Abstract
Phosphatase and tensin homolog (PTEN) is a phosphoinositide lipid phosphatase and one of the most frequently disrupted tumor suppressors in many forms of cancer, with even small reductions in the expression levels of PTEN promoting cancer development. Although the post-translational ubiquitination of PTEN can control its stability, activity, and localization, a detailed understanding of how PTEN ubiquitination integrates with other cellular regulatory processes and may be dysregulated in cancer has been hampered by a poor understanding of the significance of ubiquitination at individual sites. Here we show that Lys66 is not required for cellular activity, yet dominates over other PTEN ubiquitination sites in the regulation of protein stability. Notably, combined mutation of other sites (Lys13, Lys80, and Lys289) has relatively little effect on protein expression, protein stability, or PTEN polyubiquitination. The present work identifies a key role for Lys66 in the regulation of PTEN expression and provides both an opportunity to improve the stability of PTEN as a protein therapy and a mechanistic basis for efforts to stabilize endogenous PTEN.
Keywords: cancer, phosphatase, phosphatase and tensin homolog (PTEN), phosphoinositide, protein stability, ubiquitylation (ubiquitination), phosphoinositide 3-kinase, tumor suppressor
Introduction
The function of the phosphatase and tensin homolog (PTEN)3 tumor suppressor is disrupted in many forms of cancer by diverse mechanisms (1, 2). PTEN is a phosphatidylinositol 3,4,5-trisphosphate lipid phosphatase that acts to oppose the class I phosphoinositide 3-kinases and therefore loss of PTEN function leads to aberrant accumulation of phosphatidylinositol 3,4,5-trisphosphate and activation of downstream oncogenic PI3K-dependent signaling as well as potentially other PI3K-independent changes (2, 3). PTEN is currently known to exist in two forms, a 403-amino acid cytosolic and nuclear protein usually simply called PTEN, and a longer 576-amino acid protein, PTEN-L, which due to its ability to cross cell membranes and inhibit tumor formation when injected into in mice, has been proposed as a protein therapy (4–6).
Although in clinical data from some tumor types, notably breast cancer, reduced PTEN mRNA levels correlate well with reduced PTEN protein (7), in others including prostate cancer, reduced PTEN protein levels are frequently observed in tumors apparently retaining normal levels of PTEN mRNA (8). Among the mechanisms potentially responsible for this selective loss of PTEN protein expression, accelerated degradation by post-translational ubiquitination seems likely to contribute. This conclusion is supported by the paradigm established for the p53 protein and the ubiquitin E3 ligase MDM2 that destabilization of tumor suppressor proteins can be a significant oncogenic driver and also accumulating evidence for the dysregulation of ubiquitin ligases and proteases that control PTEN stability in several cancer types (9–12).
In addition to its stability, the activity and localization of PTEN can also be controlled by its ubiquitination, notably with PTEN monoubiquitination being linked to its nuclear accumulation (13–15). Several E3 ubiquitin ligases have been proposed to contribute to PTEN ubiquitination, including NEDD4 and the related HECT-domain E3 ligase WWP2, as well as XIAP, CHIP, RFP, SPOP, and MKRN1 (9, 10, 16–20) and ubiquitin proteases have been identified that can deubiquitinate PTEN, including HAUSP/USP7, OTUD3, and USP13 (11, 12, 21). Although many of these studies have identified important effects of manipulating these ligases and proteases on PTEN function and often PTEN stability, clear pictures are yet to emerge of the molecular details of these regulatory events, in particular the sites at which PTEN becomes ubiquitinated, the form of the ubiquitin chains generated and beyond proteasomal degradation, the mechanisms by which ubiquitination influences PTEN function, such as ubiquitin-dependent binding partners. To date, only two sites of PTEN ubiquitination have been described, lysine 13 (Lys13) and lysine 289 (Lys289), although evidence supports the existence of several more (15). Here we show that a novel site of PTEN ubiquitination identified in an unbiased proteomic screen, lysine 66 (Lys66), is polyubiquitinated in cells and dominates in the regulation of PTEN stability.
Results
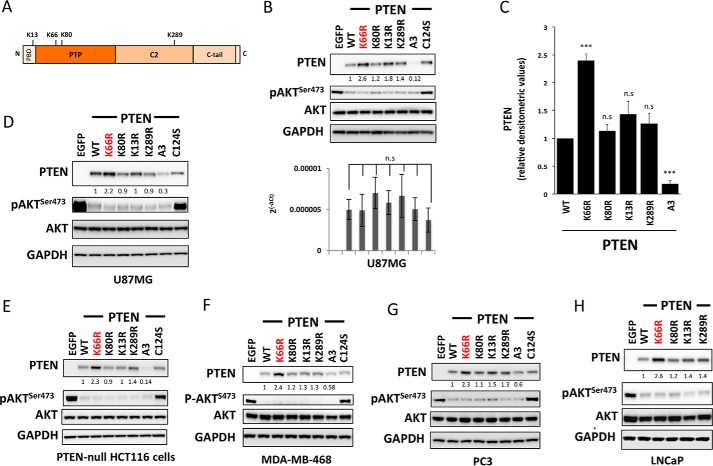
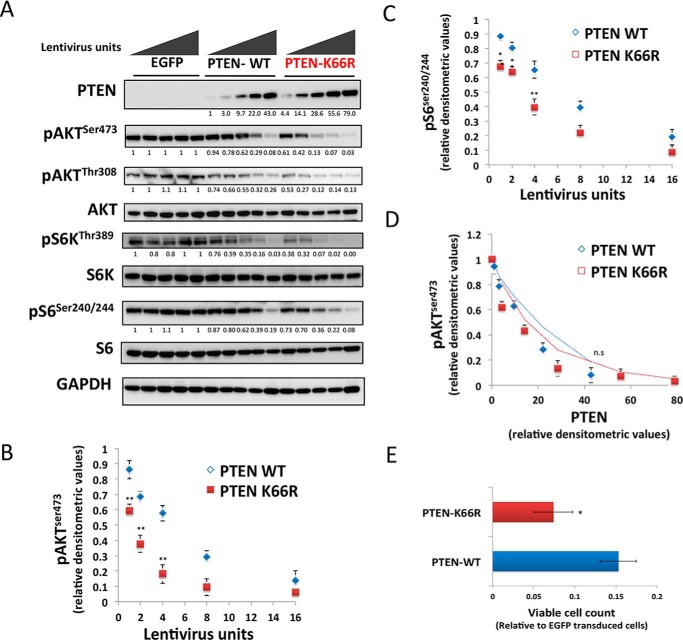
Ubiquitination site prediction software that successfully predicts PTEN Lys13 and Lys289 ubiquitination (22) also predicts sites at Lys66 and Lys147. More significantly, global tissue proteomic data (23) identifying ubiquitinated lysine residues reveals two new sites on PTEN in brain and liver at Lys66 and Lys80 (Fig. 1A). Therefore, we studied the effects on PTEN function and stability of mutating these sites to similarly charged and sized arginine (Arg) residues that cannot be ubiquitinated. Wild-type PTEN (PTEN WT) and mutants, K13R, K66R, K80R, and K289R were each expressed transiently and polyclonally using lentiviruses in PTEN null U87MG glioma cells alongside a phosphatase inactive mutant in which the active site nucleophilic cysteine is replaced (PTEN C124S) and a known unstable mutant (PTEN A3, which has 3 C-terminal phosphorylation sites mutated to alanine residues, S380A, T382A, T383A termed A3) as controls. Although levels of PTEN mRNA in each sample were not significantly different (Fig. 1B, lower panel), levels of PTEN protein expression were significantly higher for PTEN K66R (Fig. 1, B, upper panel, and C). No significant changes in PTEN protein expression levels were observed for other ubiquitination site mutants (Fig. 1C). Transfection experiments with independent plasmid expression vectors gave a similar result, showing elevated protein levels for PTEN K66R (Fig. 1D). None of these mutations greatly affected PTEN activity as assessed by the ability to suppress cellular phosphorylation of the downstream PI3K/PIP3-regulated kinase AKT, except the known phosphatase-dead PTEN C124S control (Fig. 1, B and D). To judge the consistency of this apparent effect, the experiment was repeated in several other PTEN null cell types: MDA-MB-468 breast cancer, PC3, and LNCaP prostate cancer and a PTEN-null clone of HCT116 colon cancer cells (24). In all cases, as in U87MG cells, expression of PTEN K66R was higher than wild-type and in most cases noticeably higher than the other mutants analyzed (Fig. 1, E–H). To test whether the PTEN K66R mutation and the resultant higher levels of mutant protein cause a greater effect on cell signaling and proliferation, U87MG cells were transduced with increasing doses of multiple independently prepared viruses encoding PTEN WT or PTEN K66R. PTEN K66R viruses always led to a higher PTEN expression level (Fig. 2A) and showed a concomitant greater inhibition of downstream cell signaling as seen by greater suppression of the phosphorylation of both AKT and ribosomal protein S6 (Fig. 2, A–C). PTEN K66R expression also had a greater effect on cell proliferation (Fig. 2E). The observed effects on both signaling and proliferation appear to mirror the PTEN expression level with no evidence for altered specific activity found (Fig. 2D), suggesting that the K66R mutation increases PTEN protein expression without strong independent effects on protein function.
FIGURE 1.
Mutation of PTEN lysine 66 increases PTEN expression. A, a schematic illustrating the 403-amino acid PTEN protein and the ubiquitinated lysine residues analyzed in this study. B and C, PTEN-null U87MG glioblastoma cells were transduced with similar units of lentivirus particles encoding for PTEN WT or the indicated PTEN mutants. Control cells were transduced with viruses encoding EGFP. PTEN expression and AKT phosphorylation were investigated by Western blotting of total cell lysates using total and phospho-specific antibodies. GAPDH levels were used as a loading control. A representative blot from at least three independent experiments is shown. Parallel samples from U87MG cells transduced with PTEN WT or mutants were used to analyze the levels of PTEN mRNA by quantitative PCR (B, lower panel). The PTEN transcript levels are expressed relative to GAPDH. y axis represents the average levels of transcript ± S.E. from three experiments each performed in duplicate. No significant difference in the transcript levels was observed between PTEN WT and each PTEN mutant-expressing sample. C, bars representing the relative densitometric values of PTEN expression. The quantitation is derived from three independent experiments and values are mean ± S.E. PTEN K66R was significantly more stable compared with PTEN WT (p value < 0.001). No significant changes in PTEN levels were observed with mutation of other ubiquitin sites (n.s., non significant). D, PTEN-null U87MG glioblastoma cells were transfected with plasmid expression vectors encoding for PTEN WT, PTEN mutants, or EGFP as a control. Cells were lysed 48 h post-transfection and PTEN expression and AKT phosphorylation were investigated by Western blotting analysis of total cell lysates using total and phospho-specific antibodies. GAPDH levels were used as a loading control. A representative blot from three independent experiments is shown. These plasmid transfection experiments recapitulate the increase in PTEN expression with K66R mutation relative to PTEN WT (p value < 0.001) as observed with a lentiviral transduction approach. E–H, PTEN-null clone of HCT116 colon cancer cells (E), MDA-MB-468 breast cancer cells (F), PC3 (G), and LNCaP prostate cancer cells (H) were transduced with similar units of lentivirus particles encoding for PTEN WT or the indicated PTEN mutants. Control cells were transduced with viruses encoding EGFP. PTEN expression and AKT phosphorylation were investigated by Western blotting analysis of total cell lysates using total and phosphospecific antibodies. GAPDH levels were used as a loading control. In each case a representative blot from at least three independent experiments is shown. In all cases, as in U87MG cells, expression of PTEN K66R was significantly higher than wild-type (p value < 0.01).
FIGURE 2.
Mutation of PTEN lysine 66 increases PTEN expression and shows a concomitant greater effect on downstream cell signaling and proliferation. A–D, U87MG cells were transduced with increasing doses of lentiviruses encoding EGFP, PTEN WT, or PTEN K66R (A) PTEN expression and the effect on downstream cell signaling was analyzed by immunoblotting with PTEN, phospho-AKT Ser473, phospho-AKT Thr308, AKT, phospho-S6K Thr389, S6K, phospho-S6 Ser240/244, and S6 antibodies. GAPDH levels were used as a loading control. B and C, graph representing the effect of increasing doses of PTEN WT or PTEN K66R lentiviruses on the suppression of (B) AKT Ser473 and (C) S6 Ser240/244 cellular phosphorylation, relative to EGFP-transduced cells. y axis shows the mean relative densitometric values of phosphorylation ± S.E. from three independent experiments. x axis shows the units of lentivirus used. PTEN K66R lentiviruses were significantly more effective in suppressing both AKT and S6 phosphorylation compared with similar dose of PTEN WT lentiviruses. D, the graph representing the specific activity of PTEN-WT versus PTEN K66R. The average relative densitometric values of AKT Ser473 phosphorylation from three independent experiments for PTEN WT and PTEN K66R was plotted against the relative PTEN expression levels with increasing doses of PTEN WT or PTEN K66R lentiviruses. E, PTEN-null U87MG cells were transduced with similar units of lentiviruses encoding EGFP, PTEN WT, or PTEN K66R and the viable cell number was quantified bye 5 days post-transduction using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. x axis shows the mean measured cellular metabolic activity relative to EGFP-transduced cells ± S.E. from three experiments each performed in triplicate. t test: **, p value < 0.01; *, p value < 0.05; n.s., non significant.
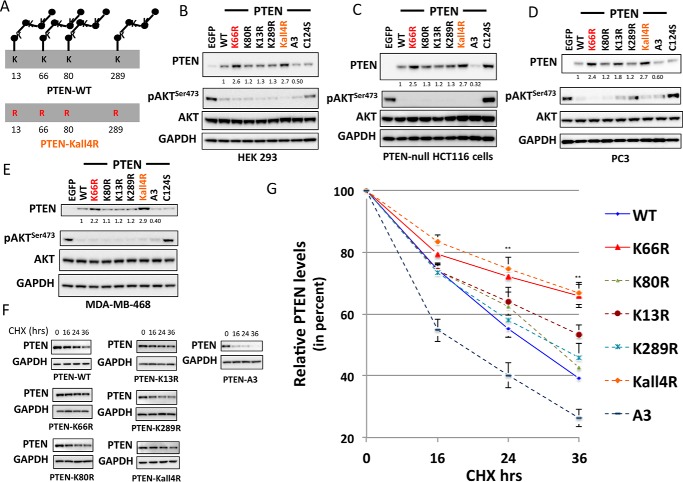
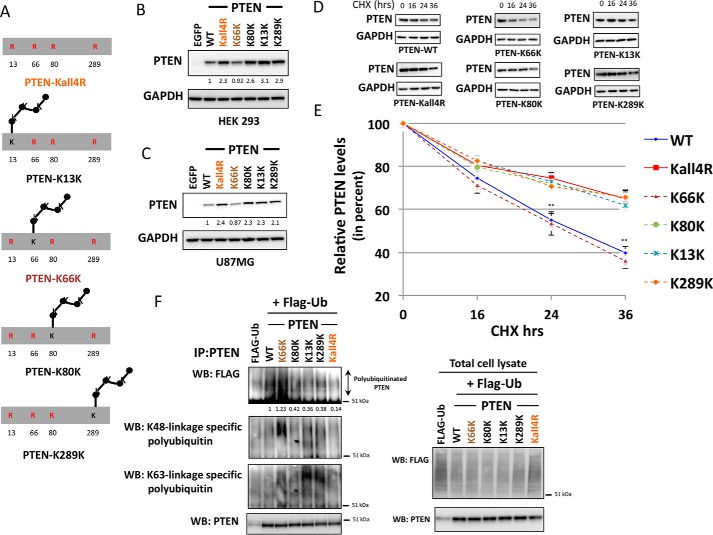
To dissect the consequences of ubiquitination at individual sites, a mutant was prepared with all the four lysine residues (Lys13, Lys66, Lys80, and Lys289) mutated to arginine, termed PTEN Kall4R (Fig. 3A). When its expression in several different cell lines was investigated, this mutation of all the four lysine residues together resulted in similar or only marginally greater PTEN expression than K66R mutation alone in all the cell lines examined (Fig. 3, B–E), further implying a major role for Lys66 in regulating PTEN turnover. To test the effects of these mutations directly on protein stability, PTEN null cells expressing the mutants were treated with the protein synthesis inhibitor cycloheximide and the decay of extant protein was observed. PTEN A3 was less stable than the wild-type enzyme, whereas PTEN K13R, K80R, and K289R were all similar to, or modestly more stable than, PTEN WT. In contrast, both PTEN K66R and Kall4R with Lys66 mutated were significantly more stable (Fig. 3, F and G).
FIGURE 3.
Dominant role of PTEN lysine 66 ubiquitination site in the regulation of PTEN expression and stability. A, a schematic illustrating the mutant PTEN Kall4R with all the four studied potential ubiquitination sites (lysine 13, 66, 80, and 289) mutated to arginine (Arg). B–E, HEK293T cells (B), PTEN-null HCT116 colon cancer cells (C), PC3 prostate cancer cells (D), and MDA-MB-468 breast cancer cells (E) were transduced with similar units of lentivirus particles encoding for PTEN WT, the indicated PTEN mutants, or control EGFP. PTEN expression and AKT phosphorylation were investigated by Western blotting analysis of cell lysates using total and phospho-specific antibodies. GAPDH levels were used as a loading control. A representative blot from three independent experiments is shown. F and G, the stability of PTEN ubiquitin-site mutants was determined using protein synthesis inhibitor cycloheximide (CHX). F, PTEN-null U87MG cells transduced with viruses encoding PTEN WT or PTEN mutants were treated with 100 μg/ml of CHX for the indicated times, followed by immunoblot analysis with anti-PTEN or anti-GAPDH antibodies. The blots shown are representative of three independent experiments. G, plot showing densitometric analysis of the CHX assay. y axis shows the relative PTEN levels (in percent) after normalizing with GAPDH levels. The quantitation is derived from three independent experiments and values are mean ± S.E. Both PTEN K66R and PTEN Kall4R, with Lys66 mutation, were significantly more stable compared with PTEN WT (p value < 0.01).
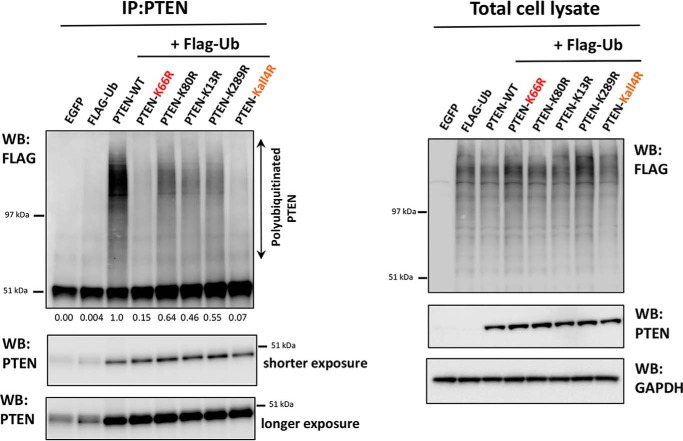
To test the hypothesis that the observed effects of lysine mutations on PTEN stability were mediated directly through changes in ubiquitination, we applied a previously established assay of cellular ubiquitination using FLAG epitope-tagged ubiquitin (13, 25). These experiments showed strong ubiquitination of wild-type PTEN, which was dramatically lower (∼95%) in PTEN Kall4R (Fig. 4), suggesting that these four lysines do represent the major sites of ubiquitination incorporation into PTEN, or at least are required for incorporation at other sites. Although PTEN K13R, K80R, and K289R appeared to display somewhat lower ubiquitination than PTEN WT, PTEN K66R had very low levels of ubiquitination, similar to Kall4R (Fig. 4). These results strongly indicate that Lys66 specifically is required for the normal levels of PTEN polyubiquitination.
FIGURE 4.
Dominant role of the PTEN lysine 66 ubiquitination site in the regulation of PTEN polyubiquitination. Cellular assay for PTEN ubiquitination. U87MG cells were transfected with an expression vector encoding FLAG-ubiquitin (FLAG-Ub). 6 h after transfection cells were transduced with viruses encoding PTEN WT or PTEN mutants. Lentiviruses were titrated prior to the experiment to ensure similar levels of PTEN protein in each sample. 48 h post-transfection cells were treated with 10 μm MG132 for 5 h. Cells were lysed, and PTEN was immunoprecipitated (IP) before Western blotting (WB) of these immunoprecipitates with antibodies raised against the FLAG epitope and PTEN. In parallel, total cell lysates were immunoblotted with antibodies raised against the FLAG epitope, PTEN, or GAPDH (left panel).
To further test the contributions of individual ubiquitination sites and the potential specific significance of Lys66, PTEN expression vectors were made in which one of the four mutated ubiquitination sites in PTEN Kall4R (K13R, K66R, K80R, K289R) was mutated back to lysine, so that each construct expresses PTEN with only one of these lysine residues available for ubiquitination. For brevity of labeling, these constructs are referred to as shown in Fig. 5A as, e.g. K13K, which is K66R,K80R,K289R with only the Lys13 residue available for ubiquitination out of these four studied ubiquitination sites. Notably, the higher PTEN expression seen in PTEN Kall4R is reduced to WT PTEN levels if lysine 66 is retained in its wild-type form in the PTEN Kall4R background (i.e. K13R,K80R,K289R-labeled PTEN K66K). In contrast, individually retaining Lys13, Lys80, or Lys289 in their wild-type form (mutants PTEN K13K, K80K, and K289K, respectively) had no effect on the PTEN expression, with these mutants still showing similar enhanced PTEN expression as seen with PTEN Kall4R (Fig. 5, B and C). Direct analysis of PTEN protein stability showed that these effects on expression levels correlated with the observed protein half-life, with PTEN proteins retaining Lys66 showing significantly lower stability than mutants that include the K66R change (i.e. PTEN Kall4R, K13K, K80K, and K289K) (Fig. 5, D and E). The low level of ubiquitination seen in PTEN Kall4R is also reverted to WT PTEN levels if Lys66 is retained in the unmutated form (i.e. PTEN K66K). In contrast, other PTEN Kall4R derivatives with K66R change (i.e. PTEN K13K, K80K, or K289K) despite the presence of Lys13, Lys80, or Lys289, respectively, show low levels of ubiquitination as seen in PTEN Kall4R (Fig. 5F). These results provide further compelling evidence for a dominant role of Lys66 in regulating PTEN expression, stability, and its polyubiquitination. Additionally, experiments using antibodies that recognize polyubiquitin chains with specific linkage topologies suggested that the polyubiquitination occurring on Lys66 of PTEN may be linked through Lys48 of ubiquitin (a polyubiquitin chain topology that is commonly involved in proteasomal degradation of target proteins), as a stronger signal was apparent with a Lys48 linkage-specific ubiquitin antibody with immunopurified PTEN K66K (Fig. 5F). Further methods would need to be used to provide conclusive evidence of chain topology specific ubiquitination.
FIGURE 5.
Contribution of individual ubiquitination sites in regulating PTEN expression, stability, and PTEN polyubiquitination. A, schematic illustrating PTEN mutants generated to assess the contribution of individual ubiquitination sites to expression and stability. B and C, HEK293T cells (B) and PTEN-null U87MG glioblastoma cells (C) were transduced with similar units of lentivirus particles encoding for PTEN WT or PTEN mutants or EGFP. PTEN expression was investigated by Western blotting (WB) analysis, GAPDH levels were used as a loading control. A representative blot from three independent experiments is shown. D and E, the stability of these PTEN mutants was determined using protein synthesis inhibitor cycloheximide (CHX). D, U87MG cells transduced with viruses encoding PTEN WT or PTEN mutants were treated with 100 μg/ml of CHX for the indicated times, followed by immunoblot analysis with anti-PTEN or anti-GAPDH antibodies. The blots shown are representative of three independent experiments. E, plot showing densitometric analysis of the CHX assay. y axis shows the relative percentage PTEN levels after normalizing to GAPDH. The quantitation is derived from three independent experiments and values are mean ± S.E. PTEN K66K, with only Lys66 ubiquitination site available, showed significantly lower stability than PTEN Kall4R (p value < 0.01). PTEN mutants that included the K66R change (i.e. PTEN Kall4R, K13K, K80K, and K289K) showed more or less similar stability with data points falling on top of each other. F, cellular assay for PTEN ubiquitination. U87MG cells were transfected to express FLAG-ubiquitin (FLAG-Ub) and 6 h later transduced with viruses encoding PTEN WT or the indicated PTEN mutants. Lentiviruses were titrated prior to the experiment to ensure similar levels of PTEN protein is expressed in each sample. 48 h post-transfection, cells were treated with 10 μm MG132 for 5 h. Cells were lysed, and PTEN was immunoprecipitated (IP) before Western blotting analysis of these immunoprecipitates with antibodies raised against the FLAG epitope, Lys48 linkage-specific polyubiquitin, Lys63 linkage-specific polyubiquitin, and PTEN. A smear of high molecular weight in the FLAG Western blot represents polyubiquitinated PTEN. A stronger signal is observed with a Lys48 linkage-specific antibody in PTEN K66K mutant that has only the Lys66 site available for ubiquitination out of the four studied ubiquitination sites. In parallel, total cell lysates were immunoblotted with antibodies raised against the FLAG epitope, PTEN, or GAPDH (F, lower panel).
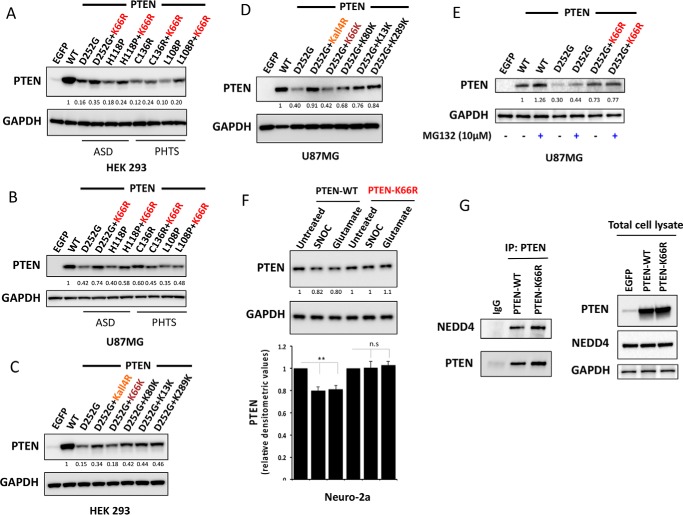
Unstable PTEN mutant proteins have been identified encoded in the germ line of patients with diverse pathologies that appear to be linked to the biological activity of these mutant enzymes (26–29). Treatment of a subset of unstable PTEN mutants found in PTEN hamartoma tumor syndrome (PHTS) patients with proteasome inhibitors could restore mutant PTEN protein levels (30) highlighting an important role for the ubiquitin proteasome pathway in controlling their stability, irrespective of the mechanism(s) by which the relevant PTEN mutations diminish protein stability. Therefore, we investigated the effects of the K66R mutation on the expression of unstable PTEN mutant proteins identified in patients with tumor and autism-related syndromes. Additional mutation of K66R increased the protein expression level of the unstable PTEN mutant proteins D252G and H118P, which have been identified in patients with autism (29) and also appeared to have a similar but more modest effect on PTEN L108P, an unstable PTEN mutant protein that has been identified in patients with severe tumor phenotypes (29, 30) (Fig. 6, A and B). In further study of the stabilization of the PTEN D252G disease-associated unstable protein, the Lys66 ubiquitination site seemed dominant over other ubiquitination sites, as all mutant PTEN proteins containing the K66R mutation showed significant stabilization of the D252G mutant enzyme and other Lys to Arg mutations had little or no effect even when combined (termed the D252G,K66K mutant, with only Lys66 retained in its wild-type form) (Fig. 6, C and D). It was also noted that the treatment with proteasome inhibitor MG132 lead to a modest increase in the protein stability of the unstable PTEN D252G mutant. Of note, the same MG132 treatment in the PTEN D252G mutant with the additional K66R mutation does not show any further significant increase in the protein levels (Fig. 6E). Finally, the PTEN K66R mutant was also found to be resistant to the small but significant NO-mediated proteasomal degradation of PTEN that has been proposed as the mechanism for PTEN inhibition in neurodegeneration (31) (Fig. 6F). Taken together, these results strongly point to a dominant role of the Lys66 ubiquitination site in controlling ubiquitin proteasome-mediated degradation of PTEN and thus in regulating PTEN stability. We found that the PTEN K66R mutant could equally well bind to PTEN ubiquitin ligase NEDD4 as PTEN WT (Fig. 6G).
FIGURE 6.
Mutating lysine 66 counteracts destabilizing PTEN mutations associated with autism and tumor syndromes and NO-mediated proteasomal degradation. HEK293T cells (A) and PTEN-null U87MG cells (B) were transfected with vectors encoding PTEN WT or unstable PTEN mutants associated with autism spectrum disorder (ASD) and tumor syndromes (PHTS) or the same unstable mutants carrying an additional K66R mutation. Cells were lysed and PTEN expression was investigated by Western blotting analysis. GAPDH levels were used as a loading control. Representative blots from three independent experiments is shown. Additional mutation of K66R significantly increased the protein expression level of the unstable PTEN mutant proteins D252G, H118P (p value < 0.001), and L108P (p value < 0.01). C and D, effects of PTEN ubiquitination site mutation in PTEN D252G mutant background. HEK293T cells (C) and U87MG cells (D) were transfected as indicated with expressing vectors encoding PTEN WT or PTEN mutants. Cells were lysed and PTEN expression was investigated by Western blotting analysis. GAPDH levels were used as a loading control. A representative blot from three independent experiments is shown. All mutant PTEN proteins containing the K66R mutation showed significant stabilization of D252G mutant enzyme (p value < 0.01). Mutant PTEN D252G-K66K retaining Lys66 in its wild-type form showed no significant effect on D252G mutant enzyme stability. E, U87MG cells transfected with the indicated expression vectors encoding PTEN WT or PTEN mutants were treated for 5 h with 10 μm MG132 proteasome inhibitor before PTEN expression was analyzed by Western blotting analysis for PTEN and GAPDH. F, mouse neuroblastoma Neuro-2a cells were transfected with quantities of the indicated expression vectors encoding PTEN WT or PTEN K66R engineered to lead to similar expression of the two proteins. Cells were treated for 4 h with 200 μm freshly prepared S-nitrosocysteine (SNOC) or 200 μm glutamate before PTEN expression was analyzed by Western blotting for PTEN and GAPDH. A representative blot from three independent experiments is shown (F, lower panel). Bar representing relative densitometric values of PTEN expression after SNOC and glutamate treatments in PTEN-WT and PTEN K66R-transfected cells. The quantitation is derived from three independent experiments and values are mean ± S.E. SNOC acts as a physiological NO donor and glutamate is neurotoxic compound, which induces NO generation. SNOC and glutamate-mediated S-nitrosylation of PTEN has been shown to lead to enhanced PTEN ubiquitination leading to its proteasome-mediated degradation (31). A modest but significant decrease in PTEN levels is observed with SNOC and glutamate treatment in PTEN WT overexpressing cells (two-tailed t test, p value < 0.01). PTEN-K66R does not show this decrease in PTEN levels. G, co-immunoprecipitation of PTEN and NEDD4 in PTEN-WT and PTEN K66R-transfected 293T cells. Quantities of expression vectors encoding PTEN WT or PTEN K66R were engineered to lead to similar expression of the two proteins. PTEN was immunoprecipitated (IP) and PTEN-bound endogenous NEDD4 was immunoblotted.
Discussion
In this study, we present the first studies of a novel PTEN ubiquitination site, lysine 66, and show that its mutation causes a large increase in PTEN stability. Our data show that lysine 66 specifically is required for the normal levels of PTEN polyubiquitination and imply that lysine 66 is the site of much of the polyubiquitination on PTEN, promoting the proteasome-mediated degradation of PTEN. Importantly, we found in multiple cell types that PTEN lysine 66 dominates over other known ubiquitination sites (lysines 13, 80, and 289) in regulating PTEN expression, stability, and PTEN polyubiquitination. Two alternate sites of PTEN ubiquitination, Lys13 and Lys289 have been previously identified as major monoubiquitination sites essential for cytoplasmic-nuclear shuttling of PTEN (15). Our data indicate that they can influence PTEN stability but play weaker roles than Lys66.
It has been previously observed that PTEN ubiquitination can suppress phosphatase activity (13). Expression of PTEN K66R did have greater effects than the wild-type enzyme on downstream AKT-mediated signaling, however, this correlates with its higher cellular concentration (Fig. 2). Along with the location of Lys66 on the surface of the PTEN phosphatase domain facing away from the active site and membrane-interacting surface, this implies that direct effects of PTEN ubiquitination on phosphatase activity are more likely to be mediated through other sites of ubiquitination. Notably, in the context of unstable mutant PTEN proteins that have been identified in sporadic tumors and germline PTEN mutation carriers, we observed that additional mutation of the Lys66 ubiquitin site could significantly improve PTEN stability.
The up-regulation of PTEN ubiquitin ligases or down-regulation of PTEN deubiquitinases has been implicated in accelerated PTEN degradation and thereby in driving tumorigenesis and has identified these E3 ligases and deubiquitinases as potential drug targets (9–12). Future studies firmly identifying the E3 ligases or deubiquitinases, which specifically modify PTEN Lys66 would be highly relevant for the development of therapies aiming to indirectly enhance PTEN expression. The potential of PTEN as a protein therapy has been raised by the discovery of a cell-penetrant form of PTEN (PTEN-long or PTEN-L), which can cause regression of tumor xenografts when purified protein is injected into mice (4). Additionally, mutations have been identified that improve the cellular activity of PTEN through increased membrane association and could be used in the context of PTEN-L (32). Particularly because increased membrane association of PTEN also leads to polyubiquitination and destabilization (13), it seems an appealing strategy to engineer the PTEN-L protein in this way with mutations leading both to enhanced activity and stability.
Experimental Procedures
Cell Culture and Lentivirus Transduction
U87MG glioblastoma, MDA-MB-468 breast cancer, PC3, and LNCaP prostate cancer and HEK293T embryonic kidney cell lines were procured from the European Collection of Animal Cell Cultures (Public Health England, Salisbury, UK) and maintained in the recommended medium supplemented with 10% fetal bovine serum (FBS). PTEN-null clone of HCT116 colon cancer cells were kindly provided by Todd Waldman (Georgetown University) and cultured in McCoy's 5A medium with 10% FBS. Standard cell culture medium, additives, and sera were from Invitrogen. Lentiviral particles were prepared as described previously (33). Cells were transduced with lentiviruses in medium supplemented with Polybrene (16 μg/ml; Sigma). The medium was changed 24 h after transduction, and polyclonal cell populations were processed without selection 48 h after transduction.
Preparation of Whole Cell Extracts, SDS-PAGE, and Western Blotting
Cells were washed twice with ice-cold phosphate-buffered saline and lysed in ice-cold lysis buffer (25 mm Tris-HCl, pH 7.4), 150 mm NaCl, 1% Nonidet P-40, 1 mm EGTA, 1 mm EDTA, 5 mm sodium pyrophosphate, 10 mm β-glycerophosphate, and 50 mm sodium fluoride) containing 0.1% 2-mercaptoethanol and protease inhibitors (0.2 mm phenylmethylsulfonyl fluoride, 1 mm benzamidine, 10 μg/ml of aprotinin, and 10 μg/ml of leupeptin). Equal amounts of proteins were separated by SDS-PAGE using precast 4–12% gradient gels (Invitrogen) and blotted onto polyvinylidene difluoride membranes (Polyscreen, PerkinElmer Life Sciences). Most reagents for electrophoresis and blotting were purchased from Invitrogen, and standard manufacturers' protocols were followed. Mouse anti-PTEN monoclonal antibody (clone A2B1) was purchased from Santa Cruz Biotechnology. Antibodies against phospho-AKT (Ser-473), phospho-AKT (Thr-308), total AKT, phospho-S6K (Thr389), total S6K, phospho-S6 (Ser240/244), total S6, Lys48 linkage-specific polyubiquitin, Lys63 linkage-specific polyubiquitin and NEDD4 were obtained from Cell Signaling Technologies. Anti-GAPDH antibody was from Merck Millipore. Anti-FLAG antibody M2 was purchased from Sigma.
In Cell PTEN Ubiquitination Assay
Cellular assays to measure PTEN polyubiquitination were performed as described previously (13, 25). Briefly, where indicated, cells were transfected with vectors encoding PTEN and FLAG epitope-tagged ubiquitin. Whole cell extracts were prepared using lysis buffer containing fresh protease and phosphatase inhibitors and 40 mm N-ethylmaleimide (Sigma). PTEN was immunoprecipitated from an equal volume of the diluted cell lysates containing 1 mg of soluble protein using monoclonal antibody A2B1 pre-coupled with protein G-Sepharose beads. The proteins bound to beads were released by boiling in SDS-PAGE sample buffer (Invitrogen) for 10 min. The samples were then resolved by 4–12% SDS-PAGE, followed by immunoblot analysis using anti-FLAG antibody M2 or mouse monoclonal A2B1 anti-PTEN antibody.
Author Contributions
A. G. and N. R. L. conceived and designed the experiments; A. G. performed the experiments; A. G. and N. R. L. analyzed the data; and A. G. and N. R. L. wrote the paper.
Acknowledgments
We thank Todd Waldman (Georgetown University) for providing us PTEN-null clone of HCT116 colon cancer cells and the members of the NRL for their support and constructive discussions.
This work was supported by the Medical Research Council Grant G0801865/2 and SU2P. The authors declare that they have no conflicts of interest with the contents of this article.
- PTEN
- phosphatase and tensin homolog
- EGFP
- enhanced green fluorescent protein.
References
- 1. Leslie N. R., and Foti M. (2011) Non-genomic loss of PTEN function in cancer: not in my genes. Trends Pharmacol. Sci. 32, 131–140 [DOI] [PubMed] [Google Scholar]
- 2. Song M. S., Salmena L., and Pandolfi P. P. (2012) The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 13, 283–296 [DOI] [PubMed] [Google Scholar]
- 3. Worby C. A., and Dixon J. E. (2014) PTEN. Annu. Rev. Biochem. 83, 641–669 [DOI] [PubMed] [Google Scholar]
- 4. Hopkins B. D., Fine B., Steinbach N., Dendy M., Rapp Z., Shaw J., Pappas K., Yu J. S., Hodakoski C., Mense S., Klein J., Pegno S., Sulis M. L., Goldstein H., Amendolara B., et al. (2013) A secreted PTEN phosphatase that enters cells to alter signaling and survival. Science 341, 399–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang H., He S., Yang J., Jia X., Wang P., Chen X., Zhang Z., Zou X., McNutt M. A., Shen W. H., and Yin Y. (2014) PTENα, a PTEN isoform translated through alternative initiation, regulates mitochondrial function and energy metabolism. Cell Metab. 19, 836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang H., Zhang P., Lin C., Yu Q., Wu J., Wang L., Cui Y., Wang K., Gao Z., and Li H. (2015) Relevance and therapeutic possibility of PTEN-long in renal cell carcinoma. PloS One 10, e114250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saal L. H., Johansson P., Holm K., Gruvberger-Saal S. K., She Q. B., Maurer M., Koujak S., Ferrando A. A., Malmström P., Memeo L., Isola J., Bendahl P. O., Rosen N., Hibshoosh H., Ringnér M., et al. (2007) Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc. Natl. Acad. Sci. U.S.A. 104, 7564–7569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen M., Pratt C. P., Zeeman M. E., Schultz N., Taylor B. S., O'Neill A., Castillo-Martin M., Nowak D. G., Naguib A., Grace D. M., Murn J., Navin N., Atwal G. S., Sander C., Gerald W. L., et al. (2011) Identification of PHLPP1 as a tumor suppressor reveals the role of feedback activation in PTEN-mutant prostate cancer progression. Cancer Cell 20, 173–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee M. S., Jeong M. H., Lee H. W., Han H. J., Ko A., Hewitt S. M., Kim J. H., Chun K. H., Chung J. Y., Lee C., Cho H., and Song J. (2015) PI3K/AKT activation induces PTEN ubiquitination and destabilization accelerating tumourigenesis. Nat. Commun. 6, 7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X., Trotman L. C., Koppie T., Alimonti A., Chen Z., Gao Z., Wang J., Erdjument-Bromage H., Tempst P., Cordon-Cardo C., Pandolfi P. P., and Jiang X. (2007) NEDD4–1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuan L., Lv Y., Li H., Gao H., Song S., Zhang Y., Xing G., Kong X., Wang L., Li Y., Zhou T., Gao D., Xiao Z. X., Yin Y., et al. (2015) Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis. Nat. Cell Biol. 17, 1169–1181 [DOI] [PubMed] [Google Scholar]
- 12. Zhang J., Zhang P., Wei Y., Piao H. L., Wang W., Maddika S., Wang M., Chen D., Sun Y., Hung M. C., Chen J., and Ma L. (2013) Deubiquitylation and stabilization of PTEN by USP13. Nat. Cell Biol. 15, 1486–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maccario H., Perera N. M., Gray A., Downes C. P., and Leslie N. R. (2010) Ubiquitination of PTEN (phosphatase and tensin homolog) inhibits phosphatase activity and is enhanced by membrane targeting and hyperosmotic stress. J. Biol. Chem. 285, 12620–12628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh G., and Chan A. M. (2011) Post-translational modifications of PTEN and their potential therapeutic implications. Curr. Cancer Drug Targets 11, 536–547 [DOI] [PubMed] [Google Scholar]
- 15. Trotman L. C., Wang X., Alimonti A., Chen Z., Teruya-Feldstein J., Yang H., Pavletich N. P., Carver B. S., Cordon-Cardo C., Erdjument-Bromage H., Tempst P., Chi S. G., Kim H. J., Misteli T., Jiang X., and Pandolfi P. P. (2007) Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128, 141–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed S. F., Deb S., Paul I., Chatterjee A., Mandal T., Chatterjee U., and Ghosh M. K. (2012) The chaperone-assisted E3 ligase C terminus of Hsc70-interacting protein (CHIP) targets PTEN for proteasomal degradation. J. Biol. Chem. 287, 15996–16006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee J. T., Shan J., Zhong J., Li M., Zhou B., Zhou A., Parsons R., and Gu W. (2013) RFP-mediated ubiquitination of PTEN modulates its effect on AKT activation. Cell Res. 23, 552–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li G., Ci W., Karmakar S., Chen K., Dhar R., Fan Z., Guo Z., Zhang J., Ke Y., Wang L., Zhuang M., Hu S., Li X., Zhou L., Li X., et al. (2014) SPOP promotes tumorigenesis by acting as a key regulatory hub in kidney cancer. Cancer Cell 25, 455–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maddika S., Kavela S., Rani N., Palicharla V. R., Pokorny J. L., Sarkaria J. N., and Chen J. (2011) WWP2 is an E3 ubiquitin ligase for PTEN. Nat. Cell Biol. 13, 728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Themsche C., Leblanc V., Parent S., and Asselin E. (2009) X-linked Inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J. Biol. Chem. 284, 20462–20466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song M. S., Salmena L., Carracedo A., Egia A., Lo-Coco F., Teruya-Feldstein J., and Pandolfi P. P. (2008) The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature 455, 813–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Z., Chen Y. Z., Wang X. F., Wang C., Yan R. X., and Zhang Z. (2011) Prediction of ubiquitination sites by using the composition of k-spaced amino acid pairs. PloS One 6, e22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wagner S. A., Beli P., Weinert B. T., Schölz C., Kelstrup C. D., Young C., Nielsen M. L., Olsen J. V., Brakebusch C., and Choudhary C. (2012) Proteomic analyses reveal divergent ubiquitylation site patterns in murine tissues. Mol. Cell. Proteomics 11, 1578–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee C., Kim J. S., and Waldman T. (2004) PTEN gene targeting reveals a radiation-induced size checkpoint in human cancer cells. Cancer Res. 64, 6906–6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gupta A., Maccario H., Kriplani N., and Leslie N. R. (2016) In cell and in vitro assays to measure PTEN ubiquitination. Methods Mol. Biol. 1388, 155–165 [DOI] [PubMed] [Google Scholar]
- 26. Eng C. (2003) PTEN: one gene, many syndromes. Hum. Mutat. 22, 183–198 [DOI] [PubMed] [Google Scholar]
- 27. Han S. Y., Kato H., Kato S., Suzuki T., Shibata H., Ishii S., Shiiba K., Matsuno S., Kanamaru R., and Ishioka C. (2000) Functional evaluation of PTEN missense mutations using in vitro phosphoinositide phosphatase assay. Cancer Res. 60, 3147–3151 [PubMed] [Google Scholar]
- 28. Rodríguez-Escudero I., Oliver M. D., Andrés-Pons A., Molina M., Cid V. J., and Pulido R. (2011) A comprehensive functional analysis of PTEN mutations: implications in tumor- and autism-related syndromes. Hum. Mol. Genet. 20, 4132–4142 [DOI] [PubMed] [Google Scholar]
- 29. Spinelli L., Black F. M., Berg J. N., Eickholt B. J., and Leslie N. R. (2015) Functionally distinct groups of inherited PTEN mutations in autism and tumour syndromes. J. Med. Genet. 52, 128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He X., Arrotta N., Radhakrishnan D., Wang Y., Romigh T., and Eng C. (2013) Cowden syndrome-related mutations in PTEN associate with enhanced proteasome activity. Cancer res. 73, 3029–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kwak Y. D., Ma T., Diao S., Zhang X., Chen Y., Hsu J., Lipton S. A., Masliah E., Xu H., and Liao F. F. (2010) NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol. Neurodegener. 5, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen H. N., Yang J. M., Afkari Y., Park B. H., Sesaki H., Devreotes P. N., and Iijima M. (2014) Engineering ePTEN, an enhanced PTEN with increased tumor suppressor activities. Proc. Natl. Acad. Sci. U.S.A. 111, E2684–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davidson L., Maccario H., Perera N. M., Yang X., Spinelli L., Tibarewal P., Glancy B., Gray A., Weijer C. J., Downes C. P., and Leslie N. R. (2010) Suppression of cellular proliferation and invasion by the concerted lipid and protein phosphatase activities of PTEN. Oncogene 29, 687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]