Abstract
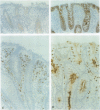
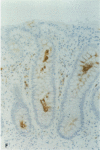
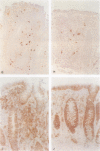
AIMS: To assess quantitatively both the morphological changes in the rectal mucosa and the changes in the relative frequency of IgA and IgG subclass producing cells found in the rectal mucosa during the acute phase of shigellosis and at convalescence. METHODS: Rectal biopsies from 25 Shigella dysenteriae 1 infected patients, 10 Shigella flexneri infected patients, and 40 uninfected controls were studied. Morphological changes in the mucosa were graded. The frequency of IgA and IgG subclass producing cells was assessed. In addition, immunostaining for secretory component in epithelial cells was analysed. RESULTS: Using morphological grading, 20% of the 35 patients studied had advanced inflammation (grade 3) in the acute phase of the disease. At convalescence, grade 1 inflammation was seen in 37% of the patients and in 10% of the controls. In the acute phase, as well as at convalescence, the number of IgA1, IgA2, and IgG2 positive cells was significantly higher than in the controls. The results were related to the histopathological degree of inflammation. CONCLUSIONS: In shigellosis, there is evidence for a prolonged humoral response residing in the mucosa long after the clinical symptoms have resolved, suggesting that shigellosis induces persisting mucosal humoral immune and inflammatory responses, remaining at least until 30 days after the infection.
Full text
PDF
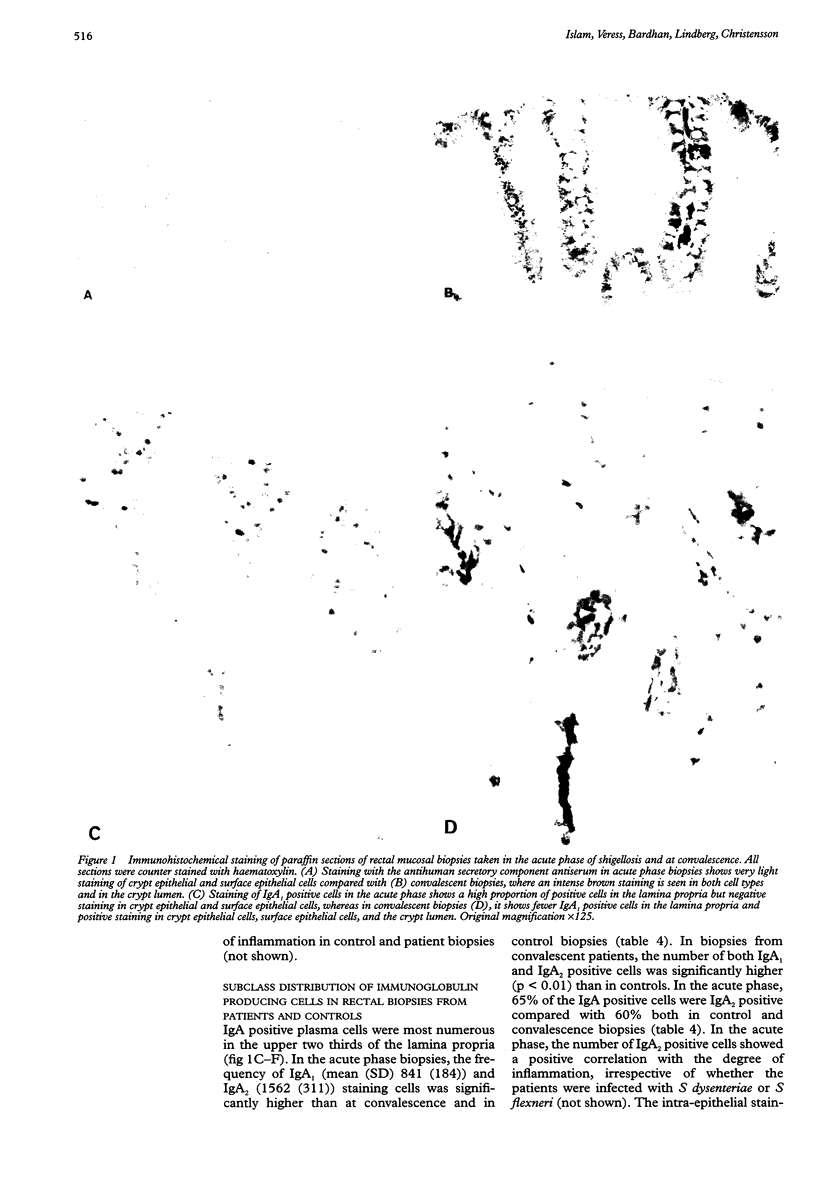




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amigorena S., Bonnerot C., Fridman W. H., Teillaud J. L. Recombinant interleukin 2-activated natural killer cells regulate IgG2a production. Eur J Immunol. 1990 Aug;20(8):1781–1787. doi: 10.1002/eji.1830200824. [DOI] [PubMed] [Google Scholar]
- Bjerke K., Brandtzaeg P., Rognum T. O. Distribution of immunoglobulin producing cells is different in normal human appendix and colon mucosa. Gut. 1986 Jun;27(6):667–674. doi: 10.1136/gut.27.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Halstensen T. S., Huitfeldt H. S., Krajci P., Kvale D., Scott H., Thrane P. S. Epithelial expression of HLA, secretory component (poly-Ig receptor), and adhesion molecules in the human alimentary tract. Ann N Y Acad Sci. 1992;664:157–179. doi: 10.1111/j.1749-6632.1992.tb39758.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Halstensen T. S., Kett K., Krajci P., Kvale D., Rognum T. O., Scott H., Sollid L. M. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989 Dec;97(6):1562–1584. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Kett K., Rognum T. O. Subclass distribution of IgG- and IgA-producing cells in secretory tissues and alterations related to gut diseases. Adv Exp Med Biol. 1987;216A:321–333. doi: 10.1007/978-1-4684-5344-7_37. [DOI] [PubMed] [Google Scholar]
- Butler T., Dunn D., Dahms B., Islam M. Causes of death and the histopathologic findings in fatal shigellosis. Pediatr Infect Dis J. 1989 Nov;8(11):767–772. doi: 10.1097/00006454-198911000-00008. [DOI] [PubMed] [Google Scholar]
- High N., Mounier J., Prévost M. C., Sansonetti P. J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992 May;11(5):1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam D., Wretlind B., Hammarström L., Christensson B., Lindberg A. A. Semiquantitative estimation of Shigella antigen-specific antibodies: correlation with disease severity during shigellosis. APMIS. 1996 Jul-Aug;104(7-8):563–574. doi: 10.1111/j.1699-0463.1996.tb04912.x. [DOI] [PubMed] [Google Scholar]
- Islam D., Wretlind B., Ryd M., Lindberg A. A., Christensson B. Immunoglobulin subclass distribution and dynamics of Shigella-specific antibody responses in serum and stool samples in shigellosis. Infect Immun. 1995 May;63(5):2054–2061. doi: 10.1128/iai.63.5.2054-2061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. M., Azad A. K., Bardhan P. K., Raqib R., Islam D. Pathology of shigellosis and its complications. Histopathology. 1994 Jan;24(1):65–71. doi: 10.1111/j.1365-2559.1994.tb01272.x. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Faludy J. Immunohistochemical demonstration of IgA and secretory component in relation to epithelial cell differentiation in normal colorectal mucosa and metaplastic polyp: a semiquantitative study. Histochem J. 1985 Mar;17(3):373–380. doi: 10.1007/BF01004598. [DOI] [PubMed] [Google Scholar]
- Kett K., Scott H., Fausa O., Brandtzaeg P. Secretory immunity in celiac disease: cellular expression of immunoglobulin A subclass and joining chain. Gastroenterology. 1990 Aug;99(2):386–392. doi: 10.1016/0016-5085(90)91020-7. [DOI] [PubMed] [Google Scholar]
- Mathan M. M., Mathan V. I. Morphology of rectal mucosa of patients with shigellosis. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 4):S314–S318. doi: 10.1093/clinids/13.supplement_4.s314. [DOI] [PubMed] [Google Scholar]
- Mathan M. M., Mathan V. I. Ultrastructural pathology of the rectal mucosa in Shigella dysentery. Am J Pathol. 1986 Apr;123(1):25–38. [PMC free article] [PubMed] [Google Scholar]
- Mathew M., Mathan M. M., Mani K., George R., Jebakumar K., Dharamsi R., Kirubakaran C., Pereira S., Mathan V. I. The relationship of microbial pathogens to acute infectious diarrhoea of childhood. J Trop Med Hyg. 1991 Aug;94(4):253–260. [PubMed] [Google Scholar]
- Matsueda K., Yamada G., Tsuji T. Immunohistochemical characterization of the lymphocyte and the immunoglobulin-containing cell in the epithelium and the lamina propria of normal human intestines. Acta Med Okayama. 1991 Jun;45(3):161–169. doi: 10.18926/AMO/32203. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Russell M. W. IgA subclasses. Monogr Allergy. 1986;19:277–301. [PubMed] [Google Scholar]
- Ménard R., Sansonetti P. J., Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993 Sep;175(18):5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991 May 1;77(9):1859–1870. [PubMed] [Google Scholar]
- Pellat-Deceunynck C., Bataille R., Robillard N., Harousseau J. L., Rapp M. J., Juge-Morineau N., Wijdenes J., Amiot M. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood. 1994 Oct 15;84(8):2597–2603. [PubMed] [Google Scholar]
- Punnonen J., Aversa G., Cocks B. G., McKenzie A. N., Menon S., Zurawski G., de Waal Malefyt R., de Vries J. E. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R., Lindberg A. A., Björk L., Bardhan P. K., Wretlind B., Andersson U., Andersson J. Down-regulation of gamma interferon, tumor necrosis factor type I, interleukin 1 (IL-1) type I, IL-3, IL-4, and transforming growth factor beta type I receptors at the local site during the acute phase of Shigella infection. Infect Immun. 1995 Aug;63(8):3079–3087. doi: 10.1128/iai.63.8.3079-3087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R., Lindberg A. A., Wretlind B., Bardhan P. K., Andersson U., Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995 Jan;63(1):289–296. doi: 10.1128/iai.63.1.289-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R., Ljungdahl A., Lindberg A. A., Andersson U., Andersson J. Local entrapment of interferon gamma in the recovery from Shigella dysenteriae type 1 infection. Gut. 1996 Mar;38(3):328–336. doi: 10.1136/gut.38.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R., Wretlind B., Andersson J., Lindberg A. A. Cytokine secretion in acute shigellosis is correlated to disease activity and directed more to stool than to plasma. J Infect Dis. 1995 Feb;171(2):376–384. doi: 10.1093/infdis/171.2.376. [DOI] [PubMed] [Google Scholar]
- Reinholt F. P., Veress B., Lindquist K., Liljeqvist L. Qualitative assessment and morphometry in the study of the ileal reservoir after restorative proctocolectomy. APMIS. 1989 Feb;97(2):97–104. doi: 10.1111/j.1699-0463.1989.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Rognum T. O., Elgjo K., Fausa O., Brandtzaeg P. Immunohistochemical evaluation of carcinoembryonic antigen, secretory component, and epithelial IgA in ulcerative colitis with dysplasia. Gut. 1982 Feb;23(2):123–133. doi: 10.1136/gut.23.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognum T. O., Fausa O., Brandtzaeg P. Immunohistochemical evaluation of carcinoembryonic antigen, secretory component, and epithelial IgA in tubular and villous large-bowel adenomas with different grades of dysplasia. Scand J Gastroenterol. 1982 Apr;17(3):341–348. doi: 10.3109/00365528209182065. [DOI] [PubMed] [Google Scholar]
- Sachdev H. P., Chadha V., Malhotra V., Verghese A., Puri R. K. Rectal histopathology in endemic Shigella and Salmonella diarrhea. J Pediatr Gastroenterol Nutr. 1993 Jan;16(1):33–38. doi: 10.1097/00005176-199301000-00007. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. G., Nahm M. H., Macke K., Nash G. S., Bertovich M. J., MacDermott R. P. Spontaneous secretion of IgG subclasses by intestinal mononuclear cells: differences between ulcerative colitis, Crohn's disease, and controls. Clin Exp Immunol. 1986 Oct;66(1):209–215. [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., McIntyre T. M., Mandler R., Pecanha L. M., Finkelman F. D., Lees A., Mond J. J. Induction of IgG3 secretion by interferon gamma: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J Exp Med. 1992 May 1;175(5):1367–1371. doi: 10.1084/jem.175.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Speelman P., Kabir I., Islam M. Distribution and spread of colonic lesions in shigellosis: a colonoscopic study. J Infect Dis. 1984 Dec;150(6):899–903. doi: 10.1093/infdis/150.6.899. [DOI] [PubMed] [Google Scholar]
- Speelman P., McGlaughlin R., Kabir I., Butler T. Differential clinical features and stool findings in shigellosis and amoebic dysentery. Trans R Soc Trop Med Hyg. 1987;81(4):549–551. doi: 10.1016/0035-9203(87)90402-0. [DOI] [PubMed] [Google Scholar]
- Struelens M. J., Patte D., Kabir I., Salam A., Nath S. K., Butler T. Shigella septicemia: prevalence, presentation, risk factors, and outcome. J Infect Dis. 1985 Oct;152(4):784–790. doi: 10.1093/infdis/152.4.784. [DOI] [PubMed] [Google Scholar]
- Valnes K., Brandtzaeg P., Elgjo K., Stave R. Quantitative distribution of immunoglobulin-producing cells in gastric mucosa: relation to chronic gastritis and glandular atrophy. Gut. 1986 May;27(5):505–514. doi: 10.1136/gut.27.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valnes K., Brandtzaeg P. Subclass distribution of mucosal IgG-producing cells in gastritis. Gut. 1989 Mar;30(3):322–326. doi: 10.1136/gut.30.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veress B., Reinholt F. P., Lindquist K., Liljeqvist L. Different types of mucosal adaptation in the ileal reservoir after restorative proctocolectomy. A two-year follow-up study. APMIS. 1990 Sep;98(9):786–796. doi: 10.1111/j.1699-0463.1990.tb04999.x. [DOI] [PubMed] [Google Scholar]
- van Spreeuwel J. P., Lindeman J., Meijer C. J. A quantitative study of immunoglobulin containing cells in the differential diagnosis of acute colitis. J Clin Pathol. 1985 Jul;38(7):774–777. doi: 10.1136/jcp.38.7.774. [DOI] [PMC free article] [PubMed] [Google Scholar]