Abstract
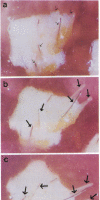
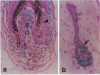
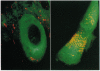
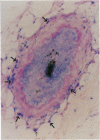
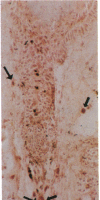
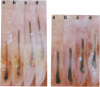
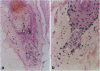
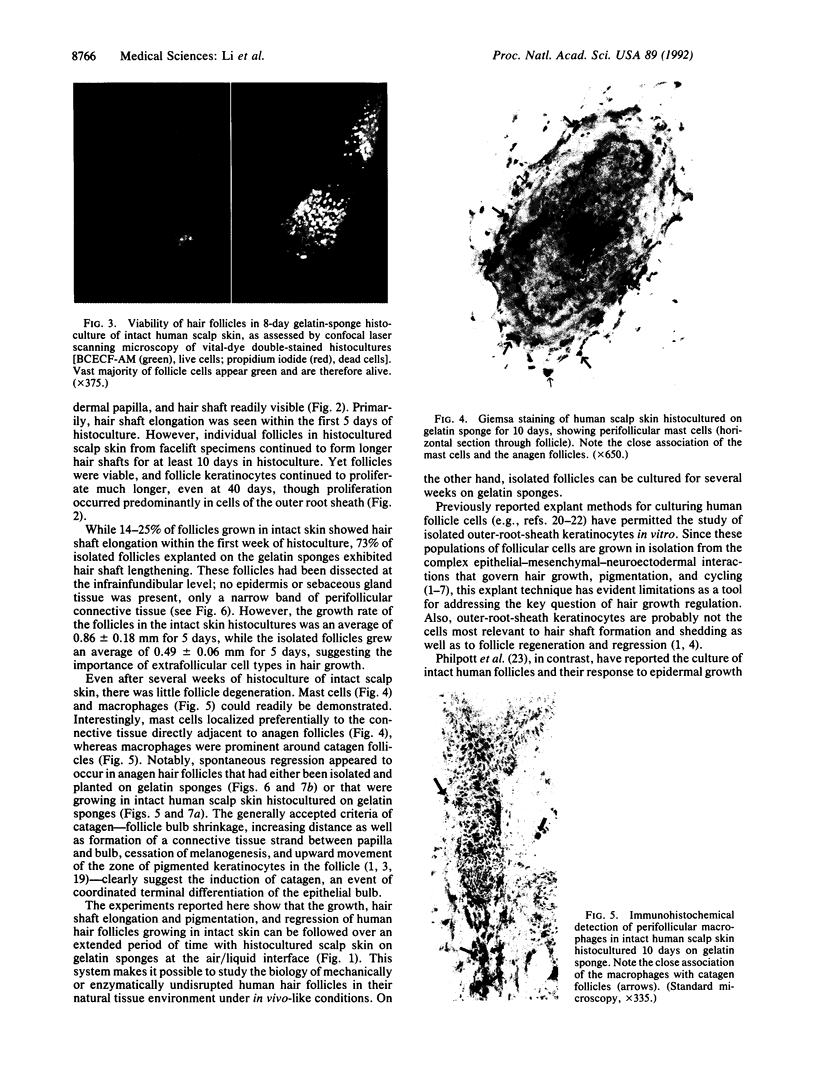
In order to better understand the molecular mechanisms of human hair growth control and to test hair growth-modulatory drugs, appropriate in vitro models are required. Here, we report the long-term growth, shaft elongation, and spontaneous regression of human hair follicles in histoculture of intact scalp skin. Human scalp skin with abundant hair follicles in various stages of the hair growth cycle was grown for up to 40 days in a gelatin sponge-supported histoculture system at the air/liquid interface. Isolated follicles placed in the gelatin-sponge matrix also supported hair shaft elongation, with the hair follicle cells remaining proliferative and viable for very long periods. Hair shaft elongation occurred mainly during the first 10 days of histoculture of both intact skin and isolated follicles. However, hair follicles were viable and follicle keratinocytes continued to incorporate [3H]thymidine for up to several weeks after shaft elongation had ceased as shown by fluorescent-dye double staining, measured by confocal laser scanning microscopy, and by histological autoradiography of [3H]thymidine incorporation, respectively. Hair follicles could continue their cycle in histoculture; for example, apparent spontaneous catagen induction was observed both histologically and by the actual regression of the hair follicle. In addition, vellus follicles were shown to be viable at day 40 after initiation of culture. In the histocultured human scalp we demonstrated the association of mast cells with anagen follicles and macrophages with catagen follicles, suggesting a role of these cells in the hair cycle. This histoculture technique should serve as a powerful tool for future hair research in the human system as well as a screening assay for compounds that can perturb the hair cycle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billingham R. E., Silvers W. K. A biologist's reflections on dermatology. J Invest Dermatol. 1971 Oct;57(4):227–240. doi: 10.1111/1523-1747.ep12261543. [DOI] [PubMed] [Google Scholar]
- CHASE H. B. Growth of the hair. Physiol Rev. 1954 Jan;34(1):113–126. doi: 10.1152/physrev.1954.34.1.113. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G., Sun T. T., Lavker R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990 Jun 29;61(7):1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Ebling F. J. The biology of hair. Dermatol Clin. 1987 Jul;5(3):467–481. [PubMed] [Google Scholar]
- Hardy M. H. The secret life of the hair follicle. Trends Genet. 1992 Feb;8(2):55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Hoffman R. M. Three-dimensional histoculture: origins and applications in cancer research. Cancer Cells. 1991 Mar;3(3):86–92. [PubMed] [Google Scholar]
- Jahoda C. A., Horne K. A., Oliver R. F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984 Oct 11;311(5986):560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- LEIGHTON J. A sponge matrix method for tissue culture; formation of organized aggregates of cells in vitro. J Natl Cancer Inst. 1951 Dec;12(3):545–561. [PubMed] [Google Scholar]
- Li L. N., Margolis L. B., Hoffman R. M. Skin toxicity determined in vitro by three-dimensional, native-state histoculture. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1908–1912. doi: 10.1073/pnas.88.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limat A., Noser F. K. Serial cultivation of single keratinocytes from the outer root sheath of human scalp hair follicles. J Invest Dermatol. 1986 Oct;87(4):485–488. doi: 10.1111/1523-1747.ep12455548. [DOI] [PubMed] [Google Scholar]
- Parakkal P. F. Role of macrophages in collagen resorption during hair growth cycle. J Ultrastruct Res. 1969 Nov;29(3):210–217. doi: 10.1016/s0022-5320(69)90101-4. [DOI] [PubMed] [Google Scholar]
- Paus R., Czarnetzki B. M. Neue Perspektiven in der Haarforschung: auf der Suche nach der "biologischen Uhr" des Haarzyklus. Hautarzt. 1992 May;43(5):264–271. [PubMed] [Google Scholar]
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991 May;124(5):415–422. doi: 10.1111/j.1365-2133.1991.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Paus R., Link R. E. The psoriatic epidermal lesion and anagen hair growth may share the same "switch-on" mechanism. Yale J Biol Med. 1988 Sep-Oct;61(5):467–476. [PMC free article] [PubMed] [Google Scholar]
- Paus R., Stenn K. S., Elgjo K. The epidermal pentapeptide pyroGlu-Glu-Asp-Ser-GlyOH inhibits murine hair growth in vivo and in vitro. Dermatologica. 1991;183(3):173–178. doi: 10.1159/000247664. [DOI] [PubMed] [Google Scholar]
- Paus R., Stenn K. S., Link R. E. Telogen skin contains an inhibitor of hair growth. Br J Dermatol. 1990 Jun;122(6):777–784. doi: 10.1111/j.1365-2133.1990.tb06266.x. [DOI] [PubMed] [Google Scholar]
- Paus R., Stenn K. S., Link R. E. The induction of anagen hair growth in telogen mouse skin by cyclosporine A administration. Lab Invest. 1989 Mar;60(3):365–369. [PubMed] [Google Scholar]
- Philpott M. P., Green M. R., Kealey T. Human hair growth in vitro. J Cell Sci. 1990 Nov;97(Pt 3):463–471. doi: 10.1242/jcs.97.3.463. [DOI] [PubMed] [Google Scholar]
- Rogers G., Martinet N., Steinert P., Wynn P., Roop D., Kilkenny A., Morgan D., Yuspa S. H. Cultivation of murine hair follicles as organoids in a collagen matrix. J Invest Dermatol. 1987 Oct;89(4):369–379. doi: 10.1111/1523-1747.ep12471760. [DOI] [PubMed] [Google Scholar]
- Slominski A., Paus R., Costantino R. Differential expression and activity of melanogenesis-related proteins during induced hair growth in mice. J Invest Dermatol. 1991 Feb;96(2):172–179. doi: 10.1111/1523-1747.ep12460956. [DOI] [PubMed] [Google Scholar]
- Slominski A., Paus R., Mazurkiewicz J. Proopiomelanocortin expression in the skin during induced hair growth in mice. Experientia. 1992 Jan 15;48(1):50–54. doi: 10.1007/BF01923606. [DOI] [PubMed] [Google Scholar]
- Vermorken A. J., Bloemendal H. Human hair follicle cells in culture: the development of a new culture system and its potential applications. Mol Biol Rep. 1986;11(1):3–12. doi: 10.1007/BF00417588. [DOI] [PubMed] [Google Scholar]
- Westgate G. E., Craggs R. I., Gibson W. T. Immune privilege in hair growth. J Invest Dermatol. 1991 Sep;97(3):417–420. doi: 10.1111/1523-1747.ep12481002. [DOI] [PubMed] [Google Scholar]
- Weterings P. J., Vermorken A. J., Bloemendal H. A method for culturing human hair follicle cells. Br J Dermatol. 1981 Jan;104(1):1–5. doi: 10.1111/j.1365-2133.1981.tb01704.x. [DOI] [PubMed] [Google Scholar]