Abstract
β-Carotene is an important source of vitamin A for the mammalian embryo, which depends on its adequate supply to achieve proper organogenesis. In mammalian tissues, β-carotene 15,15′-oxygenase (BCO1) converts β-carotene to retinaldehyde, which is then oxidized to retinoic acid, the biologically active form of vitamin A that acts as a transcription factor ligand to regulate gene expression. β-Carotene can also be cleaved by β-carotene 9′,10′-oxygenase (BCO2) to form β-apo-10′-carotenal, a precursor of retinoic acid and a transcriptional regulator per se. The mammalian embryo obtains β-carotene from the maternal circulation. However, the molecular mechanisms that enable its transfer across the maternal-fetal barrier are not understood. Given that β-carotene is transported in the adult bloodstream by lipoproteins and that the placenta acquires, assembles, and secretes lipoproteins, we hypothesized that the aforementioned process requires placental lipoprotein biosynthesis. Here we show that β-carotene availability regulates transcription and activity of placental microsomal triglyceride transfer protein as well as expression of placental apolipoprotein B, two key players in lipoprotein biosynthesis. We also show that β-apo-10′-carotenal mediates the transcriptional regulation of microsomal triglyceride transfer protein via hepatic nuclear factor 4α and chicken ovalbumin upstream promoter transcription factor I/II. Our data provide the first in vivo evidence of the transcriptional regulatory activity of β-apocarotenoids and identify microsomal triglyceride transfer protein and its transcription factors as the targets of their action. This study demonstrates that β-carotene induces a feed-forward mechanism in the placenta to enhance the assimilation of β-carotene for proper embryogenesis.
Keywords: carotenoid, metabolism, placenta, retinoid, vitamin A, apocarotenoid
Introduction
The importance of vitamin A as a critical modulator of mammalian embryonic development has been known for decades (1). This essential nutrient exerts its function mainly through its active form, retinoic acid. Retinoic acid binds to retinoic acid receptors and retinoid X receptors (RXRs)3 and regulates, in a spatial and temporal manner, the transcription of numerous genes vital to development (2–6).
The mammalian embryo obtains retinoids (vitamin A and its derivatives) and provitamin A carotenoids from the maternal bloodstream. Among dietary carotenoids, β-carotene (BC) is the main source of vitamin A for the majority of the world population (7). Intact BC from the maternal circulation crosses the placenta and reaches the developing embryo where the cytoplasmic β-carotene 15,15′-oxygenase (BCO1) cleaves BC symmetrically to yield retinaldehyde, which in turn is oxidized to retinoic acid (8, 9). Asymmetric cleavage of BC by β-carotene 9′,10′-oxygenase (BCO2) also occurs, generating β-ionone and β-apo-10′-carotenal (apo10AL) (9). The latter, as well as other β-apocarotenoids of various chain lengths generated from oxidative breakdown of BC in food and animal tissues (10), can be converted into one molecule of retinaldehyde by BCO1 (9, 11). However, BCO2 does not contribute significantly to the generation of retinoids from BC at least in adult tissues (9). Instead, BCO2, which has a broader substrate specificity than BCO1 (7), seems to prevent toxic accumulation of carotenoids, including BC, in mitochondria where the asymmetric cleavage enzyme is localized (8, 12). Interestingly, β-apocarotenoids have also been recently found to function as transcriptional regulators, specifically as nuclear receptor antagonists, exerting anti-retinoic acid activities (13–17).
The placenta is a critical organ that during gestation transports BC, among other nutrients, from the maternal bloodstream to the fetal circulation. Syncytiotrophoblast cells of the blood-placental barrier fulfill this transport function (18, 19). Despite the importance of intact BC as a source of retinoids for the developing tissues (20–23), the molecular mechanisms of BC transfer from mother to fetus and the factors that influence this process are largely unknown. Given that BC is a highly hydrophobic molecule transported in the adult bloodstream by lipoproteins, mainly low density lipoproteins (LDL) (24–26), and that the placenta acquires, assembles, and secretes lipoproteins (27, 28), we hypothesized that the transfer of BC from the placenta to the fetal circulation occurs via placental lipoprotein biosynthesis. The latter is predominantly regulated by apolipoprotein B (apoB), a structural component of the lipoproteins (29), and microsomal triglyceride transfer protein (MTP) that binds and chaperones lipids to the nascent apoB to prevent aberrant folding and degradation by proteasomes and assists in the intracellular assembly of apoB lipoproteins, such as chylomicrons and very low density lipoproteins (VLDL) (30, 31).
Here we present evidence that the transport of intact BC from the placenta to the fetal bloodstream occurs via lipoproteins. We also show that placental lipoprotein production is regulated by BC availability. Our results identify β-apocarotenoids, such as apo10AL generated by BCO2, and placental MTP as critical players in this process. Specifically, they indicate that when BC is available the generation of apo10AL in placenta modulates Mttp gene expression and functions to enable transport of intact BC to the embryo.
Results
Placental Mttp Transcription and Activity Are Influenced by BC Availability
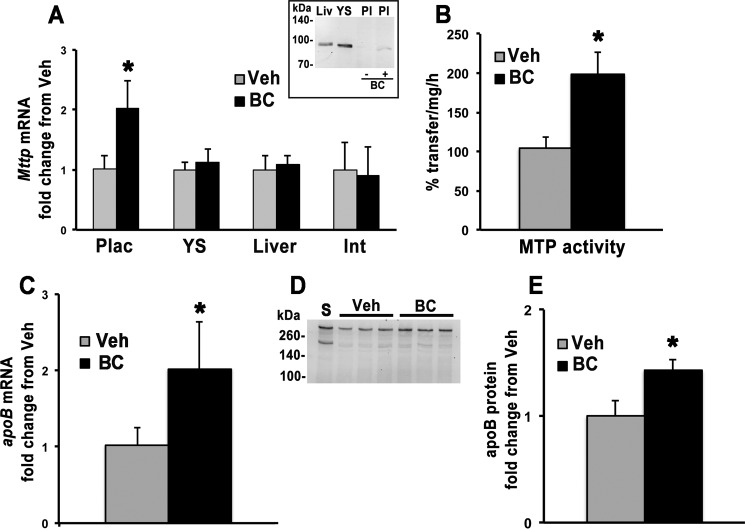
Despite the fact that the exact cellular localization of all the key regulators of lipoprotein metabolism in placenta remain elusive, it is known that placenta assembles and secretes apoB-containing lipoproteins both in vivo and in vitro (27, 32). To establish whether these processes are influenced by BC availability, wild-type (WT) pregnant mice maintained during gestation on a purified diet containing 14 IU vitamin A/g of diet were intraperitoneally (i.p.) injected at 13.5 days postcoitum (dpc) with vehicle or BC (∼35 μg of BC/g of body weight) and sacrificed 24 h later. We showed earlier that this BC administration protocol results in significant uptake of BC by the placenta without signs of embryonic toxicity (22, 23, 25). Dams were then sacrificed after 24 h. qPCR analysis showed that placental gene expression of Mttp was significantly increased in dams administered BC as compared with vehicle-injected dams (Fig. 1A). This transcriptional response was tissue-specific as no significant difference in Mttp mRNA levels was observed in the yolk sac, liver, or intestinal mucosa of these dams (Fig. 1A). Placental MTP protein levels were undetectable (compared with liver and yolk sac) but increased upon maternal BC administration (Fig. 1A, inset). In agreement with the increase in mRNA and protein levels, placental MTP activity was also increased in the BC-treated female mice compared with vehicle-treated controls (Fig. 1B). Furthermore, placental mRNA and protein levels of apoB were similarly increased upon BC administration (Fig. 1, C, D, and E). Overall, these data suggest that BC may up-regulate placental lipoprotein production.
FIGURE 1.
Placental MTP and apoB expression. Placentas were collected from WT dams 24 h post-i.p. injection of vehicle (Veh) or BC. A, qPCR analysis of mRNA levels of Mttp in placenta (n = 1–2 placentas/dam from four vehicle-treated and six BC-treated dams), yolk sac (n = 1–2 yolk sac/dam from four dams for each group), liver (n = 4 vehicle-treated and n = 6 BC-treated dams), and intestinal mucosa (n = 3 dams/treatment group). Inset, Western blot of placental protein extracts probed with a mouse anti-MTP antibody (BD Biosciences). A representative Western blot is shown. B, placental MTP activity expressed as percent transfer of lipids/mg/h (n = 1–3 placentas/dam from four vehicle-treated and three BC-treated dams). C, qPCR analysis of apoB expression in placenta (n = 1–2 placentas/dam from four vehicle-treated and six BC-treated dams). Data are presented as mean ± S.D. (error bars) of duplicate determinations and are representative of two to three independent determinations. D, representative Western blot of placental protein extracts probed with a rabbit anti-mouse polyclonal apoB antibody (AbCam, Cambridge, UK). E, placental apoB protein levels normalized to protein loading (n = 1 placenta/dam from three dams per treatment group). Normalization to albumin signal yielded similar results. Statistical analysis was by Student's t test. *, p < 0.05. Int, intestinal mucosa; Liv, liver; Plac and Pl, placenta; YS, yolk sac; S, serum.
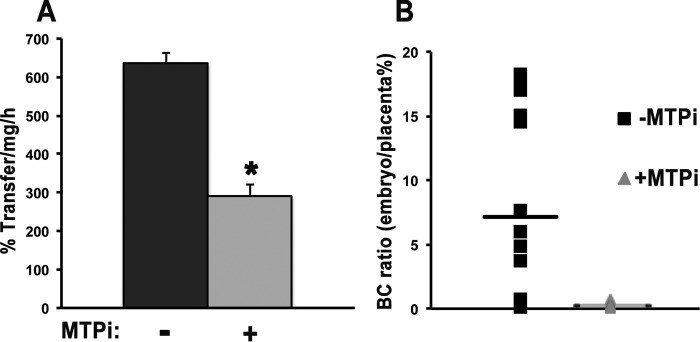
The role of MTP was further tested by daily gavage of pregnant WT mice with an MTP inhibitor, lomitapide (40 μl of 1 mm stock in DMSO (33)) starting from 12.5 dpc. This inhibitor blocks synthesis and secretion of lipoproteins in all the apoB lipoprotein-producing organs (33). At 14.5 dpc, BC was injected directly into the placenta (5 μl/placenta of ∼6.5 μg/μl BC), and mice were sacrificed after 30 min (34). Effectiveness of lomitapide treatment was confirmed by reduced MTP activity in the liver of the dams injected with the inhibitor (Fig. 2A). Furthermore, BC transfer from placenta to embryo, assessed as the ratio between BC content in each placenta and its corresponding embryo, was severely attenuated in the dams administered lomitapide compared with controls (Fig. 2B). Overall, these results support the hypothesis that placental MTP plays a key role in the transport of BC from the maternal circulation to the embryo.
FIGURE 2.
Lomitapide attenuates the transfer of BC from placenta to embryo. BC was directly injected into the placentas of WT dams at 14.5 dpc. Dams pretreated with the MTP inhibitor lomitapide (+MTPi) are compared with non-treated dams (−MTPi). A, maternal liver MTP activity expressed as percent transfer of triglyceride/mg/h. n = 4 samples (mice)/group. Data are presented as mean ± S.D. (error bars) of triplicate determination. Statistical analysis was by Student's t test. *, p < 0.05. B, ratio of BC content in each placenta and its corresponding embryo expressed as percent. BC levels in placenta and embryos were measured by HPLC. Individual values are shown. n = 1–7 placentas/dam from four WT dams per treatment group.
BC Administration Influences Expression of Transcription Factors That Regulate Mttp Expression
We hypothesized that the increase in Mttp mRNA in BC-administered placenta was due to transcriptional up-regulation and quantified changes in the mRNA levels of known transcription factors that regulate Mttp expression (30). In adult tissues, 19 transcription factors bind to cis-regulatory elements less than 600 base pairs upstream of the transcriptional start site of this gene, acting as transcriptional repressors or activators (Fig. 3A) (30). To gain insights into the potential mechanism of BC-mediated placental Mttp regulation, mRNA levels of these 19 transcription factors in the placentas of WT dams administered either BC or vehicle and sacrificed after 24 h were measured by qPCR. Maternal BC administration significantly increased placental expression of the Mttp activator Hnf4α, and slightly but significantly decreased mRNA levels of the Mttp repressors chicken ovalbumin upstream promoter transcription factor I (Coup-TFI), Coup-TFII, and Srebp1a (Fig. 3B). Of the 15 remaining transcription factors, Hnf1α, Lrh-1, Shp, and Pparγ showed expression levels below the limit of detection of the qPCR analysis. FoxA2, FoxO1, Pgc1α/β, Srebp1c, Srebp2, Hnf1β, Rxrα, Pparα/β, and nuclear receptor co-repressor 1 (Ncor1) did not show significant changes in mRNA levels when BC was administered to the dams (data not shown). These data indicate that increases in Hnf4α activator and reductions in Coup-TFI/II repressors might contribute to increased Mttp transcripts in BC-administered dams.
FIGURE 3.
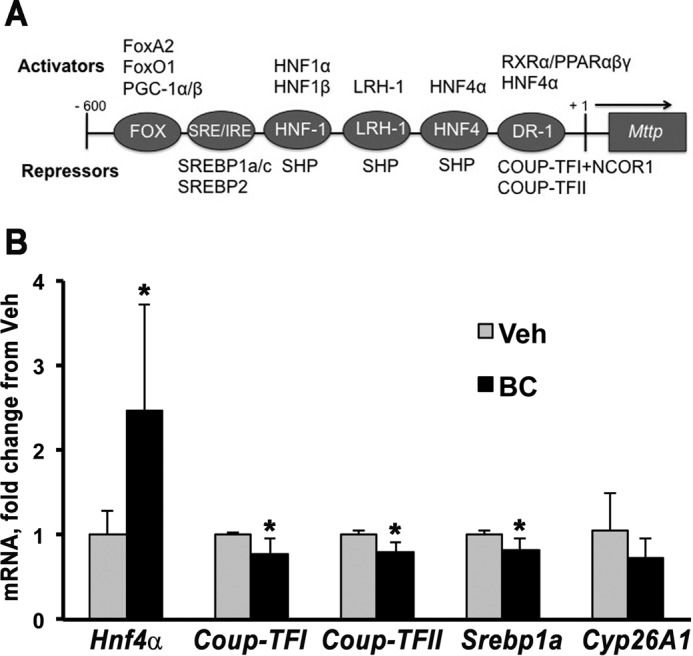
Placental Mttp transcription factors regulated by BC. A, depiction of Mttp promoter cis-elements and factors known to bind this promoter region in maternal tissues (adapted from Ref. 30). B, placental qPCR analysis of four Mttp transcription factors and Cyp26A1 in WT dams administered vehicle (Veh) or BC by i.p. injection at midgestation and sacrificed after 24 h. Data are presented as mean ± S.D. (error bars) of duplicate determinations and are representative of three independent determinations. n = 1–2 placentas/dam from four to six WT dams per group. Statistical analysis was by Student's t test. *, p < 0.05. SRE, sterol-responsive element; IRE, insulin-responsive element.
BC-mediated Transcriptional Response of Placental Mttp and Its Transcription Factors Is Retinoic Acid-independent
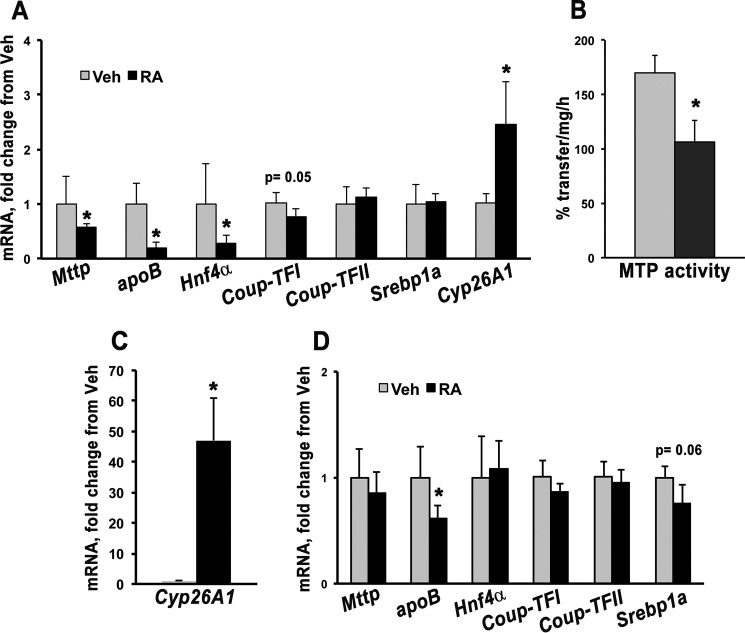
BC is not a transcriptional regulator per se, but it serves as a precursor of transcriptional regulators, such as retinoic acid and β-apocarotenoids, that originate upon its enzymatic cleavage (8). Placental expression of Cyp26A1, a direct target of retinoic acid (35), was not significantly different in WT dams administered vehicle versus BC after 24 h (Fig. 3B). This finding suggests that administration of BC does not lead to significant increase in placenta retinoic acid levels at 24 h. Hence, it is likely that changes in Mttp mRNA and its transcription factors observed 24 h post-BC administration were retinoic acid-independent.
To further prove this hypothesis, retinoic acid (25 mg/kg of body weight (36, 37)) was i.p. injected into WT dams at 13.5 dpc. After 24 h, the dams were sacrificed, and placental mRNA was analyzed by qPCR. As expected, Cyp26A1 expression increased 1.5-fold upon retinoic acid administration, demonstrating that our delivery method was effective (Fig. 4A). However, placental expression of Mttp and its activator Hnf4α were significantly decreased in dams injected with retinoic acid as compared with vehicle (Fig. 4A). Coup-TFII and Srebp1a mRNA levels were similar in the two groups of dams, whereas Coup-TFI showed a slight decrease (p = 0.05) in the presence of retinoic acid (Fig. 4A). apoB mRNA levels were also significantly decreased upon retinoic acid treatment (Fig. 4A). In agreement with the changes in mRNA, MTP activity was significantly attenuated by retinoic acid (Fig. 4B). These data indicate that retinoic acid down-regulates Mttp expression and that it is not the mediator of the BC-induced increase in MTP transcription and activity.
FIGURE 4.
Placental Mttp transcriptional regulation and activity upon maternal retinoic acid administration. A, placental qPCR analysis of apoB, Cyp26A1, Mttp and its transcription factors in WT dams injected with vehicle (Veh) (n = 4, i.e. two placentas/dam from two WT dams) or retinoic acid (RA) (n = 7, i.e. one to two placentas/dam from four WT dams) and sacrificed after 24 h. B, MTP activity (expressed as percent triglyceride transfer/mg/h) in placentas from dams analyzed in A administered vehicle (n = 4, i.e. two placentas/dam from two WT dams) or retinoic acid (n = 5, i.e. one to two placentas/dam from four WT dams). qPCR analysis of Cyp26A1 (C) and apoB, Mttp, and its transcription factors (D) in WT dams administered vehicle (n = 4, i.e. two placentas/dam from two dams) or retinoic acid (n = 6, i.e. two placentas/dam from three dams) and sacrificed after 4 h. Data are presented as mean ± S.D. (error bars) of triplicate determinations. Statistical analysis was by Student's t test. *, p < 0.05.
To rule out that retinoic acid might increase Mttp expression at an earlier time, WT dams were sacrificed 4 h after retinoic acid administration. This time point was chosen based on an experiment in which WT dams were administered BC and sacrificed at different times post-treatment. Placenta BC accumulation peaked at ∼4 h and declined at later times in this experiment (data not shown). As expected, Cyp26A1 expression was dramatically increased (∼50-fold) 4 h post-retinoic acid administration (Fig. 4C). However, no difference in the expression of Mttp and its transcription factors was observed with the exception of a trend toward a decrease for Srebp1a (p = 0.06) (Fig. 4D). apoB mRNA levels were slightly but significantly reduced (Fig. 4D). Thus, retinoic acid suppresses MTP transcription and activity, and another BC metabolite might be involved in its up-regulation after BC administration.
BC-dependent Transcriptional Up-regulation of Placental Mttp Is Mediated by Apo10AL
Upon asymmetric cleavage catalyzed by BCO2, BC generates apo10AL (8, 9). This cleavage product can ultimately yield one molecule of retinoic acid; however, it can also function itself as a transcriptional regulator by antagonizing retinoic acid actions (13–17). We previously showed that 24 h after a single maternal administration of BC at midgestation, Bco2 mRNA levels increased, whereas Bco1 transcription was down-regulated in the placentas of WT dams (25). Hence, we hypothesized that such transcriptional changes could result in an overall increase in β-apocarotenoids that could, in turn, mediate the transcriptional up-regulation of Mttp. To establish the role of apo10AL in this regulatory mechanism, Bco2 knock-out (Bco2−/−) dams were injected with BC or vehicle at 13.5 dpc and sacrificed after 24 h. Placental mRNA levels of Mttp, Hnf4α, Coup-TFI, Coup-TFII, and Cyp26A1 were not different in the pregnant Bco2−/− mice administered vehicle or BC (Fig. 5). Only Srebp1a showed a slight significant decrease (Fig. 5). These results strongly suggest that a product generated by BCO2 is involved in the BC-dependent up-regulation of Mttp.
FIGURE 5.
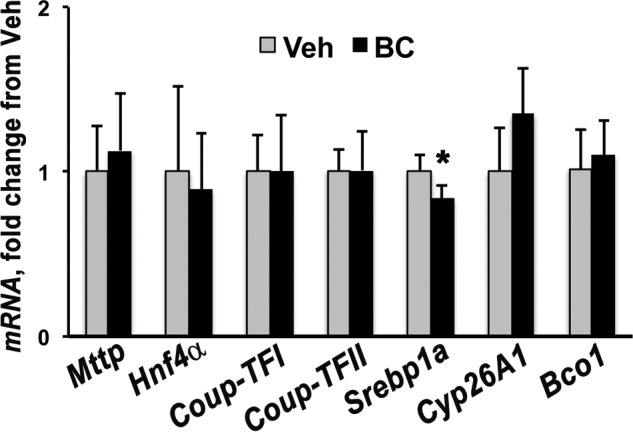
Effect of BC maternal administration in placenta from Bco2−/− dams. Shown is qPCR analysis of placental Bco1, Cyp26A1, Mttp, and genes regulating expression of Mttp in Bco2−/− dams i.p.-injected with vehicle (Veh) or BC (n = 4, i.e. one placenta/dam from four dams/group) and sacrificed after 24 h. Data are presented as mean ± S.D. (error bars) of triplicate determinations. Statistical analysis was by Student's t test. *, p < 0.05.
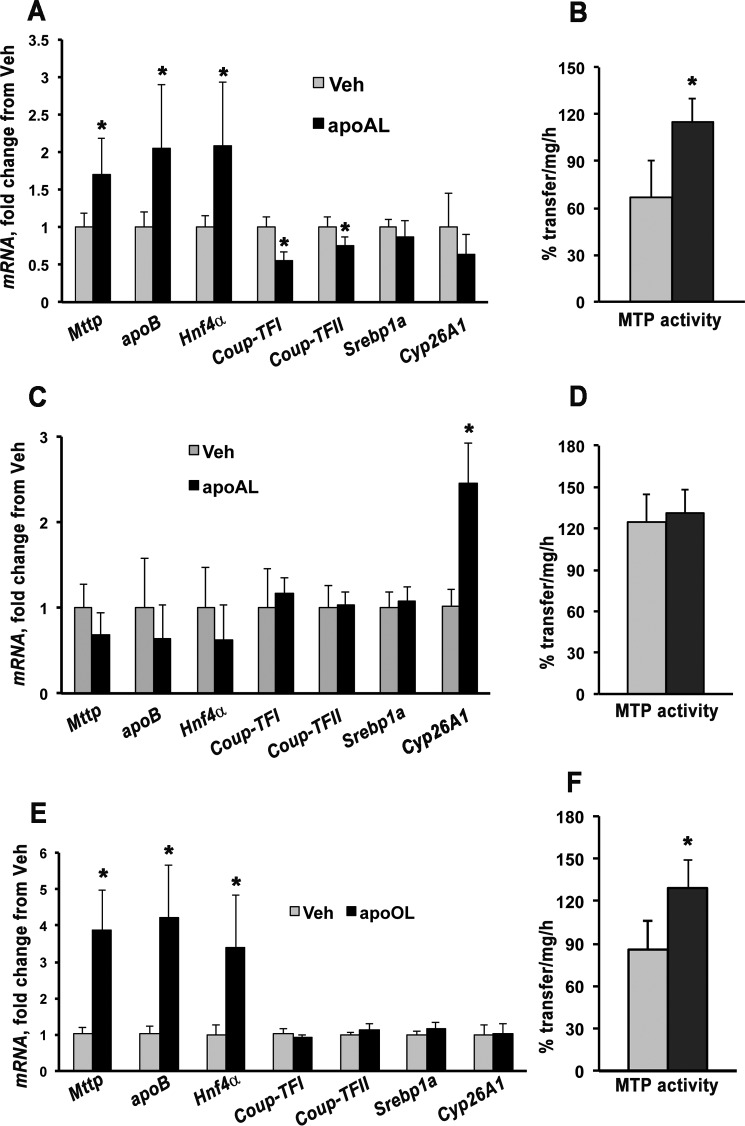
To identify a possible mediator, WT dams were administered apo10AL (35 μg/g of body weight) via i.p. injection at 13.5 dpc and sacrificed after 4 or 24 h. Apo10AL was detectable in placenta and maternal liver at both time points (Table 1), indicating that the β-apocarotenoid was effectively delivered to maternal and developing tissues. The placental transcriptional response to apo10AL was similar to that observed in the case of BC supplementation. Specifically, 24 h after maternal administration of apo10AL, Mttp and its activator Hnf4α as well as apoB mRNA levels were significantly increased, whereas expression of the repressors Coup-TFI and Coup-TFII was significantly decreased (Fig. 6A). No significant changes were observed in Srebp1a (Fig. 6A). Placental mRNA levels of Cyp26A1 were not different between the two groups of dams (Fig. 6A). In agreement with the transcriptional changes, MTP activity was also significantly enhanced in the placentas of females administered apo10AL (Fig. 6B). These results strongly support our hypothesis that apo10AL could be the mediator of the Mttp transcriptional activation induced by BC. Four hours after the administration of apo10AL, the transcription of the above mentioned genes (Fig. 6C) as well as MTP activity (Fig. 6D) did not change. Interestingly, placental transcription of the retinoic acid target gene Cyp26A1 significantly increased 4 h after maternal administration of apo10AL (Fig. 6C), suggesting that, in placenta, β-apocarotenoids may initially be converted into retinoic acid likely via BCO1 (to retinal first followed by oxidation to retinoic acid) (9, 11). Overall, Mttp showed a late transcriptional regulation by this β-apocarotenoid, strongly supporting the possibility that Mttp is not a direct target of apo10AL action.
TABLE 1.
Serum, liver, and placenta concentrations of apo10AL in pregnant WT dams sacrificed 4 or 24 h post-i.p. injection of apo10AL
Analysis was performed by reverse phase HPLC. n, number of dams/group. For placenta, at 4 h, one to two placentas/dam (three dams total), n = 4 placentas analyzed; at 24 h, one to two placentas/dam (two dams total), n = 3 placentas analyzed. Data are presented as mean ± S.D. ND, not-detectable (below detection limits).
| Treatment period | n | Serum | Liver | Placenta |
|---|---|---|---|---|
| h | μg/dl | μg/g | ng/g | |
| 4 | 3 | 341.3 ± 0.1 | 6.2 ± 4.2 | 28.5 ± 22.1 |
| 24 | 3 | N.D. | 1.3 ± 0.1 | 107.1 ± 68.0 |
FIGURE 6.
Placental Mttp transcriptional regulation and activity upon maternal apo10AL or apo10OL administration. A and B, qPCR analysis of placental Cyp26A1, apoB, Mttp, and transcription factors of Mttp (A) and placental MTP activity (B) of WT dams i.p. injected with vehicle (Veh) (n = 5, i.e. one to two placentas/dam from four dams) or apo10AL (n = 6, i.e. two placentas/dam from three dams) and sacrificed after 24 h. C and D, qPCR analysis of placental Cyp26A1, apoB, Mttp, and transcription factors of Mttp (C) and placental MTP activity (D) of WT dams i.p. injected with vehicle (n = 5, one to two placentas/dam from three dams) or apo10AL (n = 6, i.e. two placentas/dam from three dams) and sacrificed after 4 h. E and F, qPCR analysis of placental Cyp26A1, apoB, Mttp, and transcription factors of Mttp (E) and placental MTP activity (F) of WT dams i.p. injected with vehicle (n = 5, i.e. one to two placentas/dam from four dams) or apo10OL (n = 4, i.e. one to two placentas/dam from three dams) and sacrificed after 24 h. Data are expressed as mean ± S.D. (error bars) of triplicate determinations. Statistical analysis was by Student's t test. *, p < 0.05. apoAL, apo10AL; apoOL, apo10OL.
β-Apo-10′-carotenol Also Increases MTP Transcription and Activity
Although it is not known which enzyme(s) could catalyze the conversion of apo10AL to β-apo-10′-carotenol (apo10OL), the latter was detected in tissues of mice fed BC-containing diets (9). Thus, to gain some insights into whether the aldehyde or the alcohol form of the β-apo-10′-carotenoid may be the active compound responsible for regulating placental MTP function, we administered apo10OL (35 μg/g of body weight) via i.p. injection to WT dams at 13.5 dpc and sacrificed them after 24 h. Detection of apo10OL in the maternal tissues (serum, 568.0 μg/dl, n = 2; liver, 20.4 ± 8.6 μg/g, n = 3) confirmed effective delivery of the compound. Apo10OL enhanced placental Mttp, apoB, and Hnf4α transcription even more dramatically than did apo10AL (Fig. 6E). Accordingly, MTP activity was also increased (Fig. 6F), suggesting that both β-apo-10′-carotenoid forms (apo10AL and apo10OL) could mediate the BC-induced regulation of MTP function.
Discussion
BC circulating in the maternal bloodstream is an important source of vitamin A for the developing mammalian embryo (20–23). Nevertheless, the mechanisms that enable and regulate maternal-fetal transfer of the provitamin A carotenoids are still unknown despite the knowledge that BC is transported in the circulation in association with lipoprotein particles (24–26). Our studies show that BC transport from placenta to the fetal circulation is a highly regulated process that involves lipoprotein assembly and secretion. We show that BC availability in the circulation of WT pregnant female mice increases placental transcription and activity of MTP. MTP protein levels were also increased even though they were overall very low. Placental subcellular localization of MTP remains elusive, but it is possible that the protein is expressed in the syncytiotrophoblast layer where other key molecular players of lipoprotein biosynthesis have been localized (38, 39). Such restricted localization in a specific cell population relative to the whole organ could account for the overall low levels of MTP protein detected by Western blotting. An inhibitor of MTP also severely attenuates BC placental-fetal transfer in vivo. Remarkably, this effect of BC is tissue-specific as it was observed only in placenta and not in other organs known to synthesize and secrete apoB-containing lipoproteins. The reason for this tissue specificity is currently unclear. In adult tissues, Mttp expression is dependent on a conserved minimal 204-bp sequence of the promoter that contains several cis-elements (40, 41). Three critical elements are HNF1, HNF4, and direct repeat 1 (DR-1). The first two elements bind HNF1 and HNF4 family members that mainly function as transcriptional activators of Mttp (40, 41). DR-1 could bind to HNF4 and/or other transcription factors (including COUP-TFs), mostly RXR heterodimers (2). DR-1 is critical for suppression of Mttp expression. COUP-TFI binds to the DR-1 element and abolishes the synergistic activation of the Mttp promoter by HNF1 and HNF4α by recruiting the nuclear receptor co-repressor 1 (NCOR1) (40, 41). We surveyed the placental expression of the 19 transcription factors that bind to the ∼600 bp upstream of the start site of Mttp. We found that Mttp activator Hnf4α was up-regulated, whereas its repressors Coup-TFI/II and Srepb1a were down-regulated by BC availability. Thus, changes in the binding of these transcription factors to different elements in the promoter might be involved in the regulation of MTP by BC.
Despite the fact that BC is not a transcriptional regulator, it can serve as a precursor of potent transcriptional regulators, such as retinoic acid and β-apocarotenoids, that originate upon its enzymatic cleavage (8). Given that the expression of Cyp26A1, a direct target of retinoic acid (35), did not change upon maternal BC administration and that maternal administration of retinoic acid did not reproduce the changes in mRNA levels observed in Mttp and its transcription factors when BC was available, we inferred that the transcriptional effect of BC on Mttp is retinoic acid-independent. Moreover, the lack of Mttp transcriptional response in the placenta of Bco2−/− dams supplemented with BC and the similar placental transcriptional response to apo10AL and BC in WT dams strongly suggest that β-apocarotenoids are among the active compounds that regulate placental lipoprotein biosynthesis. These findings agree with the current notion that β-apocarotenoids can function as transcriptional regulators. Eroglu et al. (13) showed that β-apocarotenoids of various chain lengths and forms (aldehyde, acid, and ketone) antagonize all-trans-retinoic acid-induced transactivation of all three retinoic acid receptor isoforms at nanomolar concentrations likely by directly competing with retinoic acid for receptor binding or by modulating the RXR oligomeric state as in the case of β-apo-13-carotenone (14). β-Apocarotenoids also inhibited the all-trans-retinoic acid-induced expression of retinoid-responsive genes in HepG2 cells (13) and antagonized the activation of RXRα by 9-cis-retinoic acid (15). Also, β-apo-14′-carotenal effectively inhibited agonist-induced RXRα, PPARα, and PPARγ activation, decreasing adipogenesis (16). These cell culture-based experiments demonstrated that specific β-apocarotenoids function as nuclear receptor antagonists exerting an anti-vitamin A (retinoic acid) activity (17). Not only apo10AL (42) but also apo10OL (9) was detected in tissues of mice fed BC-containing diets even though the enzymes that could catalyze the conversion of β-apocarotenal, the primary asymmetric cleavage product of BC, into β-apocarotenol have not been identified. Our data show that apo10AL and apo10OL can both up-regulate MTP transcription and activity as well as the expression of its transcription factors. Although the transcriptional response induced by apo10OL seems more robust, the increase in enzymatic activity is comparable with that of the aldehyde form. Thus, we cannot unequivocally establish which of these β-apocarotenoids mediates the changes in gene expression or whether they need to be further metabolized to become transcriptionally active. Nevertheless, our data provide the first in vivo evidence of the transcriptional regulatory activity of these BC derivatives and identify the first in vivo targets of their action, i.e. MTP and its transcription factors.
Based on all our data, we propose that intact BC taken up by the placenta from the maternal bloodstream is transported toward the fetal circulation in association with lipoproteins likely assembled within the placenta syncytiotrophoblast cells (18, 19). Placenta production of apoB-containing lipoproteins is enhanced by BC availability through a mechanism that seems to be mediated by β-apocarotenoids. Specifically, our data suggest that when BC is available placental Bco2 transcription (and likely activity) increases, thus enhancing the generation of apo10AL/OL from BC asymmetric cleavage. This metabolite then, directly or indirectly, increases the transcription of Hnf4α and represses Coup-TFI and Coup-TFII, which in turn increase the transcription of Mttp and consequently its activity, ultimately stimulating biosynthesis and transfer of BC-containing lipoproteins toward the fetus (Fig. 7A). It is relevant that apoB, whose mRNA levels are also elevated by BC and apo10AL/OL supplementation, is a target of HNF4α transcription action (43, 44). Thus, we favor the possibility that placental apoB could also be a target of the BC/β-apocarotenoid regulatory mechanism contributing to modulating placenta lipoprotein assembly and secretion when BC is available.
FIGURE 7.
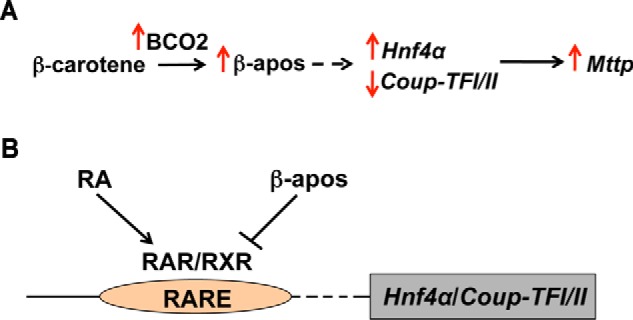
Schematic representation of the proposed model of BC regulation of lipoprotein biosynthesis in placenta. A, in the presence of BC, placental Bco2 transcription (and likely activity) increases, thus enhancing the generation of β-apocarotenoids (β-apos). This metabolite(s) then, directly or indirectly (dashed arrow), increases the transcription of Hnf4α and represses the transcription of Coup-TFI and Coup-TFII, which in turn increase the transcription of Mttp and consequently its activity, ultimately stimulating biosynthesis and transfer of BC-containing lipoproteins toward the fetus. B, β-apocarotenoids antagonize the action of retinoic acid (RA) in regulating, directly or indirectly (dashed arrow), the transcription of Hnf4α and Coup-TFI/II. RAR, retinoic acid receptor.
As discussed previously, β-apocarotenoids function by antagonizing retinoic acid activity (13–17), therefore implying the existence of a retinoic acid-responsive element (RARE) in the promoter region of their target gene(s). Given that Mttp and its transcription factors are affected by the maternal administration of BC or apo10AL only after 24 h, we favor the hypothesis that the effect of the provitamin A carotenoid is not directly on the promoter of Mttp even though the latter contains a putative RARE DR-1 element (40, 41). Therefore, we propose that suppression of Coup-TFs and activation of Hnf4α contribute to increased expression of Mttp mediated by BC (Fig. 7B).
The human Hnf4α gene has been shown to be regulated by retinoic acid (45). Whether the promoter or any other regulatory elements of the mouse Hnf4α contain a functional RARE cis-element(s) responsible for its repression has not been demonstrated. However, we showed that, as in the case of the human gene (45), retinoic acid down-regulates the expression of Hnf4α in mouse placenta. Our data do not unequivocally demonstrate whether retinoic acid regulates Hnf4α directly or indirectly. Unlike Cyp26A1 (35), a known retinoic acid direct target gene, we showed that Hnf4α is repressed by retinoic acid in vivo only 24 h after retinoid administration, thus suggesting an indirect regulation. However, genes can also respond late to a direct regulation by retinoic acid (via a RARE promoter element) (46) as the relative potency and specificity of the RAREs depend on both the configuration and nucleotide sequence of the repeats (47). This question will need to be addressed in future studies. Whether directly on the promoter of Hnf4α or indirectly on the promoter of another transcription factor upstream of Hnf4α, we hypothesize that apo10AL/OL antagonizes retinoic acid for the binding to retinoic acid receptors and/or RXRs on a RARE cis-element, ultimately turning on Hnf4α transcription. A similar reasoning could also be applied to Coup-TFI and Coup-TFII. Because the expression of these orphan receptors is regulated by retinoic acid treatment (48), it is expected (and confirmed by our data) that, in placenta, apo10AL/OL reduces the expression of these Mttp repressors and thus increases Mttp transcription.
Members of the Srebp1 family of transcription factors have also been shown to be induced by retinoic acid in few experimental systems (49, 50). Whereas the transcriptional down-regulation of the Mttp repressor Srebp1a in placenta of WT administered BC may be in agreement with our model, the lack of Srebp1a changes upon maternal supplementation with apo10AL/OL and its persistent down-regulation in the placenta of Bco2−/− dams treated with BC are inconsistent, questioning the role of SREBP1a in the regulatory mechanism proposed here.
The critical role of retinoic acid in embryogenesis is well established (1). Retinoic acid is preferentially synthesized in the developing embryo from retinoid precursors rather than transferred to the embryo as such due to its spatially and temporally restricted action (6). Indeed, maternal retinoic acid administration can rescue several but not all the developmental defects in mouse mutants for retinoic acid biosynthetic enzymes, such as RALDH2, RALDH3, and RDH10 (36). Also, retinoic acid supplementation never resulted in normal live embryos at term in these strains (37), hence the notions that the placenta favors the transfer of retinol (retinoic acid precursor) versus retinoic acid (51) and that BC could be used as a precursor of retinol in placenta (52). Contrary to this hypothesis, our data suggest that the placenta is equipped with highly regulated molecular machineries that favor the transfer of intact BC (retinoic acid precursor) versus retinoic acid under a normal maternal vitamin A status. We speculate that this mechanism might provide an advantage in acquiring intact BC, a critical “safe” source of retinoid for the developing embryo, from the maternal circulation.
We cannot exclude that the increase in MTP activity caused by the maternal BC or apo10AL/OL administration could also facilitate retinyl ester transfer to the developing fetus via placental lipoproteins. Previous data from our laboratory failed to detect changes in embryonic retinol and retinyl ester levels upon a single maternal supplementation with BC (25). However, in our experimental system, the baseline concentration of retinoids in the embryo is much higher than β-carotene (which is indeed undetectable prior to the supplementation). Thus, it is possible that a potentially enhanced (but quantitatively small) transfer of retinoids would not be measurable over a relatively high baseline level of retinoids. Further experiments will need to be conducted under different experimental conditions to address this issue. In summary, we showed that BC regulates placental lipoprotein biosynthesis via apo10AL generated by BCO2 and that the targets of this BC-mediated transcriptional regulation are Mttp and its transcription factors Hnf4α and Coup-TFI/II.
Experimental Procedures
Mice and Nutritional Manipulation
WT and Bco2−/− female mice (a generous gift from Dr. J. Von Lintig, Case Western Reserve (12)) on a mixed genetic background (C57BL/6 × Sv129) were maintained on a regular chow rodent diet (Prolab Isopro RMH3000 5p75; energy from protein, fat, and carbohydrates, 26, 14, and 60%, respectively; 18 IU of vitamin A (as retinyl palmitate)/g of diet; BC, from trace to 1.2 ppm/g of diet) manufactured by LabDiet (W. F. Fisher and Son, Inc.). At 3 months of age, females were mated with males of the same genotype. At the time of vaginal plug detection, designated as 0.5 dpc, females were placed on a purified diet containing 14 IU of vitamin A/g of diet provided as retinyl palmitate. The nutritional composition of the purified diet was similar to that of the regular chow diet described above with the exception of the slightly lower vitamin A concentration (14 IU of vitamin A (as retinyl palmitate)/g of diet). Neither BC nor its metabolites were present in the purified diet (Research Diet).
All mice were maintained on a 12:12-h light/dark cycle with the period of darkness between the hours of 7:00 p.m. and 7:00 a.m. Dams were continually fed until the end of the experiment with the purified diet and water available ad libitum. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (53) and were reviewed and approved by the Rutgers University Animal Care and Use Committee.
BC and β-Apocarotenoid Administration
BC was administered to WT and Bco2−/− dams at midgestation (13.5 dpc) when the embryo is capable of regulating its own BC and retinoid metabolism (21). The BC (Type II, Sigma-Aldrich) emulsion was prepared in a vehicle mixture of ethanol, Cremophor (Sigma), and PBS (1:11:18 ratio) under yellow light with vortexing. Spectrophotometry at 450 nm was used to determine the concentration of the solution (∼3.5 g/liter). The dams were administered a single dose of BC by i.p. injection at 13.5 dpc of ∼35 μg of BC/g of body weight in a volume of 250 μl (n = 6 WT dams; n = 4 Bco2−/− dams). Vehicle-assigned dams were injected with 250 μl of the vehicle mixture described above (n = 4 WT dams; n = 4 Bco2−/− dams). All dams were i.p. injected between the hours of 9:00 and 11:00 a.m. and sacrificed by CO2 asphyxiation followed by exsanguination 24 h postinjection at 14.5 dpc. The majority of the WT mouse tissues analyzed in the experiment described above were obtained from WT pregnant female mice i.p. injected with vehicle or BC analyzed in a recently published study (25).
The apo10AL emulsion was prepared in the same manner as BC; the powdered compound was mixed with ethanol, PBS, and Cremophor (see above), and the resulting solution was measured at 438 nm to determine the concentration. WT and Bco2−/− dams were administered vehicle or apo10AL (∼40 μg of apo10AL/g of body weight in 250 μl of vehicle) between the hours of 9:00 and 11:00 a.m. at 13.5 dpc. Tissues were collected 4 (n = 3 WT dams/treatment treated with apo10AL or vehicle) or 24 h (n = 3 WT dams treated with apo10AL; n = 4 WT dams treated with vehicle) post-i.p. injection for the WT dams and 24 h post-i.p. injection for the Bco2−/− dams (n = 3 Bco2−/− dams treated with apo10AL; n = 4 Bco2−/− dams treated with vehicle). The WT vehicle-injected dams sacrificed after 24 h described above also served as the control group for the WT dams administered apo10AL and sacrificed after 24 h.
The apo10OL emulsion was prepared similarly to the apo10AL described above. Approximately 40 μg of apo10OL/g of body weight in a 250-μl volume was administered to 3-month-old WT dams (n = 3) by i.p. injection at 13.5 dpc and sacrificed after 24 h. Dams were injected and sacrificed according to the scheme above. Tissues were compared against the aforementioned WT vehicle-injected dams sacrificed after 24 h. All tissues collected at sacrifice were stored at −80 °C until further analysis.
Retinoic Acid Administration
Retinoic acid emulsion was prepared by diluting retinoic acid (Sigma) in a mixture of DMSO and Cremophor (1:10 ratio) at a final concentration of 5 μg/μl. Each WT dam was administered 25 μg of retinoic acid/g of body weight (36, 37) or an equal volume of vehicle solution (no retinoic acid) by i.p. injection at 13.5 dpc between the hours of 9:00 and 11:00 a.m. Dams were sacrificed at 4 (n = 3 dams treated with retinoic acid; n = 2 dams treated with vehicle) or 24 h (n = 4 dams treated with retinoic acid; n = 2 dams treated with vehicle) post-i.p. injection.
Direct Injection of BC into the Placenta
Pregnant WT dams (maintained on the regular chow diet throughout life as described above; n = 8) were mated with WT males and placed on the purified diet at 0.5 dpc. Four of these dams were orally administered (gavage) 27.7 μg of lomitapide (40 μl of 1 mm in DMSO (33)), an MTP inhibitor, once daily at 12.5, 13.5, and 14.5 dpc between the hours of 9:00 and 11:00 a.m. The last gavage was performed 2 h prior to the surgery (see below). The other four dams were not treated with lomitapide but were only administered BC (see below). Following a procedure described by Bonnin et al. (34), on the morning of 14.5 dpc, dams were anesthetized using isoflurane, and the peritoneum was opened to expose the uterine horns. Approximately 32 μg of BC (5 μl of 6.5 μg of BC/μl of stock solution prepared as described above) was injected into each placenta (n = 2–5 placentas per dam not administered lomitapide (control group); n = 1–7 placentas per dam administered lomitapide) through the still intact uterine wall. After 30 min, each dam was sacrificed by exsanguination while under anesthesia, and maternal and developing tissues were collected for further analysis. BC concentrations in each injected placenta and corresponding embryo were measured by reverse phase HPLC.
Synthesis of β-Apocarotenoids
The apo10AL was synthesized from ethyl β-apo-10′-carotenoate as described previously (13). The apo10OL was prepared from ethyl β-apo-10′-carotenoate as follows. Diisobutylaluminum hydride (3.03 ml of a 1 m solution in CH2Cl2) was added to a solution of ethyl β-apo-10′-carotenoate (0.43 g; 1.02 mmol) in 30 ml of dry CH2Cl2. After stirring for 30 min under argon, a solution of 2.42 g of potassium sodium tartrate (Rochelle's salt) in 3.63 ml of water was added, and the mixture was stirred for 1.5 h. The mixture was extracted with 60 ml of diethyl ether, and this organic layer was washed with 3 × 60 ml of saturated NaCl solution. The organic layer was then dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was dissolved in a minimum amount of petroleum ether (36–56 °C; 5–7 ml) and stored at −30 °C to precipitate apo10OL as a red-orange powder (146 mg; 39% yield) with the following properties: UV (CH2Cl2) λmax 402 (ϵ 67,925), 424 nm; HPLC: tR = 29.5 min (Polaris C18 4.6 × 250-mm column with 90% methanol, water at 1 ml/min); 1H NMR (400 MHz; CDCl3) δ 1.02 (6H, s), 1.48 (2H, m), 1.62 (2H, m), 1.75 (3H, s), 1.93 (3H, s), 1.99 (3H, s) 2.04 (2H, br t), 2.19 (3H, s), 4.36 (2H, br t), 5.82–5.92 (1H, m), 6–6.38 (5H, m), 6.41–6.49 (2H, m), 6.15–6.22 (3H, m); 13C NMR (100 MHz; CDCl3) δ 12.76, 12.82, 12.86, 19.28, 21.76, 28.98, 30.9, 33.12, 34.28, 39.67, 63.89, 125.04, 125.20, 126.74, 127.41, 127.95, 129.25, 129.38, 129.44, 130.38, 130.75, 130.88, 132.03, 132.35, 133.20, 134.68, 136.01; high resolution MS (electrospray ionization) m/z calculated for C27H36O 378.2926, observed 378.2918.
HPLC Analysis of Carotenoids
Measurements of BC, apo10AL, and apo10OL in maternal and developing tissues were performed using a reverse phase HPLC analysis protocol described previously (20, 25). Briefly, BC, apo10AL, and apo10OL were separated on a 4.6 × 250-mm Denali C18 column preceded by a C18 guard column (both from Grace, Deerfield, IL) using acetonitrile:methanol:methylene chloride (70:15:15, v/v) as the mobile phase at a flow rate of 1.8 ml/min. A Dionex UltiMate 3000 HPLC system and a computerized data analysis work station with Chromeleon software were used (Thermo Scientific, Waltham, MA). BC, apo10AL, and apo10OL were identified by comparing retention times and UV absorption spectra of experimental compounds with those of authentic standards. Spectra were read at 450 and 438 nm in the case of BC and apo10AL/OL, respectively. Carotenoid concentrations were determined by comparing respective peak integrated areas against those of known amounts of purified standards. Any losses during extraction were accounted for by adjusting for the recovery of echinenone, added as an internal standard immediately prior to the extraction of the tissue homogenate or serum. Detection limits for both BC and β-apocarotenoids were as follows: serum, ∼ 1 μg/dl; tissues, ∼10 ng/g.
RNA Isolation, cDNA Synthesis, and qPCR
Total RNA isolation from maternal and developing tissues, cDNA synthesis, and qPCR were performed as described previously (22, 23, 25, 28). Data were analyzed using the ΔΔCt method, and measurements were expressed as -fold change of the calibrator, which was set as the vehicle group in each comparison. Primer sequences and amplicon sizes are as follows except for actin, Bco1, and Cyp26A1, which are reported elsewhere (22, 23, 25, 28): apoB: forward, 5′-CGTGGGCTCCAGCATTCTA-3′, reverse; 5′-TCACCAGTCATTTCTGCCTTTG-3′; amplicon size, 72 bp; Coup-TFI: forward, 5′-TGCTATTCACGTCAGATGCTTG-3′; reverse, 5′-GGATGGACATGTAAGGCCAG-3′; amplicon size, 285 bp; Coup-TFII: forward, 5′-GCCATAGTCCTGTTCACCTCA-3′; reverse, 5′-AAGCACACTGGGACTTTTCCT-3′; amplicon size; 85 bp; Hnf4α: forward, 5′-CGTGGGTAGGGGAGAATGC-3′; reverse, 5′-GTGGTTCTTCCTCACGCTCC-3′, amplicon size, 291 bp; Mttp: forward, 5′-AGCTTTGTCACCGCTGTGC-3′; reverse; 5′-TCCTGCTATGGTTTGTTGGAA-3′, amplicon size, 72 bp; Srebp1a: forward, 5′-GGCCGAGATGTGCGAACT-3′, reverse; 5′-TTGTTGATGAGCTGGAGCAT-3′, amplicon size, 69 bp.
MTP Activity Assay
This procedure has been published previously (54, 55). In brief, small pieces (0.1 g) of liver and placenta were homogenized in low salt buffer (1 mm Tris-HCl, pH 7.6, 1 mm EGTA, and 1 mm MgCl2) and centrifuged, and supernatants were used for protein determination (by Bradford assay), and triglyceride transfer activity of MTP was assayed using a commercial kit (Chylos, Inc.).
Western Blotting Analysis
Placental content of apoB and MTP was analyzed by Western blotting. For apoB, 80 μg of placental protein was run on a 4–12% gradient SDS-polyacrylamide gel. A rabbit anti-mouse polyclonal apoB antibody (AbCam, Cambridge, UK) was used for immunodetection (1:3000 dilution). For MTP, 80 μg of placental protein and 1 μg of liver or yolk sac extract were run on a 4–12% gradient SDS-polyacrylamide gel. A purified mouse MTP antibody (BD Biosciences) was used for immunodetection (1:3000 dilution). Goat anti-rabbit or goat anti-mouse secondary antibodies were used in a 1:5000 dilution. Signals were normalized to albumin, detected by the apoB antibody, and to protein loading (assessed by staining membranes using Coomassie Blue). The signals were imaged using a Bio-Rad Chemidoc XRS Molecular Imager system. Expected molecular masses were as follows: apoB100, 510 kDa; apoB48, 250 kDa; MTP, 97 kDa; albumin, 65 kDa. The quantification of the membranes was completed by densitometry analysis with Quantity One software (Bio-Rad).
Statistical Analysis
Normality of the data was established using the Shapiro-Wilk test. When data were normally distributed, statistical analysis was performed by Students' t test. For data that were not normally distributed, comparisons among groups were made using the Kruskal-Wallis test followed by Mann-Whitney test. Analyses were performed with SPSS statistical software (IBM SPSS Statistics, version 19). A p value ≤0.05 was considered significant.
Author Contributions
B. K. C., Y.-K. K., and J. I. designed and performed experiments, analyzed data, interpreted results, discussed implications, and critically evaluated the manuscript. M. V. Z. performed protein analysis of apoB. L. W. contributed the initial observation of the β-carotene-dependent transcriptional changes in placental MTP. S. N., R. W. C., and E. H. H. synthesized β-apocarotenoid compounds. R. W. C. and E. H. H. also critically evaluated the manuscript. L. Q. and M. M. H. conceived the study, supervised the project, interpreted results, discussed implications, and wrote the manuscript. B. K. C. wrote the first draft of the manuscript.
This work was supported in part by National Institutes of Health Grants R01HD057493, R01HD057493-02S1, R01HD057493-05S1, and R01HD833331 (to L. Q.); R01HL95924 and R01DK81879 (to M. M. H.); and R01HL049879 (to E. H. H. and R. W. C.) and by Veterans Affairs Merit Award BX001728 (to M. M. H.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- RXR
- retinoid X receptor
- apoB
- apolipoprotein B
- apo10AL
- β-apo-10′-carotenal
- apo10OL
- β-apo-10′-carotenol
- BC
- β-carotene
- BCO1
- β-carotene 15,15′-oxygenase
- BCO2
- β-carotene 9′,10′-oxygenase
- COUP-TFI/II
- chicken ovalbumin upstream promoter transcription factor I/II
- Cyp26A1
- cytochrome p450 family 26 subfamily A polypeptide 1
- DR-1
- direct repeat 1
- Fox
- forkhead box protein
- Lrh-1
- liver receptor homolog 1
- MTP
- microsomal triglyceride transfer protein (Mttp, MTP gene)
- NCOR1
- nuclear receptor co-repressor 1
- PPAR
- peroxisome proliferator-activated receptor
- Pgc1α/β
- peroxisome proliferator-activated receptor γ coactivator 1α/β
- qPCR
- quantitative real time PCR
- Shp
- small heterodimer partner
- SREBP
- sterol regulatory element-binding protein
- HNF
- hepatic nuclear factor
- dpc
- day(s) postcoitum
- RARE
- retinoic acid-responsive element.
References
- 1. Clagett-Dame M., and Knutson D. (2011) Vitamin A in reproduction and development. Nutrients 3, 385–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al Tanoury Z., Piskunov A., and Rochette-Egly C. (2013) Vitamin A and retinoid signaling: genomic and nongenomic effects. J. Lipid Res. 54, 1761–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chambon P. (1996) A decade of molecular biology of retinoic acid receptors. FASEB J. 10, 940–954 [PubMed] [Google Scholar]
- 4. Niederreither K., and Dollé P. (2008) Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 9, 541–553 [DOI] [PubMed] [Google Scholar]
- 5. Balmer J. E., and Blomhoff R. (2002) Gene expression regulation by retinoic acid. J. Lipid Res. 43, 1773–1808 [DOI] [PubMed] [Google Scholar]
- 6. Dollé P., and Niederreither K. (2015) The Retinoids: Biology, Biochemistry, and Disease, pp, 309–401, Wiley-Blackwell, Hoboken, NJ [Google Scholar]
- 7. von Lintig J. (2010) Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 30, 35–56 [DOI] [PubMed] [Google Scholar]
- 8. Lobo G. P., Amengual J., Palczewski G., Babino D., and von Lintig J. (2012) Mammalian carotenoid-oxygenases: key players for carotenoid function and homeostasis. Biochim. Biophys. Acta 1821, 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amengual J., Widjaja-Adhi M. A., Rodriguez-Santiago S., Hessel S., Golczak M., Palczewski K., and von Lintig J. (2013) Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J. Biol. Chem. 288, 34081–34096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eroglu A., and Harrison E. H. (2013) Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J. Lipid Res. 54, 1719–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. dela Seña C., Riedl K. M., Narayanasamy S., Curley R. W. Jr., Schwartz S. J., and Harrison E. H. (2014) The human enzyme that converts dietary provitamin A carotenoids to vitamin A is a dioxygenase. J. Biol. Chem. 289, 13661–13666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amengual J., Lobo G. P., Golczak M., Li H. N., Klimova T., Hoppel C. L., Wyss A., Palczewski K., and von Lintig J. (2011) A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 25, 948–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eroglu A., Hruszkewycz D. P., dela Sena C., Narayanasamy S., Riedl K. M., Kopec R. E., Schwartz S. J., Curley R. W. Jr., and Harrison E. H. (2012) Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 287, 15886–15895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun J., Narayanasamy S., Curley R. W. Jr., and Harrison E. H. (2014) β-Apo-13-carotenone regulates retinoid X receptor transcriptional activity through tetramerization of the receptor. J. Biol. Chem. 289, 33118–33124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eroglu A., Hruszkewycz D. P., Curley R. W. Jr., and Harrison E. H. (2010) The eccentric cleavage product of β-carotene, β-apo-13-carotenone, functions as an antagonist of RXRα. Arch. Biochem. Biophys. 504, 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ziouzenkova O., Orasanu G., Sukhova G., Lau E., Berger J. P., Tang G., Krinsky N. I., Dolnikowski G. G., and Plutzky J. (2007) Asymmetric cleavage of β-carotene yields a transcriptional repressor of RXR and PPAR responses. Mol. Endocrinol. 21, 77–88 [DOI] [PubMed] [Google Scholar]
- 17. Wang C. X., Jiang H., Yuen J. J., Lee S. A., Narayanasamy S., Curley R. W. Jr., Harrison E. H., and Blaner W. S. (2015) Actions of β-apo-carotenoids in differentiating cells: differential effects in P19 cells and 3T3-L1 adipocytes. Arch. Biochem. Biophys. 572, 2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nadeau V., and Charron J. (2014) Essential role of the ERK/MAPK pathway in blood-placental barrier formation. Development 141, 2825–2837 [DOI] [PubMed] [Google Scholar]
- 19. Simmons D. G., Natale D. R., Begay V., Hughes M., Leutz A., and Cross J. C. (2008) Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development 135, 2083–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim Y. K., Wassef L., Chung S., Jiang H., Wyss A., Blaner W. S., and Quadro L. (2011) β-Carotene and its cleavage enzyme β-carotene-15,15′-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J. 25, 1641–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spiegler E., Kim Y. K., Wassef L., Shete V., and Quadro L. (2012) Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim. Biophys. Acta 1821, 88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wassef L., Spiegler E., and Quadro L. (2013) Embryonic phenotype, β-carotene and retinoid metabolism upon maternal supplementation of β-carotene in a mouse model of severe vitamin A deficiency. Arch. Biochem. Biophys. 539, 223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wassef L., Shete V., Hong A., Spiegler E., and Quadro L. (2012) β-Carotene supplementation decreases placental transcription of LDL receptor-related protein 1 in wild-type mice and stimulates placental β-carotene uptake in a marginally vitamin A deficient mice. J. Nutr. 142, 1456–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parker R. S. (1996) Absorption, metabolism, and transport of carotenoids. FASEB J. 10, 542–551 [PubMed] [Google Scholar]
- 25. Wassef L., Shete V., Costabile B., Rodas R., and Quadro L. (2015) High preformed vitamin A intake during pregnancy prevents embryonic accumulation of intact β-carotene from the maternal circulation in mice. J. Nutr. 145, 1408–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Erdman J. W. Jr., Bierer T. L., and Gugger E. T. (1993) Absorption and transport of carotenoids. Ann. N.Y. Acad. Sci. 691, 76–85 [DOI] [PubMed] [Google Scholar]
- 27. Madsen E. M., Lindegaard M. L., Andersen C. B., Damm P., and Nielsen L. B. (2004) Human placenta secretes apolipoprotein B-100-containing lipoproteins. J. Biol. Chem. 279, 55271–55276 [DOI] [PubMed] [Google Scholar]
- 28. Wassef L., and Quadro L. (2011) Uptake of dietary retinoids at the maternal-fetal barrier: in vivo evidence for the role of lipoprotein lipase and alternative pathways. J. Biol. Chem. 286, 32198–32207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ginsberg H. N. (1998) Lipoprotein physiology. Endocrinol. Metab. Clin. North Am. 27, 503–519 [DOI] [PubMed] [Google Scholar]
- 30. Hussain M. M., Nijstad N., and Franceschini L. (2011) Regulation of microsomal triglyceride transfer protein. Clin. Lipidol. 6, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fisher E. A. (2012) The degradation of apolipoprotein B100: multiple opportunities to regulate VLDL triglyceride production by different proteolytic pathways. Biochim. Biophys. Acta 1821, 778–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamper M., Manns C. C., Plieschnig J. A., Schneider W. J., Ivessa N. E., and Hermann M. (2015) Estrogen enhances secretion of apolipoprotein B-100 containing lipoproteins by BeWo cells. Biochimie 112, 121–128 [DOI] [PubMed] [Google Scholar]
- 33. Bakillah A., Nayak N., Saxena U., Medford R. M., and Hussain M. M. (2000) Decreased secretion of apoB follows inhibition of apoB-MTP binding by a novel antagonist. Biochemistry 39, 4892–4899 [DOI] [PubMed] [Google Scholar]
- 34. Bonnin A., Goeden N., Chen K., Wilson M. L., King J., Shih J. C., Blakely R. D., Deneris E. S., and Levitt P. (2011) A transient placental source of serotonin for the fetal forebrain. Nature 472, 347–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Topletz A. R., Tripathy S., Foti R. S., Shimshoni J. A., Nelson W. L., and Isoherranen N. (2015) Induction of CYP26A1 by metabolites of retinoic acid: evidence that CYP26A1 is an important enzyme in the elimination of active retinoids. Mol. Pharmacol. 87, 430–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rhinn M., Schuhbaur B., Niederreither K., and Dollé P. (2011) Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc. Natl. Acad. Sci. U.S.A. 108, 16687–16692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rhinn M., and Dollé P. (2012) Retinoic acid signaling during development. Development 139, 843–858 [DOI] [PubMed] [Google Scholar]
- 38. Herrera E. (2002) Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 19, 43–55 [DOI] [PubMed] [Google Scholar]
- 39. Wittmaack F. M., Gåfvels M. E., Bronner M., Matsuo H., McCrae K. R., Tomaszewski J. E., Robinson S. L., Strickland D. K., and Strauss J. F. 3rd. (1995) Localization and regulation of the human very low density lipoprotein/apolipoprotein-E receptor: trophoblast expression predicts a role for the receptor in placental lipid transport. Endocrinology 136, 340–348 [DOI] [PubMed] [Google Scholar]
- 40. Dai K., Khatun I., and Hussain M. M. (2010) NR2F1 and IRE1β suppress microsomal triglyceride transfer protein expression and lipoprotein assembly in undifferentiated intestinal epithelial cells. Arterioscler. Thromb. Vasc. Biol. 30, 568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dai K., and Hussain M. M. (2012) NR2F1 disrupts synergistic activation of the MTTP gene transcription by HNF-4α and HNF-1α. J. Lipid Res. 53, 901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee S. A., Jiang H., Trent C. M., Yuen J. J., Narayanasamy S., Curley R. W. Jr., Harrison E. H., Goldberg I. J., Maurer M. S., and Blaner W. S. (2014) Cardiac dysfunction in β-carotene-15,15′-dioxygenase-deficient mice is associated with altered retinoid and lipid metabolism. Am. J. Physiol. Heart Circ. Physiol. 307, H1675–H1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ladias J. A., Hadzopoulou-Cladaras M., Kardassis D., Cardot P., Cheng J., Zannis V., and Cladaras C. (1992) Transcriptional regulation of human apolipoprotein genes apoB, apoCIII, and apoAII by members of the steroid hormone receptor superfamily HNF-4, ARP-1, EAR-2, and EAR-3. J. Biol. Chem. 267, 15849–15860 [PubMed] [Google Scholar]
- 44. Hayhurst G. P., Lee Y. H., Lambert G., Ward J. M., and Gonzalez F. J. (2001) Hepatocyte nuclear factor 4α (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21, 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qian A., Cai Y., Magee T. R., and Wan Y. J. (2000) Identification of retinoic acid-responsive elements on the HNF1α and HNF4α genes. Biochem. Biophys. Res. Commun. 276, 837–842 [DOI] [PubMed] [Google Scholar]
- 46. Panariello L., Quadro L., Trematerra S., and Colantuoni V. (1996) Identification of a novel retinoic acid response element in the promoter region of the retinol-binding protein gene. J. Biol. Chem. 271, 25524–25532 [DOI] [PubMed] [Google Scholar]
- 47. Giguère V. (1994) Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr. Rev. 15, 61–79 [DOI] [PubMed] [Google Scholar]
- 48. Pickens B. S., Teets B. W., Soprano K. J., and Soprano D. R. (2013) Role of COUP-TFI during retinoic acid-induced differentiation of P19 cells to endodermal cells. J. Cell. Physiol. 228, 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roder K., and Schweizer M. (2007) Retinoic acid-mediated transcription and maturation of SREBP-1c regulates fatty acid synthase via cis-elements responsible for nutritional regulation. Biochem. Soc. Trans. 35, 1211–1214 [DOI] [PubMed] [Google Scholar]
- 50. Li R., Chen W., Li Y., Zhang Y., and Chen G. (2011) Retinoids synergized with insulin to induce Srebp-1c expression and activated its promoter via the two liver X receptor binding sites that mediate insulin action. Biochem. Biophys. Res. Commun. 406, 268–272 [DOI] [PubMed] [Google Scholar]
- 51. Marceau G., Gallot D., Lemery D., and Sapin V. (2007) Metabolism of retinol during mammalian placental and embryonic development. Vitam. Horm. 75, 97–115 [DOI] [PubMed] [Google Scholar]
- 52. Dimenstein R., Trugo N. M., Donangelo C. M., Trugo L. C., and Anastácio A. S. (1996) Effect of subadequate maternal vitamin A status on placental transfer of retinol and β-carotene to the human fetus. Biol. Neonate 69, 230–234 [DOI] [PubMed] [Google Scholar]
- 53. National Research Council (1996) Guide for the Care and Use of Laboratory Animals, 7th Ed., National Academic Press, Washington, D. C. [Google Scholar]
- 54. Athar H., Iqbal J., Jiang X. C., and Hussain M. M. (2004) A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 45, 764–772 [DOI] [PubMed] [Google Scholar]
- 55. Rava P., Athar H., Johnson C., and Hussain M. M. (2005) Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J. Lipid Res. 46, 1779–1785 [DOI] [PubMed] [Google Scholar]