepithelial carcinoma. Arch Pathol Lab Med 2004;128:92-4. [DOI] [PubMed] [Google Scholar]
- 33.Daa T, Kashima K, Gamachi A, et al. Epithelial-myoepithelial carcinoma harboring p53 mutation. APMIS 2001;109:316-20. 10.1034/j.1600-0463.2001.d01-126.x [DOI] [PubMed] [Google Scholar]
- 34.Young J, Barker M, Robertson T, et al. A case of myoepithelial carcinoma displaying biallelic inactivation of the tumour suppressor gene APC in a patient with familial adenomatous polyposis. J Clin Pathol 2002;55:230-1. 10.1136/jcp.55.3.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibuya H, Kutomi T, Kujime K, et al. An adult case of multiple squamous papillomas of the trachea associated with human papilloma virus type 6. Intern Med 2008;47:1535-8. 10.2169/internalmedicine.47.1239 [DOI] [PubMed] [Google Scholar]
- 36.Lang TU, Khalbuss WE, Monaco SE, et al. Solitary Tracheobronchial Papilloma: Cytomorphology and ancillary studies with histologic correlation. Cytojournal 2011;8:6. 10.4103/1742-6413.77286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Go C, Schwartz MR, Donovan DT. Molecular transformation of recurrent respiratory papillomatosis: viral typing and p53 overexpression. Ann Otol Rhinol Laryngol 2003;112:298-302. 10.1177/000348940311200402 [DOI] [PubMed] [Google Scholar]
- 38.Davila DG, Dunn WF, Tazelaar HD, et al. Bronchial carcinoid tumors. Mayo Clin Proc 1993;68:795-803. 10.1016/S0025-6196(12)60641-7 [DOI] [PubMed] [Google Scholar]
- 39.Sachithanandan N, Harle RA, Burgess JR. Bronchopulmonary carcinoid in multiple endocrine neoplasia type 1. Cancer 2005;103:509-15. 10.1002/cncr.20825 [DOI] [PubMed] [Google Scholar]
- 40.Zhao J, de Krijger RR, Meier D, et al. Genomic alterations in well-differentiated gastrointestinal and bronchial neuroendocrine tumors (carcinoids): marked differences indicating diversity in molecular pathogenesis. Am J Pathol 2000;157:1431-8. 10.1016/S0002-9440(10)64780-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debelenko LV, Swalwell JI, Kelley MJ, et al. MEN1 gene mutation analysis of high-grade neuroendocrine lung carcinoma. Genes Chromosomes Cancer 2000;28:58-65. [DOI] [PubMed] [Google Scholar]
- 42.Debelenko LV, Brambilla E, Agarwal SK, et al. Identification of MEN1 gene mutations in sporadic carcinoid tumors of the lung. Hum Mol Genet 1997;6:2285-90. 10.1093/hmg/6.13.2285 [DOI] [PubMed] [Google Scholar]
- 43.Onuki N, Wistuba II, Travis WD, et al. Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer 1999;85:600-7. [DOI] [PubMed] [Google Scholar]
- 44.Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21. 10.1002/cncr.23542 [DOI] [PubMed] [Google Scholar]
- 45.Laitinen KL, Soini Y, Mattila J, et al. Atypical bronchopulmonary carcinoids show a tendency toward increased apoptotic and proliferative activity. Cancer 2000;88:1590-8. [DOI] [PubMed] [Google Scholar]
- 46.Metta H, Corti M, Redini L, et al. Endobronchial leiomyoma: an unusual non-defining neoplasm in a patient with AIDS. Rev Inst Med Trop Sao Paulo 2009;51:53-5. 10.1590/S0036-46652009000100010 [DOI] [PubMed] [Google Scholar]
- 47.Cárdenas-García J, Lee-Chang A, Chung V, et al. Bronchial leiomyoma, a case report and review of literature. Respir Med Case Rep 2014;12:59-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon YS, Kim H, Koh WJ, et al. Clinical characteristics and efficacy of bronchoscopic intervention for tracheobronchial leiomyoma. Respirology 2008;13:908-12. 10.1111/j.1440-1843.2008.01366.x [DOI] [PubMed] [Google Scholar]
- 49.Matsubara O, Tan-Liu NS, Kenney RM, et al. Inflammatory pseudotumors of the lung: progression from organizing pneumonia to fibrous histiocytoma or to plasma cell granuloma in 32 cases. Hum Pathol 1988;19:807-14. 10.1016/S0046-8177(88)80264-8 [DOI] [PubMed] [Google Scholar]
- 50.Applebaum H, Kieran MW, Cripe TP, et al. The rationale for nonsteroidal anti-inflammatory drug therapy for inflammatory myofibroblastic tumors: a Children's Oncology Group study. J Pediatr Surg 2005;40:999-1003; discussion 1003. 10.1016/j.jpedsurg.2005.03.016 [DOI] [PubMed] [Google Scholar]
- 51.Snyder CS, Dell'Aquila M, Haghighi P, et al. Clonal changes in inflammatory pseudotumor of the lung: a case report. Cancer 1995;76:1545-9. [DOI] [PubMed] [Google Scholar]
- 52.Bridge JA, Kanamori M, Ma Z, et al. Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol 2001;159:411-5. 10.1016/S0002-9440(10)61711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yousem DM, Kraut MA, Chalian AA. Major salivary gland imaging. Radiology 2000;216:19-29. 10.1148/radiology.216.1.r00jl4519 [DOI] [PubMed] [Google Scholar]
- 54.Gao H, Ding X, Wei D, Cheng P, Su X, Liu H, et al. Endoscopic management of benign tracheobronchial tumors. J Thorac Dis 2011;3:255-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lund ME, Garland R, Ernst A. Airway stenting: Applications and practice management considerations. Chest 2007;131:579-87. 10.1378/chest.06-0766 [DOI] [PubMed] [Google Scholar]
- 56.Haas AR, Vachani A, Sterman DH. Advances in diagnostic bronchoscopy. Am J Respir Crit Care Med 2010;182:589-97. 10.1164/rccm.201002-0186CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varela-Lema L, Fernandez-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156-64. 10.1183/09031936.00097908 [DOI] [PubMed] [Google Scholar]
- 58.Umezu H, Tamura M, Kobayashi S, Sawabata N, Honma K, Miyoshi S. Tracheal chondrosarcoma. Gen Thorac Cardiovasc Surg 2008;56:199-202. 10.1007/s11748-007-0218-3 [DOI] [PubMed] [Google Scholar]
- 59.Pecoraro Y, Diso D, Anile M, Russo E, Patella M, Venuta F. Primary inflammatory myofibroblastic tumor of the trachea. Respirol Case Rep 2014;2:147-9. 10.1002/rcr2.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim TS, Lee KS, Han J, Im JG, Seo JB, Kim JS, et al. Mucoepidermoid carcinoma of the tracheobronchial tree: radiographic and CT findings in 12 patients. Radiology 1999;212:643-8. 10.1148/radiology.212.3.r99se09643 [DOI] [PubMed] [Google Scholar]
- 61.Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. 10.1164/rccm.200210-1181SO [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Zhang J, Zhang N, Li D, Zou H, Zhou Y, et al. Bronchoscopic intervention as a main treatment for tracheobronchial adenoid cystic carcinoma. Minim Invasive Ther Allied Technol 2015;24:167-74. 10.3109/13645706.2014.977298 [DOI] [PubMed] [Google Scholar]
- 63.Attakkil A, Thorawade V, Jagade M, et al. Tracheal Leiomyoma: A Rare Entity & Role of Diode Laser in Its Management. IJOHNS 2014;3:143-8. 10.4236/ijohns.2014.34027 [DOI] [Google Scholar]
- 64.Murata T, Shino M, Yasuoka Y, Chikamatsu K. Subglottic Schwannoma: a report of a rare case that was treated with medial thyrotomy. Am J Otolaryngol 2013;34:569-73. 10.1016/j.amjoto.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 65.Ge X, Han F, Guan W, Sun J, Guo X. Optimal treatment for primary benign intratracheal schwannoma: A case report and review of the literature. Oncol Lett 2015;10:2273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu HH, Jao YT, Wu MH. Glomus tumor of the trachea managed by spiral tracheoplasty. Am J Case Rep 2014;15:459-65. 10.12659/AJCR.891191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghani S, Desai A, Pokharel S, Demmy T, Dy GK. Pneumonectomy-Sparing NSAID Therapy for Pulmonary Inflammatory Myofibroblastic Tumor. J Thorac Oncol 2015;10:e89-90. 10.1097/JTO.0000000000000574 [DOI] [PubMed] [Google Scholar]