Abstract
Contact-active antibacterial surfaces play a vital role in preventing bacterial contamination of artificial surfaces. In the past, numerous researches have been focused on antibacterial surfaces comprising of antifouling upper-layer and antibacterial sub-layer. In this work, we demonstrate a reversed surface structure which integrate antibacterial upper-layer and antifouling sub-layer. These surfaces are prepared by simply casting gemini quaternary ammonium salt waterborne polyurethanes (GWPU) and their blends. Due to the high interfacial energy of gemini quaternary ammonium salt (GQAS), chain segments containing GQAS can accumulate at polymer/air interface to form an antibacterial upper-layer spontaneously during the film formation. Meanwhile, the soft segments composed of polyethylene glycol (PEG) formed the antifouling sub-layer. Our findings indicate that the combination of antibacterial upper-layer and antifouling sub-layer endow these surfaces strong, long-lasting antifouling and contact-active antibacterial properties, with a more than 99.99% killing efficiency against both gram-positive and gram-negative bacteria attached to them.
Microbial contamination occurring on artificial surfaces invariably leads to the formation of biofilms which have been associated with a variety of multi-resistant bacterial strains and resilient infections1,2. Moreover, biofilms may also cause material damage due to the secretion of embedded cells can degrade most man-made materials3,4. These hazards have threaten human health and caused functional failure in the applications of medical implants and devices5,6,7,8, water purification systems9,10, and ship hulls11,12, etc. To overcome these issues, various contact-active antibacterial materials derived from antibacterial polymers have been developed3,13,14. These materials are capable of perpetuating service life while avoiding the long-term cumulative toxicity originated from the release of biocides into the surrounding environment3,15,16,17. More importantly, contact-active antibacterial agents immobilized onto these material surfaces are less likely to induce resistant microbes18.
A significant issue with most current contact-active antibacterial surfaces is that they can easily be masked by biomolecules (such as proteins) or residues of dead cells, blocking further interactions with pathogens and triggering other undesired adverse effects19. The development of environmental friendly antibacterial materials that integrate contact-active biocidal and anti-fouling properties is therefore a promising approach to combat microbial contamination20,21. Recent approaches are based on a kind of surface combining biocidal sub-layer with antifouling upper-layer which is capable of repelling bacteria and killing anchored bacteria escaping from the antifouling upper-layer22,23. Such biocidal sub-layers within these surfaces are possibly blocked from further biocidal activity by dead adherent bacteria, which even leads to subsequent biofilms formation23. Evidently, the adhesion capacity of dead bacteria is much weaker than that of living bacteria, thus it is easier to repel dead bacteria from antifouling surfaces than living bacteria1. Therefore, an alternative to the method combining contact-active antimicrobial upper-layer and antifouling sub-layer will be more effective against bacterial contamination, of which surface is able to kill bacteria on contact first, and then eliminate the dead bacteria more effectively.
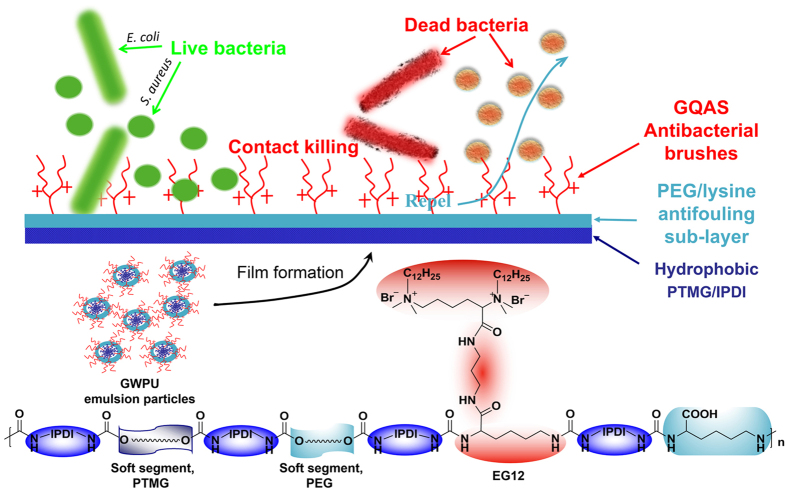
In our previous work, new antibacterial WPUs have been prepared using isophorone diisocyanate (IPDI), polytetramethylene glycol (PTMG), polyethylene glycol (PEG), a lysine-derivate of GQAS (N,N,N’,N’-tetramethyl-N,N’-bisdodecyl-2,6-bis(ammonium bromide)–L–lysine-(1′,3′- propylene diamide)–L-lysine, named EG12) and L-lysine via a simple polymerization process24. The structures of these WPUs are illustrated in Fig. 1. The samples are denoted as GWPU20, GWPU30, GWPU50, GWPU70 and GWPU100, of which the theoretical molar fraction of EG12 in chain extenders is 20, 30, 50, 70, and 100%, respectively. Also, polyurethane without EG12 as a blank is denoted as GWPU0 ((Supplementary Table S1). The chain extender EG12 applied in these WPUs possess much stronger surface activity and permanent excellent antibacterial activity than the normal single-chain quaternary ammonium salts25,26,27,28. More importantly, such antibacterial surfactant can accumulate at polymer/air interface to form antibacterial brushes because of the high interfacial energy29,30. Therefore, we demonstrate here novel contact-active antibacterial and antifouling surfaces constructed from these antibacterial GWPUs. These surfaces are comprised of GQAS antibacterial brushes and an antifouling sub-layer (Fig. 1). The antifouling sub-layer consisted of PEG and carboxyl anions of L-lysine could remove proteins and the residue from dead bacteria cells.
Figure 1. The schematic of antibacterial and antifouling gemini quaternary ammonium salt WPU (GWPU) films and the corresponding chemical structure.
Results and Discussion
Surface characterization of GWPU films
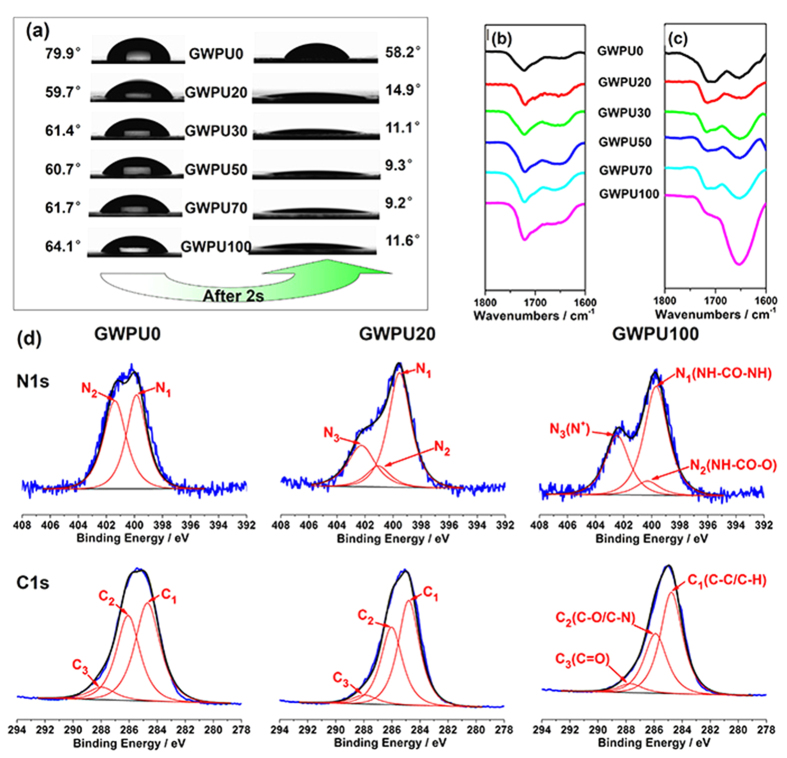
For contact-active antibacterial materials, in general, high concentrations of antibacterial agents on their surfaces are desirable. Taking full advantage of high interfacial energy of GQAS, a series of special GWPU films containing antibacterial brushes over an antifouling layer are prepared by simply casting GWPU emulsions on the bottoms of siliconized culture dishes, followed by air-drying at room temperature. As a result, the GQAS in GWPUs could spontaneously migrate and aggregate onto the surfaces of the films during forming process. Therefore, the water contact angles (WCAs) of GWPUs are in the range of 59–64° at the beginning of contact, and rapidly decrease with contact time, falling to around 9–15° after 20 s (Fig. 2a, Supplementary Fig. S1). In contrast, the WCAs of the control (GWPU0) change from 80° to 58° during the observation period (Fig. 2a, Supplementary Fig. S1). Water could spread out on the surfaces of these GWPU films, indicating that the hydrophilicity of GWPU surfaces is significantly increased by the incorporation of GQAS31,32. These phenomena are mainly attributed to cationic GQAS migrating to the uppermost polyurethane surfaces31.
Figure 2. The surface structures of GWPU films.
(a) WCAs of GWPU films. (b) The transmission FTIR spectra of GWPU films. (c) ATR-FTIR spectra of GWPU films. (d) High resolution N1s and C1s spectra of XPS at 30° take-off angle. For more details, see Supplementary Fig. S3.
Comparing the attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) spectra representing the surface character of these GWPUs with the transmission Fourier transform infrared (FTIR) spectra representing the bulk character, the adsorption intensities of peaks around 1650 cm−1 attributed to carbonyls in urea groups in the ATR-FTIR spectra (Fig. 2c, Supplementary Fig. S2b) are much stronger than those in transmission FTIR spectra (Fig. 2b, Supplementary Fig. S2a), and are enhanced as the GQAS concentration in these polyurethanes increased (Fig. 2c).
To further verify and quantify the GQAS accumulating on the surfaces of the GWPU films, the atomic percentages of carbon, oxygen, and nitrogen on these surfaces are measured by X-ray Photoelectron Spectroscopy (XPS) (Fig. 2d, Supplementary Fig. S3, and Table S2). The atomic percentages of positive nitrogen atoms (N+) are taken to represent the amount of GQAS on the GWPU surfaces. The N1s peaks at around 402.5 eV originating from the GQAS nitrogen (N+) can be detected in GWPU20 and GWPU100 (Fig. 2d)33. The atomic percentages of N+ on these surfaces are 6.08 and 2.25 times higher than those in their corresponding bulks, which are up to 2.19% and 3.10%, respectively (Supplementary Table S2), again, suggesting that GQAS surfactants can accumulate at the film/air interfaces of GWPU films owing to their high interfacial energy34. These surfaces therefore have excellent antibacterial activity, the details will be discussed in a later section. Additionally, the atomic percentage of oxygen on the surface of GWPU0 films is slightly higher than in the bulks owing to the migration of PEG segments to the surfaces (Supplementary Table S2)35,36. For GWPU20 and GWPU100 samples, however, the reverse is true, probably because their uppermost surfaces are covered by GQAS (Supplementary Table S2). These results confirm that the surfaces of the GWPU films are formed by an upper GQAS antibacterial brush layer and an antifouling PEG and carboxyl anion sub-layer, as depicted in Fig. 1.
Antibacterial properties of GWPU film surfaces
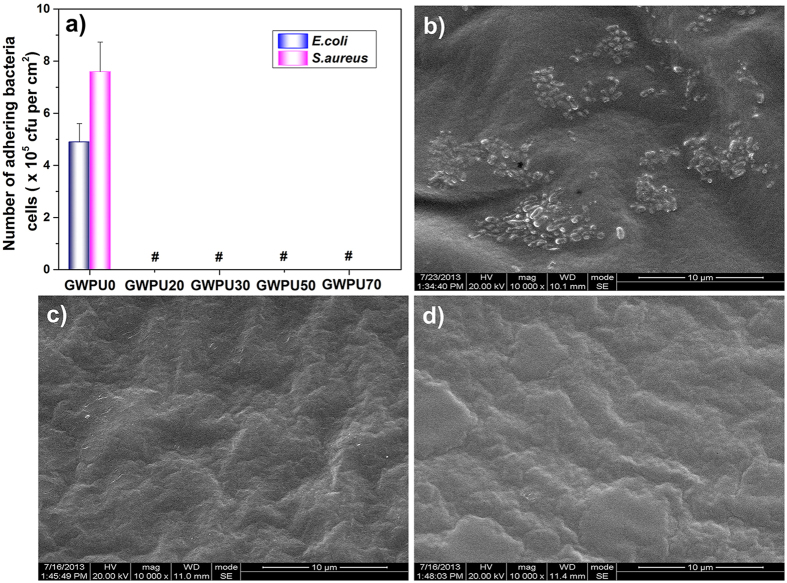
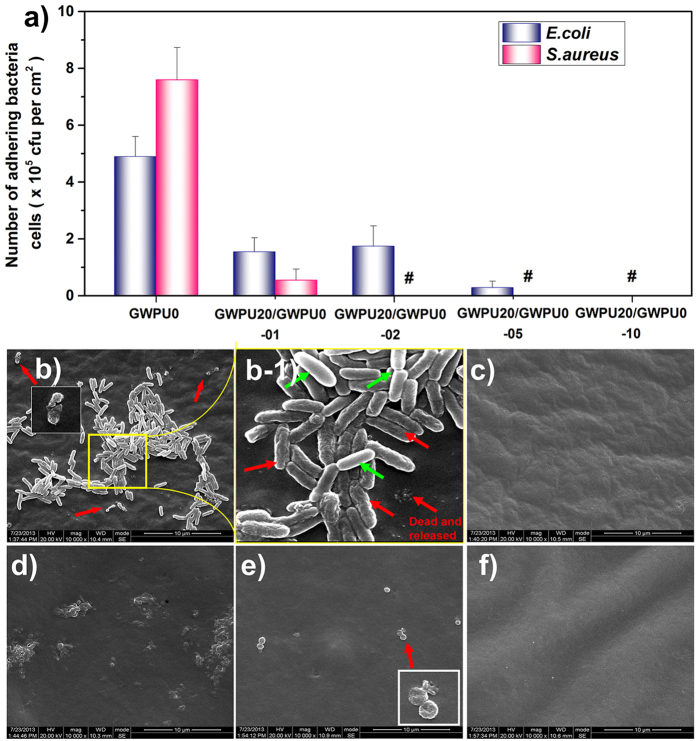
To evaluate antibacterial and antifouling activities of these surfaces, these films are subjected to a measurement conducted by the shake-flask method37,38,39. We cannot detect any living Escherichia coli (E. coli) or Staphylococcus aureus (S. aureus) cells attaching to GWPU films, even those formed by GWPU20, the one with the lowest GQAS content (Fig. 3a, Supplementary Table S3). In contrast, 4.9 × 105 CFU/ml living E. coli cells and 7.6 × 105 CFU/ml living S. aureus cells are found attaching to the surface of GWPU0 films (Supplementary Table S3). This is corroborated by scanning electron microscope (SEM) images. GWPU0 films are covered by E. coli (Fig. 3b) while no bacteria cells or their residues stay on GWPU20 films (Fig. 3c), indicating that antifouling surfaces can merely restrain bacteria residue adhesion, instead of living bacteria. The long-term antibacterial and antifouling activities of GWPU20 films are also tested, with the results showing that the antibacterial and antifouling effects are not diminished, even after washing in water (110 rpm, 37 °C) for 7 days (Fig. 3d, Supplementary Fig. S4 and Table S3). These results demonstrate that all the GQAS-containing WPU films show excellent antibacterial and antifouling properties.
Figure 3. Antifouling and antimicrobial performances of GWPU films.
(a) The number of living bacteria cells attaching on GWPU surfaces. # no bacteria cell was observed. (b) SEM image of GWPU0 film, (c) SEM image of GWPU20 film, (d) SEM image of GWPU20-7d film. Scale bars in (b–d) represent 10 μm.
Surface structure and contact-active antibacterial properties of GWPU blend films
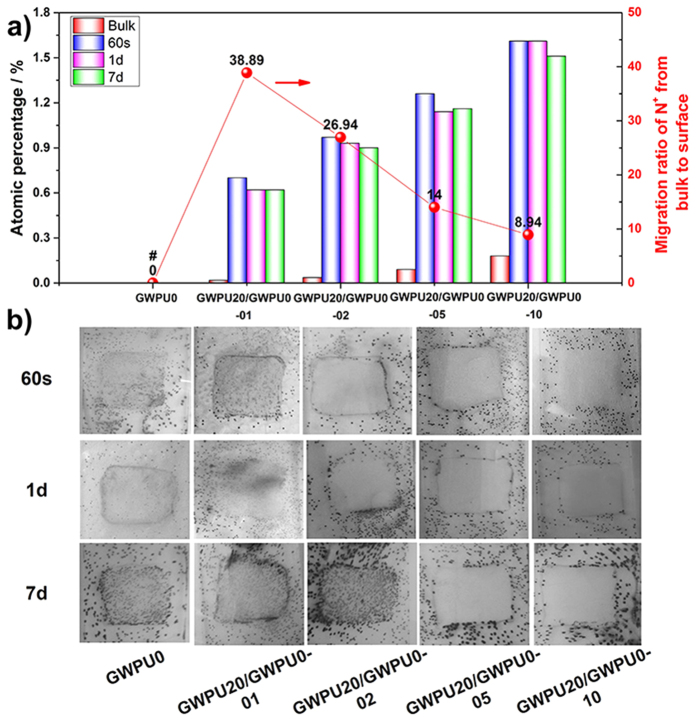
To demonstrate that blending gemini-containing WPU with GWPU0 can also form such surfaces with an upper GQAS antibacterial brush layer and an antifouling sub-layer, a series of GWPU blends with different GQAS content (GWPU20/GWPU0-01, 02, 05, and 10, of which the theoretical molar fraction of GQAS in GWPU blends is 1, 2, 5 and 10%, respectively) are prepared. Even though the GQAS content of these blend films is dramatically reduced, their surfaces retained good hydrophilicity (Supplementary Fig. S1), because the GQAS moieties can readily migrate to the surfaces of blend films with a higher migration ratio (8.94–38.89) than that of the GWPU series (2.25–6.08). Besides, XPS analysis demonstrates that the atomic percentage of N+ on the surface is merely reduced a little, even in the blends films washed in water for 7 days (Fig. 4a, Supplementary Fig. S3, and Table S2).
Figure 4. Surface structure and contact-active antibacterial activity of GWPU blends films.
(a) Atomic percentages of N+ on the surface of the GWPU blend films (after 60 s, 1 day, and 7 days of washing in water, respectively) and migration ratio of N+ from bulk to surface of GWPU blend films obtained from XPS spectra. # no N+ was detected in GWPU0. (b) Photographs of GWPU blend films coated on glass slides with the size of 1.5 cm × 1.5 cm (after 60 s, 1 day and 7 days of washing in water, respectively) and sprayed with S. aureus aqueous suspensions (106 CFU/ml) in phosphate buffer saline (PBS), air dried for 10 min, incubated with in a nutrient broth (0.8% agar) medium at 37 °C for 24 h, stained with 3 mL 5% 2, 3, 5-triphenyltetrazolium chloride (TTC). Each black dot corresponds to a bacterial colony grown from a single surviving bacteria cell.
Then, we employ a glass slide spreading method to investigate the contact-active antibacterial activity of the films made from these blends12, using GWPU0 as a negative control. No inhibition zones are observed, and their contact-active antibacterial activity enhance with increasing GWPU20 content (Fig. 4b). As a result, no S. aureus colonies are found on the films, including those which have been washed for 7 days in water prior to testing (Fig. 4b). The results suggest that effective contact-active antimicrobial activity against S. aureus requires a minimal concentration of antibacterial GQAS moieties in their bulks (0.99%, Supplementary Table S3), or a minimal N+ atomic percentage on the surfaces (0.93%, Fig. 4a, Supplementary Table S2).
To further evaluate the antibacterial and antifouling properties of blend films, the shake-flask method is employed. The GWPU20/GWPU0-01 surface containing 0.5 wt% EG12 could reduce surface-attached E. coli by 68.4%, and surface-attached S. aureus by 92.8% compared with GWPU0 film surfaces, and GWPU20/GWPU0-05 films (EG12: 2.48 wt%) exhibited a reduction of more than 99.99% in surface-attached S. aureus, and of 94.1% in surface-attached E. coli (Fig. 5a, Supplementary Table S3). As the EG12 content increase to 4.96 wt% (GWPU20/GWPU0-10), all surface-attached E. coli and S. aureus are killed (Fig. 5a, Supplementary Table S3). The result shows that the films containing more than 4.96 wt% EG12 have antifouling and antibacterial activity against both gram-positive and gram-negative bacteria. To demonstrate the mechanism through which attached bacteria are killed and their residues are dispersed, we use SEM to monitor morphological changes of the bacteria attaching to the films after 2 days of culture (Fig. 5b,f). The outcome of contact killing and shedding of attached E. coli can be observed in SEM images of GWPU20/GWPU0-05 (Fig. 5b). E. coli cell membranes first become distorted and wrinkled, and then broken, leading to cell death and the gradual shedding of residues from the film surface40. However, the E. coli adhering to other cells rather than directly attaching to films still retained their regular shape (Fig. 5b-1). Similar observations are made on S. aureus cells killed on the surface of a GWPU20/GWPU0-01 film (Fig. 5e). The contact-active antibacterial and antifouling mechanisms can thus be summarized as follows: both gram-positive and gram-negative bacterial cells contain a net negative charged outer envelope41. The main strategy for designing cationic GWPU has been determined by this common structural features. Cationic gemini ammonium salts can interact with the negative charged cell wall of gram-positive bacteria or the outer membrane of gram-negative bacteria or the cytoplasmic membrane of the both bacteria. Moreover, recent researches demonstrate that the positively charged quaternary ammonium salts on the polyurethane film surfaces first interact with the negatively charged phospholipid head groups of the bacteria cytoplasmic membrane, causing general perturbation of the lipid bilayer. The long hydrophobic alkyl chains then pierce the membranes of these surface-attached bacteria, forming holes that cause cytoplasm leakage, lysis, and death42,43. Because hydrophilic PEG chains combining with carboxyl anions exhibit a high resistance to protein adsorption21,44, the bacterial residues remaining on the film surface are shed and the contact-active antibacterial function restored (Fig. 5b,f).
Figure 5. Antibacterial and antifouling performances of GWPU blends films.
(a) Amounts of living bacteria cells attached on GWPU blend films. # no living bacteria cells were observed. Morphology of E. coli (b,c) and S. aureus (d–f) attached to GWPU blend films. (b) GWPU20/GWPU0-05, (b-1) The magnified image of (b), (c) GWPU20/GWPU0-10. (d) GWPU0, (e) GWPU20/GWPU0-01, (f) GWPU20/GWPU0-05. Red arrows indicate distortions and wrinkles in the membrane of dead bacteria after contact with the film; green arrows indicate living bacteria. Scale bars in b-f represent 10 μm.
Conclusion
In summary, a series of novel surfaces containing contact-active antibacterial upper-layer and antifouling sub-layer are prepared by simply casting GWPUs and their blends, as in which antibacterial GQAS brushes are positioned above a PEG and carboxyl anion antifouling layer. At EG12 contents above 4.96 wt%, the GWPUs surfaces possesses strong, long-lasting antifouling and contact-active antibacterial activity, with a more than 99.99% killing efficiency against attached gram-positive and gram-negative bacteria. This novel surface structure may provide new insights for the better designing of contact-active antibacterial and antifouling surfaces. The GWPUs with non-fouling and antimicrobial properties could potentially be used in a wide range of biomedical and industrial applications.
Materials and Methods
Waterborne polyurethane preparation
Waterborne polyurethane and gemini quaternary ammonium salt waterborne polyurethane (GWPU) were synthesized as described in our previous report24. Briefly, GWPU emulsions are prepared using isophorone diisocyanate (IPDI), polytetramethylene glycol (PTMG), polyethylene glycol (PEG), a lysine-derivate of GQAS (N,N,N’,N’-tetramethyl-N,N’-bisdodecyl-2,6-bis(ammonium bromide)–L–lysine-(1′,3′- propylene diamide)–L-lysine, named EG12) and L-lysine via a simple polymerization process.
Preparation of GWPU films
GWPU films were prepared through casting the GWPU emulsions on the surfaces of siliconized culture dishes and drying at room temperature for 2 days, then putting into an oven at 60 °C for 2 days, followed by 60 °C under vacuum for 2 days. The films were cut in sheets with 1 cm × 1 cm in size and approximately 0.5 mm thickness for physicochemical characterization. All GWPU films were immersed in water in a horizontal laboratory shaker (110 rpm, 37 °C) for 60 s, and then dried at 60 °C under vacuum for 2 days before testing, including antibacterial and antifouling activity, WCA, and ATR-FTIR measurements.
Preparation of the waterborne GWPU blending films
Waterborne GWPU20 and GWPU0 were blended at the ratio of 1:19, 1:9, 1:3, 1:1, thus to obtain GWPU20/GWPU0-01, GWPU20/GWPU0-02, GWPU20/GWPU0-05, GWPU20/GWPU0-10 samples of these blends, respectively. These blend films were also prepared with a similar process described above.
Samples for XPS analysis and contact-active antibacterial test
Samples were prepared through casting the emulsions (50 μl) onto cover glasses for XPS analysis and glass slides for contact-active antibacterial test (1.5 × 1.5 cm2) and drying. Then these samples were immersed in water at 37 °C for 60 s, 1 day or 7 days, respectively. The water was replaced every 12 hours in the first day, then every 2 days in the following time. Samples were taken out at the set time and dried in vacuum oven.
Water contact angle (WCA) measurement
The surface hydrophilicity of various multi-block GWPUs is determined by contact angle measurement. Water contact angles were obtained on a Drop Shape Analysis System DSA 100 (Krüss, Hamburg, Germany) and 3 μL of distilled water at room temperature. The results were the mean values of three replicates.
Fourier transform infrared (FTIR) spectra
Attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) was recorded on a Nicolet 6700 spectrometer (Thermo Electron Corporation, USA) between 4000 and 600 cm−1, with a resolution of 4 cm−1. Each sample spectrum was obtained by averaging 32 scans.
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy (XPS) was determined by a Kratos XSAM-800 Spectrometer with a Mg KR. The X-ray gun was operated at 20 kV and 10 mA current with a take-off angle of 30°. The relative atomic percentage of each element on the GWPU films and blend films was calculated by the peak areas using atomic sensitivity factors specified for the XSAM-800. C1s, O1s and N1s spectra bands were deconvoluted into sub-peaks by processing with the XPSPEAK4.0 spectrometer software.
Evaluation of antibacterial and antifouling activities of GWPU films
To eliminate the influences of water-soluble antibacterial moieties onto these films of GWPUs on evaluation of their antibacterial and antifouling activities, all the films were immersed in water in a horizontal laboratory shaker (110 rpm, 37 °C) for 1 day or 7 days to a constant weight before the antibacterial test (Supplementary Fig. S4). The antibacterial and antifouling activities of these films were assessed against both E. coli (ATCC 25922) and S. aureus (ATCC 6538) according to shaking flask methods37,38,39. Each GWPU film (1.0 × 1.0 cm2, 0.5 mm thickness) was placed in a well of 24-well plate and sterilized under UV overnight. E. coli and S. aureus strain cultures in the nutrient broth (NB) were grown overnight at 37 °C and diluted to 107 CFU/ml. This bacteria strain culture (2 mL) was added into each well with one GWPU film. The 24-well plate was then placed in a constant temperature incubator at 37 °C, 110 rpm for 2 days. After that, the films were taken out and rinsed three times with sterile deionized water, and then placed into another tube into which 2 mL of sterile water was added. The bacteria were detached in an ultrasonic cleaner for 5 min and diluted serially to proper concentration then counted by the flat colony counting method37. Each dilution was plated in triplicate on a nutrient agar plate and incubated at 37 °C for 24 h. The number of CFU at each dilution rate was counted after incubation and the average CFU/ml was determined.
Contact-active antibacterial activity
GWPU films were transferred on to glass slides for the contact-active antibacterial test. Glass slides and GWPU films on them were sterilized by UV-irradiation for 30min before contact-active antibacterial test. In the test, aqueous suspensions of bacteria were sprayed onto both glass slides and GWPU films on glass slides followed by air-drying for 10 min. Afterward, the inoculated slides were transferred into Petri dishes and then the semi-solid nutrient agar (0.8% agar in a nutrient broth, autoclaved at 121 °C for 25 min, and cooled to 40 °C) was poured into Petri dishes slowly to make sure that the spayed cells would not be washed off. Then Petri dishes were sealed and placed in the incubator at 37 °C for 24 h. This allows the initially sprayed cells on glass slides and GWPU films to grow while being in contact with the surface. Finally, CFUs were stained with 3 ml 5% TTC (0.5 mg/ml) for observation and counting43.
The antibacterial and antifouling activity of the blend GWPU films
As aforementioned in the part of evaluation of antibacterial and antifouling activities of GWPU films, the blend films were firstly washed in water (110 rpm, 37 °C) for 1 day or 7 days, and then the antibacterial and antifouling test were conducted. After 2 days incubation (110 rpm, 37 °C), the films were taken out and rinsed three times with sterile deionized water. The bacteria adhered to these films were counted by the flat colony counting method. The remaining films were immediately fixed with glutaraldehyde solution (2.5%) for 4 hours, then dehydrated by adding a graded series of aqueous ethanol (20%, 30%, and 50%) and dried in vacuum freeze drier. The bacteria adhered to the films were observed with scanning electron microscopy (SEM, Inspect F, FEI Company) for morphology changes. The numbers of live bacteria adhered to these GWPU and their blends films surface, and the antibacterial ratio of these film are shown in Supplementary Table S3.
Additional Information
How to cite this article: He, W. et al. A Novel Surface Structure Consisting of Contact-active Antibacterial Upper-layer and Antifouling Sub-layer Derived from Gemini Quaternary Ammonium Salt Polyurethanes. Sci. Rep. 6, 32140; doi: 10.1038/srep32140 (2016).
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos 51173118, 51273124, and 51273126) and the National Science Fund for Distinguished Young Scholars of China (No. 51425305).
Footnotes
Author Contributions W.H. and Y.Z. completed most of the experiments and wrote the manuscript. Y.G. synthesized the WPUs. F.L., K.W. and Q.F. provided valuable suggestions on the data analysis. F.L., J.L. and H.T. revised the manuscript. J.L. and H.T. the corresponding authors of this study, designed and supervised this project.
References
- Tuson H. H. & Weibel D. B. Bacteria-surface interactions. Soft Matter 9, 4368–4380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A. S. & Robertson G. T. Bacterial and fungal biofilm infections. Annu. Rev. Med. 59, 415–428 (2008). [DOI] [PubMed] [Google Scholar]
- Siedenbiedel F. & Tiller J. C. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers 4, 46–71 (2012). [Google Scholar]
- Macedo M. F., Miller A. Z., Dionisio A. & Saiz-Jimenez C. Biodiversity of cyanobacteria and green algae on monuments in the Mediterranean Basin: an overview. Microbiology 155, 3476–3490 (2009). [DOI] [PubMed] [Google Scholar]
- Asri L. A. T. W. et al. A Shape-Adaptive, Antibacterial-Coating of Immobilized Quaternary-Ammonium Compounds Tethered on Hyperbranched Polyurea and its Mechanism of Action. Adv. Funct. Mater. 24, 346–355 (2014). [Google Scholar]
- Kazemzadeh-Narbat M. et al. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials 34, 5969–5977 (2013). [DOI] [PubMed] [Google Scholar]
- Tang Z., Wang Y., Podsiadlo P. & Kotov N. A. Biomedical Applications of Layer-by-Layer Assembly: From Biomimetics to Tissue Engineering. Adv. Mater. 18, 3203–3224 (2006). [Google Scholar]
- Wang J. et al. Biodegradable Hydrophilic Polyurethane PEGU25 Loading Antimicrobial Peptide Bmap-28: A Sustained-release Membrane Able to Inhibit Bacterial Biofilm Formation in Vitro. Sci. Rep. 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar M. U. et al. Biopolymer-reinforced synthetic granular nanocomposites for affordable point-of-use water purification. Proc Natl Acad Sci USA 110, 8459–8464 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M. et al. Magnetically ultraresponsive nanoscavengers for next-generation water purification systems. Nat. Commun. 4, 1866 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fernandez L. et al. Antibacterial strategies from the sea: polymer-bound cl-catechols for prevention of biofilm formation. Adv. Mater. 25, 529–533 (2013). [DOI] [PubMed] [Google Scholar]
- Goddard J. M. & Hotchkiss J. H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 32, 698–725 (2007). [Google Scholar]
- Zhang Q. M. & Serpe M. J. Synthesis, Characterization, and Antibacterial Properties of a Hydroxyapatite Adhesive Block Copolymer. Macromolecules 47, 8018–8025 (2014). [Google Scholar]
- Xiong M. H. et al. Bacteria-responsive multifunctional nanogel for targeted antibiotic delivery. Adv. Mater. 24, 6175–6180 (2012). [DOI] [PubMed] [Google Scholar]
- Fuchs A. D. & Tiller J. C. Contact-active antimicrobial coatings derived from aqueous suspensions. Angew. Chem. Int. Ed. 45, 6759–6762 (2006). [DOI] [PubMed] [Google Scholar]
- Smith A. W. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv. Drug Delivery Rev. 57, 1539–1550 (2005). [DOI] [PubMed] [Google Scholar]
- Hoffman L. R. et al. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436, 1171–1175 (2005). [DOI] [PubMed] [Google Scholar]
- Waschinski C. J. et al. Design of Contact-Active Antimicrobial Acrylate-Based Materials Using Biocidal Macromers. Adv. Mater. 20, 104–108 (2008). [Google Scholar]
- Cheng G., Xue H., Zhang Z., Chen S. & Jiang S. A switchable biocompatible polymer surface with self-sterilizing and nonfouling capabilities. Angew. Chem. Int. Ed. 47, 8831–8834 (2008). [DOI] [PubMed] [Google Scholar]
- Sundaram H. S., Ella-Menye J.-R., Brault N. D., Shao Q. & Jiang S. Reversibly switchable polymer with cationic/zwitterionic/anionic behavior through synergistic protonation and deprotonation. Chem. Sci. 5, 200–205 (2014). [Google Scholar]
- Liu S. Q. et al. Antimicrobial and antifouling hydrogels formed in situ from polycarbonate and poly(ethylene glycol) via Michael addition. Adv. Mater. 24, 6484–6489 (2012). [DOI] [PubMed] [Google Scholar]
- Sui Y., Gao X., Wang Z. & Gao C. Antifouling and antibacterial improvement of surface-functionalized poly(vinylidene fluoride) membrane prepared via dihydroxyphenylalanine-initiated atom transfer radical graft polymerizations. J. Membr. Sci. 394–395, 107–119 (2012). [Google Scholar]
- Ho C. H., Tobis J., Sprich C., Thomann R. & Tiller J. C. Nanoseparated polymeric networks with multiple antimicrobial properties. Adv. Mater. 16, 957–961 (2004). [Google Scholar]
- Zhang Y. et al. Synthesis and antibacterial characterization of waterborne polyurethanes with gemini quaternary ammonium salt. Sci. Bull. 60, 1114–1121 (2015). [Google Scholar]
- Lin J., Qiu S., Lewis K. & Klibanov A. M. Bactericidal properties of flat surfaces and nanoparticles derivatized with alkylated polyethylenimines. Biotechnol. Prog 18, 1082–1086 (2002). [DOI] [PubMed] [Google Scholar]
- Tan H. & Xiao H. Synthesis and antimicrobial characterization of novel l-lysine gemini surfactants pended with reactive groups. Tetrahedron Lett. 49, 1759–1761 (2008). [Google Scholar]
- Kourai H., Yabuhara T., Shirai A., Maeda T. & Nagamune H. Syntheses and antimicrobial activities of a series of new bis-quaternary ammonium compounds. Eur. J. Med. Chem. 41, 437–444 (2006). [DOI] [PubMed] [Google Scholar]
- Menger F. M. & Keiper J. S. Gemini surfactants. Angew. Chem. Int. Ed. 39, 1906–1920 (2000). [DOI] [PubMed] [Google Scholar]
- Arnold C. et al. Surfactant distribution in waterborne acrylic films. Colloids Surf., A 374, 58–68 (2011). [Google Scholar]
- Lee W. P. et al. Distribution of surfactants in latex films: a Rutherford backscattering study. Langmuir 22, 5314–5320 (2006). [DOI] [PubMed] [Google Scholar]
- Ding M. et al. Synthesis, degradation, and cytotoxicity of multiblock poly (ε-caprolactone urethane) s containing gemini quaternary ammonium cationic groups. Biomacromolecules 10, 2857–2865 (2009). [DOI] [PubMed] [Google Scholar]
- Song N.-j. et al. The degradation and biocompatibility of waterborne biodegradable polyurethanes for tissue engineering. Chin. J. Polym. Sci. 31, 1451–1462 (2013). [Google Scholar]
- Li J. et al. Synthesis and surface properties of polyurethane end-capped with hybrid hydrocarbon/fluorocarbon double-chain phospholipid. J. Biomed. Mater. Res., A 101, 1362–1372 (2013). [DOI] [PubMed] [Google Scholar]
- Wynne J. H., Fulmer P. A., McCluskey D. M., Mackey N. M. & Buchanan J. P. Synthesis and development of a multifunctional self-decontaminating polyurethane coating. ACS Appl. Mater. Interfaces 3, 2005–2011 (2011). [DOI] [PubMed] [Google Scholar]
- Kurt P., Wood L., Ohman D. E. & Wynne K. J. Highly effective contact antimicrobial surfaces via polymer surface modifiers. Langmuir 23, 4719–4723 (2007). [DOI] [PubMed] [Google Scholar]
- Ding M. et al. Effect of PEG content on the properties of biodegradable amphiphilic multiblock poly(ε-caprolactone urethane)s. Polym. Chem. 2, 885 (2011). [Google Scholar]
- Gao G. et al. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials 32, 3899–3909 (2011). [DOI] [PubMed] [Google Scholar]
- Bakhshi H. et al. Synthesis and characterization of antibacterial polyurethane coatings from quaternary ammonium salts functionalized soybean oil based polyols. Mater. Sci. Eng., C 33, 153–164 (2013). [DOI] [PubMed] [Google Scholar]
- Jeon Y.-J., Park P.-J. & Kim S.-K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 44, 71–76 (2001). [Google Scholar]
- Li P. et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 10, 149–156 (2011). [DOI] [PubMed] [Google Scholar]
- Timofeeva L. & Kleshcheva N. Antimicrobial polymers: mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 89, 475–492 (2011). [DOI] [PubMed] [Google Scholar]
- Hoque J. et al. Broad Spectrum Antibacterial and Antifungal Polymeric Paint Materials: Synthesis, Structure–Activity Relationship, and Membrane-Active Mode of Action. ACS Appl. Mater. Interfaces 7, 1804–1815 (2015). [DOI] [PubMed] [Google Scholar]
- Bieser A. M. & Tiller J. C. Mechanistic Considerations on Contact-Active Antimicrobial Surfaces with Controlled Functional Group Densities. Macromol. Biosci. 11, 526–534 (2011). [DOI] [PubMed] [Google Scholar]
- Rana D. & Matsuura T. Surface modifications for antifouling membranes. Chem. Rev. 110, 2448–2471 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.