Abstract
Sharks have greater risk for bioaccumulation of marine toxins and mercury (Hg), because they are long-lived predators. Shark fins and cartilage also contain β-N-methylamino-l-alanine (BMAA), a ubiquitous cyanobacterial toxin linked to neurodegenerative diseases. Today, a significant number of shark species have found their way onto the International Union for Conservation of Nature (IUCN) Red List of Threatened Species. Many species of large sharks are threatened with extinction due in part to the growing high demand for shark fin soup and, to a lesser extent, for shark meat and cartilage products. Recent studies suggest that the consumption of shark parts may be a route to human exposure of marine toxins. Here, we investigated BMAA and Hg concentrations in fins and muscles sampled in ten species of sharks from the South Atlantic and Pacific Oceans. BMAA was detected in all shark species with only seven of the 55 samples analyzed testing below the limit of detection of the assay. Hg concentrations measured in fins and muscle samples from the 10 species ranged from 0.05 to 13.23 ng/mg. These analytical test results suggest restricting human consumption of shark meat and fins due to the high frequency and co-occurrence of two synergistic environmental neurotoxic compounds.
Keywords: β-N-methylamino-l-alanine, conservation, cyanobacteria, total mercury, methylmercury, neurodegenerative disease, neurotoxin, sharks
1. Introduction
Sharks are exploited in both target fisheries [1,2,3] and as bycatch (both discarded and incidental catch) that is also sold [4,5,6]. The estimates suggest total annual mortality of 100 million sharks killed in 2000 and about 97 million sharks in 2010, with a total range between 63 and 273 million per year [7]. At least 126 countries worldwide catch sharks, and the global annual value of trade in shark parts is approximately $1 billion US. [8]. Though sharks are harvested for meat consumption and/or for their cartilage used in alternative medicine products, the largest driver of shark mortality is directed fishing to obtain their fins for human consumption in shark fin soup [9,10,11,12]. Given their relatively low natural population growth rates, many sharks are undergoing population declines [5,7] rendering about 16% of the ocean’s shark species threatened with extinction [13].
Shark fin soup primarily consumed in China is also a delicacy in other Asian countries and their diaspora communities worldwide [11,12]. Records from the Chinese Song Dynasty (960–1279) describe the use of shark fin soup as a traditional banquet staple [14]. Today, shark fin soup is in increasingly high demand, popular at weddings and other celebrations across Asia [15]. Dietary supplements containing shark cartilage, the health benefits of which are purportedly bolstered by traditional Chinese medicine claims, have gained popularity in western nations. However, the U.S. Food and Drug Administration (FDA) has been unable to confirm any proclaimed benefits [16] and available reports of health benefits are questionable [17].
There is growing concern as to the potential negative health consequences associated with consuming shark parts, including fins, meat and cartilage. The neurotoxic compound methyl Hg (MeHg) has been known to bioaccumulate in sharks over their lifespans [18,19,20,21]. As such, Hg levels in shark muscle often exceed advisory guidelines for safe human consumption [21,22,23,24]. For example, the Florida Department of Health (FDOH) advises that people should not eat sharks greater than ~109 cm and further recommends that children and pregnant woman not eat any shark meat [23]. Moreover, recent studies have reported that commercial shark cartilage supplements contain pro-inflammatory compounds that could pose health risks for consumers, especially those with inflammatory diseases [17].
Recently, the cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA), has been detected in shark fins [25] and shark cartilage supplements [16]. BMAA has been linked to amyotrophic lateral sclerosis/Parkinsonism dementia complex (ALS/PDC) of Guam and has been detected in the brains of North American Alzheimer’s disease and ALS patients [26,27], suggesting that BMAA plays a role as an environmental toxin in neurodegenerative disease. Recent evidence suggests that merely living near a body of water with cyanobacterial blooms, which contaminate the water, fish, and even the air, may increase the risk of developing ALS [28]. In vitro exposures have demonstrated BMAA’s acute neurotoxicity and animal studies show that BMAA exposure leads to motor impairments in rats [29,30]. Thus, the consumption of shark fins and dietary cartilage supplements may pose a risk for human exposure to environmental neurotoxins BMAA and Hg [16,25].
A causal role for BMAA toxicity in humans is still uncertain due to a lack of epidemiological data with human intake estimated from dietary exposures. Thus, it remains unclear whether detection of BMAA in shark fin or cartilage supplements on its own poses a threat to human health. Likewise, it has been noted that the concentrations of MeHg found in fish and marine products are unlikely to cause significant adverse CNS health effects [31]. However, a synergistic toxicity between these two neurotoxic compounds has been suggested, since BMAA concentrations in a range of 10–100 μM were potentiated by MeHg (3 μM) when these were combined. BMAA and MeHg have been shown to decrease the main cellular antioxidant glutationine, which would increase vulnerability of the brain to oxidative stress [31]. Recent studies by Cox and coworkers demonstrate that vervet monkeys fed BMAA for 140 days develop neurofibrillary tangles and β-amyloid deposits in the brain similar to what is seen in patients with neurodegenerative diseases, including ALS and Alzheimer’s disease [32].
Given the potential synergistic toxicity of Hg and BMAA and their likely prevalence in top marine predators, we conducted an expanded analysis to test fin and muscle from an opportunistic sample of 10 different shark species collected from different ocean basins. Shark samples were analyzed for BMAA using high performance liquid chromatography with fluorescence detection (HPLC-FD) and Hg concentrations were quantified by cold vapor atomic fluorescence spectrometry (CVAFS). Since the total Hg (THg) that is measured in shark muscle and fin is mostly in the form of MeHg+, measures of total Hg are generally equivalent to MeHg+ [33,34,35] Here, THg concentrations in fin and muscle samples were measured and compared to MeHg+ for confirmation of levels in select samples. Our results demonstrated that all 10 shark species tested positive for both BMAA and Hg. Independent laboratory confirmation of BMAA and its isomers 2, 4-diaminobutyric acid (DAB) and N-(2-aminoethyl) glycine (AEG) was determined by ultra-performance liquid chromatography/mass spectrometry/mass spectrometry (UPLC-MS/MS).
2. Results and Discussion
A total of 55 sharks were analyzed for contaminations of BMAA and Hg in selected fin and/or muscle. Our cohort contained 10 different shark species sampled from the Atlantic and the Pacific Ocean (Table 1). These shark species sampled range in threat status from Least Concern (bonnethead shark) to Endangered (great hammerhead) by the International Union for Conservation of Nature (IUCN). Several species (tiger, great hammerhead, and bull) are known to be common in the shark fin trade [12], and the fins and meat of all species sampled are subject to exploitation (Table 1) [36].
Table 1.
A summary of shark species, sampling times and locations sites.
| Scientific Name | International Union for Conservation of Nature Red List Category | Common Name | Location | Month |
|---|---|---|---|---|
| Carharhinus acronotus | Near Threatened | Blacknose b | 25.09417oN 81.04234oW | March |
| - | - | Blacknose b | 25.00858oN 81.00089oW | April |
| - | - | Blacknose a | 25.62099oN 80.15602oW | October |
| - | - | Blacknose a | Biscayne Bay | June |
| - | - | Blacknose b | 25.09417oN 81.04234oW | December |
| - | - | Blacknose b | 25.01089oN 81.00419oW | April |
| Carcharhinus limbatus | Near Threatened | Blacktip b | 25.00644oN 80.99969oW | March |
| - | - | Blacktip b | 25.00644oN 80.99969oW | September |
| - | - | Blacktip a | 25.59968oN 80.15205oW | July |
| - | - | Blacktip b | 25.01109oN 80.99832oW | September |
| - | - | Blacktip b | 25.00644oN 80.99969oW | March |
| - | - | Blacktip a | 25.62592oN 80.15442oW | October |
| - | - | Blacktip a | 25.61905oN 80.1714oW | October |
| - | - | Blacktip a | 25.64757oN 80.1881oW | April |
| - | - | Blacktip a | 25.67199oN 80.18144oW | September |
| - | - | Blacktip b | 25.01089oN 81.00419oW | September |
| - | - | Blacktip b | 25.00976oN 81.00079oW | September |
| - | - | Blacktip b | 25.00644oN 80.99969oW | September |
| - | - | Blacktip b | 25.01715oN 81.01056oW | October |
| - | - | Blacktip b | 25.01715oN 81.01056oW | February |
| - | - | Blacktip b | 25.01089oN 81.00419oW | April |
| - | - | Blacktip b | 25.00858oN 81.00089oW | December |
| - | - | Blacktip b | 25.00623oN 80.99723oW | March |
| Sphyrna tiburo | Least concerned | Bonnethead a | 25.36711oN 80.14806oW | March |
| - | - | Bonnethead a | 25.36711oN 80.14806oW | March |
| - | - | Bonnethead a | 25.40807oN 80.21806oW | October |
| - | - | Bonnethead b | 25.36711oN 80.14806oW | March |
| Carcharhinus leucas | Near threatened | Bull b | 25.01715oN 81.01056oW | September |
| - | - | Bull b | 25.01309oN 80.00129oW | September |
| - | - | Bull b | 25.00623oN 80.99723oW | March |
| Sphyrna mokarran | Endangered | Great Hammerhead a | 25.62138oN 80.15656oW | July |
| - | - | Great Hammerhead b | 25.01715oN 81.01056oW | September |
| - | - | Great Hammerhead a | 25.740092oN 79.967258oW | May |
| - | - | Great Hammerhead b | 26.61587oN 79.96725oW | February |
| - | - | Great Hammerhead b | 26.457892oN 80.053938 oW | April |
| Negaprion brevirostris | Near threatened | Lemon b | 25.00644oN 80.99969oW | June |
| - | - | Lemon b | 25.00644oN 80.99969oW | March |
| Ginglymostoma cirraum | Data deficient | Nurse a | 25.61942oN 80.1835 oW | September |
| - | - | Nurse b | 24.88335oN 80.84475 oW | April |
| - | - | Nurse b | 25.00644oN 80.99969 oW | March |
| - | - | Nurse a | 25.62311oN 80.15626oW | August |
| - | - | Nurse a | 25.60062oN 80.15214 oW | August |
| - | - | Nurse a | 25.60569oN 80.1534 oW | August |
| - | - | Nurse a | 25.62311oN 80.15626 oW | August |
| - | - | Nurse b | 25.00858oN 80.00089 oW | September |
| - | - | Nurse b | Florida Bay | January |
| - | - | Nurse b | 25.00983oN 80.99305oW | March |
| Rhizoprionodon terraenovae | Least Concerned | Atlantic Sharpnose b | 25.00858oN 81.00089oW | April |
| - | - | Atlantic Sharpnose b | 25.10566oN 81.04757oW | April |
| - | - | Atlantic Sharpnose b | Florida Bay | April |
| Sphyrna zygaena | Vulnerable | Smooth Hammerhead a | 26.117727oN 80.09734oW | February |
| Galeocerdo cuvier | Near threatened | Tiger c | −21.12055oN 149.22416oE | January |
| - | - | Tiger c | −32.78278oN 152.41171oE | January |
| - | - | Tiger c | −24.81665oN 152.47257 oE | September |
| - | - | Tiger c | −24.81665oN 152.47257 oE | March |
a Biscayne Bay; b Florida Bay; c Pacific Ocean.
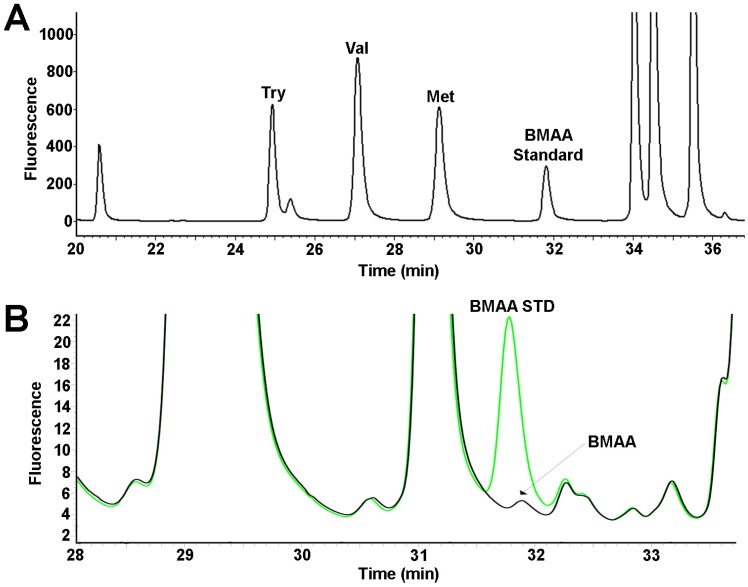
We used a rapid and sensitive HPLC-FD method for detection of 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) tagged BMAA (Figure 1) [25]. We detected BMAA in shark fins of all 10 species surveyed in this study in concentrations ranging from 34 to 2011 ng/mg (wet weight) (Table 2). The average concentration for this survey of BMAA in sharks was 366 ± 72 ng/mg (wet weight) (Table 2). The HPLC-FD method has lower sensitivity compared to LC-MS/MS methods [37,38]. The unambiguous detection and identification of BMAA in complex biological samples requires mass spectrometry validation and AQC derivatization to distinguish BMAA from its positional isomers DAB and AEG (Table 3) [16,25]. UPLC-MS/MS was used to confirm the identity of BMAA in a representative sample of shark fins (Figure 2).
Figure 1.
High performance liquid chromatography with fluorescence detection (HPLC-FD) identification of β-N-methylamino-l-alanine (BMAA) in shark fins. (A) Separation of 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) derivatized amino acid standards tyrosine (Try), valine (Val), methionine (Met), and BMAA standard; (B) representative chromatogram of Australian Tiger shark fin (black) and BMAA standard (green). Chromatogram shows BMAA has a distinct peak with a retention time of 31.8 mins.
Table 2.
β-N-methylamino-l-alanine (BMAA) concentrations detected by high performance liquid chromatography with fluorescence detection (HPLC-FD) in shark fins
| Species | Range (ng/mg) | Detected Mean ± SE (ng/mg) | BMAA/Length (ng/100 cm) |
|---|---|---|---|
| Blacknose (n = 6) a | ND–1663 | 573 ± 322 * | 473 |
| Blacktip (n = 17) a | ND–811 | 282 ± 72 * | 203 |
| Bonnethead (n = 4) a | 40–1836 | 707 ± 395 | 925 |
| Bull (n = 3) a | 43–264 | 180 ± 69 | 103 |
| Great Hammerhead (n = 5) a | 42–1528 | 576 ± 272 | 273 |
| Lemon (n = 2) a | 556–628 | 592 ± 36 | 322 |
| Nurse (n = 10) a | ND–2011 | 442 ± 315 * | 216 |
| Sharpnose (n = 3) a | 40–115 | 68 ± 24 | 47 |
| Smooth Hammerhead (n = 1) a | - | 43 | 21 |
| Tiger (n = 4) b | 34–44 | 39 ± 2 | 11 |
ND, Below limit of detection; SE: Standard Error; *: Only detected samples averaged; a: Atlantic Ocean; b: Pacific Ocean.
Table 3.
Comparison of BMAA concentrations detected by HPLC-FD and ultra-performance liquid chromatography/mass spectrometry/mass spectrometry (UPLC-MS/MS).
| Species | HPLC-FD * (ng/mg) | UPLC-MS/MS * (ng/mg) |
|---|---|---|
| Galeocerdo cuvier | - | - |
| Tiger a | 35.60 ± 1.90 | 19.20 ± 7.10 |
| Tiger a | 31.50 ± 2.60 | 20.68 ± 3.50 |
| Tiger a | 39.60 ± 4.70 | 33.15 ± 5.60 |
| Tiger a | 38.90 ± 5.10 | 20.17 ± 2.40 |
* Data presented as Mean ± Standard Error; a: Pacific Ocean; Four replicate biological samples were analyzed in triplicate to determine method reproducibility and ruggedness.
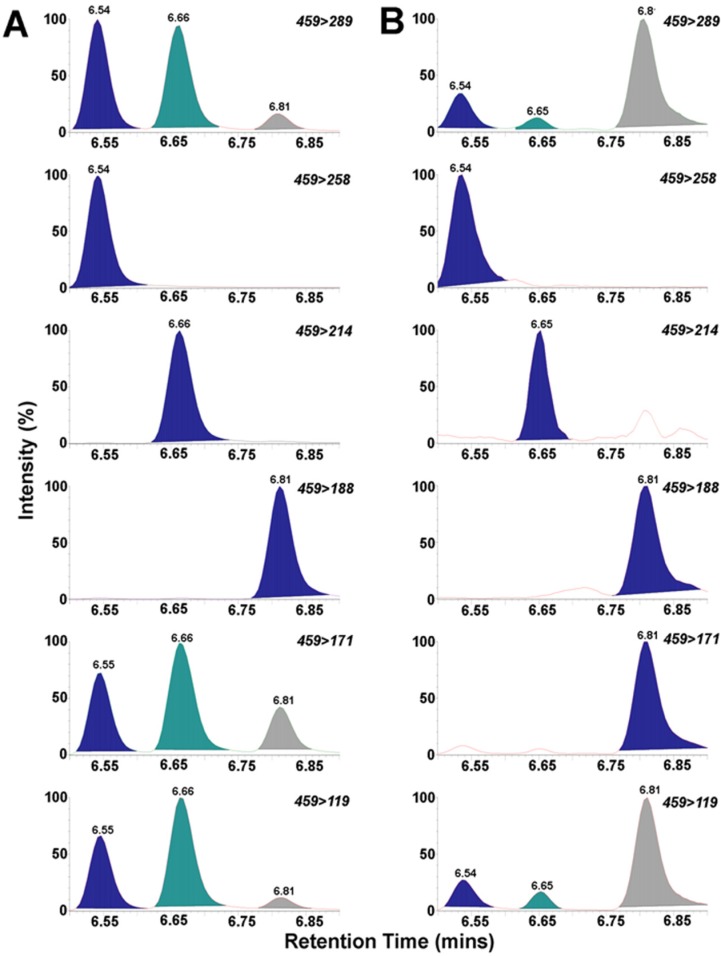
Figure 2.
Ultra-performance liquid chromatography/mass spectrometry/mass spectrometry (UPLC-MS/MS) detection and conformation of BMAA in shark fins. (A) Chromatograms depicting detection of ACQ derivatized standards of BMAA, and structural isomers N-(2-aminoethyl) glycine (AEG) and 4-diaminobutyric acid (DAB); (B) UPLC-MS/MS chromatograms of BMAA detection in fins from Australian sharks. The diagnostic selected reaction monitoring (SRM) transitions of the parent ion m/z 459 to daughter ions 289, 171 and 119 are common to all three isomers. The BMAA (blue) peak is selectively identified at 6.55 min by the transition 459 > 258. AEG (green) is selectively identified at 6.66 min by the transition 459 > 214. DAB (grey) is selectively identified at 6.81 min by the transition 459 > 188.
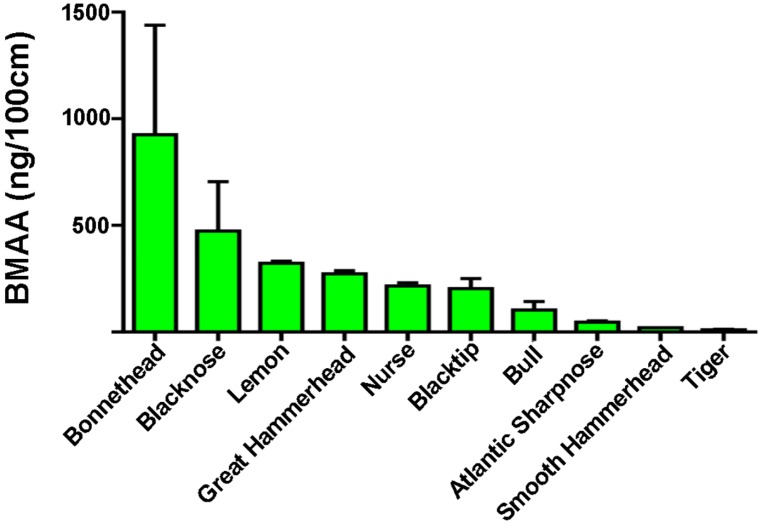
BMAA was below the level of quantitation in only 12% of shark fins tested (Table S1). The highest BMAA concentrations were measured in bonnethead sharks (Figure 3, Table 2; 707 ± 395 ng/mg wet weight and 925 ng/100 cm fin length). This result is in keeping with the elevated levels of BMAA in benthic organisms [39]. The preferred prey of bonnetheads is found in coastal inshore areas that feed primarily on blue cabs (Callinectes sapidus) and other crustaceans [40]. In an examination of cyanobacteria in South Florida and BMAA concentrations in resident fish and invertebrates [41], we found that blue crab and shrimp had among the highest concentrations of BMAA reported in animals (6976 μg/g). Macroalgal abundance per square meter in such habitats can be typically around 20 times higher on the sediment than in the water column [39]. The elevated levels of BMAA may be due to the high occurrence of benthic cyanobacteria associated with the microalgae and detritus that the blue crab and shrimp feed on [41].
Figure 3.
BMAA concentration per unit length of shark fin. Bar graphs depict the mean and standard error of BMAA concentration per 100 cm in ten shark species from the Atlantic and Pacific oceans.
Cold vapor atomic fluorescence spectroscopy (CVAFS) and thermal decomposition methods for total Hg in sharks gave positive results in all 10 species surveyed. The THg concentrations ranged from 0.048 to 13.23 ng/mg with a mean concentration of 2.3 ± 0.4 ng/mg (Table 4; Table S2). These values are higher than those reported safe for human consumption, which range from 0.3 to 1.0 µg/g wet weight based on different criteria and benchmark dose estimates reported by health organization or government agencies [42,43]. The highest THg concentrations were found in the bull sharks, averaging 7.26 ± 3.04 ng/mg. Bull sharks are large coastal apex predators with high Hg levels reported previously in agreement with our results [21,44,45]. Shark muscle samples contained nine times the amount of THg as compared to fins (Student’s t-test p < 0.0001; n = 26/20) (Table S1). THg in muscles ranged from 0.27 to 13.23 ng/mg with a mean concentration of 3.8 ± 0.6 ng/mg. In addition, MeHg was measured in a subset of sharks with concentrations ranging from 0.05 to 1.95 ng/mg and a mean concentration of 0.42 ± 0.11 ng/mg (Table 4). THg and MeHg concentration in shark samples tested were highly correlated (Spearman correlations r = 0.94, p < 0.0001; n = 18) in our shark cohort as expected [33,34,35].
Table 4.
Mercury concentrations detected in shark fin and muscle.
| Species | Range Hg (ng/mg) | THg (ng/mg) * | MeHg (ng/mg) * | BMAA:THg |
|---|---|---|---|---|
| Blacknose a | 0.05–5.65 | 1.93 ± 2.27 (n = 3) | 0.71 ± 0.02 (n = 2) | 429:1 |
| Blacktip a | 0.22–7.73 | 3.70 ± 0.69 (n = 16) | 1.40 ± 0.75 (n = 7) | 368:1 |
| Bonnethead a | 0.41–1.77 | 0.96 ± 0.32 (n = 4) | 0.56 ± 0.44 (n = 4) | 668:1 |
| Bull a | 3.24–13.23 | 7.26 ± 3.04 (n = 3) | 2.32 (n = 1) | 27:1 |
| Great Hammerhead a | - | 3.29 (n = 1) | N/A | 465:1 |
| Lemon a | 0.27–1.34 | 0.81 ± 0.54 (n = 2) | 0.26 ± 0.08 (n = 2) | 1390:1 |
| Nurse a | 0.06–0.48 | 0.24 ± 0.04 (n = 10) | N/A | 1509:1 |
| Sharpnose a | 0.44–2.41 | 1.42 ± 0.98 (n = 2) | 0.25 (n = 1) | 70:1 |
| Smooth Hammerhead a | - | 2.85 (n = 1) | N/A | 15:1 |
| Tiger b | 0.12–1.61 | 0.74 ± 0.36 (n = 4) | N/A | 23:1 |
* Data presented as mean ± standard error; a: Atlantic Ocean; b: Pacific Ocean. N/A, Samples not available for measurement.
Previous studies of MeHg in shark fins and soup reported variable levels, with higher concentrations measured in higher trophic levels species consistent with biomagnification [35]. However, a recent report suggests that THg levels are low in shark fin soup posing only a minor risk for human exposure [35]. We reported that THg and BMAA are detected in shark cartilage dietary supplements [16]. Despite the low levels of THg in shark fin soup, the co-occurrence of BMAA and elemental and Hg in shark fin and muscle should be considered a potential human health concern due to their possible synergistic toxicity to neural tissues [31].
In all shark species surveyed, BMAA levels averaged 15 to 1500 times higher than the concentration of Hg (Table 4). We correlated BMAA and Hg levels by comparing the linear mass density of each contaminant. The concentrations of Hg were positively correlated with BMAA among individuals when both values were normalized for length (concentration/100 cm; Spearman correlations r = 0.37, p = 0.02; n = 39). The positive correlation demonstrates that sharks with higher BMAA concentrations show increased Hg levels. Shark fins are often dried or cooked prior to human consumption. While these preparation methods are known to remove other marine toxins, neither BMAA nor Hg would likely be significantly affected because both are associated with stable incorporation into proteins [46]. BMAA is misincorporated into neural proteins [47,48,49] and Hg-binding proteins are a likely source of harmful accumulation of Hg in the marine food web [50].
The sharks surveyed show both inter- and intra-specific variation in BMAA and Hg concentrations. There are various biological and physical properties of the environment that may affect exposures of sharks to spatial and temporal differences in the accumulation of Hg and BMAA through the food web. In the case of Hg, exposure can be through both point source pollution and atmospheric emissions from fossil fuel combustion [51]. The bioaccumulation will depend in part on the presence of anaerobic bacteria that can convert inorganic Hg to the organic form for trophic transfer up the food web to sharks [52] Similarly, variation in the bioaccumulation and exposure of sharks to BMAA will depend on environmental levels of cyanobacteria that increase with nutrient pollution derived from land-based sources [25,41]. While evaluating patterns of spatial, temporal and even individual variation in BMAA and Hg toxicity are important for mitigation efforts to reduce exposure, our data suggest that the risk of BMAA exposure may be greatest between spring and summer seasons (Table S2). Thus, further studies are warranted based on the limited reports of BMAA in the marine food web across diverse geographical locales.
The prevalence of dementia and Alzheimer’s disease is significantly higher in certain Asian countries [53]. In China alone, the number of people with dementia has increased significantly from 3.7 million in 1990 to 9.2 million in 2010 [54]. Moreover, a report in Lancet on global disease burden found that the number of deaths in China due to Alzheimer’s disease and other dementias doubled between 1990 and 2010, while mortality rates, especially among women, fell steeply during the same period [54]. With the continuing growth of China’s aging population, these findings suggest that the nation is heading for a bigger dementia burden than anticipated [55]. The present study suggests that ingestion of shark fin and shark dietary supplements is a route for human exposure to the environmental toxins BMAA and Hg. Although there are no estimates to help benchmark exposure risk of BMAA to humans from dietary exposures, in China, male infertility has been linked to Hg exposure through consumption of seafood, including shark fins [56]. Because sharks were sampled in South Florida waters, there is a concern of BMAA exposures also to USA residents. In Florida, there is an estimated 0.5 million people over the age of 65 with Alzheimer’s disease. These numbers are anticipated to increase to 0.7 million by the 2020 [57].
Given the decline in many shark populations from overfishing, more research is needed to fully understand the potential toxicity of BMAA and Hg to the health and fitness of shark species [1]. Systemic exposure to BMAA and Hg is likely to worsen the problem and limit recovery efforts if not considered in conservation management efforts. Since sharks often occupy high trophic levels in the marine food web, they are vulnerable to bioaccumulation and biomagnification of neurotoxins and other toxic compounds. Given that humans and sharks are both top predators, the results reported here support the view that sharks serve as bioindicators of ecosystem health from human stressors and marine contaminants [58,59].
3. Materials and Methods
3.1. Sample Collection
Fin clips and muscle biopsies were collected from shark species (n = 10) sampled from areas with or without documented cyanobacterial blooms in the Atlantic and Pacific Oceans as described previously [25,26]. Small clips were sampled from archived frozen dorsal fins for analysis of BMAA (n = 55), total Hg (n = 46). Shark specimens where available were assayed for Hg concentrations in muscle (n = 26) and fins (n = 20). Tissue specimens from blacknose (Carcharhinus acronotus), blacktip (Carcharhinus limbatus), bonnethead (Sphyrna tiburo), bull (Carcharhinus leucas), great hammerhead (Sphyrna mokarran), lemon (Negaprion brevirostris), nurse (Ginglymostoma cirratum), Atlantic sharpnose (Rhizoprionodon terraenovae), smooth hammerhead (Sphyrna zygaena) and tiger (Galeocredo cuvier) sharks were included in this survey (Table 1).
3.2. HPLC Sample Preparation
BMAA in shark fin clips was detected and quantified using high performance liquid chromatography (HPLC) methods as reported previously [25]. Briefly, fin clips (50 mg) were hydrolyzed at 110 °C for 18 h in 6 N HCl (1:8 w/v) followed by filtration using centrifugation at 15,800× g for 3 min. Sample extracts were concentrated and dried in a speed-vac (Thermo-Savant SC250DDA Speed Vac Plus with a Savant refrigerator trap RVT 4104, ThermoFischer; Waltham, MA, USA). Extracts were re-suspended in 0.1 M trichloroacetic acid and washed with chloroform to remove any residual lipids. The dried extract was resuspended to 1000 µL in 20 mM HCl. A 100 µL aliquot of the sample extract was derivatized with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) using the AccQ-Fluor reagent (Waters Crop; Milford, MA, USA). The derivatized samples (20 µL resuspended HCl extract, 60 µL of borate buffer (AccQ-Fluor Reagents A and B; Waters), and 20 µL AccQ-Tag) were run in parallel with buffer and AQC blanks and BMAA, AEG, DAB and reference amino acid standards. The sample matrix was spiked with known amounts of BMAA to determine recovery of the extraction procedure and confirm peak identity. Each sample was prepared in triplicate for quantitative studies and orthogonal detection method comparisons. For the orthogonal method comparisons, the shark samples were prepared by different analysts in different labs as a measure of method ruggedness.
3.3. Fluorescence HPLC Methods for Analysis of BMAA
BMAA was separated from amino-acids by reverse-phase high pressure chromatography (Waters Nova-Pak C18 column, 3.9 mm × 300 mm; Waters Crop; Milford, MA, USA) eluted in a gradient of 140 mM sodium acetate, 5.6 mM triethylamine, pH 5.2 (mobile phase A), and 52% (v/v) acetonitrile in water (mobile phase B) at 37 °C using a flow rate of 1.0 mL/min, and 10 µL sample injection volume. The samples were eluted using a 60 min gradient: 0.0 min = 100% A; 2 min = 90% A curve 11; 5 min = 86% A curve 11; 10 min = 86% A curve 6; 18 min = 73% A curve 6; 30 min = 57% A curve 10; 35 min = 40% A curve 6; 37.5 min = 100% B curve 6; 47.5 min = 100% B curve 6; 50 min = 100% A curve 6; 60 min = 100% A curve 6. Detection of the AQC fluorescent tag was achieved using a Waters 2475 Multi λ-Fluorescence Detector (Milford, MA, USA) with excitation at 250 nm and emission at 395 nm. Experimental samples were compared with standard spiked shark fin matrix negative for endogenous BMAA and commercial BMAA reference standard (Sigma B-107; >95% purity, St. Louis, MO, USA). The limits of detection (LOD) and limits of quantification (LOQ) were 2.7 and 7.0 ng, respectively. The percentage of recovery of BMAA was 88%.
3.4. UPLC/MS/MS of BMAA
BMAA and the isomers N-(2-aminoethyl) glycine (AEG) and 2,4-diaminobutyric acid (DAB) were separated, detected and quantified by ultra-performance liquid chromatography/mass spectrometry/mass spectrometry (UPLC/MS/MS) using a fully validated method as previously described [60]. Briefly, 50 mg samples of frozen shark fin clips were accurately weighed and suspended in 1.0 mL of 6 N HCl sealed with N2 gas blown into the tubes for 30 s to displace oxygen. Samples were hydrolyzed for 18 h at 110 °C. A subsample of 400 µL was filtered (0.22 μm PVDF Ultrafree MC centrifuge filters; EMD Millipore; Billerica, MA, USA) and a 100 µL aliquot was dried overnight (Labconco Centrivap; Kansas City, MO, USA). The sample was reconstituted in 1.0 mL 20 mM HCl and a 20 µL aliquot was derivatized with 20 µL 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) in 60 µL borate buffer (AccQ-Fluor Reagents A and B; Waters, Milford, MA, USA). BMAA, AEG and DAB were separated by reverse phase C18 chromatography (BEH column 150 × 2.1 mm 1.7 μm; Waters) and eluted with a gradient of 20 mM ammonium formate with 0.2% formic acid (A) and 0.1% formic acid in acetonitrile; (B). Gradient was delivered by a Waters Acquity I-Class UPLC (Milford, MA, USA) (0 min, 95% A; 1.0 min, 95% A; 7 min, 85% A; 7.5 min, 78% A; 8 min, 15% A; 8.5 min, 15% A; 8.6 min, 95% A; 10 min, 95% A) with a flow rate of 0.7 mL/min at 52 °C. Compounds were clearly separated with BMAA elution at 6.56 min (%RSD = 0.23), AEG at 6.67 min (%RSD = 0.22) and DAB at 6.82 min (%RSD = 0.26) (see Figure 2). Triplicate measures were performed on each shark fin sample (Table 3).
Ions were detected on a triple quadrupole tandem mass spectrometer (Waters Xevo TQS, Milford, MA, USA) with the following parameters: cone voltage was 16 V. Capillary voltage was set to 2500 V with a source offset of 50 V. Desolvation temperature was 550 °C, with a corresponding gas flow of 800 L/h. and a cone gas flow of 150 L/h. Collision-induced-dissociation was performed with 99.999% pure argon pressurized to 7.0 bar with a dwell time of 0.05 s. The characteristic transitions were detected as: BMAA 459 > 258 at collision voltage 18 V, DAB 459 > 188 at collision voltage 20 V, AEG 459 > 214 at collision voltage 20 V.
3.5. Determination of Hg in Fins (CVAFS Method)
Total Hg (THg) includes inorganic and organic forms of Hg. THg and MeHg analyses were performed on shark fin clips following the Standard Operating Procedure modified from the U.S. Environmental Protection Agency (EPA) Test Method 1631 [61]. THg in a sample was isolated and oxidized to mercuric ion using acid digestion, and then reduced to elemental Hg by stannous chloride, purged from the liquid by a carrier gas (Argon). MeHg was extracted from the sample matrix with sodium hydroxide in methanol on a hot block. The Hg species on the traps were desorbed, pyrolyzed and detected by Cold Vapor Atomic Fluorescence Spectrometry (CVAFS) (Millennium Merlin 10.035, PS Analytical, Deerfield Beach, FL, USA). Briefly, the samples (about 0.2 g) were weighed into 10-mL glass ampoules to which 1 mL of deionized water and 2 mL of concentrated HNO3 were added. The ampules were then sealed and the samples were autoclaved for 1 h at 105 °C for sample digestion. The samples were diluted with 1% HCl and introduced into CVAFS, reduced with 2% (v/v) SnCl2 (in 2.5% HCl). Daily analytical runs began with an initial calibration containing 5 non-zero points and a system blank. The mean calibration factor (CFm), calculated from the calibration factor (CFx) for Hg in each of the five standards using the system blank-subtracted peak height, was used for the calculation of sample concentration. Each analytical batch included at least one method blank, a Continuing Calibration Check Samples (CCS), and a Quality Control Sample (QCS). All method blanks during analysis were below the method detection limits (MDLs). The readings of CCS were always within acceptable range (85%–115% for THg of initial calibration). Certified reference material (CRM), DORM-2, was used as a QCS sample throughout the analysis and the recoveries for the QCS samples (84%–128% for THg) were always within acceptable range specified in standard operating procedure (SOPs) (70%–130% for THg). The method limit of detection for the instrument was 0.002 mg/kg.
3.6. Determination of Total Mercury in Muscles (Thermal Decomposition Method)
Muscle samples were placed into nickel sample boats, weighed, and analyzed for THg using thermal decomposition technique with an automated direct Hg analyzer (DMA 80, Milestone Incorporated, Shelton, CT, USA) using the US EPA Method 7473 [62]. Assays were run with one sample each of two standard reference materials (DORM-3 and DOLT-4), two method blanks, and one sample blank.
Acknowledgments
The Herbert W. Hoover Foundation (Akron, OH, USA) provided the funding for this study. Guangling Liu PhD and Yong Cai PhD, Department of Chemistry and Bioinorganic and Environmental Analytics, Florida International University, Miami, FL provided contract services for Hg analyses. We acknowledge Bonnie J. Holmes PhD, University of Queensland Brisbane, for providing Australia tiger shark fins for this survey.
Abbreviations
| ALS/PDC | Amyotrophic lateral sclerosis/parkinsonism dementia complex |
| AEG | N-(2-aminoethyl) glycine |
| AQC | 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate |
| BMAA | β-N-methylamino-l-alanine |
| CVAFS | Cold vapor atomic fluorescence spectrometry |
| CCS | Calibration Check Samples |
| CFm | Calibration factor |
| CFx | Calibration factor |
| DAB | 2,4-diaminobutyric acid |
| FDOH | Florida Department of Health |
| Hg | Mercury |
| HPLC-FD | High performance liquid chromatography with fluorescence detection |
| LOD | Limits of detection |
| LOQ | Limits of quantification |
| MDLs | Method detection limits |
| MeHg | Methyl mercury |
| QCS | Quality Control Sample |
| THg | Total mercury |
| UPLC-MS/MS | Ultra-performance liquid chromatography/mass spectrometry/mass spectrometry |
Supplementary Materials
The following is available online at www.mdpi.com/2072-6651/8/8/238/s1. Table S1 BMAA and Total Hg concentrations determined by HPLC in sharks. Table S2: BMAA and Mercury concentration detected by seasons in sharks.
Author Contributions
N.H. analyzed data and wrote the manuscript; D.A.D. analyzed data and wrote the manuscript; K.M. performed experiments, analyzed data, and assisted with graphics; M.S.S. conducted the literature review and wrote the manuscript; S.J.M. performed experiments, analyzed data and wrote the manuscript; W.B.G. performed experiments and analyzed data; T.D. performed experiments and analyzed data; D.C.E. performed experiments and analyzed data; and D.C.M. conceived the project, designed experiments, analyzed data; and wrote the manuscript.
References
- 1.Baum J.K., Myers R.A., Kehler D.G., Worm B., Harley S.J., Doherty P.A. Collapse and conservation of shark populations in the Northwest Atlantic. Science. 2003;299:389–392. doi: 10.1126/science.1079777. [DOI] [PubMed] [Google Scholar]
- 2.Dulvy N.K., Baum J., Clarke S., Compagno L.J., Cortes E., Domingo A., Fordham S., Fowler S., Francis M.P., Gibson C., et al. You can swim but you can’t hide: The global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. 2008;18:459–482. doi: 10.1002/aqc.975. [DOI] [Google Scholar]
- 3.Camhi M.D., Valenti S.V., Fordham S.V., Fowler S.L., Gibson C. The Conservation Status of Pelagic Sharks and Rays: Report of the IUCN Shark Specialist Group Pelagic Shark Red List Workshop. IUCN Species Survival Commission Shark Specialist Group; Newbury, UK: 2009. [Google Scholar]
- 4.Molina J.M., Cooke S.J. Trends in shark bycatch research: Current status and research needs. Rev. Fish Biol. Fish. 2012;22:719–737. doi: 10.1007/s11160-012-9269-3. [DOI] [Google Scholar]
- 5.Gallagher A.J., Kyne P.M., Hammerschlag N. Ecological risk assessment and its application to elasmobranch conservation and management. J. Fish Biol. 2012;80:1727–1748. doi: 10.1111/j.1095-8649.2012.03235.x. [DOI] [PubMed] [Google Scholar]
- 6.Oliver S., Braccini M., Newman S.J., Harvey E.S. Global patterns in the bycatch of sharks and rays. Mar. Policy. 2015;54:86–97. doi: 10.1016/j.marpol.2014.12.017. [DOI] [Google Scholar]
- 7.Worm B., Davis B., Kettemer L., Ward-Paige C.A., Chapman D., Heithaus M.R., Kessel S.T., Gruber S.H. Global catches, exploitation rates, and rebuilding options for sharks. Mar. Pol. 2013;40:194–204. doi: 10.1016/j.marpol.2012.12.034. [DOI] [Google Scholar]
- 8.The state of world fisheries and aquaculture Rome: Food and agriculture organization of the United States. [(accessed on 15 January 2016)]. Available online: http://www.fao.org/3/a-i3720e.pdf.
- 9.Shiffman D., Hammerschlag N. Shark conservation and management policy: A review and primer for non-specialists. Anim. Consv. 2016 doi: 10.1111/acv.12265. [DOI] [Google Scholar]
- 10.Cunningham-Day R. Sharks in Danger: Global Shark Conservation Status with Reference to Management Plans and Legislation. Universal Publishers; Boca Raton, FL, USA: 2001. [Google Scholar]
- 11.Spiegel J. Even Jaws Deserves to Keep His Fins: Outlawing Shark Finning Throughout Global Waters. Boston Coll. Law Rev. 2000;24:409–437. [Google Scholar]
- 12.Clarke S.C., Milner-Gulland E.J., Bjorndal T. Social, economic, and regulatory drivers of the shark fin trade. Mar. Resour. Econ. 2007;22:305–327. doi: 10.1086/mre.22.3.42629561. [DOI] [Google Scholar]
- 13.Dulvy N.K., Fowler S.L., Musick J.A., Cavanagh R.D., Kyne P.M., Harrison L.R., Carlson J.K., Davidson L.N., Fordham S.V., Francis M.P., et al. Extinction risk and conservation of the world’s sharks and rays. Elife. 2014;3:e00590. doi: 10.7554/eLife.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Templer R. Food for Thought: Chicken Soup for the Ostentatious Soul—Tonic or Tragedy? East, West Divided Over Shark’s Fin. Asian Wall St J. 1999. The Wall Street Journel. [(accessed on 31 January 2011)]. Availble online: http://www.wsj.com/articles/SB934473109149930399.
- 15.Mahr K. Shark-Fin Soup and the Conservation Challenge. Time. 2010. Aug 9, ; Mahr K. Shark-Fin Soup and the Conservation Challenge. [(accessed on 25 June 2016)]. Available online: http://content.time.com/time/magazine/article/0,9171,2021071,00.html.
- 16.Mondo K., Broc Glover W., Murch S.J., Liu G., Cai Y., Davis D.A., Mash D.C. Environmental neurotoxins beta-N-methylamino-l-alanine (BMAA) and mercury in shark cartilage dietary supplements. Food Chem. Toxicol. 2014;70:26–32. doi: 10.1016/j.fct.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Merly L., Smith S.L. Pro-inflammatory properties of shark cartilage supplements. Immunopharmacol. Immunotoxicol. 2015;37:140–147. doi: 10.3109/08923973.2014.999160. [DOI] [PubMed] [Google Scholar]
- 18.Delshad S.T., Mousavi S.A., Islami H.R., Pazira A. Mercury concentration of the whitecheek shark, Carcharhinus dussumieri (Elasmobranchii, Chondrichthyes), and its relation with length and sex. Pan-Am. J. Aquat. Sci. 2012;7:135–142. [Google Scholar]
- 19.Escobar-Sanchez O., Galvan-Magana F., Rosiles-Martinez R. Mercury and selenium bioaccumulation in the smooth hammerhead shark, Sphyrna zygaena Linnaeus, from the Mexican Pacific Ocean. Bull. Environ. Contam Toxicol. 2010;84:488–491. doi: 10.1007/s00128-010-9966-3. [DOI] [PubMed] [Google Scholar]
- 20.Pethybridge H., Cossa D., Butler E.C. Mercury in 16 demersal sharks from southeast Australia: Biotic and abiotic sources of variation and consumer health implications. Mar. Environ. Res. 2010;69:18–26. doi: 10.1016/j.marenvres.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Rumbold D., Wasno R., Hammerschlag N., Volety A. Mercury accumulation in sharks from the coastal waters of southwest Florida. Arch. Environ. Contam. Toxicol. 2014;67:402–412. doi: 10.1007/s00244-014-0050-6. [DOI] [PubMed] [Google Scholar]
- 22.Adams D.H., Jr. Mercury levels in four species of sharks from the Atlantic coast of Florida. Fish. Bull. 1999;97:372–329. [Google Scholar]
- 23.Health FDo Seafood Consumption, Get Fresh with Florida Fish. [(accessed on 25 June 2016)]. Available online: http://www.doh.state.fl.us/floridafishadvice/
- 24.Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. [(accessed on 28 June 2016)]. Avaible online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2012.2985/abstract.
- 25.Mondo K., Hammerschlag N., Basile M., Pablo J., Banack S.A., Mash D.C. Cyanobacterial neurotoxin beta-N-methylamino-l-alanine (BMAA) in shark fins. Mar. Drugs. 2012;10:509–520. doi: 10.3390/md10020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murch S.J., Cox P.A., Banack S.A., Steele J.C., Sacks O.W. Occurrence of beta-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol. Scand. 2004;110:267–269. doi: 10.1111/j.1600-0404.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- 27.Pablo J., Banack S.A., Cox P.A., Johnson T.E., Papapetropoulos S., Bradley W.G., Buck A., Mash D.C. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol. Scand. 2009;120:216–225. doi: 10.1111/j.1600-0404.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- 28.Field N.C., Metcalf J.S., Caller T.A., Banack S.A., Cox P.A., Stommel E.W. Linking beta-methylamino-l-alanine exposure to sporadic amyotrophic lateral sclerosis in Annapolis, MD. Toxicon. 2013;70:179–183. doi: 10.1016/j.toxicon.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Shen W.B., McDowell K.A., Siebert A.A., Clark S.M., Dugger N.V., Valentino K.M., Jinnah H.A., Sztalryd C., Fishman P.S., Shaw C.A., et al. Environmental neurotoxin-induced progressive model of parkinsonism in rats. Ann. Neurol. 2010;68:70–80. doi: 10.1002/ana.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer P.S., Nunn P.B., Hugon J., Ludolph A.C., Ross S.M., Roy D.N., Robertson R.C. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- 31.Rush T., Liu X., Lobner D. Synergistic toxicity of the environmental neurotoxins methylmercury and beta-N-methylamino-l-alanine. Neuroreport. 2012;23:216–219. doi: 10.1097/WNR.0b013e32834fe6d6. [DOI] [PubMed] [Google Scholar]
- 32.Cox P.A., Davis D.A., Mash D.C., Metcalf J.S., Banack S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. R. Soc. B Biol. Sci. 2016;283 doi: 10.1098/rspb.2015.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storelli M., Ceci E., Storelli A., Marcotrigiano G. Polychlorinated biphenyl, heavy metal and methylmercury residues in hammerhead sharks: Contaminant status and assessment. Mar. Pollut. Bull. 2003;46:1035–1039. doi: 10.1016/S0025-326X(03)00119-X. [DOI] [PubMed] [Google Scholar]
- 34.Nam D., Adams D., Reyier E., Basu N. Mercury and selenium levels in lemon sharks (Negaprion brevirostris) in relation to a harmful red tide event. Environ. Monit. Assess. 2010;176:549–559. doi: 10.1007/s10661-010-1603-4. [DOI] [PubMed] [Google Scholar]
- 35.Nalluri D., Baumann Z., Abercrombie D.L., Chapman D.D., Hammerschmidt C.R., Fisher N.S. Methylmercury in dried shark fins and shark fin soup from American restaurants. Sci. Total Environ. 2014;496:644–648. doi: 10.1016/j.scitotenv.2014.04.107. [DOI] [PubMed] [Google Scholar]
- 36.Nature IUfCo THE IUCN Red List of Threatened Species. [(accessed on 28 February 2016)]. Available online: http://www.iucnredlist.org.
- 37.Al-sammak M.A., Hoagland K.D., Snow D.D., Cassada D. Toxicon Methods for simultaneous detection of the cyanotoxins BMAA, DABA, and anatoxin-a in environmental samples. Toxicon. 2013;76:316–325. doi: 10.1016/j.toxicon.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Faassen E.J., Gillissen F., Lürling M. A comparative study on three analytical methods for the determination of the neurotoxin BMAA in cyanobacteria. PLoS ONE. 2012;7:238. doi: 10.1371/journal.pone.0036667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brand L., Suzuki M. Distribution of Benthic Chlorophyll in Florida Bay Sediments; Proceedings of the 1999 Florida Bay and Adjacent Marine Systems Science Conference; Key Largo, FL, USA. 1–5 November 1999; p. 129. [Google Scholar]
- 40.Bethea D.M., Hale L., Carlson J.K., Cortés E., Manire C.A., Gelsleichter J. Geographic and ontogenetic variation in the diet and daily ration of the bonnethead shark, Sphyrna tiburo, from the eastern Gulf of Mexico. Mar. Biol. 2007;152:1009–1020. doi: 10.1007/s00227-007-0728-7. [DOI] [Google Scholar]
- 41.Brand L.E., Pablo J., Compton A., Hammerschlag N., Mash D.C. Cyanobacterial Blooms and the Occurrence of the neurotoxin beta-N-methylamino-l-alanine (BMAA) in South Florida Aquatic Food Webs. Harmful Algae. 2010;9:620–635. doi: 10.1016/j.hal.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adminstration USFaD. [(accessed on 15 June 2016)];2016 Available online: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ChemicalContaminantsMetalsNaturalToxinsPesticides/ucm077969.htm-merc.
- 43.Agency USEP. [(accessed on 20 April 2015)]; Available online: http://www.epa.gov/mercury.
- 44.Hueter R., Fong W., Henderson G., French M., Manire C. Methylmercury concentration in shark muscle by species, size and distribution of sharks in Florida coastal waters. Water Air Soil Pollut. 1995;80:893–899. doi: 10.1007/BF01189741. [DOI] [Google Scholar]
- 45.Adams D.H., Jr., Henderson G.E. Mercury Levels in Marine and Estuarine Fishes of Florida 1989–2001. Florida Marine Research Institute; Port Charlotte, FL, USA: 2003. [Google Scholar]
- 46.Holtcamp W. The emerging science of BMAA: Do cyanobacteria contribute to neurodegenerative disease? Environ. Health Perspect. 2012;120:110–116. doi: 10.1289/ehp.120-a110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie X., Basile M., Mash D.C. Cerebral uptake and protein incorporation of cyanobacterial toxin beta-N-methylamino-l-alanine. Neuroreport. 2013;24:779–784. doi: 10.1097/WNR.0b013e328363fd89. [DOI] [PubMed] [Google Scholar]
- 48.Dunlop R.A., Cox P.A., Banack S.A., Rodgers K.J. The non-protein amino acid BMAA is misincorporated into human proteins in place of l-serine causing protein misfolding and aggregation. PLoS ONE. 2013;8:238. doi: 10.1371/journal.pone.0075376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glover W.B., Mash D.C., Murch S.J. The natural non-protein amino acid N-beta-methylamino-l-alanine (BMAA) is incorporated into protein during synthesis. Amino Acids. 2014;46:2553–2559. doi: 10.1007/s00726-014-1812-1. [DOI] [PubMed] [Google Scholar]
- 50.Weiyue F., Meng W., Ming G., Yuan H., Junwen S., Bing W., Motao Z., Hong O., Yuliang Z., Zhifang C. Mercury speciation and mercury-binding protein study by HPLC-ICP-MS on the estimation of mercury toxicity between maternal and infant rats. J. Anal. At. Spectrom. 2011;26:156–164. doi: 10.1039/C0JA00111B. [DOI] [Google Scholar]
- 51.Pacyna E.G., Pacyna J.M., Sundseth K., Munthe J., Kindbom K., Wilson S., Maxson S.P. Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos. Environ. 2010;44:2487–2499. doi: 10.1016/j.atmosenv.2009.06.009. [DOI] [Google Scholar]
- 52.Compeau G.C., Bartha R. Principal Methylators. Microbiology. 1985;50:498–502. doi: 10.1128/aem.50.2.498-502.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalaria R.N., Maestre G.E., Arizaga R., Friedland R.P., Galasko D., Hall K., Luchsinger J.A., Ogunniyi A., Perry E.K., Potocnik F., et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan K.Y., Wang W., Wu J.J., Liu L., Theodoratou E., Car J., Middleton L., Russ T.C., Deary I.J., Campbell H., et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet. 2013;381:2016–2023. doi: 10.1016/S0140-6736(13)60221-4. [DOI] [PubMed] [Google Scholar]
- 55.Loef M., Walach H. Midlife obesity and dementia: Meta-analysis and adjusted forecast of dementia prevalence in the United States and China. Obesity. 2013;21:51–55. doi: 10.1002/oby.20037. [DOI] [PubMed] [Google Scholar]
- 56.Dickman M.D., Leung C.K., Leong M.K. Hong Kong male subfertility links to mercury in human hair and fish. Sci. Total Environ. 1998;214:165–174. doi: 10.1016/S0048-9697(98)00062-X. [DOI] [PubMed] [Google Scholar]
- 57.2016 Alzhemer's Disease Facts And Figures. [(accessed on 25 July 2016)]. Availble online: https://www.alz.org/documents_custom/2016-facts-and-figures.pdf.
- 58.Schindler D.W. Detecting ecosystem responses to anthropogenic stress. J. Fish. Aquat. Sci. 1987;44:S6–S25. doi: 10.1139/f87-276. [DOI] [Google Scholar]
- 59.Torres P., da Cunha R.T., Maia R., Dos Santos Rodrigues A. Trophic ecology and bioindicator potential of the North Atlantic tope shark. Sci. Total Environ. 2014;481:574–581. doi: 10.1016/j.scitotenv.2014.02.091. [DOI] [PubMed] [Google Scholar]
- 60.Glover W.B., Baker T.C., Murch S.J., Brown P.N. Determination of beta-N-methylamino-l-alanine, N-(2-aminoethyl)glycine, and 2,4-diaminobutyric acid in Food Products Containing Cyanobacteria by Ultra-Performance Liquid Chromatography and Tandem Mass Spectrometry: Single-Laboratory Validation. J. AOAC Int. 2015;98:1559–1565. doi: 10.5740/jaoacint.15-084. [DOI] [PubMed] [Google Scholar]
- 61.Agency USEP Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry. [(accessed on 17 September 2002)]; Available online: http://www.epa.gov/sites/production/files/2015-08/documents/method_1631e_2002.pdf.
- 62.EPA Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. [(accessed on 15 September 2015)]; Available online: https://www.epa.gov/homeland-security-research/epa-method-7473-sw-846-mercury-solids-and-solutions-thermal-decomposition.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.