Abstract
Background
The precise head to head relationships between Cardio-pulmonary exercise testing (CPET) parameters and patients’ daily symptoms/activities and the disease social/emotional impact are less well defined. In this study, the correlation of COPD daily symptoms and quality of life [assessed by St. George’s Respiratory Questionnaire (SGRQ)] and COPD severity index (BODE-index) with CPET parameters were investigated.
Methods
Symptom-limited CPET was performed in 37 consecutive COPD (GOLD I-III) subjects during non-exacerbation phase. The SGRQ was also completed by each patient.
Results
SGRQ-score correlated negatively with FEV1 (r=−0.49, P<0.01), predicted maximal work-rate (%WR-max) (r=−0.44, P<0.01), V’O2/WR (r=−0.52, P<0.01) and breathing reserve (r=−0.50, P<0.01). However it did not correlate with Peak-V’O2% predicted (r=−0.27, P=0.10). In 20 (54.1%) subjects in which leg fatigue was the main cause for stopping the test, Peak-V’O2, %WR-max, HR-Reserve and Breathing reserve were higher (P=0.04, <0.01, 0.04 and <0.01 respectively) than the others. There was also a significant correlation between BODE-index and ∆VO2/∆WR (r=−0.64, P<0.001) and breathing-reserve (r=−0.38, P=0.018).
Conclusions
The observed relationships between CPET parameter and daily subjective complaints in COPD were not strong. Those who discontinued the CPET because of leg fatigue were in the earlier stages of COPD. Significant negative correlation between ∆VO2/∆WR and BODE-index suggests that along with COPD progression, regardless of negative past history, other comorbidities such as cardiac/musculoskeletal problems should be sought.
Keywords: Chronic obstructive pulmonary disease (COPD), exercise capacity, ventilatory efficiency, quality of life, cardio-pulmonary exercise testing (CPET)
Introduction
It has been estimated that the burden of chronic obstructive pulmonary disease (COPD) is increasing and it is likely to be the third global leading cause of death by 2030 (1,2). In addition to impact on survival, it is also well recognized that along with increasing severity, COPD has progressive adverse effects on daily symptoms, functional capacity, and health-related quality of life (3,4). Considering these unquestionable aspects of COPD, the patient reported daily symptoms and function status in assessment of the disease has been included in the new Global Initiative on Obstructive Lung Disease (GOLD) classification (5). Although more difficult to use than other tools such as COPD Assessment Test (CAT) or COPD Clinical Questionnaire (CCQ) during routine daily practice (6), the St. George’s Respiratory Questionnaire (SGRQ) is one of the most widely used self-complete measures in research for assessing patients’ symptoms, activities and quality of life (7,8). Along with this subjective measurement, functional capacity in COPD could be measured by objective tools such as cardio-pulmonary exercise testing (CPET) or 6-min walk distance (6MWD) (9-11). Considering the fact that at rest physiologic variable such as pulmonary diffusing capacity for carbon monoxide (DLCO), body mass index (BMI) or even forced expiratory volume in one second (FEV1) could not solely predict exercise intolerance, CPET has been proposed as the gold standard for evaluating the exercise intolerance in patients with pulmonary diseases, including COPD (12). But the precise head to head comparisons of CPET variable with patients’ daily symptoms/activities and the disease social/emotional impact are less well defined. In one study among 129 COPD subjects with GOLD stage II and III, there was at best a weak correlation between Peak-V’O2 (maximum oxygen uptake) % predicted and some sub-domains of health status (13). While in another study Peak-V’O2 was to some extent a predictor of physical function (r2 increased by 0.109) and health-related quality-of-life (r2 increased by 0.044) (14).
In this study, the correlation of COPD daily symptoms and quality of life (assessed by SGRQ) and COPD severity index (BODE index) with CPET parameters were investigated.
Methods
Study subjects
From May to November 2014, during a 7-month period, consecutive COPD subjects who presented to outpatient clinic at a referral teaching hospital of Tehran University of Medical Sciences entered the study. The subjects included the study according to the following criteria: (I) clinical features of COPD and associated spirometry compatible with the GOLD criteria (FEV1/FVC <0.7 in non-exacerbation phase) (5) and FEV1 ≥30% of predicted with no concomitant evidence of Cor-pulmonale; (II) no clinical feature of asthma and/or evidence of bronchodilator responsiveness on spirometry; (III) stable COPD with no recent history of exacerbation requiring hospitalization during the last 3 months; (IV) lack of medical records or clinical examinations compatible with coronary artery disease (e.g., coronary angiography with stenting, coronary artery bypass grafting, myocardial infarction, CCU admission, etc.) or congestive heart failure; (V) no neuro-muscular, orthopaedic and/or medical diseases precluding from performing exercise testing; (VI) no cardio-pulmonary rehabilitation program within the last year. This study was approved by the Ethics Committee of Tehran University of Medical Sciences and written informed consent was obtained from each patient.
Quality of life measurement
St. George’s Respiratory Questionnaire (SGRQ) is a well-known and validated self-administered tool for measuring quality of life and well-being in patients with chronic respiratory diseases, particularly in those with COPD (7). This questionnaire consists of a total of 50 items, categorized to two separate parts: Part I: measures frequency of recent respiratory symptoms; Part II: addresses the limitation to daily activity and social/emotional impact of disease. Each item ranges from 0 (the least possible weight of disease) to 100 (the most distressing condition). Consequently, higher scores of questionnaire point to more limitations. After obtaining written informed consent and before performing CPET, in a comfortable and quiet room, the Persian version (15) of SGRQ was completed by each patient. Then total and each subcategory score (Symptoms, Activity and Impact) were calculated for analysis.
CPET
CPET was performed on a magnetically braked cycle ergometer (Ergoselect 100 p, Ergoline GmbH, Bitz, Germany) based on the recommendations of the American Thoracic Society/American College of Chest Physicians’ statement on Cardiopulmonary Exercise Testing (16). At the beginning of the test, the subjects asked for 3 minutes of unloaded cycling. Subsequently, a symptom limited, incremental exercise testing was performed at an incremental work rate of >10 W·min−1. Minute ventilation (V’E), oxygen uptake (V’O2) and rate of carbon dioxide production (V’CO2) were obtained during exercise through a face mask with less than 30 cc dead space (V2™ Oro-Nasal Mask, Hans Rudolph Inc., MO, USA). These parameters were measured via a breath-by-breath respiratory gas analysis system (Power Cube-Ergo, Ganshorn Medizin Electronic GmbH, Niederlauer, Germany). While CPET was being performed, each patient was monitored by continuous 12-lead ECG-telemetry (AT-104, Schiller, Baar, Switzerland). Arterial oxygen saturation was measured by pulse oximetry. Patient’s symptoms, especially dyspnea (using modified Borg dyspnea scale), pulse rate and blood pressure were also monitored closely during the test by a specialist.
Six-minute-walk test (6MWT)
6MWT was done according to American Thoracic Society (ATS) guideline for the 6MWT (17). This test was performed at a calm and flat indoor hallway with hard surfaced pavement. The hallway was more than 30 meter long with marked turning around point. O2 saturation and distance (6MWD) were measured every one minute till 6 minutes during patient walking on a flat place.
BODE index
The BODE index (Body mass index, airflow Obstruction, Dyspnea scale and Exercise) is multi-factorial scoring system that has been shown to have important prognostic value in patients with COPD (18). Before performing exercise test and for calculating BODE index, BMI, spirometry and dyspnea scale (by modified-MRC questionnaire) were obtained from each patient. In a session separated from CPET, 6-minute-walk test was performed according to ATS guideline for the 6MWT (see above). Them the final sum of points were calculated for these four variables: BMI (>21 kg/m2=0 point, ≤21 kg/m2=1 point), FEV1%pred (≥65% =0 point, 50–64% =1 point, 36–49% =2 point, and ≤35%=3 point), MMRC scale (0-1 =0 point, 2 =1 point, 3 =2 point and 4 =3 point) and 6MWD (≥350 m=0 point, 250–349 m=1 point, 150–249 m =2 point and <149 m =3 point). The results of BODE index were compared with clinical/CPET parameters.
Statistical analysis
Data analysis was performed by SPSS 18.0 for windows (SPSS Inc., Chicago, IL, USA). In all statistical analysis, a P value less than 0.05 and 95% confidence interval were considered significant. Pearson’s R test was used for analyzing the correlation between the continuous variables. Independent two-sample t-test was used for comparing continuous variables in those with or without leg fatigue if variables were normally distributed. Mann-Whitney test was used if distribution of the difference was skewed. Chi-square test was used for categorical variables.
Results
Finally 37 of 41 subjects completed the study. Four who did not fulfill the CPET protocol were excluded from the study. Baseline and CPET characteristics of subjects are shown in Table 1 and Table 2 respectively. The main cause for stopping the cycle exercise was leg fatigue in 20 (54.1%), dyspnea/desaturation in 14 (37.8%), rise in blood pressure in 2 (5.4%) and chest pain in 1 (2.7%) patient. Total SGRQ-score correlated negatively with FEV1 (−0.49, P=0.002), percent of predicted maximal work-rate (%WR) (r=−0.44, P=0.007), breathing reserve (r=−0.50, P=0.002), heart rate-reserve (r=−0.45, P=0.007). It also correlated positively with V’E/V’CO2 slope (r=0.45, P=0.017) and frequency of admission (r=0.44 P=0.006). However, it did not correlate with age (r=0.11, P=0.505), BMI (r=−0.20, P=0.241), Peak-V’O2% predicted (r=−0.27, P=0.103) or O2-pulse (r=−0.08, P=0.625). A separate analysis among patients with different subgroup based on GOLD classification by spirometry, also didn’t revealed a significant correlation between Peak-V’O2 and SGRQ-total score (P value=0.223 and 0.258 for GOLD I-II and GOLD III respectively).
Table 1. Basal characteristics of the patients.
| Baseline characteristics | Values |
|---|---|
| Age (year) | 56.9±9.9 |
| Sex (male/female) | 31/6 |
| BMI (kg/m2) | 26.2±4.8 |
| Smoking (pack. year) | 19.9±26.5 |
| FEV1 (% predicted) | 65.1±22.4 |
| GOLD stage | |
| Stage I (%) | 12 (32.4) |
| Stage II (%) | 15 (40.5) |
| Stage III (%) | 10 (27.0) |
| Stage IV (%) | 0 (0.0) |
| 6MWD (m) | 434.1±105.2 |
| Exacerbation with hospital admission in last year | |
| <2 | 31 |
| ≥2 | 6 |
| SGRQ-symptoms (score) | 53.0±21.1 |
| SGRQ-activity (score) | 55.6±26.1 |
| SGRQ-impact (score) | 35.7±24.5 |
| SGRQ-total | 44.5±22.1 |
Values are presented as means ± SD. BMI, body mass index; FEV1, forced expiratory volume in 1st second; SGRQ, St. George’s respiratory questionnaire; 6MWD, 6-minute walk distance.
Table 2. Basal cardiopulmonary exercise testing characteristics of patients.
| Cardio pulmonary exercise testing variables | Values |
|---|---|
| W-max (% predicted) | 55.4±20.4 |
| Peak-V’O2 (liter/min) | 1.1±0.4 |
| Peak-V’O2 (% predicted) | 53.0±18.6 |
| V’E (liter) | 39.4±15.0 |
| V’E (% MVV) | 30.90±25.1 |
| ∆V’O2/∆WR (mL·W-1·min-1) | 9.3±2.0 |
| V’E/ V’CO2 slope | 34.1±5.1 |
| O2 pulse (mL/beat) | 11.5±12.6 |
| HR-reserve | 31.4±24.1 |
| VD/VT (at rest) | 0.33±0.06 |
| VD/VT (at max load) | 0.30±0.07 |
W-max, Maximal workrate; Peak-V’O2, maximum oxygen uptake; V’E, minute ventilation; MVV, maximal voluntary ventilation; V’CO2, carbon dioxide production rate; HR-Reserve, heart rate reserve; BR, breathing reserve; WR, work rate; VD, dead space; VT, tidal volume.
Increase in V’O2 to work rate increment (∆VO2/∆WR) (mean=9.3±2.0 mL·W−1·min−1) was inversely related to the BODE index (r=−0.64, P<0.001) (Figure 1). There was also a significant correlation between BODE index and SGRQ-score (r=0.56, P<0.001), and negative correlation with CPET parameters: peak-V O2 (r=−0.56, P<0.001), WR (r=−0.55, P<0.001), breathing-reserve (r=−0.38, P=0.018) and HR-reserve (r=−0.45, P=0.006).
Figure 1.
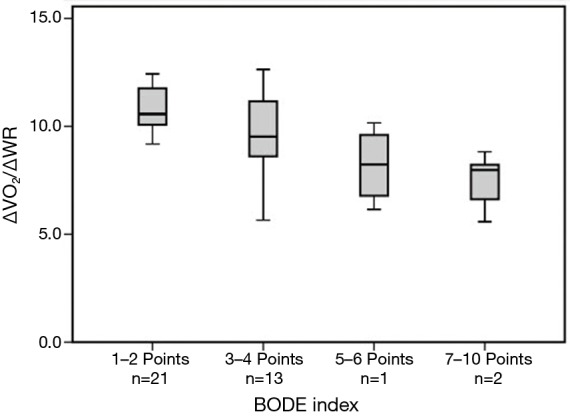
Values of ∆V’O2/∆WR in relation to chronic obstructive pulmonary disease severity index (BODE-index) scores. ∆V’O2, Increments in Oxygen uptake; ∆WR, Increments in work rate.
Before performing the test the BORG scale of dyspnea were described for the patients. The patient experienced 10/10 BORG scale were categorized as “non-leg fatigue” group and those who experienced significant leg fatigue without scoring high dyspnea scale classified as ‘leg fatigue” group. The Table 3 shows a comparison between those in which the leg fatigue caused the patient to discontinue the test with those in which other causes (non-leg fatigue) led to termination. The leg fatigue group had significantly higher FEV1 (P=0.017) and lower total-SGRQ-score (P=0.001) than non-leg fatigue group. Peak-V O2, predicted maximal WR and HR-Reserve and Breathing reserve were also higher in leg fatigue group (P=0.042, 0.005, 0.042 and 0.001 respectively). In final interpretation of CPET, the pulmonary arterial disease and cardiac disease were diagnosed in 2 and 3 subjects respectively.
Table 3. Demographic and Cardio-pulmonary exercise testing variables, between patients with and without leg fatigue at the end of the test.
| Characteristics | Leg fatigue cause (n=20) | Non-leg fatigue causes (n=17) | P value |
|---|---|---|---|
| Age | 54.5±8.8 | 59.7±10.6 | 0.114 |
| BMI | 26.7±5.2 | 25.5±4.3 | 0.446 |
| Hospital admission in last year | 0.6±1.1 | 1.0±1.3 | 0.359 |
| SGRQ-score | |||
| Total | 33.86±17.8 | 57.1±20.3 | 0.001* |
| Symptoms | 47.3±19.6 | 59.5±21.5 | 0.085 |
| Activity | 42.9±22.5 | 70.4±22.3 | 0.001* |
| Impact | 24.5±17.0 | 48.8±25.8 | 0.002* |
| FEV1 (%predicted) | 76.0±22.0 | 55.7±19.6 | 0.017* |
| 6MWD (m) | 450.5±90. 3 | 413.3±121.6 | 0.313 |
| W-max (%predicted) | 63.9±16.8 | 45. 5±20.1 | 0.005* |
| Peak-V’O2 (liter/min) | 1.2±0.3 | 0.9±0.4 | 0.042* |
| V’E (liter) | 42.6±14.7 | 33.3±16.5 | 0.081 |
| V’E (%MVV) | 43.06±25.6 | 16.6±15.4 | 0.001* |
| V’E/ V’CO2 slope | 33.6±5.4 | 35.1±4.5 | 0.465 |
| ∆V’O2/∆WR(mL·W−1·min−1) | 9.5±1.8 | 8.8±2.5 | 0.454 |
| O2 pulse (mL/beat) | 9.3±2.5 | 14.2±18.7 | 0.311 |
| HR-reserve | 38.1±27.2 | 22.4±16.1 | 0.042* |
Values are presented as means ± SD. *, significantly different (P<0.05) between patients with and without Leg fatigue. BMI, body mass index; SGRQ, St. George’s Respiratory Questionnaire; FEV1, forced expiratory volume in 1st second; W-max, Maximal workload; Peak-V’O2, maximum oxygen uptake; V’E, Minute ventilation; MVV, maximal voluntary ventilation; V’CO2, carbon dioxide production rate; HR-Reserve, heart rate reserve.
Discussion
Exercise intolerance is one of the important aspects of health related quality of life in COPD (19,20) and there is considerable evidences that pulmonary rehabilitation programs improve exercise tolerance and patients’ health related quality of life (21,22). There is little evidence about the relationship between subjective complaints in COPD and objective parameters. To the best of our knowledge, this is the first study that systematically investigated the relationships between CPET parameters and COPD symptoms/health status, assessed by SGRQ-score.
Although weak correlations between SGRQ-score and predicted maximal WR, breathing reserve and HR-reserve (r2 =0.19, 0.25 and 0.20 respectively) were found in this study, it was surprising that no significant correlation was found between SGRQ and predicted Peak-V O2%. Verhage et al. (13) studied the value of CPET parameter in relation to COPD health status. In contrast to this study which used SGRQ, they employed Nijmegen Integral Assessment Framework (NIAF) to assess the health status, including physiological functioning, complaints, functional impairments and quality of life as its main domains. While there was significant correlation between predicted Peak-V O2% and some health status sub-domains, the observed correlations were weak (highest value was r=0.36). As a final point, they concluded that Peak-VO2 is a weak indicator for health status and daily physical activities.
SGRQ is a well-known tool for measuring recent respiratory symptoms, limitation of daily activity and social/emotional impact of respiratory diseases including COPD. SGRQ mostly addresses the patient’s symptoms during ordinary daily activities (23). However the CPET parameters, notably Peak-V O2, are achieved by maximal tolerable exercise that could not be fully applicable to patient’s regular daily activities. Meanwhile, in view of complex disease pathophysiology, it has been shown that there are various potential factors that could affect the physical activity and/or quality of life in patients with COPD. These factors include skeletal muscle abnormalities (24), concomitant depression/anxiety (25,26), other co-morbidities and/or exacerbations (27).
In our study significant correlations (P<0.05) were found between six-minute walk distance and total SGRQ-score (r=−0.50) and its components including symptoms, activity and impacts (r=−0.36, −0.52 and −0.43 respectively). Some other studies have investigated the relationship between pulmonary function tests and subjective rating of patients about their exercise tolerance. By using an inter-subject assessment, Redelmeier et al. evaluated the threshold at which a difference in the 6MWD was associated with a noticeable difference in perceived walking ability for patients with COPD (28). They reported the threshold of ~54 m and, similar to our study, they also found a significant correlation between 6MWD and patients ratings of their exercise tolerance (r=0.59). However in a more systematically designed study, Puhan et al. (29) reported the threshold of ~26 m for 6MWD and ~4 W for WR obtained by CPET. This remarkable difference between achieved thresholds among various studies could also point to the fact that objective measurements are not exactly related to patient’s self-reported status.
In this study, leg fatigue was the main cause (54.1%) for stopping the CPET. These subjects had significant higher predicted FEV1% than other subjects (76.0±22.0% vs. 55.7±19.6, P<0.05). Except for “Symptoms” sub-domain of SGRQ, total score and two other sub-domains were significantly lower in leg fatigue group. W-max and Peak-V O2 were also significantly higher in these patients. Accordingly, those patients whose main cause for discontinuing the CPET was leg fatigue were at an earlier stage of COPD, with more acceptable exercise and quality of life profile. Skeletal muscle dysfunction is a common problem in patients with COPD (24). Muscle fatigability following vigorous exercise is one of the most important components of muscle dysfunction. It has been shown that even patients with earlier stages of COPD have quadriceps muscle (the most important muscle during CPET) fatigability to an extent comparable to those with more advanced stages of COPD (30). Unfortunately in this study we missed the accurate pre-test data of our patients regarding the level of usual daily physical activity. At earlier stages of COPD in which muscle fatigue is a prominent problem, pulmonary rehabilitation program with exercise training could result in more effective fatigue resistance and exercise tolerance than starting the program during a more advanced stage of disease when other irreversible components ensue. Large controlled studies are needed to determine this issue.
We also analyzed the correlation of COPD severity index (BODE index) with CPET parameter. Along with COPD progression, there are inappropriate consequences on the exercise parameter (31). In this study BODE index was inversely correlated with peak-V O2, WR and breathing-reserve. But the BODE index was also surprisingly negatively correlated with ∆VO2/∆WR (r2=0.41) and HR-reserve (r2=0.20). It has been quoted that ∆VO2/∆WR ratio is often normal in pulmonary diseases including COPD (32). In contrast, this ratio is often reduced in cardiovascular disorders. Although our subjects had negative history or physical examination compatible with such problems, referring to cardiology clinic disclosed notable cardiovascular problems (e.g., low ejection fraction) in 9 (24.3%) subjects. As a result, other comorbidities in COPD such as cardiovascular/musculoskeletal problems should be sought and managed meticulously, even with unrevealing past history. This could be an important point in patient management since in contrast to the progressive nature of underlying lung disease, many of these comorbidities are potentially treatable.
Considering the cross-sectional design and low patient number in this study, larger prospective studies are required for more precise identification of the relationships between CPET parameters and patients’ daily symptoms, exercise tolerance and health status. Meanwhile, describing these relationships following rehabilitation or other novel therapies must be defined.
Regarding the more complex course and multifactorial nature of stage-IV COPD, we excluded these subset of patients. However there is no doubt that these patients require a special attention with consideration of factors other than airway obstruction, such as muscle mass, psychosocial aspects, etc. More systematically designed studies with consideration of these aspects are required for assessment of this specific subgroup of COPD patients.
Conclusions
Regarding this study, while some correlations were found between CPET parameter and daily subjective complaints in COPD, the observed relationships were not strong. In our study, those who discontinued the CPET because of leg fatigue were in the earlier stages of COPD, and may benefit more from early rehabilitation. Finally, significant negative correlation was found between ∆VO2/∆WR and COPD severity indexes, mainly with BODE index. This finding suggests that along with COPD progression, other comorbidities such as cardiac/musculoskeletal problems should be sought and managed meticulously, even with unrevealing past history.
Acknowledgements
We appreciate the kind cooperation of our colleagues at the PFT section of ATRC.
Ethical Statement: This study was approved by the Ethics Committee of Tehran University of Medical Sciences and written informed consent was obtained from each patient.
Footnotes
Conflicts of Interest: The result of this study has been presented during upcoming ERS congress in Amsterdam, September 2015.
References
- 1.Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256-76. 10.1164/ajrccm.163.5.2101039 [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009;33:1165-85. 10.1183/09031936.00128008 [DOI] [PubMed] [Google Scholar]
- 4.Ståhl E, Lindberg A, Jansson SA, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes 2005;3:56. 10.1186/1477-7525-3-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 6.Ringbaek T, Martinez G, Lange P. A comparison of the assessment of quality of life with CAT, CCQ, and SGRQ in COPD patients participating in pulmonary rehabilitation. COPD 2012;9:12-5. 10.3109/15412555.2011.630248 [DOI] [PubMed] [Google Scholar]
- 7.Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321-7. 10.1164/ajrccm/145.6.1321 [DOI] [PubMed] [Google Scholar]
- 8.Weatherall M, Marsh S, Shirtcliffe P, et al. Quality of life measured by the St George's Respiratory Questionnaire and spirometry. Eur Respir J 2009;33:1025-30. 10.1183/09031936.00116808 [DOI] [PubMed] [Google Scholar]
- 9.Probst VS, Troosters T, Pitta F, et al. Cardiopulmonary stress during exercise training in patients with COPD. Eur Respir J 2006;27:1110-8. 10.1183/09031936.06.00110605 [DOI] [PubMed] [Google Scholar]
- 10.Chandra D, Wise RA, Kulkarni HS, et al. Optimizing the 6-min walk test as a measure of exercise capacity in COPD. Chest 2012;142:1545-52. 10.1378/chest.11-2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiraga T, Maekura R, Okuda Y, et al. Prognostic predictors for survival in patients with COPD using cardiopulmonary exercise testing. Clin Physiol Funct Imaging 2003;23:324-31. 10.1046/j.1475-0961.2003.00514.x [DOI] [PubMed] [Google Scholar]
- 12.ERS Task Force , Palange P, Ward SA, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J 2007;29:185-209. [DOI] [PubMed] [Google Scholar]
- 13.Verhage TL, Vercoulen JH, van Helvoort HA, et al. Maximal exercise capacity in chronic obstructive pulmonary disease: a limited indicator of the health status. Respiration 2010;80:453-62. 10.1159/000295920 [DOI] [PubMed] [Google Scholar]
- 14.Berry MJ, Adair NE, Rejeski WJ. Use of peak oxygen consumption in predicting physical function and quality of life in COPD patients. Chest 2006;129:1516-22. 10.1378/chest.129.6.1516 [DOI] [PubMed] [Google Scholar]
- 15.Fallah Tafti S, Cheraghvandi A, Marashian M, et al. Measurement of the validity and reliability of the persian translation of the saint george respiratory questionnaire for patients with chronic obstructive pulmonary disease. Open Respir Med J 2009;3:107-11. 10.2174/1874306400903010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Thoracic Society. American College of Chest Physicians . ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211-77. 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 17.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 18.Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 2009;374:704-11. 10.1016/S0140-6736(09)61301-5 [DOI] [PubMed] [Google Scholar]
- 19.Peruzza S, Sergi G, Vianello A, et al. Chronic obstructive pulmonary disease (COPD) in elderly subjects: impact on functional status and quality of life. Respir Med 2003;97:612-7. 10.1053/rmed.2003.1488 [DOI] [PubMed] [Google Scholar]
- 20.Wijkstra PJ, TenVergert EM, van der Mark TW, et al. Relation of lung function, maximal inspiratory pressure, dyspnoea, and quality of life with exercise capacity in patients with chronic obstructive pulmonary disease. Thorax 1994;49:468-72. 10.1136/thx.49.5.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cambach W, Chadwick-Straver RV, Wagenaar RC, et al. The effects of a community-based pulmonary rehabilitation programme on exercise tolerance and quality of life: a randomized controlled trial. Eur Respir J 1997;10:104-13. 10.1183/09031936.97.10010104 [DOI] [PubMed] [Google Scholar]
- 22.Wijkstra PJ, Ten Vergert EM, van Altena R, et al. Long term benefits of rehabilitation at home on quality of life and exercise tolerance in patients with chronic obstructive pulmonary disease. Thorax 1995;50:824-8. 10.1136/thx.50.8.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones PW1, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med 1991;85 Suppl B:25-31; discussion 33-7. [DOI] [PubMed]
- 24.Kim HC, Mofarrahi M, Hussain SN. Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2008;3:637-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev 2014;23:345-9. 10.1183/09059180.00007813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eiser N, Harte R, Spiros K, et al. Effect of treating depression on quality-of-life and exercise tolerance in severe COPD. COPD 2005;2:233-41. 10.1081/COPD-57596 [DOI] [PubMed] [Google Scholar]
- 27.Tsiligianni I, Kocks J, Tzanakis N, et al. Factors that influence disease-specific quality of life or health status in patients with COPD: a review and meta-analysis of Pearson correlations. Prim Care Respir J 2011;20:257-68. 10.4104/pcrj.2011.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redelmeier DA, Bayoumi AM, Goldstein RS, et al. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med 1997;155:1278-82. 10.1164/ajrccm.155.4.9105067 [DOI] [PubMed] [Google Scholar]
- 29.Puhan MA, Chandra D, Mosenifar Z, et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J 2011;37:784-90. 10.1183/09031936.00063810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mador MJ, Kufel TJ, Pineda LA, et al. Effect of pulmonary rehabilitation on quadriceps fatiguability during exercise. Am J Respir Crit Care Med 2001;163:930-5. 10.1164/ajrccm.163.4.2006125 [DOI] [PubMed] [Google Scholar]
- 31.Neder JA, Arbex FF, Alencar MC, et al. Exercise ventilatory inefficiency in mild to end-stage COPD. Eur Respir J 2015;45:377-87. 10.1183/09031936.00135514 [DOI] [PubMed] [Google Scholar]
- 32.Balady GJ, Arena R, Sietsema K, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010;122:191-225. 10.1161/CIR.0b013e3181e52e69 [DOI] [PubMed] [Google Scholar]


