Abstract
GABAA receptors are pentameric ligand-gated channels mediating inhibitory neurotransmission in the CNS. α4βxδ GABAA receptors are extrasynaptic receptors important for tonic inhibition. The functional properties and subunit arrangement of these receptors are controversial. We predefined subunit arrangement by using subunit concatenation. α4, β2, and δ subunits were concatenated to dimeric, trimeric, and, in some cases, pentameric subunits. We constructed in total nine different receptor pentamers in at least two different ways and expressed them in Xenopus oocytes. The δ subunit was substituted in any of the five positions in the α1β2 receptor. In addition, we investigated all receptors with the 2:2:1 subunit stoichiometry for α4, β2, and δ. Several functional receptors were obtained. Interestingly, all of these receptors had very similar EC50 values for GABA in the presence of the neurosteroid 3α, 21-dihydroxy-5α-pregnan-20-one (THDOC). All functional receptors containing δ subunits were sensitive to 4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridin-3-yl]benzamide (DS2). Moreover, none of the receptors was affected by ethanol up to 30 mm. These properties recapitulate those of non-concatenated receptors expressed from a cRNA ratio of 1:1:5 coding for α4, β2, and δ subunits. We conclude that the subunit arrangement of α4β2δ GABAA receptors is not strongly predefined but is mostly satisfying the 2:2:1 subunit stoichiometry for α4, β2, and δ subunits and that several subunit arrangements result in receptors with similar functional properties tuned to physiological conditions.
Keywords: electrophysiology, GABA, ion channel, neurotransmitter receptor, Xenopus, γ-aminobutyric acid type A, δ subunit, GABAA receptor
Introduction
GABAA receptors are the most abundant inhibitory neurotransmitter receptors in the CNS and are a therapeutic target for several drugs. They form heteropentamers made up of distinct subunit combinations selected from the following different subunit isoforms: α(1–6), β(1–4), γ(1–3), δ, ϵ, θ, π, and ρ(1–3). The five subunits form a central chloride ion-selective channel (1–3). The major isoform of the GABAA receptor is composed of α1, β2, and γ2 subunits with a fixed stoichiometry of 2:2:1 (4–6), arranged as γ2β2α1β2α1 anticlockwise when viewed from the extracellular space (7–9).
GABAA receptors incorporating the γ2 subunits are considered to be localized predominantly at synaptic regions and play a crucial role in phasic inhibition, whereas those containing the δ subunit are dominantly located peri- and extrasynaptically and mediate tonic inhibition (10). δ subunits preferentially combine with either α1 (11), α4 (12, 13), or α6 (14) subunits. Without experimental evidence, the δ subunit is generally assumed to substitute for the γ2 subunit. At least in α1β3δ, α6β3δ, and α1α6β3δ receptors, the δ subunit may assume different positions but predominantly the one of the β subunit between α subunits in the major isoform of GABAA receptors (15–17).
Subunit arrangement of α4βxδ GABAA receptors is still incompletely understood. Atomic force microscopy of recombinant α4β3δ receptors expressed in either the endoplasmic reticulum membrane or in the plasma membrane of tsA 201 cells has suggested that the δ subunit is able to substitute for the γ2 subunit (18). Structural work on α4β2δ GABAA receptors expressed in HEK cells indicated that subunit stoichiometry is highly influenced by the ratio of subunit cDNAs transfected and that more than one δ subunit can assemble into a pentamer (19). It should be noted that such structural work cannot differentiate between functional and non-functional receptors. Therefore, functional strategies are of special interest.
Several groups have observed that the functional properties of α4βxδ receptors depend on the ratio of genetic information coding for different subunits used during expression (19–22), suggesting that several subunit arrangements are possible. A recent publication concluded that the subunit stoichiometry of recombinant α4β3δ GABAA receptors expressed in HEK cells is 2:2:1 (23), independent of the cDNA ratio used for expression.
Receptor concatenation predefines subunit composition and arrangement. Electrophysiological analysis provides functional properties of these receptors. So far, subunit concatenation has been applied to approach the architecture of α1β2γ2 GABAA receptors (7–9), α1β3δ (16), α6β3δ (15), and α1α6β3δ (17). In addition, the technique has contributed to the finding that the assembly of ϵ subunits is promiscuous (24). Initial attempts to use subunit concatenation to understand subunit arrangement in α4β2δ receptors have indicated that the δ subunit simply replaces the γ2 subunit in the classical arrangement (25). Later, the same group reported that various subunit arrangements may result in functional receptors (26).
In this work, we aimed to understand the function and architecture of α4β2δ receptors by combining subunit concatenation and expression in Xenopus oocytes. The receptors were functionally characterized by determining their response to GABA in the presence of THDOC3 and modulation by DS2 and ethanol. We conclude that the δ subunit, together with α4 and β2, does not form one defined receptor but can form, at least in the oocyte, more than one subunit arrangement and that these receptors display similar functional properties.
Results
Preliminary Considerations
We wanted to get insight into the subunit arrangement of α4βxδ GABAA receptors. As a first step, we had to choose the β subunit subtype. To allow selection, we investigated whether expression of α4β1δ, α4β2δ, or α4β3δ would result in the largest current amplitudes, indicating a preference for subunit assembly. Comparison of these three receptor types revealed no preference (data not shown). We restricted our analysis to functional α4β2δ receptors expressed in Xenopus oocytes by applying electrophysiological techniques. Unless stated otherwise, 2.5 fmol cRNA/subunit was injected into an oocyte, an amount that prevents overloading artifacts. For such a study, it would be desirable to construct 30 pentameric concatenated receptors where all five subunits are covalently linked. In a realistic time period, this is impossible. We restricted ourselves to the construction of six dimeric subunit constructs, six trimeric subunit constructs, and two pentameric subunit constructs. We cannot exclude that receptors with alternative subunit arrangements are also functional.
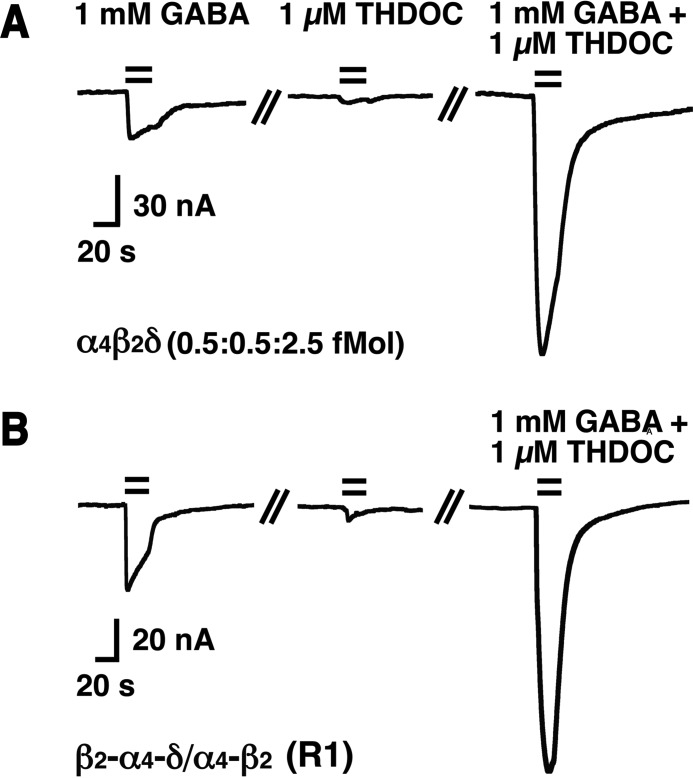
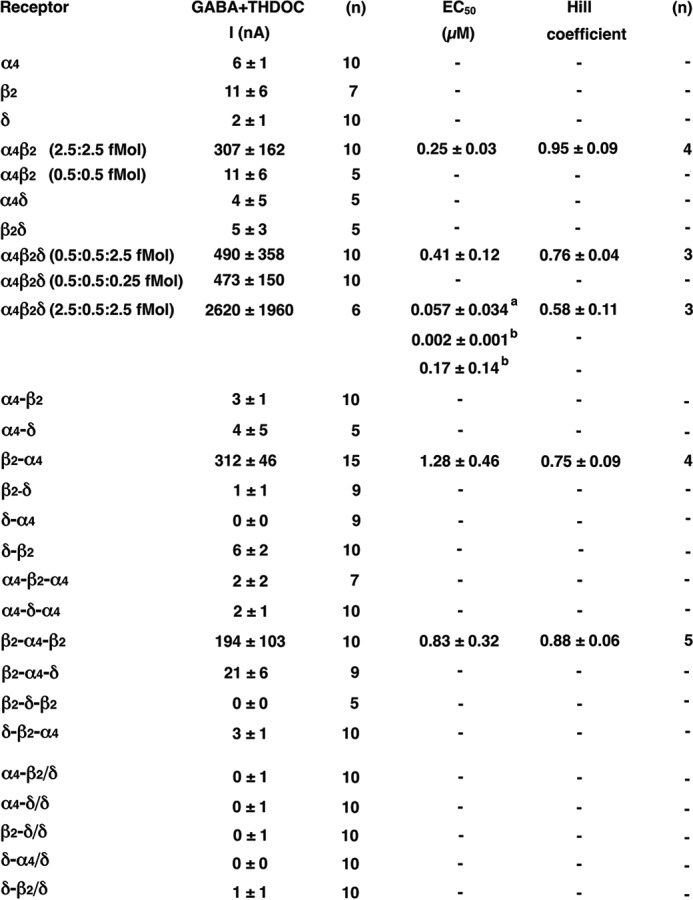
GABA acts only as a partial agonist for many δ subunit-containing receptors (15, 16, 27, 28). We made similar observations in non-concatenated α4β2δ receptors expressed at a subunit ratio of 1:1:5. Fig. 1A shows current traces from an application of 1 mm GABA followed by application of 1 μm THDOC and, subsequently, by the combination of the two. 1 mm GABA elicited 17% ± 3% (mean ± S.D., n = 5) of the current elicited in the combined presence of 1 mm GABA/1 μm THDOC. Fig. 1B shows a similar experiment with the concatenated receptor β2-α4-δ/α4-β2 (R1). Here the relative current was 32% ± 13% (mean ± S.D., n = 5). For P1 with the same subunit arrangement but all five subunits covalently linked, this value amounted to 19% ± 3% (mean ± S.D., n = 5). THDOC has been reported to potentiate current mediated by δ subunit-containing receptors with no or maximally a 2.6-fold decrease of the EC50 for GABA (16, 29, 30). Additionally, it has been reported that, in the presence of THDOC, receptors are uncovered that are silent in its absence (16, 30). The concentration of endogenous neurosteroids near the GABAA receptors in the brain is not known, but it may be assumed that at least part of the receptors are activated by these compounds. For all experiments shown below, except when DS2 was present, we included 1 μm THDOC in the solutions containing GABA. In the following, we first report our observations on the expression of non-concatenated single subunits and combinations of two or three subunits, followed by observations on concatenated receptors.
FIGURE 1.
Current traces recorded from oocytes expressing either non-concatenated α4β2δ receptors or β2-α4-δ/α4-β2 (R1) receptors. A, current traces recorded upon application of 1 mm GABA, followed by 1 μm THDOC and co-application of 1 mm GABA/1 μm THDOC to non-concatenated α4β2δ receptors. B, analogous experiment with R1 receptors. The bars indicate the time period of drug perfusion. Both experiments were repeated four times with similar results.
Functional expression of the individual subunits α4, β2, or δ did not result in appreciable currents (Fig. 2). The same was observed for the expression of the δ subunit in combination with α4 or β2 subunits (Fig. 2). The combination of α4 with β2 subunits resulted in robust currents, characterized by an EC50 for GABA of 0.25 ± 0.03 μm (n = 4). It is interesting to note that expression of 5-fold lower amounts of cRNA produced only very tiny currents, but inclusion of small amounts of cRNA coding for the δ subunit (0.25 fmol) to α4β2 rescued functional expression (Fig. 2).
FIGURE 2.
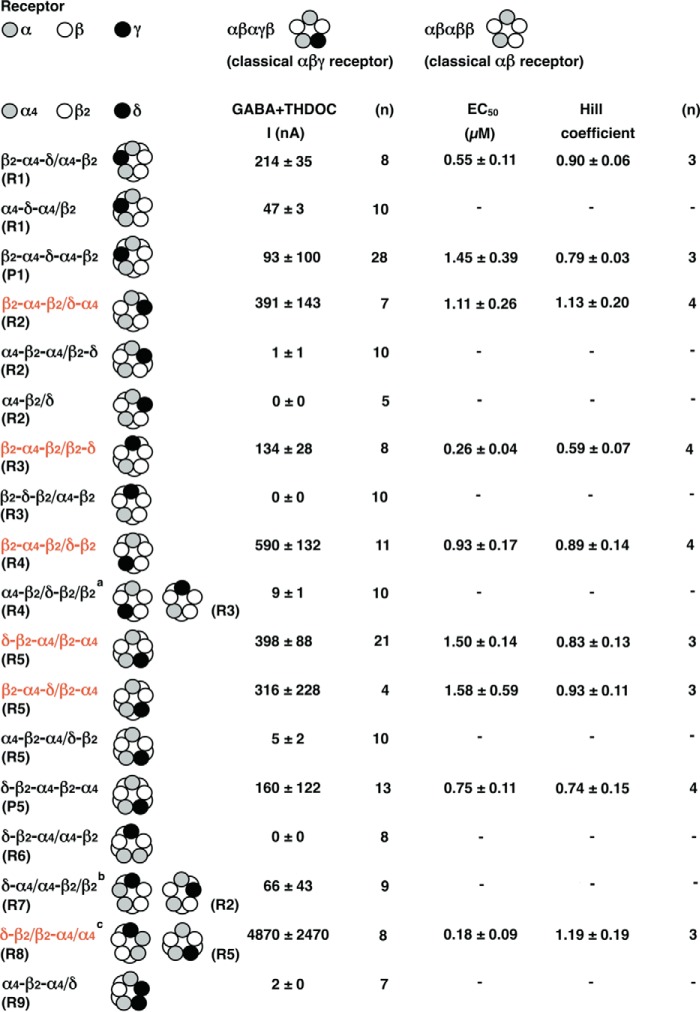
Functional expression of single subunits, concatenated subunits, and non-concatenated α4β2δ receptors in Xenopus oocytes. Unless indicated otherwise, oocytes were injected with 2.5 fmol cRNA coding for a non-concatenated subunit or a concatenated construct. The figure shows subunit composition and current amplitudes (in nanoamperes) elicited by 1 mm GABA in the presence of 1 μm THDOC (mean ± S.E.) and, in some cases, EC50 for GABA/THDOC (mean ± S.D.). n, number of oocytes; −, not analyzed. a and b, the α4β2δ receptor expressed at a subunit ratio of 2.5:0.5:2.5 fmol was fitted either assuming a single phasic (a) or a two-phasic (b) concentration-response curve, the two components amounting to about 31% and 69%, respectively.
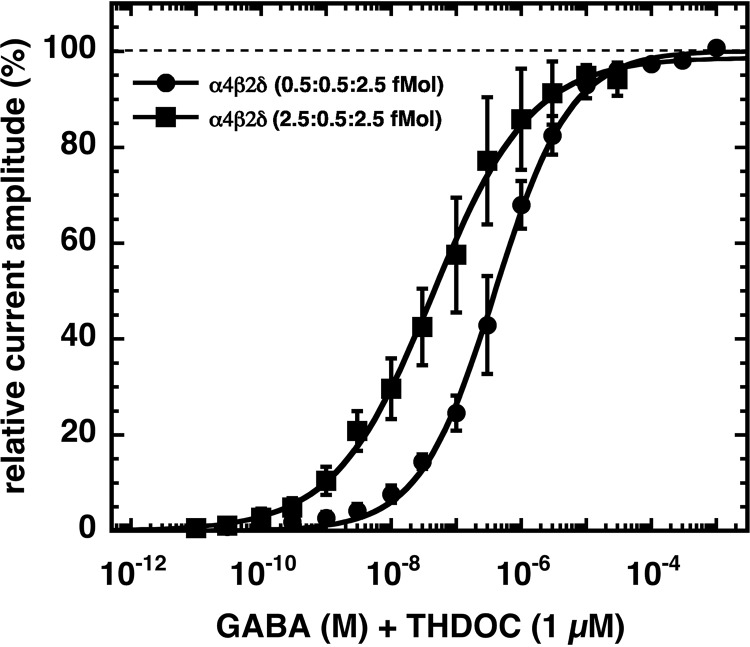
We investigated the triple subunit combination α4β2δ injected with genetic information coding for the individual subunits at two different stoichiometries that are often used in work with these receptors (21, 22, 26). Both stoichiometries, 0.5:0.5:2.5 fmol/oocyte and 2.5:0.5:2.5 fmol/oocyte, resulted in robust current expression. Although the first receptor was characterized by an EC50 for GABA of 0.41 ± 0.12 μm (n = 3), the second receptor displayed a biphasic behavior with nanomolar affinities (Fig. 2). As discussed below, we do not think that such a receptor is expressed in vivo. Detailed averaged concentration-response curves are shown in Fig. 3.
FIGURE 3.
GABA concentration dependence of non-concatenated α4β2δ receptors expressed at different subunit ratios. Oocytes were injected with x fmol of cRNA coding for α4, 0.5 fmol of β2, and 2.5 fmol of δ, where x was either 0.5 or 2.5. GABA concentration-response curves of these receptors were recorded in the presence of 1 μm THDOC. Individual curves were first normalized to the fitted maximal current amplitude and subsequently averaged. Concentration-response curves obtained from both receptors were fitted with a single phase. Data are expressed as mean ± S.D. (n = 3).
Expression of Concatenated Receptors
First we tested all dual- and triple-concatenated subunit constructs individually for current expression. In case of a subunit hanging out, pentameric receptors could be built. The dual subunit constructs α4-β2, α4-δ, β2-δ, δ-α4, and δ-β2 and the triple subunit constructs α4-β2-α4, α4-δ-α4, β2-δ-β2, and δ-β2-α4 did not result in current expression (Fig. 2). β2-α4-δ produced very small currents. Surprisingly, 2.5 fmol cRNA/oocyte coding for β2-α4 and 2.5 fmol cRNA/oocyte coding for β2-α4-β2 both resulted in expression of robust currents, possibly by squeezing out one subunit of a hexameric receptor. Therefore, all pentameric receptors where one of these two constructs forms part of the pentamer should be judged with care. It is worth mentioning that, in previous work using the dual subunit construct β2-α4, the conclusion has been reached that several subunit arrangements can be formed from α4, β2 and δ (25, 26). The authors of this study report that >40 fmol cRNA coding for β2-α4 alone did not form functional channels. The reason for the discrepancy is not clear.
None of the combinations of the δ subunit with either α4-β2, α4-δ, β2-δ, δ-α4, or δ-β2 resulted in current expression (Fig. 2). Please note that α4-β2/δ may assemble into α4-β2/α4-β2/δ (called R2 below). This fact is discussed below.
For the investigation of the putative pentameric arrangement of α4β2δ receptors, we followed two rationales. First, we assumed that the δ subunit would occupy any of the five positions in the classical subunit arrangement of α1β2 GABAA receptors (Fig. 4, top) (7–9). Second, we assumed a 2:2:1 stoichiometry of α4:β2:δ subunits. As discussed above, this stoichiometry has been found for α4β3δ receptors expressed in HEK cells and claimed to be independent of the ratio of genetic information introduced into a cell (23). These two approaches are discussed in the following.
FIGURE 4.
Functional expression of concatenated α4β2δ receptors in Xenopus oocytes. For comparison, the top line shows the classical subunit arrangements of αβγ and αβ receptors. Nine pentamers with different subunit arrangements were built, as indicated under “Results.” Pentamers were either composed of concatenated subunits, or these were combined loose subunits (R1-R9). Also, two pentameric constructs (P1 and P5) were built. The figure shows subunit composition and current amplitudes (in nanoamperes) elicited by 1 mm GABA in the presence of 1 μm THDOC (mean ± S.E.) and, in some cases, EC50 for GABA/THDOC (mean ± S.D.). n, number of oocytes; −, not analyzed. a, this subunit configuration might also assemble into R3. b, this subunit configuration might also assemble into R2. c, this subunit configuration might also assemble into R5. The receptor configurations shown in red contain one concatenated subunit resulting in current expression by itself.
For the first approach, we constructed each receptor, R1 to R5 (Fig. 4), combining free subunits and dual or triple subunit constructs in two to three different ways. For R1, where the δ subunit replaces the β subunit between the two α subunits, both combinations, β2-α4-δ/α4-β2 and α4-δ-α4/β2, could be functionally expressed. The former receptor was characterized by an EC50 for GABA of 0.55 ± 0.11 μm (n = 3). We confirmed these results by constructing the concatenated pentamer P1 and found an EC50 for GABA of 1.45 ± 0.39 μm (n = 3). An about 2-fold increased EC50 for GABA compared with the receptor composed of dual and triple subunit constructs has been observed previously in similar cases (9).
We conclude that R1 may be one of the formed receptor configurations. It should be noted that this also seems to be a preferred configuration of α1β3δ, α6β3δ, and α1α6β3δ receptors (15–17).
An example of a concentration-response curve is given for β2-α4-δ/α4-β2 (R1) as crude current traces (Fig. 5A). Averaged concentration-response curves for β2-α4-δ/α4-β2 (R1), δ-β2-α4/β2-α4 (R5), β2-α4-δ-α4-β2 (P1), and δ-β2-α4-β2-α4 (P5) are shown in Fig. 5B.
FIGURE 5.
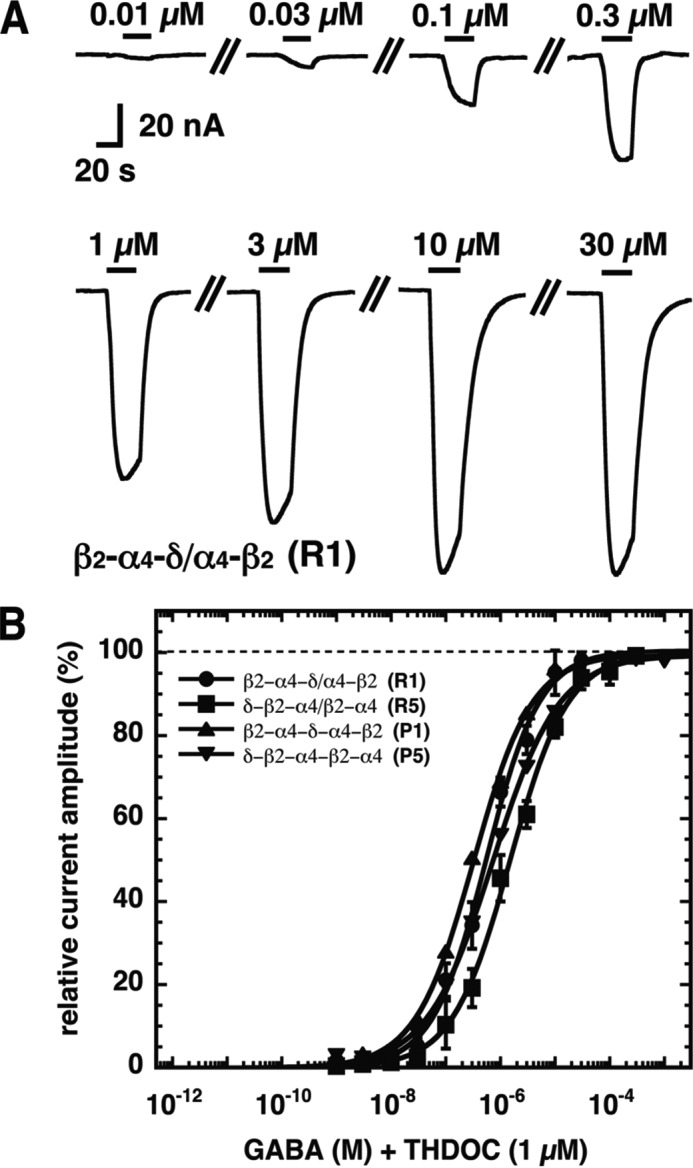
GABA concentration dependence of concatenated α4β2δ receptors. A, current traces from a GABA concentration-response curve in the presence of 1 μm THDOC obtained from a Xenopus oocyte expressing the β2-α4-δ/α4-β2 (R1) receptor. The bars indicate the time period of GABA/1 μm THDOC perfusion. Increasing concentrations of GABA were applied to the oocytes, and the corresponding current amplitudes were determined. GABA concentrations are indicated above the bars. B, averaged concentration-response curves of β2-α4-δ/α4-β2 (R1), δ-β2-α4/β2-α4 (R5), β2-α4-δ-α4-β2 (P1), and δ-β2-α4-β2-α4 (P5). Individual curves were first normalized to the fitted maximal current amplitude and subsequently averaged. Data are expressed as mean ± S.D. (n = 3–4).
For R2, where the δ subunit replaces the second β subunit of the two subsequent β subunits, two of the combinations, α4-β2-α4/β2-δ and α4-β2/δ, were silent, whereas the other combination, β2-α4-β2/δ-α4, was functionally expressed and was characterized with an EC50 for GABA of 1.11 ± 0.26 μm (n = 4). This combination contains β2-α4-β2 producing current by itself with similar functional properties as found here. As the EC50 for GABA of the combination was similar to that of the single concatenated subunit, we think that R2 may not be formed.
For R3, where the δ subunit replaces the α subunit between two subsequent β subunits and the single β subunit, one of the combinations, β2-δ-β2/α4-β2, was silent, whereas the other combination, β2-α4-β2/β2-δ, was functionally expressed. This combination contains β2-α4-β2 producing current by itself and is characterized by an EC50 for GABA of 0.26 ± 0.04 μm (n = 4). This value is significantly different (Student's t test, p < 0.02) from that characterizing the β2-α4-β2 construct. Therefore, it is not unlikely that the configuration R3 is formed. The fact that β2-δ-β2/α4-β2 is not functionally expressed may indicate that the β/α interface is assembled inefficiently. In line with this hypothesis is the observation that α4-δ-α4/β2 (R1) expresses less efficiently than β2-α4-δ/α4-β2 (R1) and α4-β2-α4/δ-β2 (R5) not at all.
For R4, where the δ subunit replaces the α subunit preceding the two subsequent β subunits, one of the combinations, which can also form R3, α4-β2/δ-β2/β2, was silent, whereas the other combination, β2-α4-β2/δ-β2, was functionally expressed. This combination contains β2-α4-β2 producing current by itself and is characterized by an EC50 for GABA of 0.93 ± 0.17 μm (n = 4), similar to the EC50 of β2-α4-β2 expressed alone. We think that R4 may either not be formed or is indistinguishable from β2-α4-β2.
For R5, where the δ subunit replaces the first of the two subsequent β subunits in the classical αβ receptor or the γ subunit in the classical αβγ receptor, α4-β2-α4/δ-β2 did not result in active channel formation, but, as discussed above, this combination requires formation of a β/α subunit interface. δ-β2-α4/β2-α4 and β2-α4-δ/β2-α4 did functionally express, but each contains β2-α4, a construct that results in current formation by itself. The two receptors were characterized by an EC50 for GABA of 1.50 ± 0.14 μm (n = 3) and 1.58 ± 0.59 μm (n = 3), respectively. Both values are similar to the EC50 of β2-α4. As this receptor has previously been claimed to represent the subunit arrangement of α4β2δ receptors (25), we decided to construct the pentameric concatenated receptor δ-β2-α4-β2-α4 (P5). To our surprise, we found functional expression of the pentameric construct with an EC50 for GABA of 0.75 ± 0.11 μm (n = 4). Successful expression of a pentameric concatenated construct suggested that R5 may be a possible receptor configuration.
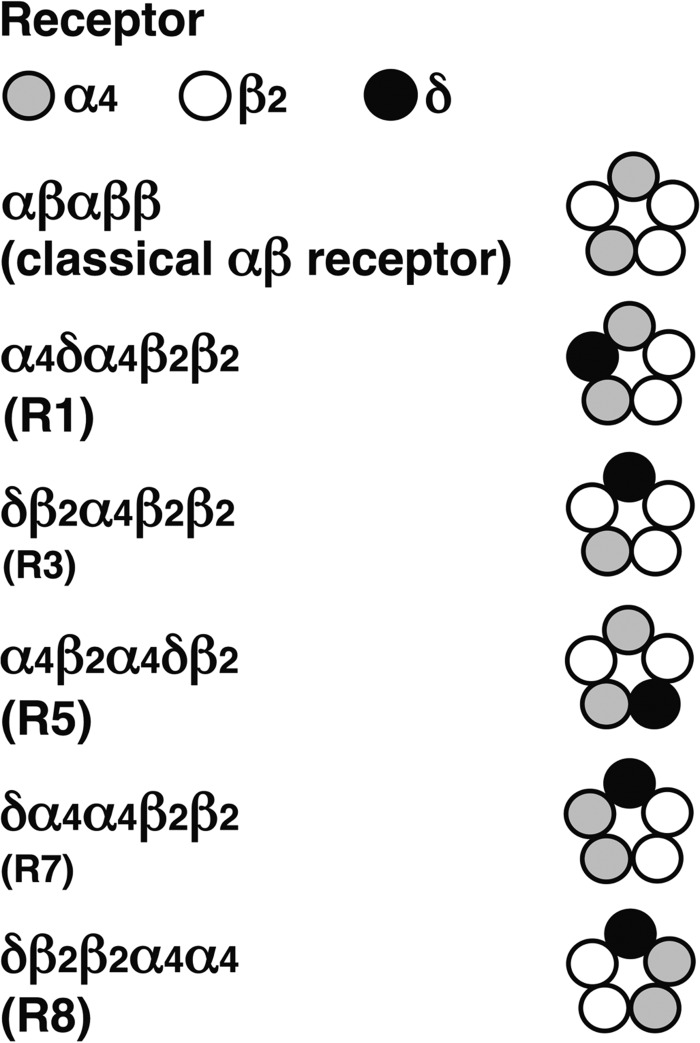
Additionally, we studied α4β2δ GABAA receptors with a 2:2:1 subunit stoichiometry of α4, β2, and δ subunits. In theory, six possible subunit arrangements exist with this stoichiometry. It should be noted that the subunit arrangements R1, R2, and R5 are identical to three of these six receptors. We did not observe functional expression of δ-β2-α4/α4-β2 (R6). A small functional expression was detected for δ-α4/α4-β2/β2 (R7). Please note that this combination may also be assembled into R2. A large current expression was observed for δ-β2/β2-α4/α4 (R8). This construct contains β2-α4, which results in current expression by itself and may also assemble to R5. The large current expression exceeds by far the one expected for β2-α4. Control experiments with β2-α4/α4 resulted in a 6-fold lower current expression and a reduced sensitivity to DS2, ensuring formation of R8 upon injection of δ-β2/β2-α4/α4. R8 was characterized by an EC50 for GABA of 0.18 ± 0.09 μm (n = 3). This EC50 differs from that of R5 and the constituent β2-α4. R8 and possibly R7 are candidates for the subunit arrangement of α4β2δ GABAA receptors. Theoretically, the components used to construct R8 could also assemble to R5. However, the formation of a β/α subunit interface would be required. An additional receptor not conforming to the above stoichiometry with two δ subunits did not result in current expression. Fig. 6 summarizes our findings on the possible subunit arrangement of concatenated α4β2δ receptors containing one or several δ subunits.
FIGURE 6.
Summary of the receptor configurations resulting in functional expression. R1, R5, and R8 are candidates, and R3 and R7 (smaller font) are likely candidates for the subunit arrangement of α4β2δ receptors.
Sensitivity to DS2
DS2 has been described to be specific for GABAA receptors containing a δ subunit (31, 32). We made similar observations. This is illustrated for α1β2γ2 and β2-α4-δ/α4-β2 (R1) (Fig. 7). The non-concatenated receptors α4β2, α1β2γ2, and α4β2δ, concatenated subunit constructs that result themselves in current expression, β2-α4 and β2-α4-β2, and the concatenated receptors β2-α4-δ/α4-β2 (R1), β2-α4-δ-α4-β2 (P1), β2-α4-β2/δ-α4 (R2), β2-α4-β2/β2-δ (R3), β2-α4-β2/δ-β2 (R4), δ-β2-α4/β2-α4 (R5), δ-β2-α4-β2-α4 (P5), δ-α4/α4-β2/β2 (R7), and δ-β2/β2-α4/α4 (R8) were tested for sensitivity to DS2 (Fig. 8). Sensitivity to DS2 was determined as potentiation by 30 μm DS2 of currents elicited by GABAEC10 and as relative current GABAEC10 + 30 μm DS2 divided by the maximal current elicited by GABA in the presence of 1 μm THDOC. Whenever the δ subunit was present, sensitivity to DS2 was high (relative current, 25–77%), and whenever the δ subunit was absent, sensitivity to DS2 was low (relative current, <2–9%).
FIGURE 7.
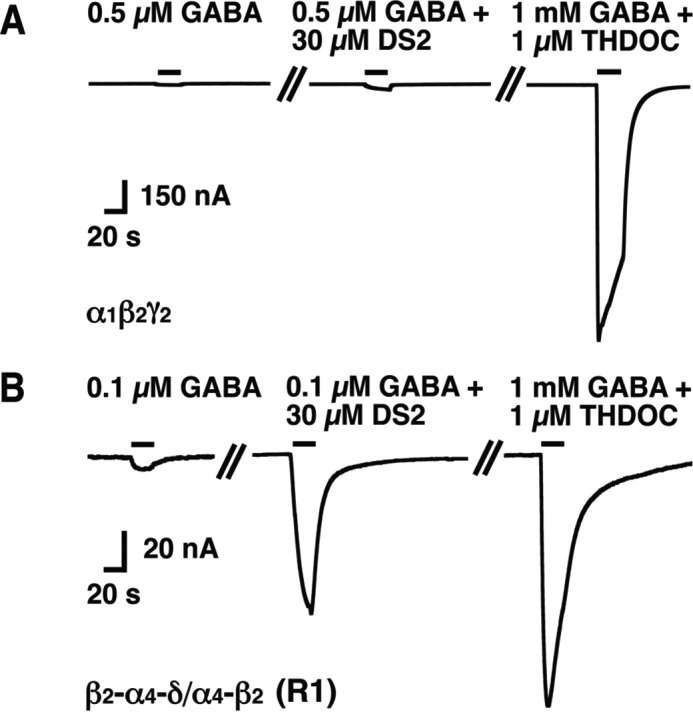
DS2 sensitivity of non-concatenated α1β2γ2 and β2-α4-δ/α4-β2 (R1) receptors. A and B, original current traces recorded upon application of 0.5 or 0.1 μm GABA alone, followed by application of 30 μm DS2 together with the same concentrations of GABA and subsequent co-application of 1 mm GABA/1 μm THDOC from a representative non δ subunit-containing GABAA receptor, α1β2γ2 (A) and a representative δ subunit-containing GABAA receptor, β2-α4-δ/α4-β2 (R1, B), respectively. The bars indicate the duration of drug application. These experiments were repeated three to four times with similar results.
FIGURE 8.
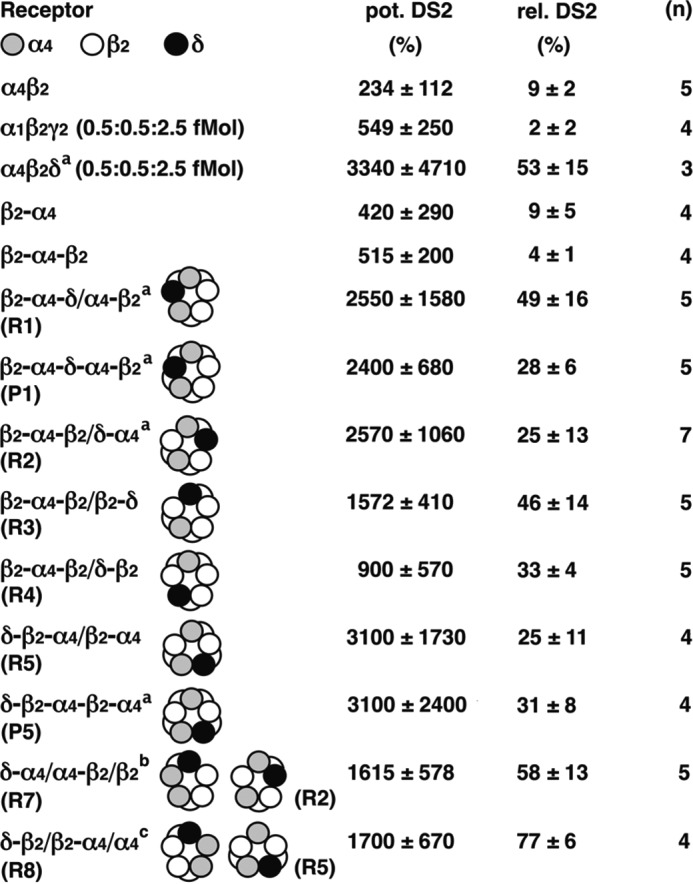
Positive allosteric modulation by DS2. The functional receptors were tested for sensitivity to DS2 by measuring the potentiation by 30 μm DS2 of current evoked by GABAEC10 (pot. DS2) and the current elicited by GABAEC10 + 30 μm DS2 relative to the maximal current elicited by 1 mm GABA in the presence of 1 μm THDOC (rel. DS2). a, the current amplitude elicited by GABAEC10 in α4β2δ (0.5:0.5:2.5 fmol), β2-α4-δ/α4-β2 (R1), β2-α4-δ-α4-β2 (P1), β2-α4-β2/δ-α4 (R2), and δ-β2-α4-β2-α4 (P5) receptors was less than 4 nA in each case. We assumed that these amplitudes amounted to 4 nA to be able to calculate potentiation by DS2. Therefore, the corresponding values represent underestimates. b, this subunit configuration might also assemble into R2. c, this subunit configuration might also assemble into R5. Data are expressed as mean ± S.D. n, number of oocytes.
Effect of Ethanol
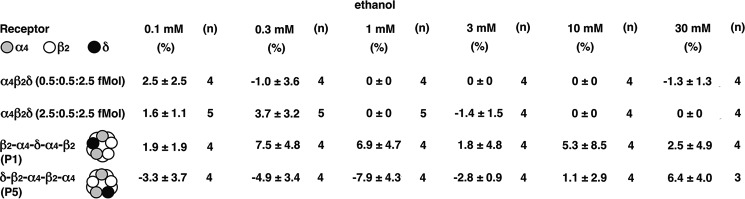
α4β2δ GABAA receptors have been claimed to be a site of action of low concentrations of ethanol (28, 33). Therefore, we were interested to investigate this with non-concatenated receptors and some of our receptors with defined subunit arrangement. A concentration dependence of ethanol was investigated in non-concatenated α4β2δ GABAA receptors expressed at two different subunit stoichiometries, 0.5:0.5:2.5 fmol and 2.5:0.5:2.5 fmol, and the two pentameric concatenated receptors P1 and P5. In the concentration range of 0.1–30 mm ethanol, no significant effect on the current amplitude by GABAEC10 was found (Fig. 9).
FIGURE 9.
Lack of an effect by physiological concentrations of ethanol. Non-concatenated α4β2δ (0.5:0.5:2.5 and 2.5:0.5:2.5), concatenated β2-α4-δ-α4-β2 (P1), and δ-β2-α4-β2-α4 (P5) receptors were expressed in Xenopus oocytes. The receptors were activated by a concentration of GABA eliciting EC10 in the presence of 1 μm THDOC, followed by application of the same concentration of GABA/THDOC subsequently in combination with 0.1, 0.3, 1, 3, 10, and 30 mm ethanol. The relative current amplitude of the responses in the presence of ethanol compared with GABA/THDOC alone is given. Neither currents mediated by the non-concatenated α4β2δ or concatenated receptors were significantly modulated by ethanol in the concentration range of 0.1–30 mm. Data are expressed as mean ± S.E. n, number of oocytes.
Discussion
In this work, we attempted to get insight into the function and subunit arrangement of α4β2δ GABAA receptors. They are located extrasynaptically and are responsible for tonic inhibition (10, 34). As a first step, we expressed mRNA coding for α4, β2, and δ at different stoichiometries. It is well known from the literature that different stoichiometries result in different functional properties, in some cases with a nanomolar EC50 value for GABA. The ambient concentration of GABA in the extracellular space is not known precisely (for a discussion, see Ref. 35). Nevertheless, the concentration is estimated to be in the range of 0.1–0.4 μm (36–38). A receptor with an EC50 for GABA in the lower nanomolar range would be always fully activated under these conditions. Thus, we think that the in vivo existence such of a receptor is unlikely (see also below). It should be noted that the receptor resulting from the expression of 2.5:0.5:2.5 fmol mRNA/oocyte, coding for α4, β2, and δ subunits, results in such properties.
It would be desirable to establish function and subunit arrangement in neuronal cells, but unfortunately we had to resort to expression systems lacking endogenous GABAA receptors and hope that the properties observed here reflect the situation in neurons. Results on function and subunit arrangement could in principle be obscured by endogenous expression of GABAA receptor subunits in the expression system used. In HEK cells, such an expression is well documented (39). To our knowledge, there are no such reports for Xenopus oocytes, which we used as an expression system.
To establish function and subunit arrangement, we used subunit concatenation. This is an elegant technique to prepare receptors with predefined subunit arrangement. The functional properties of such receptors can then be compared with the properties of non-concatenated receptors. For a detailed discussion of possible pitfalls, see Refs. 40, 41. Maybe the biggest threat to this method is proteolysis. It should be noted that, despite an intensive search, such a proteolysis of concatenated subunits has never been found. In case proteolysis should take place with a subunit, the construct is likely to be functionally silent. Therefore, we only consider proteolysis in the linker. We would like to discuss this possibility for the present case. The linkers contain no protease sites, and, in concatenated constructs, presequences were removed from all subunits except from the first one. Expression of the individual non-concatenated subunits α4, β2, and δ or α4 or β2 in combination with δ did not result in functional receptors (Fig. 2). Therefore, liberation of α4, β2, and δ each alone or δ in combination with α4 or β2 would not result in current. Exclusively, large amounts of α4 and β2 would do so. However, this current would be insensitive to DS2, unlike the current by receptor candidates listed in Fig. 7. The dual subunit constructs α4-β2, α4-δ, β2-δ, δ-α4, and δ-β2 and the triple subunit constructs α4-β2-α4, α4-δ-α4, β2-δ-β2, and δ-β2-α4 did not result in current expression (Fig. 2). Furthermore, none of the combinations of the δ subunit with either α4-β2, α4-δ, β2-δ, δ-α4, or δ-β2 and α4-β2-α4/β2-δ (R2), β2-δ-β2/α4-β2 (R3), α4-β2/δ-β2/β2 (R4), α4-β2-α4/δ-β2 (R5), δ-β2-α4/α4-β2 (R6), and α4-β2-α4/δ (R9) resulted in current expression. α4, β2, and δ must all be liberated simultaneously to produce a current sensitive to DS2. We conclude that the possibility that proteolysis affects our observations is highly unlikely.
Rearrangement of a dual construct has been documented (7). Rearrangement was quite inefficient (26%), and the resulting receptor was reported to respond rather weakly to positive allosteric modulators. Also, if rearrangement was efficient, then all configurations containing α4-β2 would be expected to result in β2-α4 and, thus, in current expression. This is not the case. Therefore, we think that subunit rearrangement is not a likely explanation for our observations.
Interpretation of our results is made more difficult by two facts. First, current expression levels are small in most cases. Second, some of the used concatenated constructs result in current expression themselves. Nevertheless, we can draw a number of conclusions. R1, R5, R8, and possibly R3 and R7, are candidates for the subunit arrangement of α4β2δ GABAA receptors. The assembly properties of α4β2δ GABAA receptors are less defined than in the case of α1β3δ, α6β3δ, and α1α6β3δ receptors, but the subunit stoichiometry of α4, β2, and δ subunits mostly satisfies 2:2:1. Remarkably, all the receptors resulting in functional expression are characterized by a similar EC50 for GABA/THDOC in the range of 0.2–1.6 μm, tuned to local physiological concentrations of GABA.
Maybe one of the most important observations is that, in the many subunit arrangements described that result in functional expression, we never encountered receptors with an EC50 for GABA in the presence of THDOC of <180 nm. As discussed above, a receptor with an EC50 for GABA of <100 nm would not make physiological sense. Observations of such receptors in recombinant systems most probably are the unnatural result of the use of large proportions of genetic information coding for the α subunit.
Our results should be compared with those obtained earlier using concatenated subunits (25, 26). The comparison is made difficult by the fact that, in our hands, the β2-α4 construct resulted in current expression by itself. In our work, oocytes were injected with 2.5 fmol cRNA coding for β2-α4 and, in the cited work, with >40 fmol (25) or 3–15 fmol (26). Despite the large quantities injected, Shu et al. (25) did not observe current expression in this case. The reason for the discrepancy is far from clear. Most of the receptors reported in the above references contain this construct. Nevertheless, the authors conclude that R2, R4, (26) and R5 (25, 26) (our nomenclature) are candidate subunit arrangements. The EC50 values for GABA in the absence of THDOC for these receptors were reported to be about 55, 1.2, and 3.5 μm, respectively. In contrast to the above conclusions, we think that R2 and R4 may not represent receptor configurations expressed from limited amounts of cRNA.
In conclusion, the processes governing assembly of α4, β2, and δ subunits to form pentameric α4β2δ GABAA receptors seem much less defined than assembly of α1, β2, and γ2 subunits. The δ subunit can assume different positions, and the resulting receptors with different subunit arrangement have remarkably similar functional properties reflecting the properties of non-concatenated receptors expressed from a ratio of genetic information of 1:1:5 coding for α4, β2, and δ subunits. While all δ subunit-containing receptors were sensitive to the positive allosteric modulator DS2, we did not find any evidence for sensitivity to low concentrations of ethanol for the tested non-concatenated and concatenated α4β2δ GABAA receptors.
Experimental Procedures
Construction of cDNAs
The cDNA coding for the rat δ subunit was generously provided by Dr. Hartmut Lüddens (Department of Psychiatry, University of Mainz, Mainz, Germany). The approach used for subunit concatenation of GABAA receptors has been described in detail previously (7–9, 40, 42). We prepared the dual constructs α4-12-β2, α4-12-δ, β2-23-α4, δ-23-α4, β2-26-δ, and δ-26-β2 and the triple subunit constructs α4-12-β2-23-α4, α4-12-δ-23-α4, β2-23-α4-12-β2, β2-23-α4-12-δ, β2-26-δ-26-β2, and δ-26-β2-23-α4. In addition, two pentameric constructs, β2-23-α4-12-δ-23-α4-12-β2 and δ-26-β2-23-α4-12-β2-23-α4, were built. The number between two subunits describes the number of amino acid residues of the introduced synthetic linker. Our strategy to design the linkers was to apply the rule that the sum of the predicted C-terminal protrusion of a preceding subunit and the artificial linker has to be minimally 23 residues in length. Constructs containing shorter linkers did not result in receptor expression (7, 8). The linkers were Q6TGQ4 for α4-β2 and α4-δ, Q5A3PTGQA3PA2Q5 for β2-α4 and δ-α4, and Q5A3PTGQ2AQA3PA2Q5 for β2-δ and δ-β2.
Expression in Xenopus Oocytes
The cDNA was subcloned into a eukaryotic expression pcDNA3.1 vector (Invitrogen). Capped cRNAs were synthesized (Ambion) from the linearized vectors containing different non-concatenated and concatenated subunits. A poly-A tail of about 400 residues was added to each transcript using yeast poly-A polymerase (USB). The concentration of the cRNA was quantified on a formaldehyde-agarose gel using Radiant Red stain (Bio-Rad) for visualization of the cRNA. Known concentrations of RNA ladder (Invitrogen) were loaded as standard on the same gel. The cRNAs were dissolved in water and stored at −80 °C. Frog oocytes obtained from Xenopus laevis (stages V-VI) were isolated, injected, and defolliculated as described earlier (43, 44). All animal experiments have been reviewed and approved by the Kantonstierarzt, Kantonaler Veterinärdienst Bern (BE85/15). cRNA coding for each dual and triple subunit concatemer was injected either alone or in different combinations in oocytes. Oocytes were injected with 50 nl of solution containing RNA. In the case of non-concatenated α4β2δ receptors, cRNAs coding for α4, β2, and δ subunits were injected at a ratio of 0.5:0.5:2.5 fmol/oocyte or 2.5:0.5:2.5 fmol/oocyte as indicated, and, in the case of α1β2γ2 receptors, cRNA coding for α1, β2, and γ2 subunits a ratio of 0.5:0.5:2.5 fmol/oocyte. In the case of concatenated receptors, oocytes were injected with cRNA coding for dual and triple subunits at 2.5 fmol each or pentameric constructs at 2.5 fmol. The injected oocytes were incubated in modified Barth's solution (43) at 18 °C for 1–2 days in case of α1β2γ2 receptors and 5–7 days for other receptors before recording.
Two-electrode Voltage Clamp Measurements
Electrophysiological studies were performed using a two-electrode voltage clamp amplifier (Oocyte Clamp OC-725C, Warner Instruments) in combination with a XY recorder (90% response time, 0.1 s) or digitized at 100 Hz using a Powerlab 2/20 (AD- Instrument GmbH, Spechbach, Germany), and data were recorded with the computer program Chart (AD Instruments GmbH). All measurements were performed in medium containing 90 mm NaCl, 1 mm MgCl2, 1 mm KCl, 1 mm CaCl2, and 5 mm HEPES (pH 7.4) at a holding potential of −80 mV. The perfusion solution (6 ml/min) was applied through a glass capillary with an inner diameter of 1.35 mm, the mouth of which was placed about 0.4 mm from the surface of the oocyte (45). In initial experiments, 1 mm GABA (Sigma-Aldrich, Switzerland) was applied alone, followed by 1 μm THDOC (Sigma-Aldrich), and then the combination of the two. Relative current potentiation by THDOC was determined as (I1 μm THDOC + 1 mm GABA/I1 μm GABA − 1) × 100%. For the determination of maximal current amplitudes, 1 mm GABA was applied in the presence of 1 μm THDOC for 20 s. THDOC was prepared as a 10 mm stock solution in DMSO and dissolved in external solution, resulting in a final DMSO concentration of 0.01%. Individual concentration-response curves for GABA in the presence of 1 μm THDOC were fitted with the equation I(c) = Imax / (1 + (EC50 / c)n), where c is the concentration of GABA, EC50 the concentration of GABA (in the presence of 1 μm THDOC) eliciting half-maximal current amplitude, Imax is the maximal current amplitude, I the current amplitude, and n the Hill coefficient. The individual curves were fitted and standardized to Imax and subsequently averaged.
Sensitivity to DS2 was measured as potentiation by 30 μm DS2 of current evoked by GABAEC10 and as GABAEC10 + 30 μm DS2 divided by the current elicited by 1 mm GABA in the presence of 1 μm THDOC. DS2 was prepared as a 10 mm stock solution in DMSO and dissolved in external solution, resulting in a final DMSO concentration of 0.3%. Potentiation by ethanol was determined at EC10 for GABA using 0.1, 0.3, 1, 3, 10, and 30 mm ethanol.
Data are given as mean ± S.E. for the Imax values for GABA in the presence of 1 μm THDOC and for analysis of properties of receptors using ethanol and as mean ± S.D. for analysis of properties of receptors using DS2. To avoid contamination, the perfusion system was cleaned between drug applications by washing with 100% DMSO.
Author Contributions
N. W. conducted most of the electrophysiological experiments, analyzed the data, prepared the figures, and wrote the manuscript. R. B. produced all cRNA constructs and performed the electrophysiological experiments. E. S. conceived the idea for this project, designed the experiments, supervised the experiments, prepared the figures, and wrote the manuscript.
This work was supported by Swiss National Science Foundation Grant 315230_156929/1. The authors declare that they have no conflicts of interest with the contents of this article.
- THDOC
- 3α, 21-dihydroxy-5α-pregnan-20-one
- DS2
- 4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridin-3-yl]benzamide.
References
- 1. Macdonald R. L., and Olsen R. W. (1994) GABAA receptor channels. Annu. Rev. Neurosci. 17, 569–602 [DOI] [PubMed] [Google Scholar]
- 2. Olsen R. W., and Sieghart W. (2008) International Union of Pharmacology: LXX: subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 60, 243–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sigel E., and Steinmann M. E. (2012) Structure, function, and modulation of GABAA receptors. J. Biol. Chem. 287, 40224–40231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang Y., Wang R., Barot S., and Weiss D. S. (1996) Stoichiometry of a recombinant GABAA receptor. J. Neurosci. 16, 5415–5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farrar S. J., Whiting P. J., Bonnert T. P., and McKernan R. M. (1999) Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J. Biol. Chem. 274, 10100–10104 [DOI] [PubMed] [Google Scholar]
- 6. Tretter V., Ehya N., Fuchs K., and Sieghart W. (1997) Stoichiometry and assembly of a recombinant GABAA receptor subtype. J. Neurosci. 17, 2728–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baumann S. W., Baur R., and Sigel E. (2001) Subunit arrangement of γ-aminobutyric acid type A receptors. J. Biol. Chem. 276, 36275–36280 [DOI] [PubMed] [Google Scholar]
- 8. Baumann S. W., Baur R., and Sigel E. (2002) Forced subunit assembly in α1β2γ2 GABAA receptors. Insight into the absolute arrangement. J. Biol. Chem. 277, 46020–46025 [DOI] [PubMed] [Google Scholar]
- 9. Baur R., Minier F., and Sigel E. (2006) A GABAA receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Lett. 580, 1616–1620 [DOI] [PubMed] [Google Scholar]
- 10. Farrant M., and Nusser Z. (2005) Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 6, 215–229 [DOI] [PubMed] [Google Scholar]
- 11. Glykys J., Peng Z., Chandra D., Homanics G. E., Houser C. R., and Mody I. (2007) A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat. Neurosci. 10, 40–48 [DOI] [PubMed] [Google Scholar]
- 12. Sur C., Farrar S. J., Kerby J., Whiting P. J., Atack J. R., and McKernan R. M. (1999) Preferential coassembly of α4 and δ subunits of the γ-aminobutyric acidA receptor in rat thalamus. Mol. Pharmacol. 56, 110–115 [DOI] [PubMed] [Google Scholar]
- 13. Peng Z., Hauer B., Mihalek R. M., Homanics G. E., Sieghart W., Olsen R. W., and Houser C. R. (2002) GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J. Comp. Neurol. 446, 179–197 [DOI] [PubMed] [Google Scholar]
- 14. Jones A., Korpi E. R., McKernan R. M., Pelz R., Nusser Z., Mäkelä R., Mellor J. R., Pollard S., Bahn S., Stephenson F. A., Randall A. D., Sieghart W., Somogyi P., Smith A. J., and Wisden W. (1997) Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J. Neurosci. 17, 1350–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baur R., Kaur K. H., and Sigel E. (2009) Structure of α6β3δ GABAA receptors and their lack of ethanol sensitivity. J. Neurochem. 111, 1172–1181 [DOI] [PubMed] [Google Scholar]
- 16. Kaur K. H., Baur R., and Sigel E. (2009) Unanticipated structural and functional properties of δ subunit-containing GABAA receptors. J. Biol. Chem. 284, 7889–7896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baur R., Kaur K. H., and Sigel E. (2010) Diversity of structure and function of α1α6β3δ GABAA receptors: comparison with α1β3δ and α6β3δ receptors. J. Biol. Chem. 285, 17398–17405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrera N. P., Betts J., You H., Henderson R. M., Martin I. L., Dunn S. M., and Edwardson J. M. (2008) Atomic force microscopy reveals the stoichiometry and subunit arrangement of the α4β3δ GABAA receptor. Mol. Pharmacol. 73, 960–967 [DOI] [PubMed] [Google Scholar]
- 19. Wagoner K. R., and Czajkowski C. (2010) Stoichiometry of expressed α4β2δ γ-aminobutyric acid type A receptors depends on the ratio of subunit cDNA transfected. J. Biol. Chem. 285, 14187–14194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. You H., and Dunn S. M. (2007) Identification of a domain in the δ subunit (S238-V264) of the α4β3δ GABAA receptor that confers high agonist sensitivity. J. Neurochem. 103, 1092–1101 [DOI] [PubMed] [Google Scholar]
- 21. Karim N., Wellendorph P., Absalom N., Bang L. H., Jensen M. L., Hansen M. M., Lee H. J., Johnston G. A., Hanrahan J. R., and Chebib M. (2012) Low nanomolar GABA effects at extrasynaptic α4β1/β3δ GABAA receptor subtypes indicate a different binding mode for GABA at these receptors. Biochem. Pharmacol. 84, 549–557 [DOI] [PubMed] [Google Scholar]
- 22. Hartiadi L. Y., Ahring P. K., Chebib M., and Absalom N. L. (2016) High and low GABA sensitivity α4β2δ GABAA receptors are expressed in Xenopus laevis oocytes with divergent stoichiometries. Biochem. Pharmacol. 103, 98–108 [DOI] [PubMed] [Google Scholar]
- 23. Patel B., Mortensen M., and Smart T. G. (2014) Stoichiometry of δ subunit containing GABAA receptors. Br. J. Pharmacol. 171, 985–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bollan K. A., Baur R., Hales T. G., Sigel E., and Connolly C. N. (2008) The promiscuous role of the ϵ subunit in GABAA receptor biogenesis. Mol. Cell. Neurosci. 37, 610–621 [DOI] [PubMed] [Google Scholar]
- 25. Shu H. J., Bracamontes J., Taylor A., Wu K., Eaton M. M., Akk G., Manion B., Evers A. S., Krishnan K., Covey D. F., Zorumski C. F., Steinbach J. H., and Mennerick S. (2012) Characteristics of concatemeric GABAA receptors containing α4/δ subunits expressed in Xenopus oocytes. Br. J. Pharmacol. 165, 2228–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eaton M. M., Bracamontes J., Shu H. J., Li P., Mennerick S., Steinbach J. H., and Akk G. (2014) γ-aminobutyric acid type A α4, β2, and δ subunits assemble to produce more than one functionally distinct receptor type. Mol. Pharmacol. 86, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bianchi M. T., and Macdonald R. L. (2003) Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J. Neurosci. 23, 10934–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wallner M., Hanchar H. J., and Olsen R. W. (2003) Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc. Natl. Acad. Sci. U.S.A. 100, 15218–15223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wohlfarth K. M., Bianchi M. T., and Macdonald R. L. (2002) Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J. Neurosci. 22, 1541–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheleznova N., Sedelnikova A., and Weiss D. S. (2008) α1β2δ, a silent GABAA receptor: recruitment by tracazolate and neurosteroids. Br. J. Pharmacol. 153, 1062–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wafford K. A., van Niel M. B., Ma Q. P., Horridge E., Herd M. B., Peden D. R., Belelli D., and Lambert J. J. (2009) Novel compounds selectively enhance δ subunit-containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology 56, 182–189 [DOI] [PubMed] [Google Scholar]
- 32. Jensen M. L., Wafford K. A., Brown A. R., Belelli D., Lambert J. J., and Mirza N. R. (2013) A study of subunit selectivity, mechanism and site of action of the δ selective compound 2 (DS2) at human recombinant and rodent native GABAA receptors. Br. J. Pharmacol. 168, 1118–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sundstrom-Poromaa I., Smith D. H., Gong Q. H., Sabado T. N., Li X., Light A., Wiedmann M., Williams K., and Smith S. S. (2002) Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat. Neurosci. 5, 721–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Belelli D., Harrison N. L., Maguire J., Macdonald R. L., Walker M. C., and Cope D. W. (2009) Extrasynaptic GABAA receptors: form, pharmacology, and function. J. Neurosci. 29, 12757–12763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel B., Bright D. P., Mortensen M., Frølund B., and Smart T. G. (2016) Context-dependent modulation of GABAAR-mediated tonic currents. J. Neurosci. 36, 607–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Attwell D., Barbour B., and Szatkowski M. (1993) Nonvesicular release of neurotransmitter. Neuron 11, 401–407 [DOI] [PubMed] [Google Scholar]
- 37. Richerson G. B., and Wu Y. (2003) Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J. Neurophysiol. 90, 1363–1374 [DOI] [PubMed] [Google Scholar]
- 38. Wu Y., Wang W., Díez-Sampedro A., and Richerson G. B. (2007) Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron 56, 851–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ueno S., Zorumski C., Bracamontes J., and Steinbach J. H. (1996) Endogenous subunits can cause ambiguities in the pharmacology of exogenous γ-aminobutyric acidA receptors expressed in human embryonic kidney 293 cells. Mol. Pharmacol. 50, 931–938 [PubMed] [Google Scholar]
- 40. Minier F., and Sigel E. (2004) Techniques: Use of concatenated subunits for the study of ligand-gated ion channels. Trends. Pharmacol. Sci. 25, 499–503 [DOI] [PubMed] [Google Scholar]
- 41. Sigel E., Kaur K. H., Lüscher B. P., and Baur R. (2009) Use of concatamers to study GABAA receptor architecture and function: application to δ subunit-containing receptors and possible pitfalls. Biochem. Soc. Trans. 37, 1338–1342 [DOI] [PubMed] [Google Scholar]
- 42. Baumann S. W., Baur R., and Sigel E. (2003) Individual properties of the two functional agonist sites in GABAA receptors. J. Neurosci. 23, 11158–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sigel E. (1987) Properties of single sodium channels translated by Xenopus oocytes after injection with messenger ribonucleic acid. J. Physiol. 386, 73–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sigel E., and Minier F. (2005) The Xenopus oocyte: system for the study of functional expression and modulation of proteins. Mol. Nutr. Food. Res. 49, 228–234 [DOI] [PubMed] [Google Scholar]
- 45. Sigel E., Baur R., Trube G., Möhler H., and Malherbe P. (1990) The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron 5, 703–711 [DOI] [PubMed] [Google Scholar]