Abstract
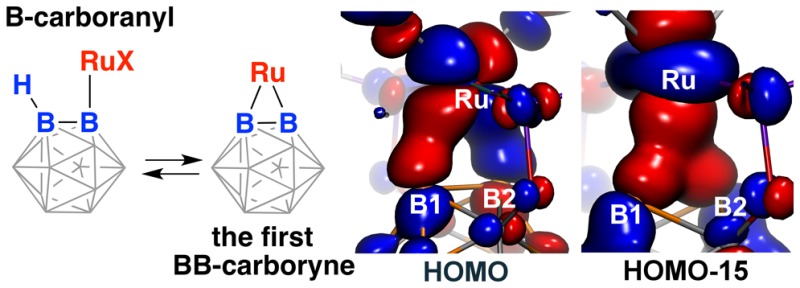
The first example of a transition metal (BB)-carboryne complex containing two boron atoms of the icosahedral cage connected to a single exohedral metal center (POBBOP)Ru(CO)2 (POBBOP = 1,7-OP(i-Pr)2-2,6-dehydro-m-carborane) was synthesized by double B–H activation within the strained m-carboranyl pincer framework. Theoretical calculations revealed that the unique three-membered (BB)>Ru metalacycle is formed by two bent B–Ru σ-bonds with the concomitant increase of the bond order between the two metalated boron atoms. The reactivity of the highly strained electron-rich (BB)-carboryne fragment with small molecules was probed by reactions with electrophiles. The carboryne–carboranyl transformations reported herein represent a new mode of cooperative metal–ligand reactivity of boron-based complexes.
Introduction
The remarkable progress in the use of well-defined transition metal complexes for a multitude of challenging organic transformations led to the exploration and introduction of numerous new ligand platforms.1 Rigid cyclometalated tridentate pincer systems possess a promising combination of reactivity, stability, and modularity and are used extensively in fundamental research and catalysis.1 The traditional PCP system featuring an aryl or alkyl backbone and phosphine arms is perhaps the most studied variation of these ligands. In addition to carbon-backbone multidentate ligands, a number of heteroatom-based systems have been reported.2 Among those, borane, borate, and boryl complexes recently attracted an increased attention, due in part to the versatility and flexibility of metal–boron interactions.3 Polyhedral boron clusters, such as icosahedral carboranes C2B10H12, have been employed as ligands to transition metals providing a combination of high steric hindrance and unique electronic effects with either C–M, B–M, or B–H···M coordination to a metal center.4 Importantly, three-dimensional icosahedral boron cages provide an unusual coordination environment of five neighboring B–H/C–H bonds to an exohedral metal center covalently bound to the cage, a situation markedly different from planar aryl- and pyridine-backbone ligands. These vicinal bridging B–H···M interactions can stabilize low-coordinate metal center configurations by hemilabile coordination as well as participate in a metal-mediated interconversion between borane and boryl moieties. Beyond their application in coordination chemistry and catalysis, high interest in boron clusters and new synthetic methods of their functionalization is also driven by their potential for application in polymers, energy storage, medicine, electronic devices, luminescent materials, liquid crystals, and ceramics.5
Transition metal benzyne complexes have been extensively studied because of their rich small-molecule activation chemistry and synthetic utility in organic synthesis.6 Metal-coordinated benzynes participate in insertion reactions with a wide range of unsaturated molecules, including nitriles, ketones, olefins, and acetylenes. The 1,2-dehydro-o-carborane C2B10H10 ((CC)-carboryne) can be considered as a three-dimensional analogue to the benzyne with transition metal carboryne complexes possessing three-membered (CC)>M cycles (Chart 1).7 The general synthetic strategy, involving the deprotonation of two relatively acidic C–H bonds of o-C2B10H12 and a reaction with transition metal halides, afforded a series of (CC)-carboryne complexes of Ni, Pd, Pt, Ti, Zr, and Hf.8 These compounds undergo regioselective insertion and multicomponent cycloaddition reactions with unsaturated substrates providing an access to numerous carborane cage derivatives.9 Recently, 1,3-dehydro-o-carborane complexes that contain the (BC)>M cycle have been implicated in certain cycloaddion reactions, however, no such intermediates could be isolated.10 Finally, one can envisage carboryne analogues containing two boron atoms connected to an exohedral metal center (the three-membered (BB)>M cycle); yet no synthetic strategy or any evidence of existence of such complexes have been reported.
Chart 1. Metal Complexes of 1,2-Dehydro-o-carborane ((CC)-Carboryne), 1,3-Dehydro-o-carborane ((BC)-Carboryne), and 2,6-Dehydro-m-carborane ((BB)-Carboryne).
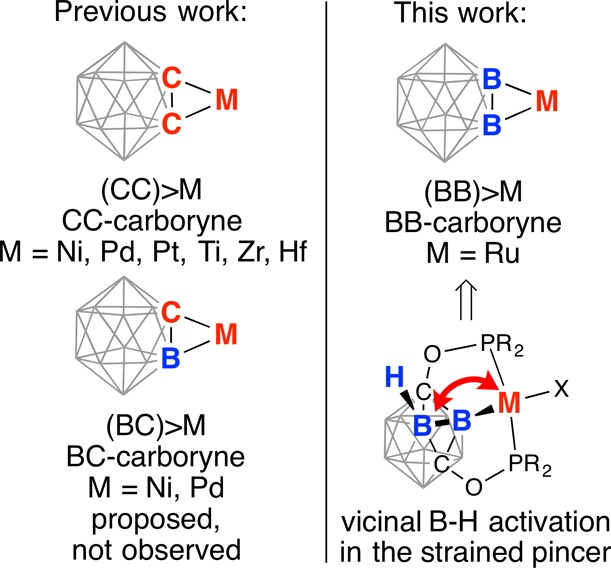
Herein, we present the first example of a transition metal (BB)-carboryne complex with its synthesis, structure, bonding analysis, and preliminary reactivity studies. The amalgamation of concepts of carboryne chemistry and pincer ligands led to the synthesis of a (BB)>Ru metalacycle upon double B–H activation at the ruthenium center within the strained pincer framework. Theoretical calculations revealed the presence of two highly strained B–Ru σ-bonds in the coordinated (BB)-carboryne. These nucleophilic bonds are accessible for reactions with electrophiles.
Metalation of boron vertices of icosahedral carborane clusters generally occurs upon coordination of a late transition metal to a directing donor group,11 although rare examples of unsupported exohedral metal–boron bonds have been reported as well.12 We have recently prepared rhodium complexes of the novel POBOP (POBOP = 1,7-OP(i-Pr)2-m-carboranyl) pincer ligand containing strained exohedral metal–boron bonds, which were enforced by the unusual geometry of the three-dimensional carborane cage and chelating phosphinite arms.4p The close contact between the metal center and the vicinal B–H bond of the carborane cage led to a cascade transformation, which resulted in the transfer of the metal center to the adjacent boron atom of the cage. This facile migration led us to a hypothesis that the POBOP m-carboranyl pincer framework could enforce the metal center to be in the vicinity of two boron atoms of the cluster at the same time and lead to two B–H activation events with the formation of the (BB)>M metalacycle (Chart 1). This synthetic strategy was successfully realized in this work.
Results and Discussion
Synthesis and Structure of the Ruthenium (BB)-Carboryne
The reaction of the ligand precursor (POBOP)H (1) and [Ru(CO)3Cl2]2 for 8 h at 90 °C in C6D6 in a J. Young valve NMR tube resulted in the initial formation of multiple products according to 31P NMR spectroscopy. Addition of an excess of NEt3 and heating the reaction mixture at 90 °C for 16 h resulted in clean conversion to a predominant product (95%) with a signal at 217.1 ppm in 31P NMR spectra (Scheme 1). The most prominent spectral feature of 2 was the 2:2:4:2 signal pattern in the 11B{1H} NMR spectrum suggesting the C2v symmetry of the complex. A signal at −2.8 ppm, corresponding to two boron atoms remained a singlet in the 11B NMR spectrum indicating two vertices of the cage being metalated. The 11B–1H HSQC NMR spectrum exhibited analogous pairwise correlation signals from three types of boron atoms and three types of hydrogen atoms (2:4:2 integral ratio) with the remaining type of boron atoms (the signal at −2.8 ppm in the 11B NMR spectrum) not showing correlation to any hydrogens. The 1H and 1H{11B} NMR spectra of 2 contained no signals in the region from 0 ppm to −20 ppm typically associated with B–H···Ru or Ru–H fragments.13 Attempts to acquire 11B–11B COSY NMR spectrum of 2 were unsuccessful due to very short relaxation times for all types of boron nuclei (2–3 ms). The IR spectrum of 2 contained two strong bands corresponding to the carbonyl stretches. One carbonyl carbon signal at 202.8 ppm was observed in the 13C NMR spectrum. On the basis of spectral characterization data, compound 2 was proposed to be a symmetric complex of Ru(II) featuring two adjacent boron atoms connected to the metal center. Single-crystal X-ray diffraction study confirmed the proposed motif and revealed the distorted octahedral coordination environment around Ru with two Ru–B bonds, two phosphinite arms, and two CO ligands (Figure 1). Importantly, the hydrogen atoms of the carborane cage were located using the electron density map and were found on all boron atoms except B1 and B2.
Scheme 1. Synthesis of (POBBOP)Ru(CO)2 Complex (POBBOP = 1,7-OP(i-Pr)2-2,6-dehydro-m-carborane) by Double B–H Activation.
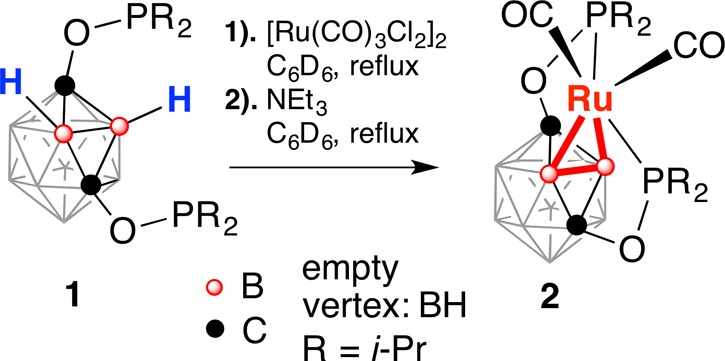
Figure 1.
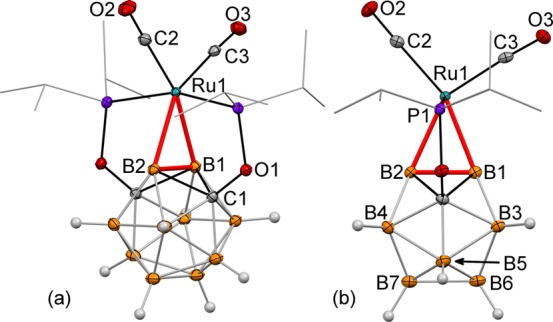
Displacement ellipsoid plot (50% probability) of the (POBBOP)Ru(CO)2 complex (POBBOP = 1,7-OP(i-Pr)2-2,6-dehydro-m-carborane) (2). (a) A general view; (b) a view perpendicular to the (B1–B2–Ru1–C2–C3) plane. Atoms belonging to isopropyl groups of the ligand arms have been omitted for clarity. Selected bond distances (Å) and angles (deg): Ru1–B1 = 2.174(3), Ru1–B2 = 2.221(3), B1–B2 = 1.720(4), Ru1–C2 = 1.939(3) Ru1–C3 = 1.915(3), B2–B4 = 1.811(3), B1–B3 = 1.796(3), B4–B7 = 1.781(3), B3–B6 = 1.785(3), B7–B6 = 1.812(4), B2–B1–Ru1 = 68.4(1), B1–B2–Ru1 = 65.5(1), and C2–Ru1–C3 = 98.6(1).
The metalated boron atoms, the metal center and the carbon atoms of carbonyl ligands are coplanar. The Ru–B bond lengths are 2.174(3) and 2.221(3) Å, which are longer than exohedral Ru–B bond lengths reported for heteroboranes (2.066(3)–2.159(3) Å).13 The B1–B2–Ru and B2–B1–Ru1 angles are 65.5(1)° and 68.4(1)°, which are the smallest exohedral B–B–X angle values for icosahedral boron clusters reported to date, indicating a high degree of bond strain. These angle values should be compared with the unstrained B–H bonds in the POBOP-H ligand precursor 1, which exhibits the B2–B1–H1 angle of 116.1(1)°.4p The B1–B2 distance in 2 is 1.720(4) Å, which is shorter than that in 1 (1.788(3) Å). Such shortening indicates the possibility of an additional bonding interaction between the metalated boron atoms. All other B–B bond lengths in 2 are in the range from 1.754(5) Å to 1.811(3) Å while corresponding B–B distances in 1 are in the range from 1.762(3) Å to 1.786(3) Å. Two CO ligands are located trans- to the carboryne ligand with C2–Ru–C3 angle of 98.6(1)°.
The values of υ(CO) = 2010 and 1958 cm–1 (υ(CO)average = 1984 cm–1) for 2 can be compared with reported IR spectral data for mononuclear Ru(II) and Ru(0) cis-dicarbonyl complexes. The values of υ(CO)average for five-coordinate Ru(0) complexes of the type Ru(CO)2L3 (L = a neutral donor ligand, in the majority of cases it is a phosphine)14a−14g are in the range from 1863 to 1976 cm–1 while Ru(II) complexes Ru(CO)2L2X2 (X = an anionic ligand)14h−14l exhibit υ(CO)average in the range from 1981 to 2060 cm–1. Interestingly, the υ(CO)average value of 2 is close to υ(CO)average values for Ru(0) η2-alkene and η2-alkyne complexes Ru(CO)2L2(η2-L′),14f,14m−14o which are in the range from 1927 to 2003 cm–1.
Compound 2 is the first example of a metal complex of the η2-coordinated 2,6-dehydro-m-carborane ((BB)-carboryne), which bears an analogy to 1,2-dehydro-o-carborane ((CC)-carborynes) and benzyne complexes. Formation of 2 occurs within the POBOP pincer framework, which geometrically imposes the close contact of two adjacent boron atoms with the chelated metal center. The crystal structure of the major intermediate product (POBOP)RuCl(CO)2 (3), which was observed in the crude reaction mixture before addition of NEt3 (δ = 214.9 ppm in the 31P NMR spectrum), was obtained. Complex 3 contained only one B–Ru bond, the chloride ligand, and two carbonyl ligands, indicating that two B–H activation events en route to 2 are likely to occur sequentially (see SI for details).
Analysis of Bonding in the Ruthenium (BB)-Carboryne
The bonding in the first (BB)-carboryne complex 2 was examined using DFT calculations followed by MO and topological analysis of electron density (ED) in the framework of QTAIM as well as with the analysis of the electron localization function (ELF).15 The highest occupied canonical molecular orbital (HOMO) and a lower lying occupied molecular orbital (HOMO–15) closely resemble the bonding arrangement in a metal olefin/alkyne complex according to the Dewar–Chatt–Duncanson model. Specifically, the HOMO corresponds to the π-type back-donation interaction between a d-orbital of the metal center and the π*-orbital of the (BB)-carboryne unit (Figure 2a). On the other hand, the HOMO–15 represents the σ-type bonding interaction between a d-orbital of the metal center and the π-orbital of the (BB)-carboryne (Figure 2b). Analysis of Pipek–Mezey15i localized orbitals revealed the presence of two similar orbitals with predominant Ru1–B1 and Ru1–B2 contributions (Figure 2c, only one localized orbital for the Ru1–B1 bond is shown, the orbital corresponding to the Ru1–B2 bond is nearly identical in shape and is shown in SI), corresponding to two localized Ru–B σ-bonds. QTAIM and ELF analysis also confirm this description. The molecular graph in the (BB)>Ru plane is triangular with individual B–B and Ru–B bond paths (Figure 2d). Remarkably, Ru–B bond paths are significantly outward-bent near boron atoms following the position of ED concentration regions. Delocalization indices (DI, defined as the number of electron pairs shared between two atoms, which is an analogue of the bond order in QTAIM) for each Ru–B bond are 0.69. Laplacian of ED at the bond critical point is positive, which is normal for transition metal–ligand bonding,15f,15j whereas the total energy density is negative, which is a sign of covalent bonding. In the ELF representation, Ru–B bonding is described by two disynaptic valence basins, V(Ru,B) (shown in green in Figure 2e), which are drastically shifted from Ru–B connectivity lines. Each of V(Ru,B) basins has the population of 1.69 e, with 0.45 e and 1.21 e being contributed by Ru and B, respectively. These results indicate that metal–ligand bonding in 2 is dominated by two individual two-electron covalent Ru–B bonds with considerable electron density bent outward of the strained (BB)>Ru cycle.
Figure 2.
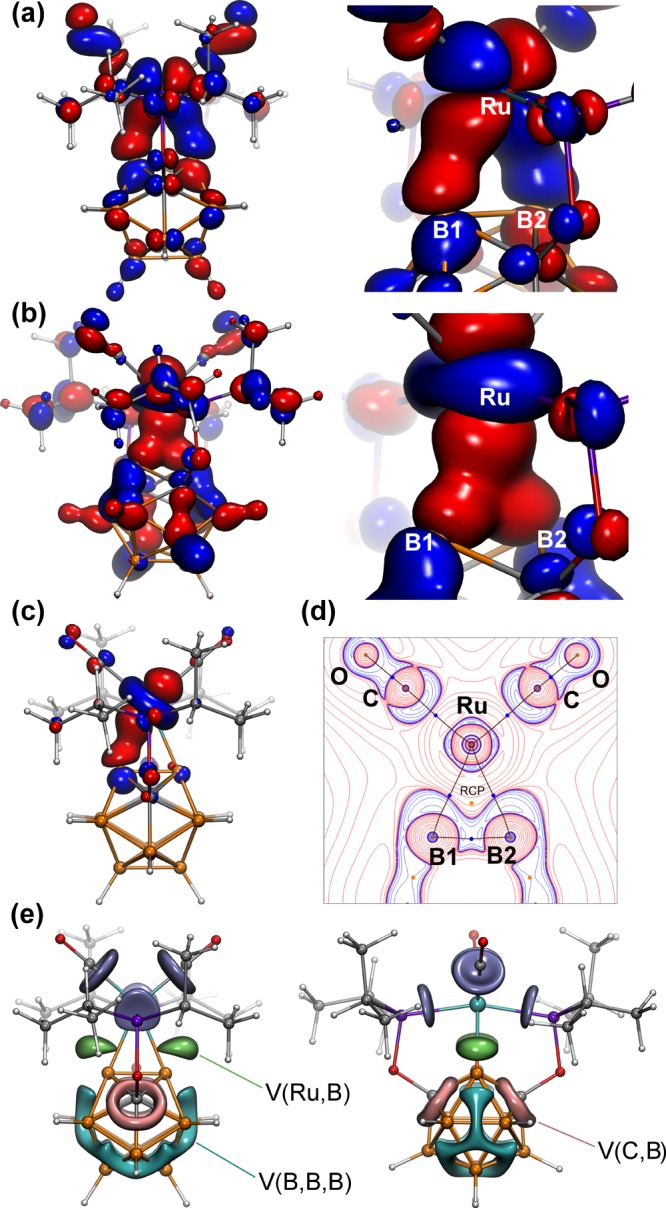
Results of theoretical calculations for the (POBBOP)Ru(CO)2 (BB)-carboryne complex (2). (a) The highest occupied molecular orbital (HOMO) and its enlarged region containing (BB)>Ru cycle. (b) The lower energy occupied molecular orbital (HOMO–15) and its enlarged region containing (BB)>Ru cycle. (c) One of the two Pipek–Mezey localized Ru–B bonding orbitals. (d) The contour map of electron density Laplacian in the (B1–B2–Ru1) plane (red curves denote ED depletion, blue curves denote ED concentration; blue dots are bond critical points). (e) The ELF isosurface at the level η = 0.80 shown in two projections; V(Ru,B) basins are shown in green, trisynaptic V(B,B,B) basins are shown in cyan, disynaptic V(C,B) basins are shown in pink, and V(Ru,C) and V(Ru,P) basins are shown in violet. Other basins are omitted for clarity; note that V(Ru,B,B) basin with smaller attractor value (η = 0.67) is not shown at this level.
The structural parameters and the bonding situation in 2 should be compared with those in benzyne and (CC)-carboryne complexes. For the benzyne complexes, a slight decrease in the bond length between two metalated carbon atoms is usually observed. For example, the first isolated and structurally characterized (Cp*)Ta(Me)2(η2-C6H4) Schrock benzyne complex exhibits the C–C bond length of 1.364(8) Å, which is shorter than the typical C–C bond between two aromatic carbon atoms (ca. 1.40 Å).6c The structurally characterized benzyne complex of ruthenium, Ru(PMe3)4(η2-C6H4), exhibits the C–C bond length of 1.355(3) Å in the (CC)>Ru metallacycle.6d This concept of the multiple bond character in benzyne complexes can be carefully applied to the carborane cages taking into account that carbon and boron atoms of the cluster engage in the delocalized bonding with bond orders smaller than one.7f,16 Thus, the (CC)-carboryne complexes of the group 4 and group 8 metals feature slight changes in the C–C bond length in the (CC)>M cycle in comparison with the unsubstituted o-C2B10H12 carborane. For example, the C–C distances in (CC)>Ni carboryne complexes are in the range from 1.551(4) Å to 1.590(10) Å, which should be compared to the C–C distance of ca. 1.63 Å in o-C2B10H12.8c For (CC)>Zr carboryne complexes, the C–C distances of the carboryne ligand (1.62(1) Å – 1.708(7) Å are often longer than in the parent o-C2B10H12.7d,8d The shortening of the B–B bond length in the (BB)>Ru metallacycle in 2 (1.720(4) Å) in comparison to that in the ligand precursor 1 (1.788(3) Å) prompted us to investigate the effect of η2-coordination of two boron atoms to the metal center on the bonding within the boron cage in more detail.
Interestingly, the B1–B2 bonding in the ligand precursor 1 is very weak with DI value of only 0.11 and no bond path between these atoms16a,16b (cf. DI of 0.43 for B9–B10 bond on the opposite side of the cage, see SI for details). The reason for such a peculiar bonding situation is the distortion of the delocalized interactions between boron atoms induced by carbons. The bonding between boron atoms in 1 is predominantly three-center-two-electron (3c-2e), which can be seen as a combination of fused trisynaptic V(B,B,B) basins in the ELF representation. When carbon substitutes boron at a vertex, the B–C bonding becomes essentially 2c-2e (hence the appearance of disynaptic V(B,C) basins fused into torus-shaped superbasins). As a result, B–B bonding is weakened, and the effect is especially pronounced for the B1–B2 bond with two adjacent carbon atoms. Comparison between the ligand precursor 1 and the (BB)>Ru cycle in 2 shows that coordination of two boron atoms to Ru increases direct B1–B2 bonding with the appearance of the bond critical point and the DI value of 0.23 in the carboryne complex 2, which is consistent with the shorter experimental B1–B2 bond length in 2 than that in 1. Furthermore, ELF analysis of 2 localized a three-center V(Ru,B,B) basin with smaller attractor value (η = 0.67, see SI for details). This interaction can be interpreted as bonding between Ru and the B–B bond in the (BB)>Ru cycle thus consistent with the description of the complex 2 as a (BB)-carboryne. In contrast to 2, the B1–B2 bonding in the mono-B-metalated complex 3 is weak with DI = 0.12 and no direct bond path (see SI for details), indicating that it is the formation of the (BB)>Ru cycle that leads to the increase in bonding between two boron atoms, not merely metalation of one boron vertex.
Hoffmann et al.16c predicted that the formal deprotonation of two adjacent boron atoms of the related closo-B12H122– boron cluster with the formation of two anionic boron vertices would lead to elongation of the B–B distance between deprotonated borons (2.035 Å vs the 1.790 Å in the parent B12H122–), while the formal removal of two hydrogen atoms from two adjacent borons to form a carboryne analogue would lead to shortening of that B–B distance (1.674 Å vs the 1.790 Å in the parent B12H122–). Consistent with these predictions and our computational results, the B1–B2 distances in mono-B-metalated carboranyl complexes 3–7 (see below), which can be considered as anionic boryl compounds, are in the range from 1.791(3) Å to 1.815(4) Å and are longer than those in 2 (1.720(4) Å) and in the parent neutral 1 (1.788(3) Å).
These computational results indicate that two types of bonding interactions are present in the complex 2. One, likely the predominant one, is the ruthenacycloborapropene structure with two σ bonds from two boron atoms connected to the metal center. Those bonds are two separate two-center B–Ru interactions. Another type of bonding that was found by the analysis of the electron localization function is the three-center interaction between two boron atoms and the metal center representing the benzyne/olefin-like coordination of the ligand to the metal. This bonding pattern is characterized by the smaller attractor value of the ELF basin, however, it is clearly present. The overall picture of bonding in the (BB)>Ru cycle is a combination of two extreme cases; however, it is apparent that the double B–H activation of the carborane cage led to the increase in bonding between two metalated boron atoms with some degree of backdonation from the metal center to the B–B bond.
Reactivity Studies of the Ruthenium (BB)-Carboryne. Carboryne to Carboranyl Transformations
The (BB)-carboryne complex 2 is also related to the growing family of the complexes of transition metals containing diborane, diborylene, and diborene ligands.17 In contrast to noncluster boryls, the exohedral metal–boron bonds in the majority of icosahedral carborane complexes are generally considered to be stable due in part to the strong steric shielding provided by the boron cage. We hypothesized that the highly strained, electron-rich,3a Ru–B bonds in the (BB)-carboryne 2 can themselves serve as nucleophilic reaction centers, thus diverging from the other B-carboranyl complexes (Scheme 2). This preliminary exploration of the reactivity of the novel (BB)>Ru metallacycle was mainly focused on the reactions typical for benzyne complexes. At the same time, the presence of chelating donor arms was anticipated to retain the metal center in the products.
Scheme 2. Transformation of the (BB)-Carboryne Complex 2 to B-Carboranyl Complexes.
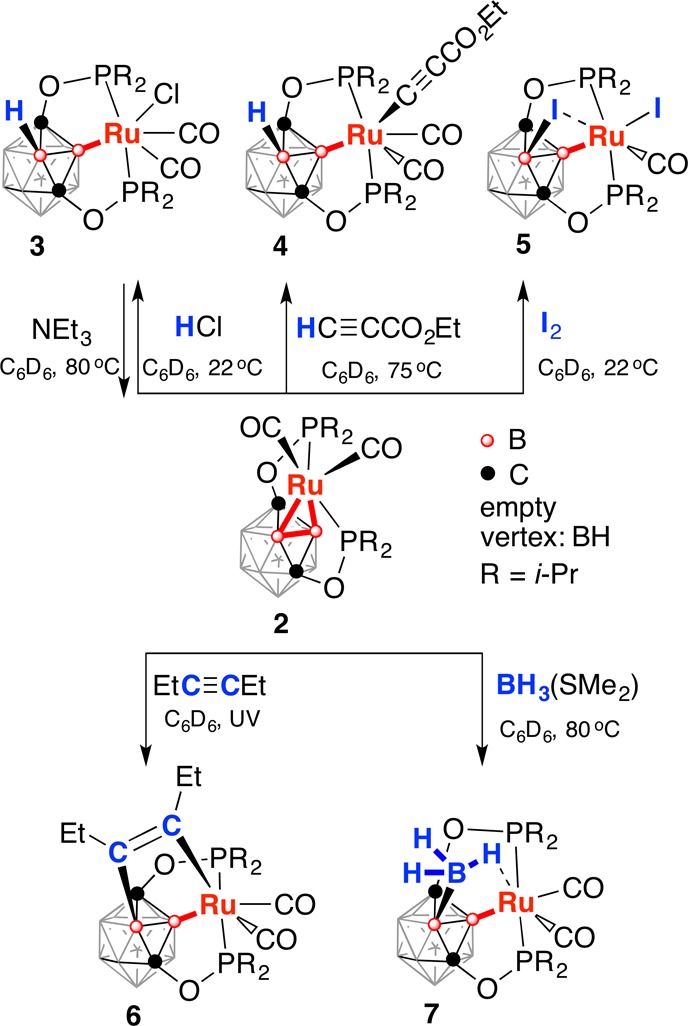
Addition of HCl to a solution of 2 in C6D6 at room temperature resulted in the selective facile conversion to the B-carboranyl complex 3, which was identified earlier (see above). Only one metal–boron bond participated in the reaction, even if a large excess of HCl was used. Importantly, this transformation can be reversed in the reaction of 3 and excess NEt3 at 80 °C in C6D6, which led to the reformation of the (BB)-carboryne complex 2 according to 31P and 11B NMR data.
The reaction of 2 and the terminal alkyne HC≡CCO2Et in C6D6 solution at 75 °C resulted in the formal oxidative addition of the substrate across one of the metal–boron bonds leading to the selective formation of B-carboranyl acetylide Ru(II) complex 4. The product was isolated in 94% yield and was structurally characterized (see SI for details). The acetylide ligand is located syn- relative to the vicinal B–H bond. The υ(C≡C) value in 4 is 2109 cm–1. Notably, both carbonyl ligands remained bound to the metal center in 4.
The reaction of 2 and I2 at 22 °C resulted in formation of the B-carboranyl complex 5 containing a vicinal B–I bond and one iodide and one carbonyl ligand on the metal (Figure 3). Interestingly, the (B2)–I2···Ru1 distance in the crystal structure of 5 is 2.884(1) Å, which is comparable to the Ru1–I1 bond of 2.777(1) Å in the same complex. In addition, the B2–B1–Ru1 angle is 98.2(1)°, which indicates a metal–boron bond strain, likely caused by the probable (B)–I···Ru interaction. The complex 5 represents the rare example of the (B)–I···M bridging geometry;18a the analogous coordination of iodocarbons to ruthenium is also rare.18b The relative stability of the bridging (B)–I···M interactions has a potential to influence the regioselectivity in metal-catalyzed coupling reactions of B-iodocarborane clusters.
Figure 3.
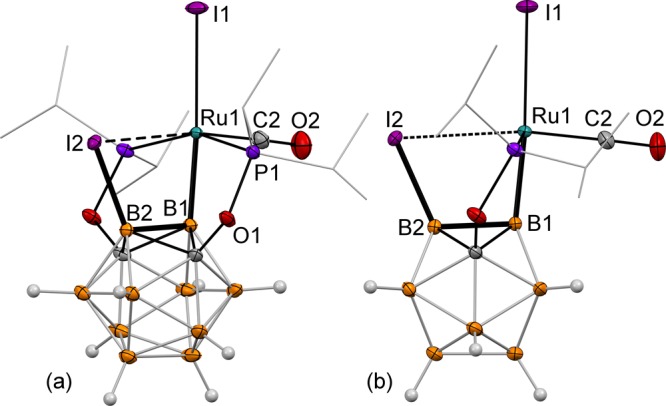
Displacement ellipsoid plot (50% probability) of the (POB(I)OP)Ru(I) (CO) complex (5). (a) A general view; (b) a view perpendicular to the (B2–B1–Ru1–C2–I1) plane. Atoms belonging to isopropyl groups of the ligand arms have been omitted for clarity. The C6D6 solvent molecule is not shown. Selected bond distances (Å) and angles (deg): Ru1–B1 = 2.059(2), Ru1–I1 = 2.777(1), Ru1···I2 = 2.884(1), B1–B2 = 1.791(3), B2–I2 = 2.156(2), B2–B1–Ru1 = 98.2(1), B1–B2–I2 = 111.8(1), I2···Ru1–C2 = 170.9(1), and B1–Ru1–I1 = 175.2(1).
One of the important reactions of metal benzyne complexes or (CC)-carboryne complexes is [2 + 2] cycloaddition reactions with unsaturated substrates such as internal alkynes. The reaction of 2 and 3-hexyne under UV irradiation led to the facile selective formation of the cycloaddition complex 6, which contained the bridging B—CEt=CEt—Ru fragment. The second B–Ru bond was found to be intact in addition to two carbonyl ligands, which remained coordinated to the metal center. The product was isolated in 88% yield and structurally characterized (see SI for details).
The nucleophilic character of the B–Ru bonds in the (BB)-carboryne 2 led us to the hypothesis of the possibility of their interactions with neutral Lewis acids. The reaction of 2 and excess of BH3·SMe2 in the THF/C6D6 mixture at 80 °C led to clean formation of an insertion product 7 featuring the newly formed exohedral B–B bond and the bridging exohedral (B)–B(H)2–H···Ru interaction (Figure 4). The overall structure can be formally described as either a Ru(II) complex of a borylhydroborate ligand or a Ru(II) hydride boryl complex with the (B)–B···H–Ru interaction. Hydrogen atoms of the BH3 group were located using the electron density map. The Ru1···H11A distance is 1.79(1) Å indicating a significant metal hydride character. The B11–H11A distance of 1.28(1) Å is longer than B11–H11B and B11–H11C distances (1.09(1) Å and 1.12(1) Å, respectively). The H11A···Ru1–C4 angle is 174.8(4)° suggesting the octahedral coordination of the metal center with the bridging hydride as one of the ligands. Two carbonyl groups remained bound to the metal center in 7. The 1H NMR spectrum of 7 in C6D6 at room temperature contained a broad signal with the relative integral intensity of 1H at −10.8 ppm corresponding to the bridging hydride (B)–B(H)2–H···Ru indicative of the static BH3 group behavior on the NMR time scale. The complexes containing similar bridging R–BH3···Ru interactions have been reported to exhibit dynamic (BH3···Ru signals at δ = +3 to −4 ppm) or static (bridging B–H···Ru signals at δ = −6 to −14 ppm) behavior in the 1H NMR spectra at room temperature.19 Complex 7 is a rare example of a carborane cluster with an exohedral B–B bond to a simple borane, and it is the first example of the regioselective formation of such a bond under metal-promoted conditions.20 This compound may also be considered as a snapshot of a probable intermediate in transition-metal-catalyzed synthesis of diboranes, which is an increasingly important area of organometallic research due to the rise to prominence of diborane reagents for borylation chemistry.21
Figure 4.
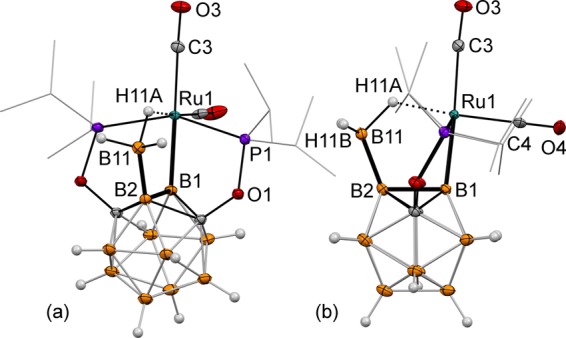
Displacement ellipsoid plot (50% probability) of the (POB(BH3)OP)Ru(CO)2 complex (7). (a) A general view; (b) a view perpendicular to the (B2–B1–Ru1–C3–C4) plane. Atoms belonging to isopropyl groups of the ligand arms have been omitted for clarity. Selected bond distances (Å) and angles (deg): Ru1–B1 = 2.136(1), Ru1···B11 = 2.700(1), B1–B2 = 1.803(1), B2–B11 = 1.680(1), Ru1···H11A = 1.79(1), B11–H11A = 1.28(1), B11–H11B = 1.09(1), B2–B1–Ru1 = 95.6(1), B1–B2–B11 = 110.8(1), and B1–Ru1–C3 = 176.8(1), and H11A···Ru1–C4 = 174.8(4).
These examples of the reactivity of 2 demonstrate that only one metal–boron bond reacts with a substrate while another Ru–B bond remains intact leading to the formation of monometalated B-carboranyl complexes. This observation further highlights the difference in the reactivity of the (BB)>Ru carboryne in comparison to (B)–Ru carboranyls/boryls.
Conclusions
In summary, the first metal complex of a closo-boron cluster containing an exohedral (BB)>M metallacycle was synthesized by taking an advantage of the unique geometry of the m-carboranyl pincer framework. The reactivity pattern of the ruthenium (BB)-carboryne complex reported herein represents a new mode of metal–ligand cooperative interaction by the transformation of the (BB)-carboryne into the B-carboranyl moiety, in some cases reversibly. The involvement of the (BB)-carboryne ligand is reminiscent of the aryne-aryl transformations of metal benzynes and aromatization–dearomatization, amine–amide, and carbene-alkyl conversion reported for pincer complexes.22 In addition, the utilization of the (BB)-carboryne motif for functionalization of boron cages can open an access to new classes of carborane-based compounds. Further studies of this novel (BB)-carboryne system and its congeners are underway.
Acknowledgments
Acknowledgment is made to the donors of the American Chemical Society Petroleum Research Fund (Award 54504-DNI3) and to ASPIRE-I funding granted through the University of South Carolina, Office of the Vice President for Research to D.V.P. We are grateful to Dr. Perry. J. Pelechia (Director of NMR Services, University of South Carolina) for immense help with 2D NMR spectroscopy studies. A.A.P. acknowledges funding by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 648295 “GraM3”). Computational resources were provided by the Center for Information Services and High Performance Computing (ZIH) at TU Dresden. The authors thank Ulrike Nitzsche for technical assistance with computational resources at IFW Dresden.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.6b05172.
The authors declare no competing financial interest.
Supplementary Material
References
- a Selander N.; Szabó K. J. Chem. Rev. 2011, 111, 2048. 10.1021/cr1002112. [DOI] [PubMed] [Google Scholar]; b Choi J.; MacArthur A. H. R.; Brookhart M.; Goldman A. S. Chem. Rev. 2011, 111, 1761. 10.1021/cr1003503. [DOI] [PubMed] [Google Scholar]; c Gunanathan C.; Milstein D. Chem. Rev. 2014, 114, 12024. 10.1021/cr5002782. [DOI] [PubMed] [Google Scholar]; d Adhikary A.; Guan H. ACS Catal. 2015, 5, 6858. 10.1021/acscatal.5b01688. [DOI] [Google Scholar]
- a Gusev D. G.; Lough A. J. Organometallics 2002, 21, 2601. 10.1021/om020355m. [DOI] [Google Scholar]; b Whited M. T.; Grubbs R. H. Acc. Chem. Res. 2009, 42, 1607. 10.1021/ar900103e. [DOI] [PubMed] [Google Scholar]; c Doyle L. E.; Piers W. E.; Borau-Garcia J. J. Am. Chem. Soc. 2015, 137, 2187. 10.1021/ja512602m. [DOI] [PubMed] [Google Scholar]; d Comanescu C. C.; Iluc V. M. Organometallics 2014, 33, 6059. 10.1021/om500682s. [DOI] [Google Scholar]; e Liang L. C. Coord. Chem. Rev. 2006, 250, 1152. 10.1016/j.ccr.2006.01.001. [DOI] [Google Scholar]; f Morgan E.; MacLean D. F.; McDonald R.; Turculet L. J. Am. Chem. Soc. 2009, 131, 14234. 10.1021/ja906646v. [DOI] [PubMed] [Google Scholar]; g Caulton K. G. Eur. J. Inorg. Chem. 2012, 2012, 435. 10.1002/ejic.201100623. [DOI] [Google Scholar]
- a Dang L.; Lin Z.; Marder T. B. Chem. Commun. 2009, 27, 3987. 10.1039/b903098k. [DOI] [PubMed] [Google Scholar]; b Segawa Y.; Yamashita M.; Nozaki K. J. Am. Chem. Soc. 2009, 131, 9201. 10.1021/ja9037092. [DOI] [PubMed] [Google Scholar]; c Chaplin A. B.; Weller A. S. Angew. Chem., Int. Ed. 2010, 49, 581. 10.1002/anie.200905185. [DOI] [PubMed] [Google Scholar]; d Harman W. H.; Peters J. C. J. Am. Chem. Soc. 2012, 134, 5080. 10.1021/ja211419t. [DOI] [PubMed] [Google Scholar]; e Lin T.-P.; Peters J. C. J. Am. Chem. Soc. 2013, 135, 15310. 10.1021/ja408397v. [DOI] [PubMed] [Google Scholar]; f Bontemps S.; Gornitzka H.; Bouhadir G.; Miqueu K.; Bourissou D. Angew. Chem., Int. Ed. 2006, 45, 1611. 10.1002/anie.200503649. [DOI] [PubMed] [Google Scholar]; g Bontemps S.; Bouhadir G.; Gu W.; Mercy M.; Chen C.-H.; Foxman B. M.; Maron L.; Ozerov O. V.; Bourissou D. Angew. Chem., Int. Ed. 2008, 47, 1481. 10.1002/anie.200705024. [DOI] [PubMed] [Google Scholar]; h Braunschweig H.; Dewhurst R. D. Dalton Trans. 2010, 40, 549. 10.1039/C0DT01181A. [DOI] [PubMed] [Google Scholar]; i Braunschweig H.; Dewhurst R. D.; Schneider A. Chem. Rev. 2010, 110, 3924. 10.1021/cr900333n. [DOI] [PubMed] [Google Scholar]; j Shih W.-C.; Gu W.; MacInnis M. C.; Timpa S. D.; Bhuvanesh N.; Zhou J.; Ozerov O. V. J. Am. Chem. Soc. 2016, 138, 2086. 10.1021/jacs.5b11706. [DOI] [PubMed] [Google Scholar]; k Hill A. F.; Lee S. B.; Park J.; Shang R.; Willis A. C. Organometallics 2010, 29, 5661. 10.1021/om100557q. [DOI] [Google Scholar]; l Hill A. F.; McQueen C. M. A. Organometallics 2014, 33, 1977. 10.1021/om5001106. [DOI] [Google Scholar]; m Owen G. R. Chem. Soc. Rev. 2012, 41, 3535. 10.1039/c2cs15346g. [DOI] [PubMed] [Google Scholar]; n Owen G. R. Chem. Commun. 2016, 10.1039/C6CC03817D. [DOI] [Google Scholar]
- a Grimes R. N. Dalton Trans. 2015, 44, 5939. 10.1039/C5DT00231A. [DOI] [PubMed] [Google Scholar]; b Popescu A. R.; Teixidor F.; Viñas C. Coord. Chem. Rev. 2014, 269, 54. 10.1016/j.ccr.2014.02.016. [DOI] [Google Scholar]; c Yinghuai Z.; Hosmane N. S. J. Organomet. Chem. 2013, 747, 25. 10.1016/j.jorganchem.2013.03.042. [DOI] [Google Scholar]; d Yao Z.-J.; Jin G.-X. Coord. Chem. Rev. 2013, 257, 2522. 10.1016/j.ccr.2013.02.004. [DOI] [Google Scholar]; e Douvris C.; Michl J. Chem. Rev. 2013, 113, PR179. 10.1021/cr400059k. [DOI] [PubMed] [Google Scholar]; f Yamashita M. Bull. Chem. Soc. Jpn. 2015, 89, 269. 10.1246/bcsj.20150355. [DOI] [Google Scholar]; g Gleeson B.; Carroll P. J.; Sneddon L. G. J. Am. Chem. Soc. 2013, 135, 12407. 10.1021/ja405977q. [DOI] [PubMed] [Google Scholar]; h Teixidor F.; Flores M. A.; Viñas C.; Sillanpää R.; Kivekäs R. J. Am. Chem. Soc. 2000, 122, 1963. 10.1021/ja992970w. [DOI] [Google Scholar]; i El-Hellani A.; Kefalidis C. E.; Tham F. S.; Maron L.; Lavallo V. Organometallics 2013, 32, 6887. 10.1021/om401001p. [DOI] [Google Scholar]; j Spokoyny A. M.; Reuter M. G.; Stern C. L.; Ratner M. A.; Seideman T.; Mirkin C. A. J. Am. Chem. Soc. 2009, 131, 9482. 10.1021/ja902526k. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Kirchmann M.; Wesemann L. Dalton Trans. 2008, 4, 444. 10.1039/B715305H. [DOI] [PubMed] [Google Scholar]; l Himmelspach A.; Finze M.; Raub S. Angew. Chem., Int. Ed. 2011, 50, 2628. 10.1002/anie.201007239. [DOI] [PubMed] [Google Scholar]; m El-Zaria M. E.; Arii H.; Nakamura H. Inorg. Chem. 2011, 50, 4149. 10.1021/ic2002095. [DOI] [PubMed] [Google Scholar]; n Lavallo V.; Wright J. H.; Tham F. S.; Quinlivan S. Angew. Chem., Int. Ed. 2013, 52, 3172. 10.1002/anie.201209107. [DOI] [PubMed] [Google Scholar]; o Tsang M. Y.; Viñas C.; Teixidor F.; Planas J. G.; Conde N.; SanMartin R.; Herrero M. T.; Domínguez E.; Lledós A.; Vidossich P.; Choquesillo-Lazarte D. Inorg. Chem. 2014, 53, 9284. 10.1021/ic5013999. [DOI] [PubMed] [Google Scholar]; p Eleazer B. J.; Smith M. D.; Peryshkov D. V. Organometallics 2016, 35, 106. 10.1021/acs.organomet.5b00807. [DOI] [Google Scholar]; q Spokoyny A. M. Pure Appl. Chem. 2013, 85, 903. 10.1351/PAC-CON-13-01-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected recent examples and reviews, see:; a Núñez R.; Romero I.; Teixidor F.; Viñas C. Chem. Soc. Rev. 2016, 10.1039/C6CS00159A. [DOI] [PubMed] [Google Scholar]; b Wee K.-R.; Cho Y.-J.; Jeong S.; Kwon S.; Lee J.-D.; Suh I.-H.; Kang S. O. J. Am. Chem. Soc. 2012, 134, 17982. 10.1021/ja3066623. [DOI] [PubMed] [Google Scholar]; c Koshino M.; Tanaka T.; Solin N.; Suenaga K.; Isobe H.; Nakamura E. Science 2007, 316, 853. 10.1126/science.1138690. [DOI] [PubMed] [Google Scholar]; d Schwartz J. J.; Mendoza M. A.; Wattanatorn N.; Zhao Y.; Nguyen V. T.; Spokoyny A. M.; Mirkin C. A.; Baše T.; Weiss P. S. J. Am. Chem. Soc. 2016, 138, 5957. 10.1021/jacs.6b02026. [DOI] [PubMed] [Google Scholar]; e Kirlikovali K. O.; Axtell J. A.; Gonzalez A.; Phung A. C.; Khan S. I.; Spokoyny A. M. Chem. Sci. 2016, 7, 5132. 10.1039/C6SC01146B. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Tutusaus O.; Mohtadi R.; Arthur T. S.; Mizuno F.; Nelson E. G.; Sevryugina Y. V. Angew. Chem., Int. Ed. 2015, 54, 7900. 10.1002/anie.201412202. [DOI] [PubMed] [Google Scholar]; g Scholz M.; Hey-Hawkins E. Chem. Rev. 2011, 111, 7035. 10.1021/cr200038x. [DOI] [PubMed] [Google Scholar]; h McArthur S. G.; Geng L.; Guo J.; Lavallo V. Inorg. Chem. Front. 2015, 2, 1101. 10.1039/C5QI00171D. [DOI] [Google Scholar]; i Kracke G. R.; VanGordon M. R.; Sevryugina Y. V.; Kueffer P. J.; Kabytaev K.; Jalisatgi S. S.; Hawthorne M. F. ChemMedChem 2015, 10, 62. 10.1002/cmdc.201402369. [DOI] [PubMed] [Google Scholar]; j Kaszynski P. Collect. Czech. Chem. Commun. 1999, 64, 895. 10.1135/cccc19990895. [DOI] [Google Scholar]; k Dash B. P.; Satapathy R.; Maguire J. A.; Hosmane N. S. New J. Chem. 2011, 35, 1955. 10.1039/c1nj20228f. [DOI] [Google Scholar]
- a Buchwald S. L.; Nielsen R. B. Chem. Rev. 1988, 88, 1047. 10.1021/cr00089a004. [DOI] [Google Scholar]; b Jones W. M.; Klosin J. Adv. Organomet. Chem. 1998, 42, 147. 10.1016/S0065-3055(08)60543-2. [DOI] [Google Scholar]; c McLain S. J.; Schrock R. R.; Sharp P. R.; Churchill M. R.; Youngs W. J. J. Am. Chem. Soc. 1979, 101, 263. 10.1021/ja00495a067. [DOI] [Google Scholar]; d Hartwig J. F.; Bergman R. G.; Andersen R. A. J. Am. Chem. Soc. 1991, 113, 3404. 10.1021/ja00009a028. [DOI] [Google Scholar]; e Retboll M.; Edwards A. J.; Rae A. D.; Willis A. C.; Bennett M. A.; Wenger E. J. Am. Chem. Soc. 2002, 124, 8348. 10.1021/ja0264091. [DOI] [PubMed] [Google Scholar]
- a Sayler A. A.; Beall H.; Sieckhaus J. F. J. Am. Chem. Soc. 1973, 95, 5790. 10.1021/ja00798a074. [DOI] [Google Scholar]; b Gingrich H. L.; Ghosh T.; Huang Q.; Jones M. J. Am. Chem. Soc. 1990, 112, 4082. 10.1021/ja00166a080. [DOI] [Google Scholar]; c Qiu Z.; Ren S.; Xie Z. Acc. Chem. Res. 2011, 44, 299. 10.1021/ar100156f. [DOI] [PubMed] [Google Scholar]; d Wang H.; Li H.-W.; Huang X.; Lin Z.; Xie Z. Angew. Chem., Int. Ed. 2003, 42, 4347. 10.1002/anie.200351892. [DOI] [PubMed] [Google Scholar]; e King R. B. Chem. Rev. 2001, 101, 1119. 10.1021/cr000442t. [DOI] [PubMed] [Google Scholar]; f Poater J.; Solà M.; Viñas C.; Teixidor F. Angew. Chem., Int. Ed. 2014, 53, 12191. 10.1002/anie.201407359. [DOI] [PubMed] [Google Scholar]
- a Qiu Z.; Xie Z. Dalton Trans. 2014, 43, 4925. 10.1039/C3DT52711E. [DOI] [PubMed] [Google Scholar]; b Ren S.; Qiu Z.; Xie Z. Organometallics 2013, 32, 4292. 10.1021/om400458r. [DOI] [Google Scholar]; c Qiu Z.; Deng L.; Chan H.-S.; Xie Z. Organometallics 2010, 29, 4541. 10.1021/om100669x. [DOI] [Google Scholar]; d Ren S.; Deng L.; Chan H.-S.; Xie Z. Organometallics 2009, 28, 5749. 10.1021/om900504g. [DOI] [Google Scholar]
- a Deng L.; Chan H.-S.; Xie Z. J. Am. Chem. Soc. 2006, 128, 7728. 10.1021/ja061605j. [DOI] [PubMed] [Google Scholar]; b Ren S.; Qiu Z.; Xie Z. Angew. Chem., Int. Ed. 2012, 51, 1010. 10.1002/anie.201106212. [DOI] [PubMed] [Google Scholar]; c Wang S. R.; Qiu Z.; Xie Z. J. Am. Chem. Soc. 2011, 133, 5760. 10.1021/ja201126h. [DOI] [PubMed] [Google Scholar]; d Zhao D.; Zhang J.; Xie Z. Angew. Chem., Int. Ed. 2014, 53, 12902. 10.1002/anie.201409141. [DOI] [PubMed] [Google Scholar]; e Zhao D.; Zhang J.; Xie Z. J. Am. Chem. Soc. 2015, 137, 13938. 10.1021/jacs.5b09074. [DOI] [PubMed] [Google Scholar]; f Cheng R.; Zhang J.; Zhang J.; Qiu Z.; Xie Z. Angew. Chem., Int. Ed. 2016, 55, 1751. 10.1002/anie.201507952. [DOI] [PubMed] [Google Scholar]
- a Qiu Z.; Xie Z. J. Am. Chem. Soc. 2010, 132, 16085. 10.1021/ja1058789. [DOI] [PubMed] [Google Scholar]; b Zhao D.; Zhang J.; Xie Z. Angew. Chem., Int. Ed. 2014, 53, 8488. 10.1002/anie.201405023. [DOI] [PubMed] [Google Scholar]
- a Fey N.; Haddow M. F.; Mistry R.; Norman N. C.; Orpen A. G.; Reynolds T. J.; Pringle P. G. Organometallics 2012, 31, 2907. 10.1021/om201198s. [DOI] [Google Scholar]; b Yao Z.-J.; Yu W.-B.; Lin Y.-J.; Huang S.-L.; Li Z.-H.; Jin G.-X. J. Am. Chem. Soc. 2014, 136, 2825. 10.1021/ja4115665. [DOI] [PubMed] [Google Scholar]; c Estrada J.; Lee S. E.; McArthur S. G.; El-Hellani A.; Tham F. S.; Lavallo V. J. Organomet. Chem. 2015, 798, 214. 10.1016/j.jorganchem.2015.05.008. [DOI] [Google Scholar]; d Adams R. D.; Kiprotich J.; Peryshkov D. V.; Wong Y. O. Inorg. Chem. 2016, 55, 8207. 10.1021/acs.inorgchem.6b01403. [DOI] [PubMed] [Google Scholar]
- a Hoel E. L.; Hawthorne M. F. J. Am. Chem. Soc. 1975, 97 (22), 6388. 10.1021/ja00855a017. [DOI] [Google Scholar]; b Bregadze V. I.; Usiatinsky A. Y.; Godovikov N. N. J. Organomet. Chem. 1985, 292 (1), 75. 10.1016/0022-328X(85)87322-8. [DOI] [Google Scholar]; c Mirabelli M. G. L.; Sneddon L. G. J. Am. Chem. Soc. 1988, 110, 449. 10.1021/ja00210a023. [DOI] [Google Scholar]; d Spokoyny A.; Saleh L.; Dziedzic R.; Khan S. Chem. - Eur. J. 2016, 22, 8466. 10.1002/chem.201601292. [DOI] [PubMed] [Google Scholar]; e Adams R. D.; Kiprotich J.; Peryshkov D. V.; Wong Y. O. Chem. - Eur. J. 2016, 22, 6501. 10.1002/chem.201601075. [DOI] [PubMed] [Google Scholar]
- a Herberhold M.; Yan H.; Milius W.; Wrackmeyer B. J. Organomet. Chem. 2000, 604, 170. 10.1016/S0022-328X(00)00221-7. [DOI] [PubMed] [Google Scholar]; b Yao Z. J.; Xu B.; Su G.; Jin G. X. J. Organomet. Chem. 2012, 721, 31. 10.1016/j.jorganchem.2012.05.004. [DOI] [Google Scholar]; c McGrath T. D.; Stone F. G. A.; Sukcharoenphon K. Dalton Trans. 2005, 15, 2500. 10.1039/b505243b. [DOI] [PubMed] [Google Scholar]; d Wu D. H.; Wu C. H.; Li Y. Z.; Guo D. D.; Wang X. M.; Yan H. Dalton Trans. 2009, 2, 285. 10.1039/B810831E. [DOI] [PubMed] [Google Scholar]; e Xu Z. W.; Han L.; Ji C.; Zhang R.; Shen X. J.; Yan H. Dalton Trans. 2011, 40, 6992. 10.1039/c1dt10291e. [DOI] [PubMed] [Google Scholar]; f Green M.; Howard J. A.; Jelfs A. N. D. M.; Johnson O.; Stone F. G. A. J. Chem. Soc., Dalton Trans. 1987, 1, 73. 10.1039/DT9870000073. [DOI] [Google Scholar]; g Herberhold M.; Yan H.; Milius W.; Wrackmeyer B. Chem. - Eur. J. 2002, 8, 388.. [DOI] [PubMed] [Google Scholar]; h Hu J.; Liu G.; Jiang Q.; Zhang R.; Huang W.; Yan H. Inorg. Chem. 2010, 49, 11199. 10.1021/ic101817u. [DOI] [PubMed] [Google Scholar]; i Wu C. H.; Wu D. H.; Liu X.; Guoyiqibayi G.; Guo D. D.; Lv G.; Wang X. M.; Yan H.; Jiang H.; Lu Z. H. Inorg. Chem. 2009, 48, 2352. 10.1021/ic900009j. [DOI] [PubMed] [Google Scholar]; j Ellis D. D.; Couchman S. M.; Jeffery J. C.; Malget J. M.; Stone F. G. A. Inorg. Chem. 1999, 38, 2981. 10.1021/ic990057q. [DOI] [PubMed] [Google Scholar]; k Liu D.; Dang L.; Sun Y.; Chan H. S.; Lin Z.; Xie Z. J. Am. Chem. Soc. 2008, 130, 16103. 10.1021/ja8067098. [DOI] [PubMed] [Google Scholar]; l Lebedev V. N.; Mullica D. F.; Sappenfield E. L.; Stone F. G. A. Organometallics 1996, 15, 1669. 10.1021/om950725p. [DOI] [Google Scholar]; m Hu J.; Wen J.; Wu D.; Zhang R.; Liu G.; Jiang Q.; Li Y.; Yan H. Organometallics 2010, 30, 298. 10.1021/om1010275. [DOI] [Google Scholar]; n Liao Y. H.; Mullica D. F.; Sappenfield E. L.; Stone F. G. A. Organometallics 1996, 15, 5102. 10.1021/om9603629. [DOI] [Google Scholar]; o Polyakov A. V.; Yanovsky A. I.; Struchkov Y. T.; Kalinin V. N.; Usatov A. V.; Zakharkin L. I. Koord. Chem. 1988, 14, 1278. [Google Scholar]
- a Sentets S.; Rodriguez-Martinez M. C.; Vendier L.; Donnadieu B.; Huc V.; Lugan N.; Lavigne G. J. Am. Chem. Soc. 2005, 127, 14554. 10.1021/ja055066e. [DOI] [PubMed] [Google Scholar]; b Benhamou L.; Wolf J.; César V.; Labande A.; Poli R.; Lugan N.; Lavigne G. Organometallics 2009, 28, 6981. 10.1021/om900813p. [DOI] [Google Scholar]; c Salem H.; Shimon L. J. W.; Diskin-Posner Y.; Leitus G.; Ben-David Y.; Milstein D. Organometallics 2009, 28, 4791. 10.1021/om9004077. [DOI] [Google Scholar]; d Jaunky P.; Schmalle H. W.; Alfonso M.; Fox T.; Berke H. J. Organomet. Chem. 2004, 689, 801. 10.1016/j.jorganchem.2003.11.013. [DOI] [Google Scholar]; e Jaunky P.; Schmalle H. W.; Blacque O.; Fox T.; Berke H. J. Organomet. Chem. 2005, 690, 1429. 10.1016/j.jorganchem.2004.11.055. [DOI] [Google Scholar]; f Nakajima Y.; Okamoto Y.; Chang Y.-H.; Ozawa F. Organometallics 2013, 32, 2918. 10.1021/om400126v. [DOI] [Google Scholar]; g Ogasawara M.; Maseras F.; Gallego-Planas N.; Kawamura K.; Ito K.; Toyota K.; Streib W. E.; Komiya S.; Eisenstein O.; Caulton K. G. Organometallics 1997, 16, 1979. 10.1021/om9700775. [DOI] [Google Scholar]; h Moreno M. A.; Haukka M.; Jääskeläinen S.; Vuoti S.; Pursiainen J.; Pakkanen T. A. J. Organomet. Chem. 2005, 690, 3803. 10.1016/j.jorganchem.2005.05.016. [DOI] [Google Scholar]; i Adams J. J.; Gruver B. C.; Donohoue R.; Arulsamy N.; Roddick D. M. Dalton Trans. 2012, 41, 12601–12611. 10.1039/c2dt31234d. [DOI] [PubMed] [Google Scholar]; j Riley L. E.; Chan A.; Taylor J.; Man W.; Ellis D.; Rosair G.; Welch A. J.; Sivaev I. B. Dalton Trans. 2015, 45, 1127. 10.1039/C5DT03417E. [DOI] [PubMed] [Google Scholar]; k Rickard C. E. F.; Roper W. R.; Williamson A.; Wright L. J. Organometallics 2000, 19, 4344. 10.1021/om0004601. [DOI] [Google Scholar]; l Amoroso D.; Jabri A.; Yap G. P. A.; Gusev D. G.; Santos E. N.; Fogg D. E. Organometallics 2004, 23, 4047. 10.1021/om040025x. [DOI] [Google Scholar]; m Helliwell M.; Vessey J. D.; Mawby R. J. J. Chem. Soc., Dalton Trans. 1994, 1193. 10.1039/dt9940001193. [DOI] [Google Scholar]; n Ogasawara M.; Macgregor S. A.; Streib W. E.; Folting K.; Eisenstein O.; Caulton K. G. J. Am. Chem. Soc. 1996, 118, 10189. 10.1021/ja960967w. [DOI] [Google Scholar]; o Hill A. F.; Schultz M.; Willis A. C. Organometallics 2004, 23, 5729. 10.1021/om0400954. [DOI] [Google Scholar]
- PBE0/def2-TZVP level with ZORA correction in ORCA package.; a Neese F. WIREs Comput. Mol. Sci. 2012, 2, 73. 10.1002/wcms.81. [DOI] [Google Scholar]; b Pantazis D. A.; Chen X.-Y.; Landis C. R.; Neese F. J. Chem. Theory Comput. 2008, 4, 908. 10.1021/ct800047t. [DOI] [PubMed] [Google Scholar]; c Bader R. F. W.Atoms in Molecules—A Quantum Theory; Oxford University Press: Oxford, 1990. [Google Scholar]; d Keith T. A.AIMAll package, version 16.01.09; TK Gristmill Software: KS, USA, 2016.; e Multiwfn package, version 3.3.8;Lu T.; Chen F. J. Comput. Chem. 2012, 33, 580. 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]; f Matito E.; Sola M. Coord. Chem. Rev. 2009, 253, 647. 10.1016/j.ccr.2008.10.003. [DOI] [Google Scholar]; g Silvi B.; Savin A. Nature 1994, 371, 683. 10.1038/371683a0. [DOI] [Google Scholar]; h TopMod package:Noury S.; Krokidis X.; Fuster F.; Silvi B. Comput. Chem. 1999, 23, 597. 10.1016/S0097-8485(99)00039-X. [DOI] [Google Scholar]; i Pipek J.; Mezey P. G. J. Chem. Phys. 1989, 90, 4916. 10.1063/1.456588. [DOI] [Google Scholar]; j Bader R. F. W.; Matta C. F. Inorg. Chem. 2001, 40, 5603. 10.1021/ic010165o. [DOI] [PubMed] [Google Scholar]
- a Oliva J. P.; Schleyer P. R.; Aullon G.; Burgos J. I.; Fernandez-Barbero A.; Alkorta I. Phys. Chem. Chem. Phys. 2010, 12, 5101. 10.1039/b924322d. [DOI] [PubMed] [Google Scholar]; b Bader R. F. W.; Legare D. A. Can. J. Chem. 1992, 70, 657. 10.1139/v92-089. [DOI] [Google Scholar]; c Pancharatna P. D.; Balakrishnarajan M. M.; Jemmis E. D.; Hoffman R. J. Am. Chem. Soc. 2012, 134, 5916. 10.1021/ja212055x. [DOI] [PubMed] [Google Scholar]
- a Segawa Y.; Yamashita M.; Nozaki K. Science 2006, 314, 113. 10.1126/science.1131914. [DOI] [PubMed] [Google Scholar]; b Braunschweig H.; Damme A.; Dewhurst R. D.; Kramer T.; Östreicher S.; Radackis K.; Vargas A. J. Am. Chem. Soc. 2013, 135, 2313. 10.1021/ja310895w. [DOI] [PubMed] [Google Scholar]; c Braunschweig H.; Dewhurst R. D. Angew. Chem., Int. Ed. 2013, 52, 3574. 10.1002/anie.201208189. [DOI] [PubMed] [Google Scholar]; d Braunschweig H.; Ye Q.; Vargas A.; Dewhurst R. D.; Radacki K.; Damme A. Nat. Chem. 2012, 4, 563. 10.1038/nchem.1379. [DOI] [PubMed] [Google Scholar]; e Bissinger P.; Braunschweig H.; Damme A.; Kupfer T.; Vargas A. Angew. Chem., Int. Ed. 2012, 51, 9931. 10.1002/anie.201204449. [DOI] [PubMed] [Google Scholar]; f Brand J.; Braunschweig H.; Sen S. S. Acc. Chem. Res. 2014, 47, 180. 10.1021/ar400106u. [DOI] [PubMed] [Google Scholar]
- a Moxham G. L.; Douglas T. M.; Brayshaw S. K.; Kociok-Köhn G.; Lowe J. P.; Weller A. S. Dalton Trans. 2006, 46, 5492. 10.1039/B612049K. [DOI] [PubMed] [Google Scholar]; b Kulawiec R. J.; Faller J. W.; Crabtree R. H. Organometallics 1990, 9, 745. 10.1021/om00117a033. [DOI] [Google Scholar]
- For representative examples of R–B(H)2–H···Ru interactions, see:; a Gusev D. G.; Dolgushin F. M.; Antipin M. Y. Organometallics 2000, 19, 3429. 10.1021/om0003842. [DOI] [Google Scholar]; b Merle N.; Koicok-Köhn G.; Mahon M. F.; Frost C. G.; Ruggerio G. D.; Weller A. S.; Willis M. C. Dalton Trans. 2004, 22, 3883. 10.1039/B413650K. [DOI] [PubMed] [Google Scholar]; c Sellmann D.; Hille A.; Heinemann F. W.; Moll M.; Reiher M.; Hess B. A.; Bauer W. Chem. - Eur. J. 2004, 10, 4214. 10.1002/chem.200400120. [DOI] [PubMed] [Google Scholar]; d Chantler V. L.; Chatwin S. L.; Jazzar R. F. R.; Mahon M. F.; Saker O.; Whittlesey M. K. Dalton Trans. 2008, 19, 2603. 10.1039/b719373d. [DOI] [PubMed] [Google Scholar]; e Hesp K. D.; Kannemann F. O.; Rankin M. A.; McDonald R.; Ferguson M. J.; Stradiotto M. Inorg. Chem. 2011, 50, 2431. 10.1021/ic1022328. [DOI] [PubMed] [Google Scholar]; f Ledger A. E. W.; Ellul C. E.; Mahon M. F.; Williams J. M. J.; Whittlesey M. K. Chem. - Eur. J. 2011, 17, 8704. 10.1002/chem.201100101. [DOI] [PubMed] [Google Scholar]; g Zhang J.; Balaraman E.; Leitus G.; Milstein D. Organometallics 2011, 30, 5716. 10.1021/om200595m. [DOI] [Google Scholar]; h Choi J. H.; Schloerer N. E.; Berger J.; Prechtl M. H. G. Dalton Trans. 2013, 43, 290. 10.1039/C3DT52037D. [DOI] [PubMed] [Google Scholar]; i Algarra A. G.; Sewell L. J.; Johnson H. C.; Macgregor S. A.; Weller A. S. Dalton Trans. 2014, 43, 11118. 10.1039/C3DT52771A. [DOI] [PubMed] [Google Scholar]; j Xu Y.; Rettenmeier C. A.; Plundrich G. T.; Wadepohl H.; Enders M.; Gade L. H. Organometallics 2015, 34, 5113. 10.1021/acs.organomet.5b00699. [DOI] [Google Scholar]; k Hooper T. N.; Weller A. S.; Beattie N. A.; Macgregor S. A. Chem. Sci. 2016, 7, 2414. 10.1039/C5SC04150C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples of exohedral 2c-2e B–B bonds to simple borane/boryl substituents, see:; a Zhang J.; Chan H.-S.; Xie Z. Chem. Commun. 2011, 47, 8082. 10.1039/c1cc12023a. [DOI] [PubMed] [Google Scholar]; b Saulys D. A.; Morrison J. A. Inorg. Chem. 1990, 29, 4174. 10.1021/ic00346a004. [DOI] [Google Scholar]; c Gaines D. F.; Heppert J. A.; Coons D. E.; Jorgenson M. W. Inorg. Chem. 1982, 21, 3662. 10.1021/ic00140a015. [DOI] [Google Scholar]; For the examples of 2c-2e B–B bonds between two icosahedral boron clusters, see:; d Zakharkin L. I.; Kovredov A. I. Izv. Akad. Nauk SSSR, Ser. Khim. 1973, 1428. [Google Scholar]; e Wiersema R. J.; Middaugh R. L. J. Am. Chem. Soc. 1967, 89, 5078. 10.1021/ja00995a067.4294620 [DOI] [Google Scholar]; f Wiersema R. J.; Middaugh R. L. Inorg. Chem. 1969, 8, 2074. 10.1021/ic50080a009. [DOI] [Google Scholar]; g Kobayashi Y.; Ivanov S. V.; Popov A. A.; Miller S. M.; Anderson O. P.; Solntsev K. A.; Strauss S. H. Heteroat. Chem. 2006, 17, 181. 10.1002/hc.20220. [DOI] [Google Scholar]
- a Braunschweig H.; Guethlein F. Angew. Chem., Int. Ed. 2011, 50, 12613. 10.1002/anie.201104854. [DOI] [PubMed] [Google Scholar]; b Johnson H. C.; McMullin C. L.; Pike S. D.; Macgregor S. A.; Weller A. S. Angew. Chem., Int. Ed. 2013, 52, 9776. 10.1002/anie.201304382. [DOI] [PubMed] [Google Scholar]; c Braunschweig H.; Claes C.; Guethlein F. J. Organomet. Chem. 2012, 706, 144. 10.1016/j.jorganchem.2012.02.004. [DOI] [Google Scholar]
- a Gunanathan C.; Milstein D. Acc. Chem. Res. 2011, 44, 588. 10.1021/ar2000265. [DOI] [PubMed] [Google Scholar]; b Schneider S.; Meiners J.; Askevold B. Eur. J. Inorg. Chem. 2012, 2012, 412. 10.1002/ejic.201100880. [DOI] [Google Scholar]; c van der Vlugt J. I. Eur. J. Inorg. Chem. 2012, 3, 363. 10.1002/ejic.201100752. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


