Abstract
Objectives:
Behavioral and psychological symptoms of dementia in individuals with Alzheimer’s disease and caregiver characteristics may influence the decision to provide care at home or in a nursing home, though few studies examine this association near the actual time of nursing home placement. Using a matched case–control design, this study investigates the association between (1) total Neuropsychiatric Inventory score, (2) the Neuropsychiatric Inventory-4 (an agitation/aggression subscale), and (3) individual domains of the Neuropsychiatric Inventory and nursing home placement.
Methods:
Data from the South Carolina Alzheimer’s disease Registry provides an opportunity to expand the literature by looking at cases at the time of nursing home care eligibility/placement and allowing for propensity-score-matched controls. Cases (n = 352) entered a nursing home within 6 months of study initiation; controls (n = 289) remained in the community. Registry data were combined with caregiver survey data, including the Neuropsychiatric Inventory. Conditional logistic regression was applied.
Results:
A 10% increase in the Neuropsychiatric Inventory score implied a 30% increase in odds of nursing home admission (odds ratio: 1.30; 95% confidence interval: 1.14–1.50), having married or male caregivers predicted nursing home placement. Cases versus controls were significantly more likely to have behavioral and psychological symptoms of dementia related to agitation/aggression 1 month prior to nursing home admission.
Conclusion:
Interventions targeting behavioral and psychological symptoms of dementia without available effective interventions in individuals with Alzheimer’s disease and caregiver support services are necessary to prevent or delay nursing home admission.
Keywords: Dementia, long-term care, Neuropsychiatric Inventory, propensity score matching, conditional logistic regression
Introduction
In 2014, an estimated 5.2 million Americans have Alzheimer’s disease (AD). The majority of those affected are diagnosed later in life, with recent statistics indicating that one in nine people aged 65 years and older and about one-third of people aged 85 years and older have AD.1,2 Since the US population aged 65 years and older is projected to drastically increase by 2030, encompassing approximately 20% of the total population, there is serious concern about the availability and cost of health and social services available for those with AD in the near future.1,3 Due to the progressive nature of AD, those diagnosed with the disease must often rely on informal caregivers to provide supervision and personal care. In 2013, the family and friends of those with AD provided an estimated 17.7 billion hours of unpaid care, valued at over US$220.2 billion.1 Even with the financial, physical, and emotional demands placed on informal caregivers, 80% of caregivers report that they are willing to sacrifice “quite a bit” or “practically anything” to care for their loved one at home for as long as possible.4 This is in accordance with the desires of most elderly individuals who wish to remain independent and live out their days at home. For many individuals with AD, nursing home placement is an inevitable and costly outcome in many situations. The average cost for nursing home care is US$42,000 per year but can exceed US$70,000 per year in some areas of the country.1 Therefore, preventing or delaying nursing home placement for individuals with AD is an important part of ensuring a sustainable long-term care system.
Reasons for nursing home placement of individuals with AD are typically multi-faceted.5 Sociodemographic variables, including older age, female gender, non-White race/ethnicity, not being married, having a lower socioeconomic status, and living alone, have been identified as indicators of nursing home placement for individuals with AD.6 Severities of memory impairment, activities of daily living (ADL) dependencies, and behavioral and psychological symptoms of dementia (BPSD) have also been identified as predictors of nursing home placement.6,7 A systematic review further supports BPSD as key predictors of institutionalization.8 BPSD include, but are not limited to, hallucinations, delusions, aggression, and nighttime behavioral disturbances, including wandering.9 Research examining stage-specific prevalence of behavioral pathology has shown that BPSD are extremely common, occurring in 98% of the sample participants.10 In particular, BPSD such as depression, apathy, agitation, and aggressiveness are known to be more stressful to caregivers than the cognitive and functional problems of the individual with AD.11 The presence of BPSD has been shown to increase the physical and emotional challenges experienced by families and caregivers.12–14 For caregivers, the stress related to coping with BPSD is the strongest predictor of caregiver burden, depressive symptoms, and physical health issues.15,16 As a result, the risk of nursing home placement for individuals with AD increases. Fully characterizing the association between BPSD and nursing home placement is challenging. Although there is some evidence that BPSD persist in some individuals with AD, others may not exhibit symptoms to the same degree over time.17 Prospective longitudinal studies assess individuals at set intervals that may not coincide with nursing home placement, whereas assessment at the actual time of nursing home placement typically includes only more general behavioral assessments, such as those included in the Minimum Data Set.18 Ideally, a full profile of a person’s cognitive and BPSD would be ascertained at the time of nursing home entry to help in the exploration of possible thresholds of clinically meaningful scores and quantify the extent of AD symptomatology at the time of entry. This study makes a substantial contribution to the available literature in this area because few previous studies have attempted to characterize and quantify symptom severity in close temporal relation to actual nursing home placement.
This article describes one approach to capturing additional insights into the BPSD of individuals with AD in close proximity to the actual date of nursing home placement. Using a matched case–control design, researchers used the Neuropsychiatric Inventory (NPI) and caregiver-focused survey instruments to assess BPSD in individuals with AD and to determine the influence of caregiver characteristics in the nursing home placement process. In particular, the researchers aimed to investigate the association between (1) total NPI score, (2) the NPI-4 (an agitation/aggression subscale), and (3) individual domains of the NPI and nursing home placement.
Design and methods
Participants
The study sample consisted of individuals with AD identified by the South Carolina AD Registry. The Office for the Study of Aging in the Arnold School of Public Health at the University of South Carolina is the home of the South Carolina AD Registry, a population-based statewide registry of South Carolina residents diagnosed with Alzheimer’s disease or related disorders (ADRD). It is the most comprehensive registry of its kind in the United States. The South Carolina AD Registry has maintained a record of diagnosed cases of ADRD in South Carolina since 1988 and has identified over 225,000 cases of ADRD.19 The registry includes multiple sources containing administrative data including in-patient hospitalizations, mental health records, Medicaid claims, emergency department visits, memory clinics, vital records, and sources that contain clinical data such as long-term care evaluations.
Inclusion criteria for each study participant identified from the registry included the following: (1) a long-term care evaluation with a self/caregiver-reported diagnosis of AD; (2) an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coded medical record indicating AD (ICD-9-CM code 331.0), pre-senile dementia (ICD-9-CM code 290.1), senile dementia (ICD-9-CM codes 290.0, 290.2–290.3), or a dementia not otherwise specified (ICD-9-CM codes 294.10–294.11); and (3) an informal or family caregiver available for interview. The study team chose to verify AD diagnosis using ICD-9 codes identified by the South Carolina AD Registry.19 Individuals with known vascular dementia or dementias caused by other medical conditions were excluded. The long-term care evaluation is an assessment made by a registered nurse on an annual basis during a home visit to a client who is enrolled in a Medicaid waiver program. The assessment may be done more frequently if the participant has a major change in their health that will affect their need for services. It includes date of assessment, demographics, the modified Mental Status Questionnaire score, measures of ADL, self-reported physician-diagnosed medical and psychiatric morbidities, an evaluation of BPSD, and caregiver contact information. If individuals are deemed eligible for nursing home care, they or their caregivers can choose between nursing home placement or receiving additional care services and case management while still residing within the community. All individuals with AD included in this study were eligible for nursing home level of care and are financially eligible for Medicaid.
Study participants were identified as cases if they had entered a nursing home within 6 months of study initiation or as controls if they remained in the community. For the purpose of this study, a caregiver was defined as a person spending at least 4 h per day and at least 4 days per week with the individual with AD. Caregivers of cases and controls were mailed a letter with information about the study, along with a US$2 bill which was an incentive for participation in the survey. Verbal informed consent was obtained from all subjects before the study. Interviewers called caregivers and conducted the interview or scheduled an appointment to conduct the interview at a later time. Interviews were completed by trained interviewers in the Office for the Study of Aging, Arnold School of Public Health, University of South Carolina. At interview completion, caregivers were mailed a letter thanking them for their participation and additional incentive, a US$10 gift card, for their time and assistance. Multiple attempts (eight or more calls) were made to contact a caregiver for an interview before a replacement case or control was considered. If the caregiver of a case was unreachable or unwilling to participate in the study, a replacement case was added to the list and the caregiver was contacted. Controls and caregivers of controls were handled similarly. Demographic characteristics and reasons for non-participation were recorded for the evaluation of potential selection bias for the caregivers of cases and controls who were reached but elected not to participate in the study. Ethical approval for this study was obtained from the University of South Carolina Institutional Review Board (registration number: 00000240).
Matching scheme
The effect of age, gender, and race on dementia incidence has been well-documented in the literature,20–30 in an effort to control for these factors; these were used as matching variables in this study. Prior to the interview, individuals diagnosed with Alzheimer’s identified as cases and controls were matched on race (African American versus White), sex, age (within 5 years), and long-term care assessment date (within 120 days). The long-term care assessment date was chosen as a matching variable to assure that cases and controls had a level of care assessment within a similar time frame; in addition, ADL dependencies and cognitive impairment, both part of the assessment, have been found to be the strongest predictors of nursing home admission.31 We were not able to find suitable controls for all cases using these matching criteria, and so two more cycles were added to the matching process. Criteria were relaxed in each cycle to obtain more matches. When these matching cycles failed to yield enough cases and controls, stratified random sampling based on the matching variables of interest was used to closely maintain overall similarities between cases and controls.
Subsequent to the completion of all interviews, any previous matches between cases and controls were discarded. Matching was redone using propensity scores in conjunction with matching with replacement since interviews resulted in more cases than controls.32–37 Propensity scores were calculated using the original matching variables of interest: race, gender, date of birth, and assessment date. Propensity scores were matched to within 0.81 of one standard deviation (SD) of the logit of the propensity score.35,38 Rosenbaum and Rubin39 and Cavuto et al.40 recommended matching within one-quarter of a SD value of the logit.39 Our matching interval (0.81 × 1 SD value) reflects a balance between achieving the best match possible and maximizing the number of case and control matched sets available for analysis. We also adjusted for the matching variables in the statistical analysis to limit any possible residual confounding. A total of 512 individuals (256 pairs) were matched one case to one control; 9 individuals (3 matched sets) were matched one case to two controls; and 120 individuals (30 matched sets) were matched one case to three controls. The final study sample used for analysis included 352 cases and 289 controls matched with replacement using propensity scores.
The authors chose to match on patient characteristics and not to the patient–caregiver dyads for several reasons. For example, since matching is one of the methods to address confounding, based on the definition of a confounder, the matching variables should be associated with both outcome and exposure without being an intermediate cause in the causal pathway between exposure and outcome. In our case, patient’s characteristics are related to both presence of behavioral disturbances and nursing home placement. However, caregiver’s characteristics are only associated with the decision of nursing home placement. In addition, matching on both caregivers and patients would have reduced our sample size leading to inefficient power in the analysis and the potential of overmatching, or loss of validity, stemming from a control group that is so closely matched to the case group that the exposure distributions differ very little.41–43
Interview instrument
Five trained interviewers used a structured interview format to survey caregivers over the phone. The interviews lasted between 30 min and 1 h and took place between January and September 2010. The interview survey consisted of questions about the caregivers’ relationship to the care recipient, household size, and other demographics. Caregivers of cases and controls were asked to answer the questions using two different reference time frames. Caregivers of cases were asked to think back to the month before admitting their care recipient with AD to a nursing facility. Caregivers of controls were asked to think back to the month prior to the interviewer’s call. The primary instrument used during the survey was the NPI, which consists of 12 domains of BPSD and assesses individuals with AD with respect to the severity, frequency, and distress experienced by the caregiver. The caregiver is also asked to provide the assessment of severity and frequency. Each domain of the NPI is scored based on severity and frequency, and the overall NPI score is the sum of scores across all 12 domains.44 Prior factor analyses suggest that a smaller subset of four NPI items including agitation, irritability, disinhibition, and aberrant motor behavior describe a unique and important cluster of symptoms.45,46 Confirmation of this agitation/aggression symptom cluster (NPI-4-A/A) was seen in the current data set, with further data to demonstrate a correlation with caregiver burden and higher (more severe) scores in nursing home residents.47
Interview data were merged with the registry and long-term care evaluation data to create the analytic data set. All identifiable data are held by the University of South Carolina in compliance with applicable regulations.
Statistical analysis
Preliminary analyses included evaluation of the associations between the binary outcome variable, nursing home placement or community residence, and a host of variables of interest. The host variables of interest included more than 50 variables from the long-term care evaluation related to the client’s health, demographic, lifestyle, and living situation as well as caregiver demographics. In the building of the multivariable models, a step-wise regression technique was implemented, based on results from the preliminary analyses and the researchers’ institutional knowledge of the study population and variables. Conditional logistic regression48–50 was used to model the relationship between the binary outcome variable related to nursing home placement and the NPI and the NPI-4. In total, 14 models of interest were considered. Each model featured the NPI score, the NPI-4 score, or the domain score for one of the 12 domains of the NPI as the main effect. For interpretation purposes, the NPI, NPI-4, and 12 individual domain scores were rescaled by a factor of 10. This means that each study participant’s score was divided by 10% of the total possible score for the main effect of interest. As a result of the rescaling, the odds ratio (OR) of the main effects of the NPI, NPI-4, and 12 individual domain scores represents a 10% increase in the respective score, providing a more substantive interpretation of the OR. The final set of covariates for the models consisted of variables related to morbidity (arthritis, skin decubiti, bladder or bowel incontinence, Charlson comorbidity index, body mass index (BMI), total number of psychiatric conditions, and Mental Status Questionnaire score) and personal care needs of individuals with AD (total number of ADL dependencies and indication of non-human assistive device). All models were adjusted for potentially confounding variables such as patient’s age, gender, race, BMI, and characteristics of the caregivers such as gender, marital status, education, and employment. There was no missing data in our main variables of interest because trained interviewers maintained data completeness. All statistical results were evaluated at the 0.05 level of significance. Results related to the NPI, the NPI-4, and the 12 domains of the NPI are included. Due to methodology of sample collection in this study, a sample size calculation was not appropriate but a power analysis was performed with p and alpha set to 0.5 and 0.05, respectively; the results confirmed that ORs of at least 1.54 could be detected with 80% power. Statistical Analysis System (SAS) version 9.3 software was used for all analyses.
Results
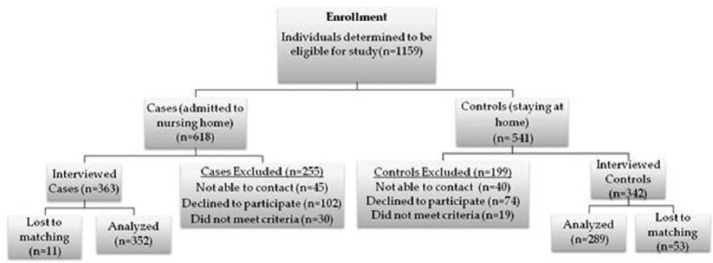
A total of 1159 individuals were initially deemed eligible for the study. Of those, 705 had caregivers who agreed to participate in the study. The study had a high overall response rate of 72%. A refusal rate of 16% was determined by combining outright refusals and appointments not kept, 9% of the callers were never reached, 3% declined due to the recent death of the person they were caring for, and 1% were unable to complete the survey due to hearing impairment, a hospitalization, or surgery. Figure 1 presents a flow chart of the study enrollment process and arrival at the final set of study participants. Characteristics of the 705 individuals whose caregivers completed interviews were compared to the 454 individuals whose caregivers did not complete interviews. Comparisons were made based on the gender, race, and age of the individual with AD. No statistically significant differences were found. The final analytic data set consisted of 641 individuals matched with replacement using propensity scores based on race, age, gender, and assessment date. Of the 641 individuals, 352 were cases and 289 were controls. Table 1 shows the demographics of the cases and controls, as well as the results of the test for differences between the two groups. No statistically significant differences were found between the two groups with respect to the demographic variables. Similar comparisons were made for the caregivers of cases and controls (Table 2). Caregivers of cases and controls were found to differ with respect to gender, marital status, and education. In an effort to reduce confounding, these variables were adjusted for in the final model.
Figure 1.
Project flow diagram.
Table 1.
Demographics of study participants (cases and controls).
| Cases (n = 352) | Controls (n = 289) | p value | |
|---|---|---|---|
| Age (mean) | 84.15 | 83.13 | 0.4403 |
| Race | |||
| White | 195 | 126 | 0.1005 |
| African American | 157 | 163 | |
| Gender | |||
| Female | 253 | 211 | 0.5077 |
| Male | 99 | 78 | |
| Marital status | |||
| Non-married | 268 | 213 | 0.0927 |
| Married | 73 | 75 | |
| Unknown | 1 | 1 | |
| Education | |||
| High school or more | 185 | 149 | 0.3101 |
| Less than high school | 124 | 131 | |
| Unknown | 43 | 9 | |
Table 2.
Caregiver demographics (cases and controls).
| Cases (n = 352) | Controls (n = 289) | p value | |
|---|---|---|---|
| Age (mean) | 60.72 | 58.07 | 0.0876 |
| Race | |||
| White | 196 | 126 | 0.0907 |
| African American | 156 | 163 | |
| Gender | |||
| Female | 270 | 248 | 0.007 |
| Male | 82 | 41 | |
| Marital status | |||
| Non-married | 227 | 136 | <0.0001 |
| Married | 125 | 153 | |
| Education | |||
| High school or more | 284 | 208 | 0.0021 |
| Less than high school | 61 | 81 | |
| Refused | 7 | 0 | |
The frequency of reported NPI domains and mean frequency times severity scores of the BPSD are reported in Table 3. All BPSD were reported more frequently in cases except for elation that was more frequently reported in controls. Mean frequency and severity scores were higher in cases except, again, for elation where the score was higher in the control group. Agitation, irritability, and apathy were the most commonly reported BPSD in both cases and controls. Agitation and apathy had the highest mean frequency and severity score in both groups. Hallucinations and aberrant motor behavior were reported equally in the cases and controls. The ORs and confidence intervals (CIs) for the main effects for each of the study models of interest are presented in Table 4. The main effect of the NPI score was statistically significant (OR: 1.30; 95% CI: 1.14–1.50) and implied that a 10% increase in the total NPI score represented a 30% increase in the odds of nursing home placement. The NPI-4 score was also statistically significant in the model where it was the main effect, with an OR of 1.21 (95% CI: 1.10–1.34), implying that a 10% increase in the NPI-4 score yielded a 21% increase in the odds of nursing home placement. The NPI-4 score proved to have a stronger effect on the odds of nursing home placement than any one of its components (agitation/aggression, irritability, disinhibition, and aberrant motor behavior). Of the four NPI-4 components, agitation/aggression, disinhibition, and irritability were significant main effects in their respective models, but aberrant motor behavior was not. The ORs of agitation/aggression, disinhibition, and irritability were 1.10 (95% CI: 1.06–1.23), 1.18 (95% CI: 1.07–1.29), and 1.10 (95% CI: 1.03–1.18), respectively. Other statistically significant domains of the NPI were delusions (OR: 1.10; 95% CI: 1.03–1.19), sleep (OR: 1.12; 95% CI: 1.05–1.19), and appetite and eating (OR: 1.10; 95% CI: 1.02–1.18).
Table 3.
Frequency of NPI domains and mean frequency times severity scores.
| Cases |
Controls |
|||
|---|---|---|---|---|
| Frequency (%) | Mean F × S score (SD) | Frequency (%) | Mean F × S score (SD) | |
| Delusion | 158 (45%) | 2.89 (4.01) | 104 (36%) | 1.86 (3.09) |
| Depression | 197 (56%) | 2.76 (3.53) | 141 (49%) | 2.35 (3.49) |
| Disinhibition | 161 (46%) | 2.09 (3.10) | 91 (31%) | 1.35 (2.64) |
| Elation | 47 (13%) | 0.46 (1.48) | 58 (20%) | 0.67 (1.62) |
| Hallucination | 159 (45%) | 2.57 (3.74) | 130 (45%) | 2.02 (3.08) |
| Irritability | 232 (66%) | 3.96 (3.98) | 158 (55%) | 2.81 (3.59) |
| Motor | 174 (49%) | 3.38 (4.29) | 143 (49%) | 2.96 (3.83) |
| Sleep | 203 (58%) | 3.91 (4.23) | 126 (44%) | 2.65 (3.87) |
| Agitation | 274 (78%) | 4.66 (4.09) | 185 (64%) | 3.21 (3.68) |
| Anxiety | 136 (39%) | 2.25 (3.56) | 104 (36%) | 1.74 (3.03) |
| Apathy | 222 (63%) | 4.01 (4.18) | 157 (54%) | 3.09 (3.81) |
| Appetite | 167 (48%) | 2.97 (3.93) | 108 (38%) | 2.08 (3.34) |
NPI: Neuropsychiatric Inventory; SD: standard deviation; F: frequency; S: severity.
Table 4.
Main effects of study models of interest.
| Model | Main effect | OR | 95% CI |
|---|---|---|---|
| 1 | NPI | 1.30 | (1.14–1.50) |
| 2 | NPI-4 | 1.21 | (1.10–1.34) |
| 3 | Agitation/aggression | 1.10 | (1.06–1.23) |
| 4 | Disinhibition | 1.18 | (1.07–1.29) |
| 5 | Irritability | 1.10 | (1.03–1.18) |
| 6 | Aberrant motor behavior | 1.06 | (0.99–1.13) |
| 7 | Delusions | 1.10 | (1.03–1.19) |
| 8 | Hallucinations | 1.07 | (0.99–1.15) |
| 9 | Depression | 1.03 | (0.95–1.10) |
| 10 | Anxiety | 1.04 | (0.97–1.12) |
| 11 | Elation | 0.93 | (0.80–1.08) |
| 12 | Apathy | 1.04 | (0.98–1.12) |
| 13 | Sleep | 1.12 | (1.05–1.19) |
| 14 | Appetite | 1.10 | (1.02–1.18) |
NPI: Neuropsychiatric Inventory; OR: odds ratio; CI: confidence interval.
For each of the models featuring either the NPI score, NPI-4 score, or one of the 12 domain scores as a main effect, the use of a non-human assistive device, having bladder incontinence, having bowel incontinence, the total number of psychiatric conditions, having arthritis, having skin decubiti, and the marital status of the caregiver were statistically significant covariates. Analysis revealed that male caregivers had significantly greater odds of placing their individual with AD in a nursing home for all models, except for the model where elation was the main effect. Married caregivers were more likely to admit their individual with AD to a nursing home than their non-married counterparts across all models. The odds of nursing home placement were higher for individuals using non-human assistive devices than those with bowel incontinence, skin decubiti, and psychiatric conditions.
Discussion
The primary focus of our study was to assess BPSD in individuals with AD and their effect on nursing home placement, while also accounting for the characteristics of the caregiver in the long-term care decision-making process. This study makes a substantial contribution to the available literature in this area because few previous studies have attempted to characterize and quantify symptom severity in close temporal relation to actual nursing home placement. A prior review of predictors for nursing home placement in dementia patients highlighted that many different types of factors influence nursing home placement, including demographic factors (e.g. age and marital status), caregiver characteristics, and disease characteristics (e.g. greater dementia severity, functional impairment, and neuropsychiatric symptoms).8 The intervals of assessment and follow-up time vary considerably, and so the temporal association between disease characteristics may account for some of the variability. In this study, caregivers of cases were asked about BPSD in the month prior to nursing home placement of their individual with AD, although caregivers of controls were asked about the month prior to the interview. In addition, this study allows exploration of the role of caregiver characteristics in the decision to place an individual with AD and BPSD in a nursing home.
As the results of other studies have indicated, cases were significantly more likely to have BPSD 1 month prior to nursing home admission when compared to the same assessment of behavior disturbances in the previous month for controls.14,51,52 Phillips and Diwan53 has also shown that individuals with dementia and BPSD would enter nursing homes nearly 2 years earlier than those without. As the AD patient deteriorates throughout the course of the disease and BPSD worsen or become more frequent, caregivers may become more distressed leading to more difficulties providing care. Results of several studies have suggested that hallucinations, depression, sleeping disorders, and agitation are related to higher levels of caregiver’s self-perceived stress, depression, and anxiety,12,54,55 while Risco et al.51 found that delusion, anxiety, apathy, motor disturbances, and eating difficulties were underlying factors in the decision of nursing home admission. Fauth and Gibbons56 have indicated that eight of the NPI indices including delusions, agitation, depression, anxiety, apathy, disinhibition, irritability, and aberrant motor behavior indirectly affect the nursing home placement process by depressing the caregiver. Our study results showed the overall NPI score to be a predictor of nursing home placement. In our main effect models, we found six of the NPI indices—agitation/aggression, disinhibition, irritability, delusions, sleep disorder, and appetite and eating disorders—as statistically significant predictors of nursing home placement. Many of these BPSD were cited in the studies above as factors related to caregiver stress, depression, and anxiety, once again substantiating the relationship between the caregiver’s mental well-being and nursing home placement. Our results also indicated that the NPI-4, which assesses four of the 12 NPI BPSD, is also a predictor of nursing home placement. The predictive ability of the NPI-4 provides evidence for its use as a shorter alternative to the NPI.47 One component of the NPI-4, aberrant motor behavior, was not statistically significant. This could be due to the fact that one of the requirements for long-term care for the study participants is serious limitations in ADL. With respect to caregiver characteristics, male caregivers were more likely to decide on nursing home placement. Results also indicated that married caregivers were more likely to decide on nursing home placement. The majority of the married caregivers were married to individuals other than the care recipient with AD (of 705 caregivers, only 75 were married to the care recipient).
Additional research should explore why certain symptoms of BPSD are stronger predictors of nursing home placement. In part, these differences may be attributable to treatment options for at-home caregivers. For example, there are many safe and effective pharmacological treatments available for the management of symptoms of anxiety and depression. On the other hand, symptoms of agitation and delusions may be somewhat alleviated with behavioral interventions, but no pharmacological interventions are available with indications for these symptoms. Rather, regulatory agencies have issued black box warnings for antipsychotic use in the elderly with dementia. When combined with the present analyses, this suggests that there are still areas of unmet need in the successful management of patients with AD and BPSD. Exploring the association between these areas of unmet need, perceived caregiver burden, and potential treatment solutions would help move research forward in this area.
A major strength of this study was that the sample was recruited from a registry of individuals with AD. The South Carolina AD Registry includes a wide range of assessment and historical information about an individual with AD, including caregiver contact information; due to the availability of multiple data sources, the registry is able to cross-validate medical diagnoses. The South Carolina AD Registry successfully facilitates studies of this nature due to its history as a state-legislated registry, its long-standing relationships with entities which supply secondary data, and the strict measures it has in place to protect the confidentiality and identity of those in the registry. The registry was particularly helpful in the recruitment of African Americans, who have historically been difficult to recruit to such studies and have subsequently not been included or been included in limited numbers.57–59 Despite the cultural differences between Whites and African Americans with respect to nursing home admission, we were able to recruit a representative sample of African Americans into our study. An additional strength of this study was the high overall response rate of 72%, which was due, in part, to the persistence of the trained interviewers and their ability to schedule appointments to do interviews at convenient times for the caregivers, including nights and weekends. Because of this, we were able to achieve a final study sample size much larger than similar studies on individuals with AD and BPSD.
The study did have some limitations. Our ability to obtain suitable matches did not go as specified in our original protocol. However, we were able to mitigate the effect of this limitation by re-matching cases and controls prior to statistical analysis using propensity scores in conjunction with matching with a replacement. With respect to the interview instrument, careful consideration was given to which survey instruments to include in the interview, while other survey instruments had to be excluded. Still, from a practical standpoint, the interview was lengthy for some caregivers, with most interviews lasting from 30 to 60 min. Additionally, since caregivers could have participated in the interview 6 months after their loved one was admitted to a nursing home there is a possibility for recall bias. It is possible that caregivers may not recall all BPSD present a week before admission to the nursing home. Furthermore, in this study a caregiver is defined as a person spending at least 4 h per day at least 4 days per week with the individual with AD. Caregivers were identified by case managers who capture the names of any caregivers for the individual with AD and the specific tasks that they assist the participant with during their assessment for community long-term care program. The names of these caregivers are collected from the individuals themselves or the responsible party for the individual who is in the home while the case manager is doing the assessment. The authors believe they have the closest available proxy for a caregiver but these individuals may have other family caregivers in addition to those interviewed who may not be living in close proximity or may not be spending as much time with the individual with AD. These caregivers may or, more importantly, may not have made similar decisions about nursing home placement and unfortunately we were not able to contact these caregivers to record their opinions. It should also be noted that this study was conducted only in South Carolina. Given state differences in Medicaid policies related to nursing home placement, generalizability to other states should be considered with caution prior to replication of findings. Similarly, findings should be replicated in non-US countries to inform which factors may be patient/caregiver versus systemically related to nursing home placement.
Our results indicate that to delay or prevent nursing home admissions among individuals with AD, additional interventions targeting behavior disturbances will need to be developed and more widely implemented. Caregiver support services also remain vital to sustaining care in the community for individuals with AD. In addition, previous research has found that certain BPSD may be more troublesome for some caregivers but not for others. According to de Vugt et al., caregivers differ in their emotional responses even when facing similar problems. Thus a caregiver’s emotional reaction to BPSD is more important than the problem behavior in the decision to institutionalize an individual with AD.11 Future research should further investigate the caregiver’s perception of BPSD as assessed by indicators such as burden and depression and their effect on the decision for nursing home placement of individuals with AD.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: During the time this study was conducted, Dr Candace Porter was an employee of the Office for the Study of Aging, Arnold School of Public Health, University of South Carolina and Dr Sarsour was an employee of Eli Lilly and Company.
Ethical approval: Ethical approval for this study was obtained from the University of South Carolina Institutional Review Board (registration number: 00000240).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Eli Lilly and Company.
Informed consent: Verbal informed consent was obtained from all subjects before the study.
References
- 1. Alzheimer’s Association. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement 2011; 7: 208–244. [DOI] [PubMed] [Google Scholar]
- 2. Hebert LE, Weuve J, Scherr PA, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80: 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vincent GK, Velkoff VA. The next four decades: the older population in the United States: 2010 to 2050. U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau, 2010. [Google Scholar]
- 4. Archbold P, Stewart B. Family caregiving inventory. Portland, OR: Oregon Health & Science University, 1996. [Google Scholar]
- 5. Lieberman MA, Fisher L. The effects of nursing home placement on family caregivers of patients with Alzheimer’s disease. Gerontologist 2001; 41: 819–826. [DOI] [PubMed] [Google Scholar]
- 6. Gaugler JE, Kane RL, Kane RA, et al. Caregiving and institutionalization of cognitively impaired older people: utilizing dynamic predictors of change. Gerontologist 2003; 43: 219–229. [DOI] [PubMed] [Google Scholar]
- 7. Coehlo DP, Hooker K, Bowman S. Institutional placement of persons with dementia: what predicts occurrence and timing? J Fam Nurs 2007; 13: 253–277. [DOI] [PubMed] [Google Scholar]
- 8. Luppa M, Luck T, Brähler E, et al. Prediction of institutionalisation in dementia. Dement Geriatr Cogn Disord 2008; 26: 65–78. [DOI] [PubMed] [Google Scholar]
- 9. Müller-Spahn F. Behavioral disturbances in dementia. Dialogues Clin Neurosci 2003; 5: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen JC, Borson S, Scanlan JM. Stage-specific prevalence of behavioral symptoms in Alzheimer’s disease in a multi-ethnic community sample. Am J Geriatr Psychiatry 2000; 8: 123–133. [PubMed] [Google Scholar]
- 11. De Vugt ME, Stevens F, Aalten P, et al. A prospective study of the effects of behavioral symptoms on the institutionalization of patients with dementia. Int Psychogeriatr 2005; 17: 577–589. [DOI] [PubMed] [Google Scholar]
- 12. Gaugler JE, Wall MM, Kane RL, et al. The effects of incident and persistent behavioral problems on change in caregiver burden and nursing home admission of persons with dementia. Med Care 2010; 48: 875–883. [DOI] [PubMed] [Google Scholar]
- 13. Steinberg M, Sheppard J-M, Tschanz JT, et al. The incidence of mental and behavioral disturbances in dementia: the cache county study. J Neuropsychiatry Clin Neurosci 2003; 15: 340–345. [DOI] [PubMed] [Google Scholar]
- 14. Chase GA, Folstein M. Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am J Psychiatry 1990; 147: 1049–1105. [DOI] [PubMed] [Google Scholar]
- 15. Pinquart M, Sörensen S. Associations of caregiver stressors and uplifts with subjective well-being and depressive mood: a meta-analytic comparison. Aging Ment Health 2004; 8: 438–449. [DOI] [PubMed] [Google Scholar]
- 16. Pinquart M, Sörensen S. Correlates of physical health of informal caregivers: a meta-analysis. J Gerontol B Psychol Sci Soc Sci 2007; 62: P126–P137. [DOI] [PubMed] [Google Scholar]
- 17. Ballard CG, Margallo-Lana M, Fossey J, et al. A 1-year follow-up study of behavioral and psychological symptoms in dementia among people in care environments. J Clin Psychiatry 2001; 62: 631–636. [DOI] [PubMed] [Google Scholar]
- 18. Aalten P, De Vugt ME, Jaspers N, et al. The course of neuropsychiatric symptoms in dementia. Part I: findings from the two-year longitudinal Maasbed study. Int J Geriatr Psychiatry 2005; 20: 523–530. [DOI] [PubMed] [Google Scholar]
- 19. Office for the Study of Aging (OSA). Annual Report South Carolina Alzheimer’s Disease Registry, Arnold School of Public Health, Office for the Study of Aging, University of South Carolina. 2016. [Google Scholar]
- 20. Ruitenberg A, Ott A, van Swieten JC, et al. Incidence of dementia: does gender make a difference? Neurobiol Aging 2001; 22: 575–580. [DOI] [PubMed] [Google Scholar]
- 21. Letenneur L, Gilleron V, Commenges D, et al. Are sex and educational level independent predictors of dementia and Alzheimer’s disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry 1999; 66: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andersen K, Launer LJ, Dewey ME, et al. Gender differences in the incidence of AD and vascular dementia: the EURODEM studies. Neurology 1999; 53: 1992–1997. [DOI] [PubMed] [Google Scholar]
- 23. Fratiglioni L, Viitanen M, von Strauss E, et al. Very old women at highest risk of dementia and Alzheimer’s disease: incidence data from the Kungsholmen Project, Stockholm. Neurology 1997; 48: 132–138. [DOI] [PubMed] [Google Scholar]
- 24. Launer L, Andersen K, Dewey M, et al. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. Neurology 1999; 52: 78–84. [DOI] [PubMed] [Google Scholar]
- 25. Mayeda ER, Glymour MM, Quesenberry CP, et al. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016; 12: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayeda ER, Karter AJ, Huang ES, et al. Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the diabetes and aging study. Diabetes Care 2014; 37: 1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weiner MF, Hynan LS, Beekly D, et al. Comparison of Alzheimer’s disease in American Indians, whites, and African Americans. Alzheimers Dement 2007; 3: 211–216. [DOI] [PubMed] [Google Scholar]
- 28. Chen H-Y, Panegyres PK. The role of ethnicity in Alzheimer’s disease: findings from the C-PATH online data repository. J Alzheimers Dis 2016; 51: 515–523. [DOI] [PubMed] [Google Scholar]
- 29. Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord 2011; 25: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Livney MG, Clark CM, Karlawish JH, et al. Ethnoracial differences in the clinical characteristics of Alzheimer’s disease at initial presentation at an urban Alzheimer’s disease center. Am J Geriatr Psychiatry 2011; 19: 430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaugler JE, Duval S, Anderson KA, et al. Predicting nursing home admission in the U.S: a meta-analysis. BMC Geriatr 2007; 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Degenholtz HB, Rosen J, Castle N, et al. The association between changes in health status and nursing home resident quality of life. Gerontologist 2008; 48: 584–592. [DOI] [PubMed] [Google Scholar]
- 33. Krakoff J, Funahashi T, Stehouwer CD, et al. Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care 2003; 26: 1745–1751. [DOI] [PubMed] [Google Scholar]
- 34. Ming K, Rosenbaum PR. Substantial gains in bias reduction from matching with a variable number of controls. Biometrics 2000; 56: 118–124. [DOI] [PubMed] [Google Scholar]
- 35. Kosanke J, Bergstralh E. SAS macro: DIST. 2004. http://www.mayo.edu/research/documents/distsas/doc-10027802
- 36. Kosanke J, Bergstralh E. SAS macro: VMATCH. 2004. http://www.mayo.edu/research/documents/vmatchsas-05-14-14/doc-20094471
- 37. Olsson A, Kromhout H, Agostini M, et al. A case-control study of lung cancer nested in a cohort of European asphalt workers. Environ Health Perspect 2010; 118: 1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feng W, Jun Y, Xu R. A method/macro based on propensity score and Mahalanobis distance to reduce bias in treatment comparison in observational study. In: Proceedings of the SAS pharmaSUG 2006 conference, Bonita Springs, FL, 22 May 2006. [Google Scholar]
- 39. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985; 39: 33–38. [Google Scholar]
- 40. Cavuto S, Bravi F, Grassi M, et al. Propensity score for the analysis of observational data: an introduction and an illustrative example. Drug Develop Res 2006; 67: 208–216. [Google Scholar]
- 41. De Graaf MA, Jager KJ, Zoccali C, et al. Matching, an appealing method to avoid confounding? Nephron Clin Pract 2011; 118: c315–c318. [DOI] [PubMed] [Google Scholar]
- 42. Pourhoseingholi MA, Baghestani AR, Vahedi M. How to control confounding effects by statistical analysis. Gastroenterol Hepatol Bed Bench 2012; 5: 79–83. [PMC free article] [PubMed] [Google Scholar]
- 43. Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 44. Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997; 48: S10–S16. [DOI] [PubMed] [Google Scholar]
- 45. Aalten P, Verhey F, Boziki M, et al. Neuropsychiatric syndromes in dementia. Results from the European Alzheimer Disease Consortium. Dement Geriatr Cogn Disord 2007; 24: 457–463. [DOI] [PubMed] [Google Scholar]
- 46. Aalten P, Verhey FR, Boziki M, et al. Consistency of neuropsychiatric syndromes across dementias: results from the European Alzheimer Disease Consortium. Dement Geriatr Cogn Disord 2008; 25: 1–8. [DOI] [PubMed] [Google Scholar]
- 47. Dennehy EB, Kahle-Wrobleski K, Sarsour K, et al. Derivation of a brief measure of agitation and aggression in Alzheimer’s disease. Int J Geriatr Psychiatry 2013; 28: 182–189. [DOI] [PubMed] [Google Scholar]
- 48. Connolly MA, Liang K-Y. Conditional logistic regression models for correlated binary data. Biometrika 1988; 75: 501–506. [Google Scholar]
- 49. Breslow N, Day N. Conditional logistic regression for matched sets. In: Breslow N, Day N. (eds) Statistical methods in cancer research, Lyon, France: vol. 1 IARC Publications, 1980, pp. 248–279. [Google Scholar]
- 50. Kleinbaum DG, Klein M. Analysis of matched data using logistic regression. In: Kleinbaum DG, Klein (eds) Logistic regression. New York: Springer, 2010, pp.389–428. [Google Scholar]
- 51. Risco E, Cabrera E, Jolley D, et al. The association between physical dependency and the presence of neuropsychiatric symptoms, with the admission of people with dementia to a long-term care institution: a prospective observational cohort study. Int J Nurs Stud 2015; 52: 980–987. [DOI] [PubMed] [Google Scholar]
- 52. Gaugler JE, Yu F, Krichbaum K, et al. Predictors of nursing home admission for persons with dementia. Med Care 2009; 47: 191–198. [DOI] [PubMed] [Google Scholar]
- 53. Phillips VL, Diwan S. The incremental effect of dementia-related problem behaviors on the time to nursing home placement in poor, frail, demented older people. J Am Geriatr Soc 2003; 51: 188–193. [DOI] [PubMed] [Google Scholar]
- 54. Vellone E, Piras G, Sansoni J. Stress, anxiety, and depression among caregivers of patients with Alzheimer’s disease. Ann Ig 2001; 14: 223–232. [PubMed] [Google Scholar]
- 55. Sansoni J, Anderson KH, Varona L, et al. Caregivers of Alzheimer’s patients and factors influencing institutionalization of loved ones: some considerations on existing literature. Ann Ig 2013; 25: 235–246. [DOI] [PubMed] [Google Scholar]
- 56. Fauth E, Gibbons A. Which behavioral and psychological symptoms of dementia are the most problematic? Variability by prevalence, intensity, distress ratings, and associations with caregiver depressive symptoms. Int J Geriatr Psychiatry 2014; 29: 263–271. [DOI] [PubMed] [Google Scholar]
- 57. Corbie-Smith G, Thomas SB, Williams MV, et al. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med 1999; 14: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ejiogu N, Norbeck JH, Mason MA, et al. Recruitment and retention strategies for minority or poor clinical research participants: lessons from the Healthy Aging in Neighborhoods of Diversity across the Life Span study. Gerontologist 2011; 51: S33–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol 2002; 12: 248–256. [DOI] [PubMed] [Google Scholar]