Abstract
We tested whether treatment with a cholinergic antagonist could reduce insulin levels in early postnatal life and attenuate metabolic dysfunctions induced by early overfeeding in adult male rats. Wistar rats raised in small litters (SLs, 3 pups/dam) and normal litters (NLs, 9 pups/dam) were used in models of early overfeeding and normal feeding, respectively. During the first 12 days of lactation, animals in the SL and NL groups received scopolamine butylbromide (B), while the controls received saline (S) injections. The drug treatment decreased insulin levels in pups from both groups, and as adults, these animals showed improvements in glucose tolerance, insulin sensitivity, vagus nerve activity, fat tissue accretion, insulinemia, leptinemia, body weight gain and food intake. Low glucose and cholinergic insulinotropic effects were observed in pancreatic islets from both groups. Low protein expression was observed for the muscarinic M3 acetylcholine receptor subtype (M3mAChR), although M2mAChR subtype expression was increased in SL-B islets. In addition, beta-cell density was reduced in drug-treated rats. These results indicate that early postnatal scopolamine butylbromide treatment inhibits early overfeeding-induced metabolic dysfunctions in adult rats, which might be caused by insulin decreases during lactation, associated with reduced parasympathetic activity and expression of M3mAChR in pancreatic islets.
The number of individuals who suffer from metabolic diseases is increasing worldwide in both wealthy and poor countries1. Although high caloric intake, sedentary behavior and genetics are the main risk factors for these diseases, stressful conditions during perinatal life, such as nutritional, metabolic and hormonal alterations, also contribute to the development of metabolic diseases in adult life. These observations support the Barker theory and the idea of metabolic programming, i.e., the developmental origins of health and disease concept, which is that early-life conditions can affect later health and development2,3,4,5.
Insulin and other hormones, such as leptin, are peripheral regulators of food intake and body adiposity; furthermore, insulin plays a key role in producing the neural connections that regulate feeding and glucose metabolism in adulthood. The central nervous system (CNS) and autonomic nervous system (ANS) govern important components of metabolism. In humans, the end of pregnancy is a crucial phase for developing neuronal connections6; however, in rodents, CNS development extends further, into the beginning of lactation7.
Insulin secretion is modulated by glucose and several neurotransmitters released from the peripheral autonomic nerves. The major neurotransmitter of the peripheral parasympathetic nervous system (PNS), i.e., acetylcholine (ACh), is known to facilitate the release of insulin in a glucose-dependent manner. This activity has been shown to be mediated by the activation of muscarinic acetylcholine receptors (mAChRs) located on the plasma membrane of pancreatic beta-cells8,9. ANS imbalances, including high parasympathetic and low sympathetic activity, are frequently observed in human and rodent models of overweight and obesity. In addition, these obese individuals exhibit high fasting insulinemia and insulin resistance10,11,12. Indeed, ANS imbalances have been suggested to be one cause of pancreatic beta-cell dysfunction13.
Neuroendocrine disruptions are induced by programming events during rat perinatal life, when the CNS is developing. For example, elevated insulin concentrations during critical periods of development might lead to malprogramming of the hypothalamic networks that regulate body weight and metabolism. In babies born to mothers with gestational diabetes, hyperinsulinemia has been shown to have a high correlation with the development of obesity during later life14. Similarly, transient hyperinsulinism during lactation led to rats becoming overweight and having an increased susceptibility to diabetes in adult life15.
Early hyperinsulinemia also occurs as a result of postnatal overnutrition and can lead to the development of permanent insulin resistance, obesity and diabetes16. In rodents, early postnatal overfeeding can be induced by reducing litter size17. Small litter (SL) rats present with early hyperinsulinemia; develop hyperphagia, high parasympathetic activity18 and impaired glucose tolerance; and become overweight in adulthood more than do normal litter (NL) rats19. Interestingly, early hypoinsulinemia observed in postnatally undernourished rats is associated with low vagus activity and a lean phenotype in later life20,21. In addition, suckling rats whose mothers were exposed to protein restriction during lactation exhibited low insulin levels and low body weights in adulthood22. In addition to its influence on metabolic homeostasis control via the brain and peripheral nervous system during perinatal life, insulin appears to play an important role in metabolic programming23,24.
It has been shown that an intravenous infusion of scopolamine butylbromide, also known as Buscopan, may attenuate the release of insulin in healthy men by competing with ACh for the M3mAChR subtype25,26. Binding M3mAChRs using muscarinic antagonists also inhibits insulin release in rodents25.
Therefore, in the current study, we hypothesized that decreasing insulin levels during lactation could attenuate later metabolic dysfunction induced by early postnatal overfeeding. We administered Buscopan during early lactation to decrease insulin levels and assess the effects on overfeeding-induced metabolism malfunction onset in adult SL rats.
Results
Insulin plasma levels throughout the suckling phase
During the lactation period, the blood insulin levels of SL rats treated with saline (SL-S) were higher than their NL-S counterparts (PL < 0.05), as shown in Fig. 1. The scopolamine butylbromide (B) treatment decreased the insulin levels of NL-B and SL-B rats (PT < 0.01, Fig. 1).
Figure 1. Effect of scopolamine butylbromide on offspring insulinemia throughout the suckling phase.
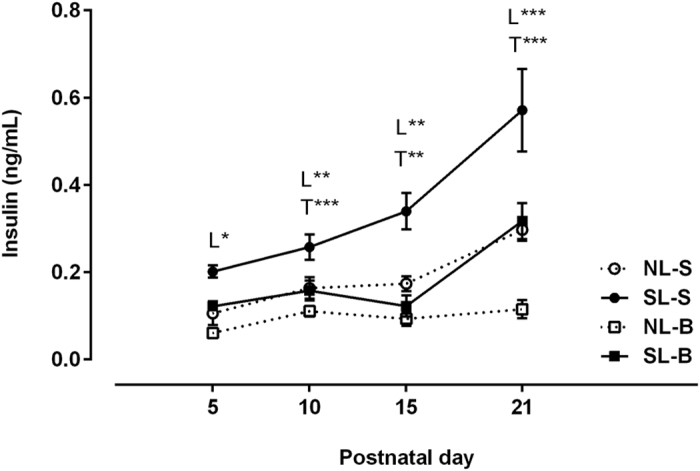
NL-S, normal litter rats treated with saline solution; SL-S, small litter rats treated with saline solution; NL-B, normal litter rats treated with scopolamine butylbromide; SL-B, small litter rats treated with scopolamine butylbromide. L, litter size factor; T, treatment factor. *P < 0.05, **P < 0.01, ***P < 0.001, based on a two-way analysis of variance of 9–12 rats from each experimental group.
Scopolamine butylbromide treatment effects on biometrics, biochemical parameters and insulin sensitivity
As shown in Table 1, reducing litter size caused increases in the body weight (bw) of SL-S rats at weaning (21 days old) and in adulthood (90 days old) of 38% and 10%, respectively (P < 0.05). However, NL-B and SL-B rats showed 20% and 16% reductions in bw at weaning, respectively (P < 0.05), and a 10% reduction in bw at adulthood (both P < 0.05). Food intake increased by 5% in SL-S rats compared with that of NL-S rats (P < 0.05), while NL-B and SL-B rats showed a 10% reduction in food consumption (P < 0.05). Animals in the SL-S group showed weight increases of approximately 20% in all three pads (retroperitoneal, periepididymal and mesenteric) compared with that of animals in the NL-S group (PL < 0.05). The drug treatment induced approximately 15% and 20% decreases in fats pads weight in NL-B and SL-B rats, respectively (PT < 0.05). Leptin blood concentration was doubled in SL-S rats compared with that in NL-S rats (PL < 0.01), and the level of leptinemia was decreased in NL-B and SL-B rats (PT < 0.01). Fasting insulinemia was increased by 71% in SL-S rats compared with NL-S rats; in contrast, NL-B and SL-B rats showed decreases in fasting insulinemia of 42% and 70%, respectively, indicating an interaction (PI < 0.05). SL-S rats presented an 11% increase in fasting glycemia compared with that of NL-S rats; while NL-B rats exhibited an 8% increase in glycemia compared with that of NL-S rats (P < 0.05), no effect was observed in SL-B animals. The rate constant for disappearance of plasma glucose (Kitt) was decreased by 28% in SL-S rats compared with NL-S rats (P < 0.05). Although no significant difference was detected in NL-B rats, SL-B rats exhibited a 40% increase in Kitt. The homeostasis model assessment of insulin resistance (HOMA-IR) was increased by 88% in SL-S rats compared with NL-S rats (P < 0.05). Although no significant difference was detected between NL-S and NL-B rats, SL-B animals exhibited a 68% decrease in HOMA-IR compared with that of SL-S rats, P < 0.05.
Table 1. Effect of scopolamine butylbromide on biometric and biochemical parameters.
| Parameters | NL-S | SL-S | NL-B | SL-B | Factors | ||
|---|---|---|---|---|---|---|---|
| L | T | I | |||||
| Body weight at 21 days of age (g) | 46.2 ± 1.13 | 63.81 ± 1.39 | 36.9 ± 0.81 | 53.42 ± 0.78 | **** | **** | ns |
| Body weight at 90 days of age (g) | 382.3 ± 6.8 | 421.8 ± 7.7 | 345.1 ± 10.1 | 383.5 ± 8.6 | **** | *** | ns |
| Retroperitoneal fat pad (g/100 g bw) | 1.34 ± 0.06 | 1.61 ± 0.07 | 1.12 ± 0.03 | 1.27 ± 0.05 | *** | **** | ns |
| Periepididymal fat pad (g/100 g bw) | 1.13 ± 0.04 | 1.30 ± 0.06 | 0.98 ± 0.01 | 1.00 ± 0.02 | * | **** | ns |
| Mesenteric fat pad (g/100 g bw) | 0.79 ± 0.03 | 0.97 ± 0.06 | 0.65 ± 0.02 | 0.78 ± 0.02 | *** | **** | ns |
| Food intake (g; AUC) | 214.1 ± 2.49 | 236.6 ± 1.92 | 192.0 ± 4.8 | 214.3 ± 5.86 | *** | *** | ns |
| Fasting glycemia (mg/dL) | 90.3 ± 2.05 | 100.3 ± 3.9 | 98.4 ± 1.39 | 101.2 ± 0.58 | ** | * | ns |
| Fasting insulinemia (ng/mL) | 0.21 ± 0.02 | 0.36 ± 0.03 | 0.12 ± 0.07 | 0.11 ± 0.06 | ns | **** | * |
| Fasting leptinemia (pg/mL) | 421.5 ± 88.4 | 843.4 ± 140.7 | 120.7 ± 72.7 | 314.2 ± 165.5 | ** | ** | ns |
| Kitt (%/min) | 3.11 ± 0.34 | 2.22 ± 0.08 | 2.78 ± 0.19 | 3.11 ± 0.28 | ns | ns | * |
| HOMA-IR | 1.19 ± 0.22 | 2.24 ± 0.35 | 0.90 ± 0.13 | 0.71 ± 0.13 | ns | *** | * |
All data are expressed as the mean ± SEM of 15–25 rats from 5 different litters. NL-S, normal litter rats treated with saline solution; SL-S, small litter rats treated with saline solution; NL-B, normal litter rats treated with scopolamine butylbromide; SL-B, small litter rats treated with scopolamine butylbromide. L, litter size factor; T, treatment factor; and I, interaction between litter and treatment factors. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 and ns, no significant difference, based on a two-way analysis of variance.
Plasma glycemia and insulinemia during the intravenous glucose tolerance test (ivGTT)
During the ivGTT, glycemia (Fig. 2a) and insulinemia (Fig. 2b) were increased by 13% and 36%, respectively, in SL-S rats compared with NL-S rats (P < 0.001), as indicated by the area under the curve (AUC) values. Although early drug treatment affected glycemia in the NL-B group (Fig. 2a), insulinemia was reduced by 40% and 50% in the NL-B and SL-B groups, respectively (P < 0.001).
Figure 2.
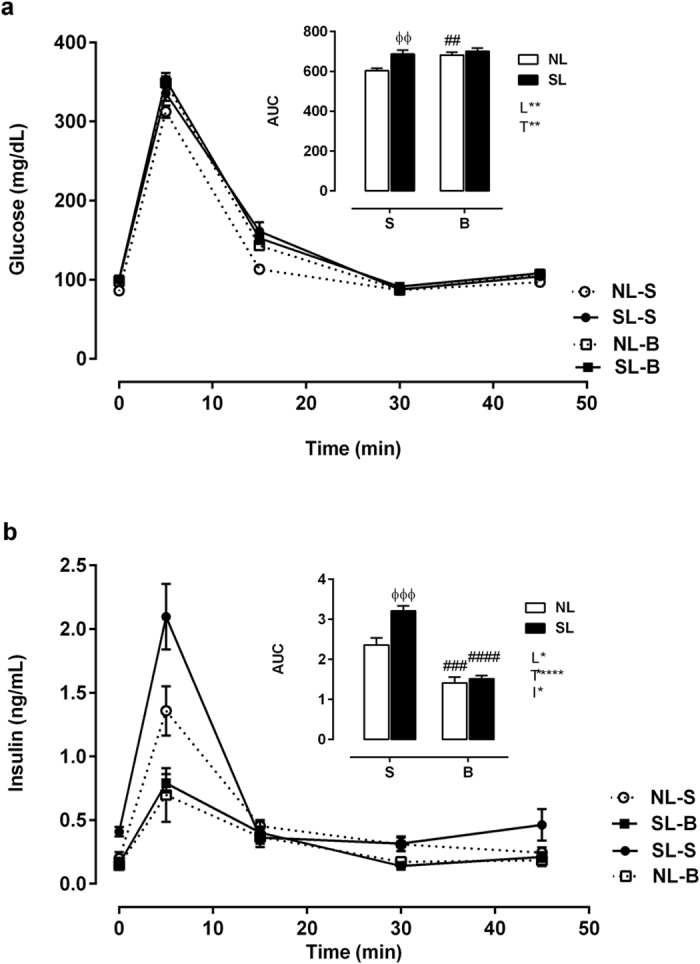
Blood glucose (a) and insulin (b) levels during the ivGTT. The upper panel of each figure represents the area under the curve (AUC). NL-S, normal litter rats treated with saline solution; SL-S, small litter rats treated with saline solution; NL-B, normal litter rats treated with scopolamine butylbromide; SL-B, small litter rats treated with scopolamine butylbromide. Data are expressed as the mean ± SEM of 9–17 rats from each experimental group. L, litter size factor; T, treatment factor; and I, interaction between litter size and treatment factors. *P < 0.05, **P < 0.01, ****P < 0.0001, based on a two-way analysis of variance. ɸɸP < 0.01 and ɸɸɸP < 0.001, statistical significance of differences between NL and SL; ##P < 0.01, ###P < 0.001 and ####P < 0.0001, statistical significance of saline versus scopolamine butyl bromide animals, based on the Tukey multiple comparisons test.
Pancreatic islet function and insulin secretion
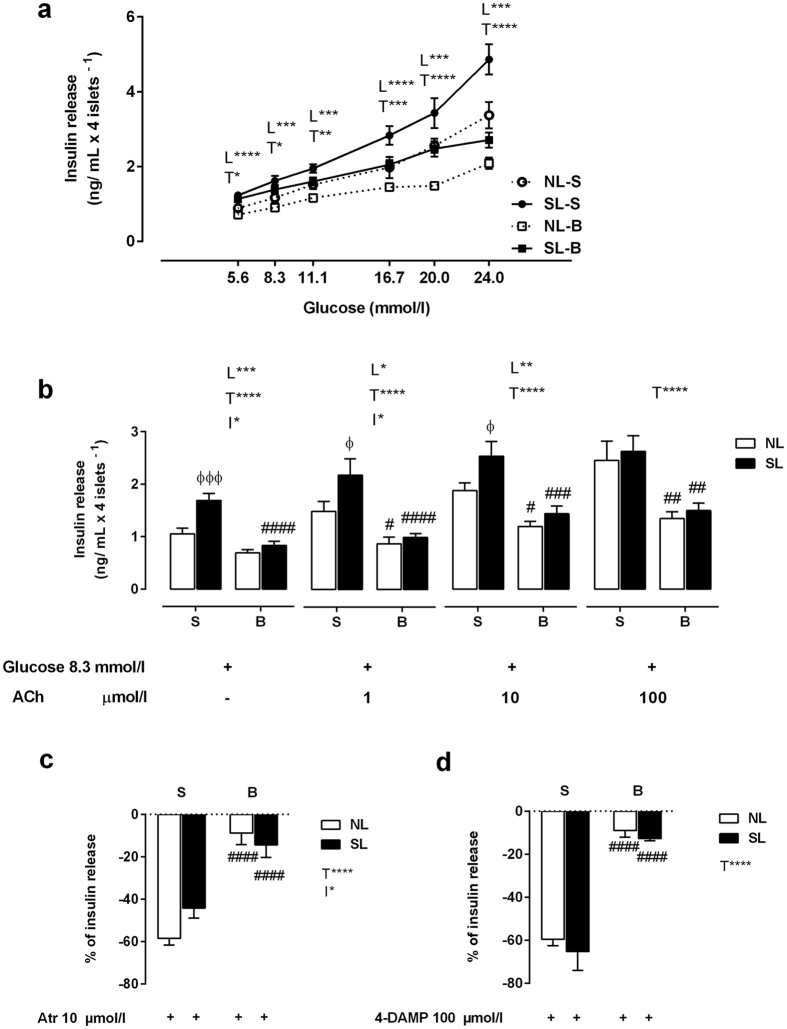
In all groups, pancreatic islets showed a dose-dependent increase in insulin secretion in response to glucose stimulation (Fig. 3a). Islets from SL-S rats secreted more insulin than islets from NL-S rats when stimulated by all the tested glucose concentrations (PL < 0.001). The drug treatment reduced the glucose-induced insulin secretion of islets from NL-B and SL-B animals (PT < 0.05). In addition, islets from rats in all groups showed dose-dependent increases in cholinergic response (Fig. 3b). However, incubation with increasing ACh concentrations showed that SL-S, SL-B and NL-B islets were less responsive to cholinergic action at all ACh concentrations than were NL-S islets (P < 0.05, Fig. 3b). Atropine (Atr), a nonselective muscarinic antagonist, inhibited the cholinergic response of islets isolated from all groups (P < 0.0001); however, this effect was reduced in NL-B and SL-B islets (Fig. 3c). Additionally, a muscarinic antagonist selective to the M3 subtype, 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP), decreased insulin secretion in islets from NL-S and SL-S rats; however, NL-B and SL-B islets were inhibited to a lesser extent (P < 0.0001, Fig. 3d).
Figure 3. Pancreatic islet insulin secretion.
Insulin secretion stimulated by different glucose concentrations (a). Insulin secretion stimulated by 8.3 mmol/l glucose and potentiated by 1, 10 or 100 μmol/l ACh (b). Inhibition of ACh insulinotropic effect (8.3 mmol/l + 10 μmol/l ACh in the presence of 10 μmol/l neostigmine) by the non-selective antagonist Atr (10 μmol/l) (c) and the antagonist selective to M3mAChR, 4-DAMP (100 μmol/l) (d). The results are presented as a percentage of insulin secretion. The line at 0 (c,d) represents 100% ACh insulinotropic action. The presence ( + ) and absence (–) of glucose, ACh, Atr and 4-DAMP is indicated below each corresponding bar. The data were obtained from the pancreatic islets of 6 rats from three different litters of each experimental group. NL-S, normal litter rats treated with saline solution; SL-S, small litter rats treated with saline solution; NL-B, normal litter rats treated with scopolamine butylbromide; SL-B, small litter rats treated with scopolamine butylbromide. L, litter size factor; T, treatment factor; and I, interaction between litter size and treatment factors. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, based on a two-way analysis of variance. ɸP < 0.05 and ɸɸɸɸP < 0.0001, statistical significance of the differences between NL and SL; ##P < 0.01 and ####P < 0.0001, statistical significance of saline versus scopolamine butylbromide animals, based on the Tukey multiple comparisons test.
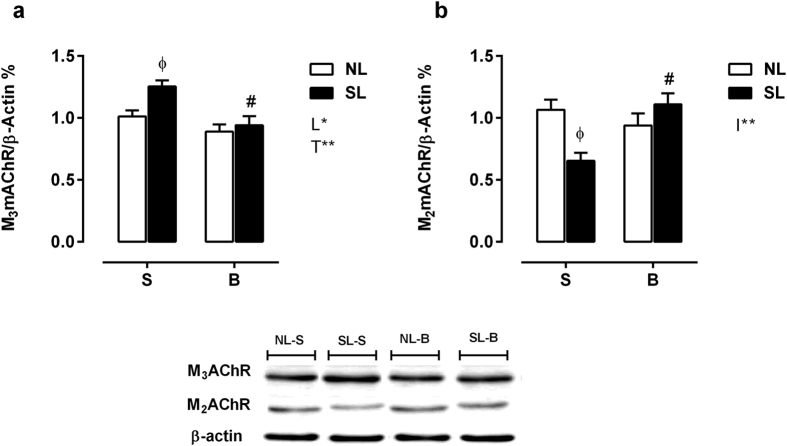
M3mAChR and M2mAChR protein expression in pancreatic islets
M3mAChR expression was 23% higher in SL-S islets than in NL-S islets (P < 0.05), and SL-B islets presented a 24% reduction in M3mAChR expression compared with that of SL-S islets, P < 0.05; however, no differences were observed between NL-B and NL-S islets (Fig. 4a). Compared with NL-S islets, SL-S islets showed a 38% reduction in M2mAChR protein expression (P < 0.05). M2mAChR expression was increased in SL-B islets by 69% compared with that in SL-S islets (P < 0.05), while no changes were observed in NL-B islets (Fig. 4b).
Figure 4.
Measurement of M3 (a) and M2 (b) muscarinic receptor subtype protein levels in pancreatic islets. NL-S, normal litter rats treated with saline solution; SL-S, small litter rats treated with saline solution; NL-B, normal litter rats treated with scopolamine butylbromide treatment; SL-B, small litter rats treated with scopolamine butylbromide treatment. L, litter size factor; T, treatment factor; and I, interaction between litter size and treatment factors. *P < 0.05, **P < 0.01, based on a two-way analysis of variance. ɸP < 0.05, statistical significance of the differences between NL and SL; #P < 0.05, statistical significance of saline versus scopolamine butylbromide animals, based on the Tukey multiple comparisons test (n = 3 different experiments using 3–4 rats from each experimental group).
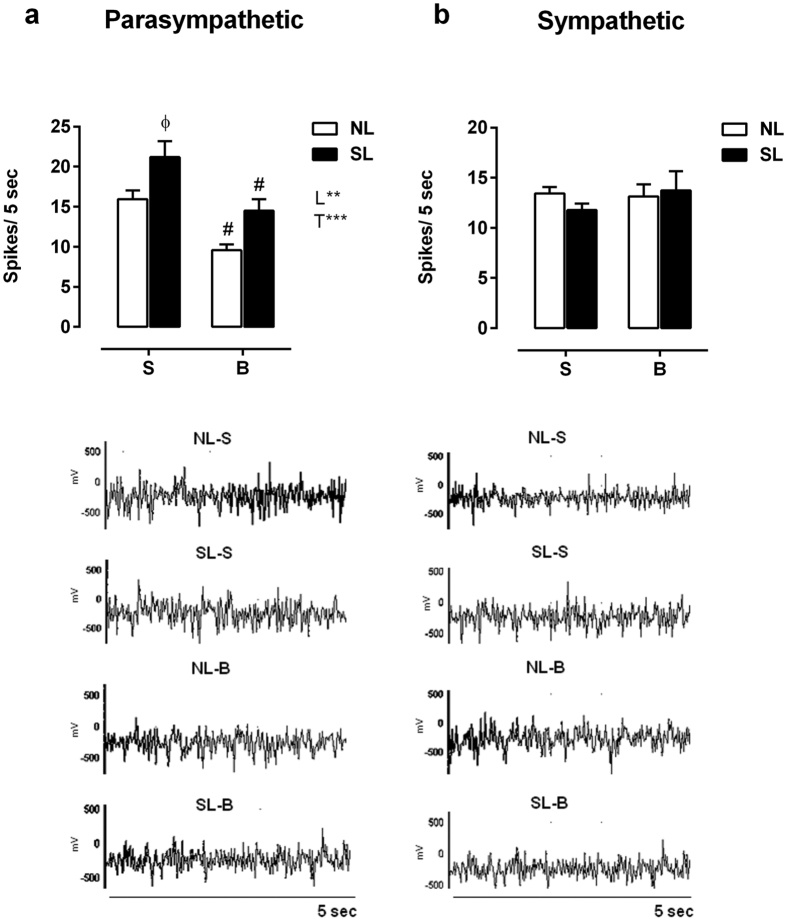
ANS activity
The SL-S animals showed a 32% augmentation in vagus nerve electrical activity when compared with NL-S rats (P < 0.05), while the NL-B and SL-B groups showed reduced vagus nerve activity (PT < 0.05, Fig. 5a). There were no changes in sympathetic electrical nerve activity in any group (Fig. 5b).
Figure 5.
Parasympathetic (a) and sympathetic (b) electrical nerve activity. Data are expressed as the mean ± SEM of 15–17 rats from each experimental group. Lower panel graphs are representative records of nerve discharges of each group. NL-S, normal litter rats treated with saline solution; SL-S, small litter rats treated with saline solution; NL-B, normal litter rats treated with scopolamine butylbromide; SL-B, small litter rats treated with scopolamine butylbromide. L, litter size factor; and T, treatment factor. **P < 0.01, ***P < 0.001, based on a two-way analysis of variance. ɸP < 0.05, statistical significance of the differences between NL and SL; #P < 0.05, statistical significance of saline versus scopolamine butylbromide animals, based on the Tukey multiple comparisons test.
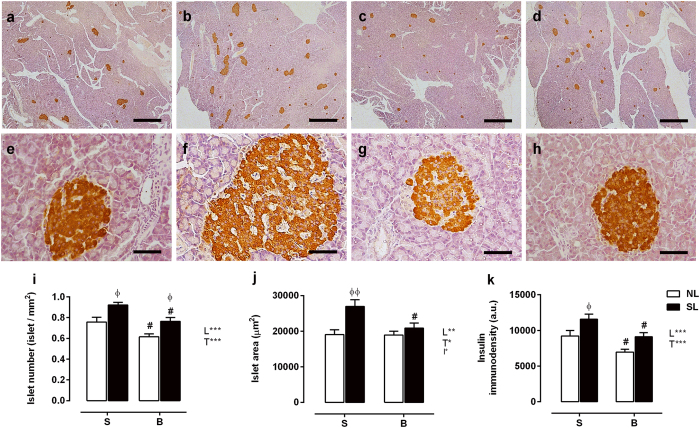
Pancreatic morphology
Figure 6 shows the immunohistochemistry staining results. Pancreatic islet number, area and insulin immunodensity were significantly increased by 22%, 41% and 25%, respectively, in the SL-S group (P < 0.05) compared with the NL-S group (Fig. 6i–k). In contrast, the drug treatment induced an 18% decrease (P < 0.01) in islet number and a 25% decrease (P < 0.05) in insulin immunodensity in the NL-B group compared with the NL-S group. There were also morphometric reductions of 17%, 20% and 21% in islet number, area and insulin immunodensity (P < 0.05) in the SL-B group compared with the SL-S group.
Figure 6. Effect of scopolamine butylbromide on histological and morphometric analysis of the pancreas.
Representative images (a,d) × 20 magnification, scale bars = 1000 μm; (e,h) × 400 magnification, scale bars = 50 μm) of pancreatic sections immunostained with an anti-insulin antibody. NL-S, normal litter rats treated with saline solution (a,e); SL-S, small litter rats treated with saline solution (b,f); NL-B, normal litter rats treated with scopolamine butylbromide (c,g); SL-B, small litter rats treated with scopolamine butylbromide (d,h). A quantitative analysis of islet number, area and insulin immunodensity is shown in (i–k), respectively. Data are presented as the mean ± SEM, obtained from 4 rats in each experimental group. A total of 160 islets were analyzed per group. L, litter size factor; T, treatment factor; and I, interaction between litter size and treatment factors. *P < 0.05, **P < 0.01 and ***P < 0.001, based on a two-way analysis of variance. ɸP < 0.05 and ɸɸP < 0.01, statistical significance of the differences between NL and SL; #P < 0.05, statistical significance of saline versus scopolamine butylbromide animals, based on the Tukey multiple comparisons test.
Discussion
The current study shows for the first time that a short scopolamine butylbromide treatment at the beginning of lactation is capable of preventing early overfeeding-induced metabolic dysfunction. These findings suggest that non-physiological insulin levels during lactation could be one cause of metabolic control disruption. In addition, the drug intervention induced a lean phenotype in rats from normal litters. Early treatment with scopolamine butylbromide induced a drop in insulin levels during the lactation period in both overfed and normal pups. An excess of insulin during the critical period of early development has been shown to change the function and structure of the hypothalamus. Indeed, inducing temporary hyperinsulinism at the beginning of lactation has been shown to lead to permanent metabolic alterations associated with disorganization in the hypothalamic nucleus, such as overweight, glucose intolerance and elevated blood pressure26. The most neural connections in primates are made at the end of pregnancy, while brain development continues throughout the lactation phase in rodents. Similarly to the brain, the pancreas initiates embryogenesis early, in the first trimester of pregnancy in primates24. However, in rodents, the pancreas begins to develop in mid-pregnancy, and islet cell proliferation and growth continue during the lactation phase27,28, which may indicate that decreasing insulin levels by using an insulin secretion inhibitor may modify normal beta-cell function. Insulin may also directly influence the programming of peripheral tissues, such as pancreatic tissue. In addition, insulin has been shown to contribute to the modulation of beta-cell function in inducing cell proliferation29.
As shown in the current work, low parasympathetic activity may also contribute to the deficit in beta-cell proliferation. Pancreatic islet quantity and volume were reduced in the NL-B and SL-B groups, both of which could be caused by low vagal activity in beta-cells30. Indeed, the scopolamine butylbromide-treated rats showed low insulin levels when fasting. The idea that the perinatal inhibition of insulin secretion can program adult offspring to develop hypoinsulinemia is reinforced by the fact that islets isolated from those animals responded poorly to glucose and ACh, which can be caused, at least partially, by vagal hypotonia. Interestingly, protein restriction during lactation induced a lean phenotype in normal adult rats; coincidently, those animals were hypoinsulinemic and exhibited low insulinotropic responses to glucose and ACh20. As expected, untreated SL rats, who were predisposed to metabolic dysfunction, had high parasympathetic activities, increased islet numbers and sizes, and high beta-cell densities; these findings are in agreement with data from the literature and from other obesity models18,31. High parasympathetic activity is associated with high glucose-induced insulin secretion and beta-cell proliferation in adult rats13, while vagotomy reduces glucose-induced insulin secretion and beta-cell proliferation30.
Low vagal activity may compromise intestinal motility, which can alter enteric hormone release. This process may contribute to the reduced insulinotropic response of beta-cells; however, the evidence of the vagal control of incretin hormone release is controversial32,33.
Scopolamine butylbromide is an anticholinergic drug that blocks mAChRs (via a high affinity for M3mAChRs); among other effects, this inhibits smooth muscle contraction and mitigates abdominal pain, such as pain in the uterus, intestines, kidneys, urinary bladder, and other tissues34. Many tissues respond to cholinergic stimuli35, and cells from these tissues, including pancreatic beta-cells, have muscarinic receptors that transduce cholinergic signals. Cholinergic stimulation via the PNS is pivotal to control oscillating insulin levels in the blood and maintain constant glycemia. ACh acts simultaneously with many other secretagogues, such as glucose, amino acids, metabolites, blood fluxes, temperature, and catecholamines, among others, which allows insulin levels to rise and fall depending on metabolic demands36.
The pancreatic beta-cell membrane expresses four subtypes of the mAChR: M1, M2, M3 and M437. The odd mAChR subtypes are G protein-coupled receptors responsible for the cholinergic insulinotropic response, while the even mAChR subtypes attenuate the insulinotropic effects of the odd receptor subtypes. The M3mAChR is mostly recruited during cholinergic stimulation in beta-cells8. The current study shows that M3mAChR protein expression in pancreatic islets is reduced when SL animals are treated with scopolamine butylbromide in early life. We suggest that this drug, by unknown mechanisms, programs beta-cells to express low M3mAChR levels with low insulin secretion. In addition, islets from SL animals showed low M2mAChR protein expression; however, when these SL animals were treated with the antimuscarinic drug, M2mAChR protein expression increased to a level close to that observed in untreated NL rats. Upon binding ACh, the M2mAChR blocks its own insulinotropic effect, which controls cholinergic responses in pancreatic beta-cells38 and in other cells39. These results reinforce the role of muscarinic receptors in the insulin secretion activity of pancreatic beta-cells. Interestingly, knockout mice lacking the M3mAChR either throughout the whole body or specifically in beta-cells present a lean phenotype. In addition, these transgenic animals are hypophagic and hypoinsulinemic, and their pancreatic islets respond poorly to glucose and ACh8,40. Those hallmarks are similar to those found in the adult rat offspring that were perinatally treated with the antimuscarinic drug.
A lean phenotype can also be obtained by restricting protein during the first two-thirds of the lactation phase. These adult rat offspring also exhibit hypophagia, hypoinsulinemia, low body weight, low insulin secretion and low M3mAChR protein expression41. These findings support the observation that dietary restrictions during the first 14 days of lactation caused decreased insulin levels.
Both infants and adults have been treated with scopolamine butylbromide to alleviate pain. As a quaternary ammonium derivative, this drug does not appear to affect the CNS because it does not cross the blood-brain barrier (BBB)34. The BBB has been shown to not have completely matured by the early postnatal period in rodents42. However, mechanisms linked to peripheral changes, such as low insulin concentration, which also affects brain function, may be considered. Furthermore, we do not know whether scopolamine butylbromide can cross the BBB in the conditions of this study.
Considering that scopolamine butylbromide may affect other tissues, which eventually could contribute to metabolic programming, pancreatic beta-cells must be a distinct step in the mechanisms of metabolic dysfunctions. In particular, mAChRs could play an important role in the programming of a lean phenotype.
Independent of the programming mechanisms, it is important to consider that fluctuations in insulin concentration during perinatal life, including lactation, are a defining factor of later health and metabolic diseases.
Materials and Methods
Ethical approval
All animal procedures were performed according to the standards established by the Brazilian Association for Animal Experimentation (COBEA) and were approved by the Ethics Committee in Animal Research of the State University of Maringa (9117110914).
Animal model and experimental design
Sets of 3 adult female Wistar rats and 1 male were mated. After 2 weeks, the pregnant females were separated and placed in individual cages with free access to water and food. At birth, all litters were adjusted to 9 pups for each dam (preferentially male). To induce early overfeeding, some litters were adjusted to 3 male pups per dam at the third day after birth, i.e., the SL group16,43,44. Litters containing 9 pups per dam served as controls, i.e., the NL group. Treatment with scopolamine butylbromide (Buscopan®, Boehringer) occurred in both groups from the first to the twelfth day of life via an intraperitoneal injection at a dose of 0.5 mg/kg bw/day, which was diluted with saline solution (i.e., the NL-B and SL-B groups). The dose was determined by the dose recommendation for the treatment of children less than 12 years old34. Other rats from both groups received a 0.9% saline solution in the same volume and period (i.e., the NL-S and SL-S groups). The offspring groups were weaned at day 21. We used five litters for each experimental group. The animals received water and a commercial diet (Nuvital®; Curitiba/PR, Brazil) ad libitum. During all phases of the protocol, the animals were placed in an environmentally controlled room (23 ± 3 °C; 12-hour light/dark cycle, from 07:00–19:00 h).
Body weight, food intake and fat pad weight evaluation
Body weight (bw) was recorded at weaning or at 90 days of age. Food intake was determined every 2 days from weaning until 90 days of age and quantified by weighing the remaining food, subtracting that amount from the ration offered to the animals the day before, and dividing by the number of animals in the cage45. Then, AUC was calculated. At 90 days of life, the rats were anesthetized with thiopental (45 mg/kg bw) and decapitated. Fat pad stores (retroperitoneal, periepididymal and mesenteric) were removed and weighed to assess the state of obesity.
Assessment of insulin plasma levels throughout the suckling phase
Blood samples of offspring from both groups that had fasted overnight were taken from the jugular vein to determine insulin concentrations on days 5, 10, 15 and 21 of life.
ivGTT
A batch of 90-day-old rats underwent a surgical procedure for the ivGTT, as previously described20. Blood samples collected before (0 min) the ivGTT were used to assess fasting glycemia, insulinemia and leptinemia. The glucose and insulin responses during the GTT were calculated by estimating the total area under the glucose curve using the trapezoidal method46. The HOMA-IR was used as a physiological index of insulin resistance. This index was determined from fasting glucose and fasting insulin concentrations using the following formula: HOMA-IR = (fasting insulin [ng/ml] × fasting glucose [mg/dl])/22.5)47.
Intraperitoneal insulin tolerance test
Another group of rats that had cannulas implanted, as described above, were fasted for 6 h prior to an intraperitoneal insulin tolerance test (1 U/kg bw), performed as previously reported48. Thereafter, the Kitt was calculated as previously described49.
Pancreatic islet isolation and insulin secretion stimulation
Pancreatic islets were isolated by collagenase digestion and washed with Hank’s solution, as previously described50. The islets (four islets per well) were preincubated for 60 min at 37 °C in Krebs solution containing 5.6 mmol/l glucose in a mixture of 95% O2: 5% CO2 at pH 7.4 to stabilize insulin secretion. To study the responses to the insulinotropic effects of different glucose concentrations, after the preincubation, the batch of islets was incubated for an additional 60 min under the experimental conditions with 5.6, 8.3, 11.1, 16.7, 20.0 or 24.0 mmol/l glucose. To study mAChRs function, following preincubation (glucose, 5.6 mmol/l), another batch of islets was incubated for an additional 60 minutes in Krebs solution containing either 8.3 mmol/l glucose or 8.3 mmol/l glucose plus 1, 10 or 100 μmol/l ACh in the presence of 10 μmol/l neostigmine to block acetylcholinesterase action in the islets. To block mAChRs function, one of the two following cholinergic antagonists was added to Krebs-Ringer solution containing 8.3 mmol/l glucose + 10 μmol/l ACh in the presence of neostigmine 10 μmol/l: the non-selective mAChR antagonist Atr (10 μmol/l) or the selective mAChR subtype M3 antagonist, 4-DAMP (100 μmol/l)41.
Blood glucose levels
Glucose concentrations was measured by the glucose oxidase method51 with a commercial kit (Gold Analisa®, Belo Horizonte, MG, Brazil).
Insulin level analyses
Insulin concentration, in plasma samples and islet cultures, was determined by radioimmunoassay52. The insulin intra- and interassay variation coefficients were 9.8 and 12.2%, respectively.
Leptin level quantification
At 90 days of life, leptin plasma levels were determined using an ELISA kit (Enzo® Life Sciences, Plymouth Meeting, PA, USA). The leptin intra- and interassay variation coefficients were 5.9 and 7.2%, respectively.
Western blotting analyses
The M2mAChR and M3mAChR protein contents in pancreatic islets isolated from 90-day-old rats were determined by immunoblotting. A pool of islets from each experimental group was collected, frozen, and subjected to maceration in Radio Immunoprecipitation Assay (RIPA) buffer (50 mmol/l Tris; 150 mmol/l NaCl; 1.0% Triton X-100; 0.1% SDS; 5 mmol/l EDTA; 50 mmol/l NaF; 30 mmol/l sodium pyrophosphate; 1 mmol/l sodium orthovanadate) containing a protease inhibitor cocktail (Roche®). The homogenate was centrifuged at 10000 rpm for 5 min at 4 °C. Total protein content was determined using a BCATM Protein Assay Kit (Thermo Scientific®, Rockford, IL, USA) and a microplate reader (Multi-Mode Reader, FlexStation® 3 Benchtop, Molecular Devices, Sunnyvale, CA, USA). The samples were treated with Laemmli sample buffer (w/v: glycerol, 20%; β-mercaptoethanol, 10%; 10% sodium dodecyl sulfate (SDS), 40%; and 0.5 mol/l Tris at pH 6.8, 0.5%; plus deionized water and bromophenol blue)53.
Total protein extracts (30 μg) from the islets were separated by 12% SDS-PAGE at 150 V for 60 min. The proteins were then transferred from the gel to a polyvinylidene difluoride (PVDF) membrane by a Trans-Blot® turbo system (Bio-Rad® Laboratories, Hercules, CA, USA) and were then blocked with 5% BSA in Tween-Tris-buffered saline (TTBS; Tris-HCl, 1 mol/l; NaCl, 5 mol/l; and Tween 20, 0.05%, v/v) for 90 min under continuous shaking. The primary antibodies anti-M3AChR (1:1000) and anti-M2AChR (1:1000) were obtained from Sigma Aldrich. PVDF membranes were washed three times with Tween–TBS (0.1%) and were incubated for 1 h with the appropriate secondary antibodies conjugated to biotin (Santa Cruz Biotechnology, Inc). Then, the membranes were incubated with streptavidin-conjugated HRP (Caltag Laboratories, Burlingame, CA, USA). The immunoreactive proteins were visualized using an ECL Prime kit and Image Quant LAS (GE Healthcare, Buckingham, Shire, UK). The bands were quantified by densitometry using ImageJ 1.4 software (Wayne Rasband, National Institutes of Health, Bethesda, MA, USA). β-Actin protein content (Santa Cruz Biotechnology®, Santa Cruz, CA, USA) was utilized for data normalization41.
Autonomic nerve activity assessment
At 90 days of life, after an overnight fast, a batch of rats from all of the experimental groups was anesthetized with thiopental (45 mg/kg bw). A longitudinal surgical incision was made in the anterior cervical region. The left vagus superior and sympathetic branch nerves were isolated from the superior cervical ganglia, and a pair of silver recording electrodes (0.6 mm in diameter) was placed underneath; the electrodes were then covered with silicone oil to prevent dehydration. The electrodes were connected to an electronic device (Bio-Amplificator; Insight Equipamentos, Ribeirão Preto, Brazil) that amplified the electrical signal prior to filtering frequencies lower than 1 kHz and higher than 80 kHz. The signal output was acquired using Insight software (Insight software) and stored on a computer. The animals were placed in a Faraday cage to avoid any electromagnetic interference during the data-acquisition period54,55.
Immunostaining and morphometric analysis of the pancreas
Pancreatic samples from 90-day-old rat offspring were fixed in 10% dehydrated formalin, cleared and embedded in histological paraffin. The fixed samples were sectioned into semi-serial, 5-μm-thick slices using a microtome. After deparaffinization, the sections were rehydrated and blocked against endogenous peroxidase activity with 3% H2O2. The sections were then washed in 0.01 M phosphate-buffered saline (PBS, pH 7.4) and incubated with 10% non-immune goat serum (Histostain-Plus®, Invitrogen) for 10 min. After blocking, the sections were incubated with an anti-insulin monoclonal antibody diluted at 1:500 (Sigma®, St. Louis, MO, USA) for 1 h at room temperature. After washing with 0.01 M PBS, the sections were incubated with a specific biotinylated secondary antibody (Histostain-Plus®, Invitrogen) for 10 min. Then, the sections were incubated with the enzyme streptavidin-peroxidase conjugate (Histostain-Plus®, Invitrogen) for 10 min and washed twice for 5 min. The streptavidin–biotin complexes were detected with diaminobenzidine chromogen solution (Histostain-Plus® Invitrogen). Counterstaining with hematoxylin was performed for 15 s.
The number of pancreatic islets in the pancreas (number of islets/cm2) was quantified by insulin-immunostained pancreatic cuts (2 cuts/animal) in a sectional area of 40 islets/animal/group. Images were obtained in TIFF format using an optical microscope with 2 × and 40 × objectives coupled with a QColor 3 camera (Olympus, Tokyo, Japan). Analyses were performed using Image Pro Plus® version 4.5 software 56.
Statistical analysis
The results are expressed as the mean and the standard error (SEM), and the results were subjected to a D’Agostino Pearson normality test to assess the Gaussian distribution. Data were submitted to variance analysis by two-way ANOVA. Differences between the means were evaluated by Tukey’s post hoc test. A P value < 0.05 was considered significant regarding the main effects of litter size (L), treatment (T), their interaction (I; litter size vs treatment) and the differences between groups. Tests were performed using GraphPad Prism version 6.0 for Windows (GraphPad Software, Inc. San Diego, CA, USA).
Additional Information
How to cite this article: Malta, A. et al. Neonatal treatment with scopolamine butylbromide prevents metabolic dysfunction in male rats. Sci. Rep. 6, 30745; doi: 10.1038/srep30745 (2016).
Acknowledgments
This work was supported by the Brazilian Federal Foundation, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Paraná Science Foundation (Fundação Araucária). We must thanks to M. Maroly Pinto and M. Marli Lícero for helping to care of rats.
Footnotes
Author Contributions A.M. and P.C.d.F.M were responsible for the conception and design of the experiments. A.A.d.S., T.A.R., F.A.F., A.P., K.V.P., L.P.T., R.A.M., J.C.d.O., I.P.M., C.P., R.M.G., C.C.S.F., M.R.M.N. and K.P.-R. were responsible for the collection, analysis and interpretation of the data. All authors were involved in drafting the article and critically revising it for intellectual content. All authors approved the final version of the manuscript submitted for publication.
References
- Felisbino-Mendes M. S., Villamor E. & Velasquez-Melendez G. Association of maternal and child nutritional status in Brazil: a population based cross-sectional study. PloS one 9, e87486, doi: 10.1371/journal.pone.0087486 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers M. H. Developmental programming of the metabolic syndrome - critical windows for intervention. World journal of diabetes 2, 137–148, doi: 10.4239/wjd.v2.i9.137 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P. J. et al. Thrifty metabolic programming in rats is induced by both maternal undernutrition and postnatal leptin treatment, but masked in the presence of both: implications for models of developmental programming. BMC genomics 15, 49, doi: 10.1186/1471-2164-15-49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. et al. The early origins of chronic heart failure: impaired placental growth and initiation of insulin resistance in childhood. European journal of heart failure 12, 819–825, doi: 10.1093/eurjhf/hfq069 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A. Programming by early nutrition: an experimental approach. The Journal of nutrition 128, 401S–406S (1998). [DOI] [PubMed] [Google Scholar]
- Koutcherov Y., Mai J. K. & Paxinos G. Hypothalamus of the human fetus. Journal of chemical neuroanatomy 26, 253–270 (2003). [DOI] [PubMed] [Google Scholar]
- Lesage J. et al. Perinatal maternal undernutrition programs the offspring hypothalamo-pituitary-adrenal (HPA) axis. Stress 9, 183–198, doi: 10.1080/10253890601056192 (2006). [DOI] [PubMed] [Google Scholar]
- Gautam D. et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell metabolism 3, 449–461, doi: 10.1016/j.cmet.2006.04.009 (2006). [DOI] [PubMed] [Google Scholar]
- Wess J. Molecular biology of muscarinic acetylcholine receptors. Critical reviews in neurobiology 10, 69–99 (1996). [DOI] [PubMed] [Google Scholar]
- Inoue S. & Bray G. A. An autonomic hypothesis for hypothalamic obesity. Life sciences 25, 561–566 (1979). [DOI] [PubMed] [Google Scholar]
- Scheurink A. J., Balkan B., Strubbe J. H., van Dijk G. & Steffens A. B. Overfeeding, autonomic regulation and metabolic consequences. Cardiovascular drugs and therapy/sponsored by the International Society of Cardiovascular Pharmacotherapy 10 Suppl 1, 263–273 (1996). [DOI] [PubMed] [Google Scholar]
- Vozarova de Courten B. et al. Parasympathetic blockade attenuates augmented pancreatic polypeptide but not insulin secretion in Pima Indians. Diabetes 53, 663–671 (2004). [DOI] [PubMed] [Google Scholar]
- Kiba T. Relationships between the autonomic nervous system and the pancreas including regulation of regeneration and apoptosis: recent developments. Pancreas 29, e51–58 (2004). [DOI] [PubMed] [Google Scholar]
- Plagemann A., Harder T., Kohlhoff R., Rohde W. & Dorner G. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity 21, 451–456 (1997). [DOI] [PubMed] [Google Scholar]
- Harder T., Rake A., Rohde W., Doerner G. & Plagemann A. Overweight and increased diabetes susceptibility in neonatally insulin-treated adult rats. Endocrine regulations 33, 25–31 (1999). [PubMed] [Google Scholar]
- Plagemann A., Heidrich I., Gotz F., Rohde W. & Dorner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Experimental and clinical endocrinology 99, 154–158, doi: 10.1055/s-0029-1211159 (1992). [DOI] [PubMed] [Google Scholar]
- Habbout A., Li N., Rochette L. & Vergely C. Postnatal overfeeding in rodents by litter size reduction induces major short- and long-term pathophysiological consequences. The Journal of nutrition 143, 553–562, doi: 10.3945/jn.112.172825 (2013). [DOI] [PubMed] [Google Scholar]
- Rinaldi W. et al. Low-intensity and moderate exercise training improves autonomic nervous system activity imbalanced by postnatal early overfeeding in rats. Journal of the International Society of Sports Nutrition 11, 25, doi: 10.1186/1550-2783-11-25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann A. et al. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain research 836, 146–155 (1999). [DOI] [PubMed] [Google Scholar]
- de Oliveira J. C. et al. Metabolic imprinting by maternal protein malnourishment impairs vagal activity in adult rats. Journal of neuroendocrinology 23, 148–157, doi: 10.1111/j.1365-2826.2010.02095.x (2011). [DOI] [PubMed] [Google Scholar]
- Dorner G. & Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus, obesity and enhanced cardiovascular risk in later life. Hormone and metabolic research=Hormon- und Stoffwechselforschung=Hormones et metabolisme 26, 213–221, doi: 10.1055/s-2007-1001668 (1994). [DOI] [PubMed] [Google Scholar]
- Agnoux A. M. et al. Time window-dependent effect of perinatal maternal protein restriction on insulin sensitivity and energy substrate oxidation in adult male offspring. American journal of physiology. Regulatory, integrative and comparative physiology 307, R184–197, doi: 10.1152/ajpregu.00015.2014 (2014). [DOI] [PubMed] [Google Scholar]
- Aynsley-Green A. et al. Neonatal insulin secretion: implications for the programming of metabolic homeostasis. Acta paediatrica Japonica; Overseas edition 39 Suppl 1, S21–25 (1997). [PubMed] [Google Scholar]
- Steculorum S. M., Vogt M. C. & Bruning J. C. Perinatal programming of metabolic diseases: role of insulin in the development of hypothalamic neurocircuits. Endocrinology and metabolism clinics of North America 42, 149–164, doi: 10.1016/j.ecl.2012.10.002 (2013). [DOI] [PubMed] [Google Scholar]
- Renuka T. R., Robinson R. & Paulose C. S. Increased insulin secretion by muscarinic M1 and M3 receptor function from rat pancreatic islets in vitro. Neurochemical research 31, 313–320, doi: 10.1007/s11064-005-9022-6 (2006). [DOI] [PubMed] [Google Scholar]
- Harder T., Plagemann A., Rohde W. & Dorner G. Syndrome X-like alterations in adult female rats due to neonatal insulin treatment. Metabolism: clinical and experimental 47, 855–862 (1998). [DOI] [PubMed] [Google Scholar]
- Gittes G. K. Developmental biology of the pancreas: a comprehensive review. Developmental biology 326, 4–35, doi: 10.1016/j.ydbio.2008.10.024 (2009). [DOI] [PubMed] [Google Scholar]
- O’Dowd J. F. & Stocker C. J. Endocrine pancreatic development: impact of obesity and diet. Frontiers in physiology 4, 170, doi: 10.3389/fphys.2013.00170 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Jones P. M. & Persaud S. J. Autocrine anti-apoptotic and proliferative effects of insulin in pancreatic beta-cells. FEBS letters 580, 6977–6980, doi: 10.1016/j.febslet.2006.11.066 (2006). [DOI] [PubMed] [Google Scholar]
- Kiba T. et al. Ventromedial hypothalamic lesion-induced vagal hyperactivity stimulates rat pancreatic cell proliferation. Gastroenterology 110, 885–893 (1996). [DOI] [PubMed] [Google Scholar]
- Gregorio B. M., Souza-Mello V., Mandarim-de-Lacerda C. A. & Aguila M. B. Maternal high-fat diet is associated with altered pancreatic remodelling in mice offspring. European journal of nutrition 52, 759–769, doi: 10.1007/s00394-012-0382-9 (2013). [DOI] [PubMed] [Google Scholar]
- Rocca A. S. & Brubaker P. L. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140, 1687–1694, doi: 10.1210/endo.140.4.6643 (1999). [DOI] [PubMed] [Google Scholar]
- Berthoud H. R. & Jeanrenaud B. Sham feeding-induced cephalic phase insulin release in the rat. The American journal of physiology 242, E280–285 (1982). [DOI] [PubMed] [Google Scholar]
- Tytgat G. N. Hyoscine butylbromide - a review on its parenteral use in acute abdominal spasm and as an aid in abdominal diagnostic and therapeutic procedures. Current medical research and opinion 24, 3159–3173, doi: 10.1185/03007990802472700 (2008). [DOI] [PubMed] [Google Scholar]
- Post G. R. & Brown J. H. G protein-coupled receptors and signaling pathways regulating growth responses. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 10, 741–749 (1996). [DOI] [PubMed] [Google Scholar]
- Wessler I., Kirkpatrick C. J. & Racke K. The cholinergic ‘pitfall’: acetylcholine, a universal cell molecule in biological systems, including humans. Clinical and experimental pharmacology & physiology 26, 198–205 (1999). [DOI] [PubMed] [Google Scholar]
- Iismaa T. P. et al. Quantitative and functional characterization of muscarinic receptor subtypes in insulin-secreting cell lines and rat pancreatic islets. Diabetes 49, 392–398 (2000). [DOI] [PubMed] [Google Scholar]
- Miguel J. C., Abdel-Wahab Y. H., Mathias P. C. & Flatt P. R. Muscarinic receptor subtypes mediate stimulatory and paradoxical inhibitory effects on an insulin-secreting beta cell line. Biochimica et biophysica acta 1569, 45–50 (2002). [DOI] [PubMed] [Google Scholar]
- Nishimaru K., Tanaka Y., Tanaka H. & Shigenobu K. Positive and negative inotropic effects of muscarinic receptor stimulation in mouse left atria. Life sciences 66, 607–615 (2000). [DOI] [PubMed] [Google Scholar]
- Yamada M. et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature 410, 207–212, doi: 10.1038/35065604 (2001). [DOI] [PubMed] [Google Scholar]
- Oliveira J. C. et al. Impaired beta-cell function in the adult offspring of rats fed a protein-restricted diet during lactation is associated with changes in muscarinic acetylcholine receptor subtypes. The British journal of nutrition 111, 227–235, doi: 10.1017/S0007114513002213 (2014). [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalez B. & Escobar A. Altered functional development of the blood-brain barrier after early life stress in the rat. Brain research bulletin 79, 376–387, doi: 10.1016/j.brainresbull.2009.05.012 (2009). [DOI] [PubMed] [Google Scholar]
- Hou M. et al. Neonatal overfeeding induced by small litter rearing causes altered glucocorticoid metabolism in rats. PloS one 6, e25726, doi: 10.1371/journal.pone.0025726 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A. L., de Moura E. G., Passos M. C., Dutra S. C. & Lisboa P. C. Postnatal early overnutrition changes the leptin signalling pathway in the hypothalamic-pituitary-thyroid axis of young and adult rats. The Journal of physiology 587, 2647–2661, doi: 10.1113/jphysiol.2009.169045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barella L. F. et al. Early exposure to a high-fat diet has more drastic consequences on metabolism compared with exposure during adulthood in rats. Hormone and metabolic research=Hormon- und Stoffwechselforschung=Hormones et metabolisme 44, 458–464, doi: 10.1055/s-0032-1306300 (2012). [DOI] [PubMed] [Google Scholar]
- Milanski M. et al. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes 61, 1455–1462, doi: 10.2337/db11-0390 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini G. & Mari A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best practice & research. Clinical endocrinology & metabolism 17, 305–322 (2003). [DOI] [PubMed] [Google Scholar]
- Miranda R. A. et al. Insulin oversecretion in MSG-obese rats is related to alterations in cholinergic muscarinic receptor subtypes in pancreatic islets. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 33, 1075–1086, doi: 10.1159/000358677 (2014). [DOI] [PubMed] [Google Scholar]
- Lundbaek K. Intravenous glucose tolerance as a tool in definition and diagnosis of diabetes mellitus. Br Med J 1, 1507–1513 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravena C., Mathias P. C. & Ashcroft S. J. Acute effects of fatty acids on insulin secretion from rat and human islets of Langerhans. J Endocrinol 173, 73–80 (2002). [DOI] [PubMed] [Google Scholar]
- Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 22, 158–161 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. M., Atwater I. & Rojas E. A method for the simultaneous measurement of insulin release and B cell membrane potential in single mouse islets of Langerhans. Diabetologia 21, 470–475 (1981). [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970). [DOI] [PubMed] [Google Scholar]
- Malta A. et al. Low-protein diet in adult male rats has long-term effects on metabolism. The Journal of endocrinology 221, 285–295, doi: 10.1530/JOE-13-0473 (2014). [DOI] [PubMed] [Google Scholar]
- Barella L. F. et al. Vagus nerve contributes to metabolic syndrome in high-fat diet-fed young and adult rats. Experimental physiology 100, 57–68, doi: 10.1113/expphysiol.2014.082982 (2015). [DOI] [PubMed] [Google Scholar]
- Rafacho A. et al. The adaptive compensations in endocrine pancreas from glucocorticoid-treated rats are reversible after the interruption of treatment. Acta physiologica 200, 223–235, doi: 10.1111/j.1748-1716.2010.02146.x (2010). [DOI] [PubMed] [Google Scholar]