Abstract
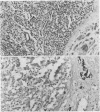
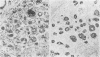
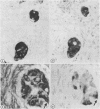
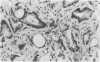
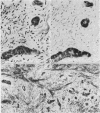
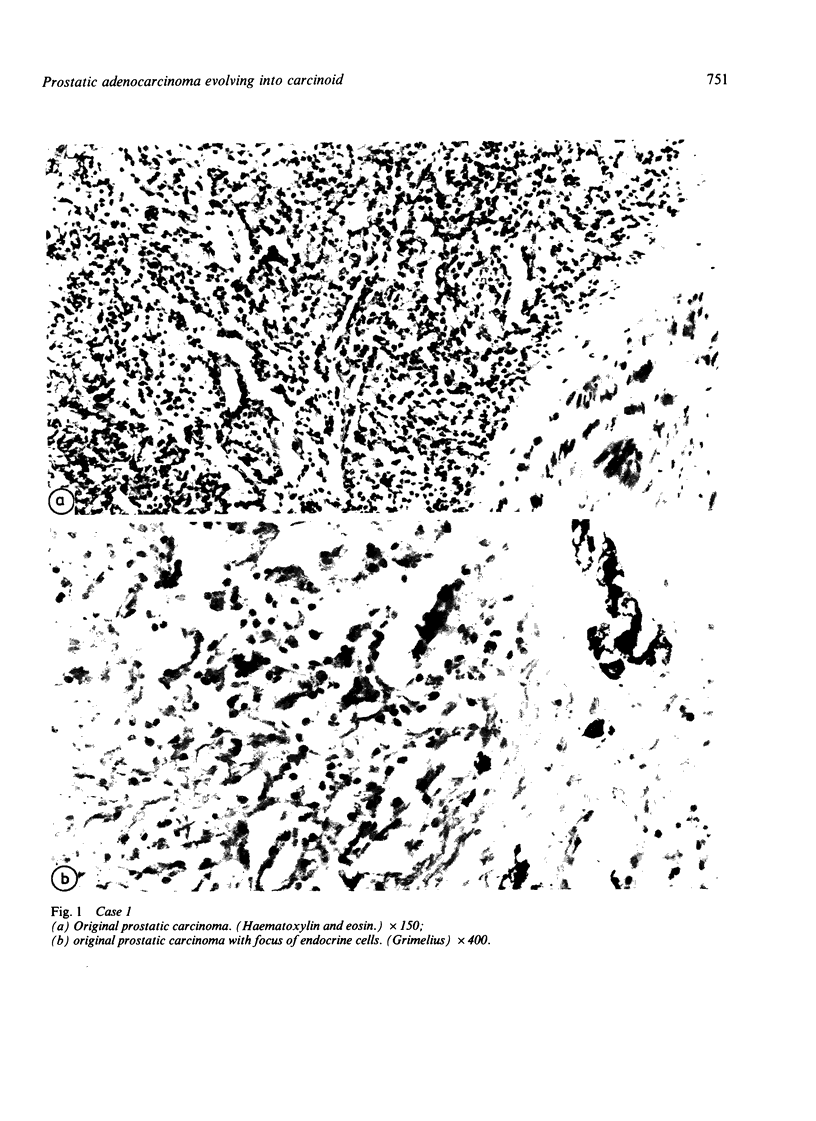
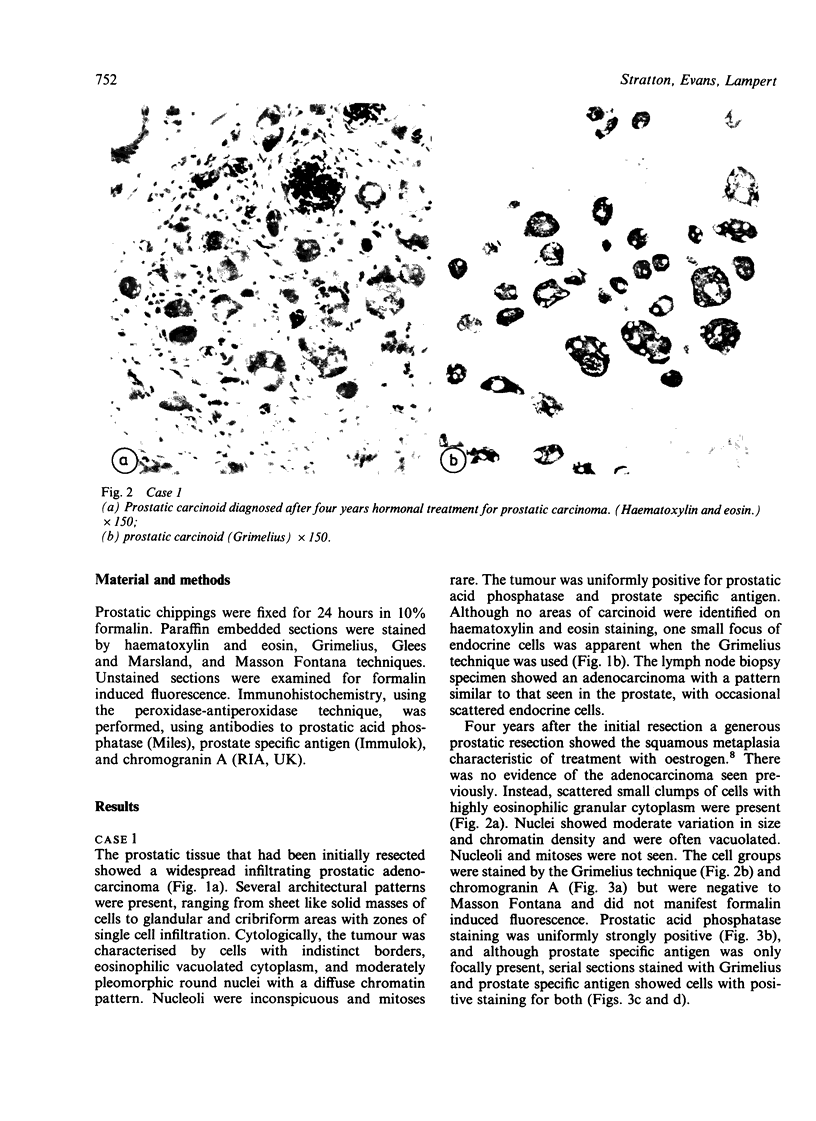
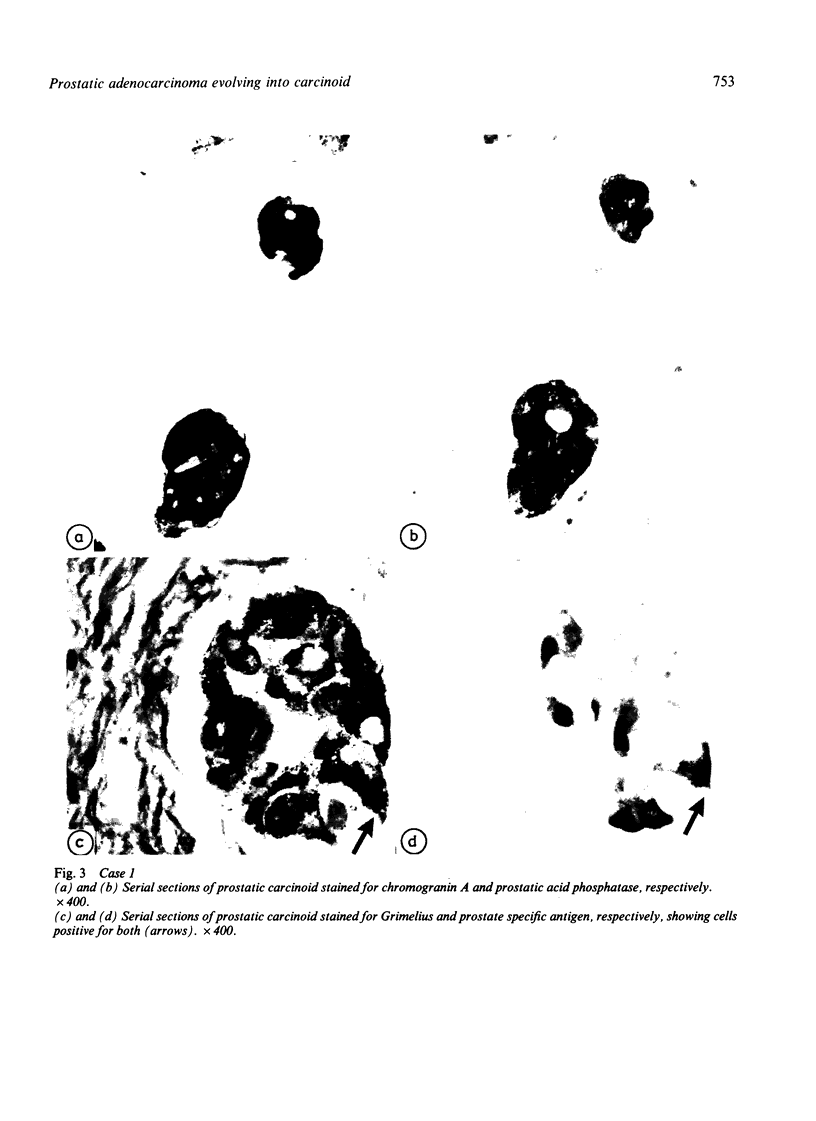
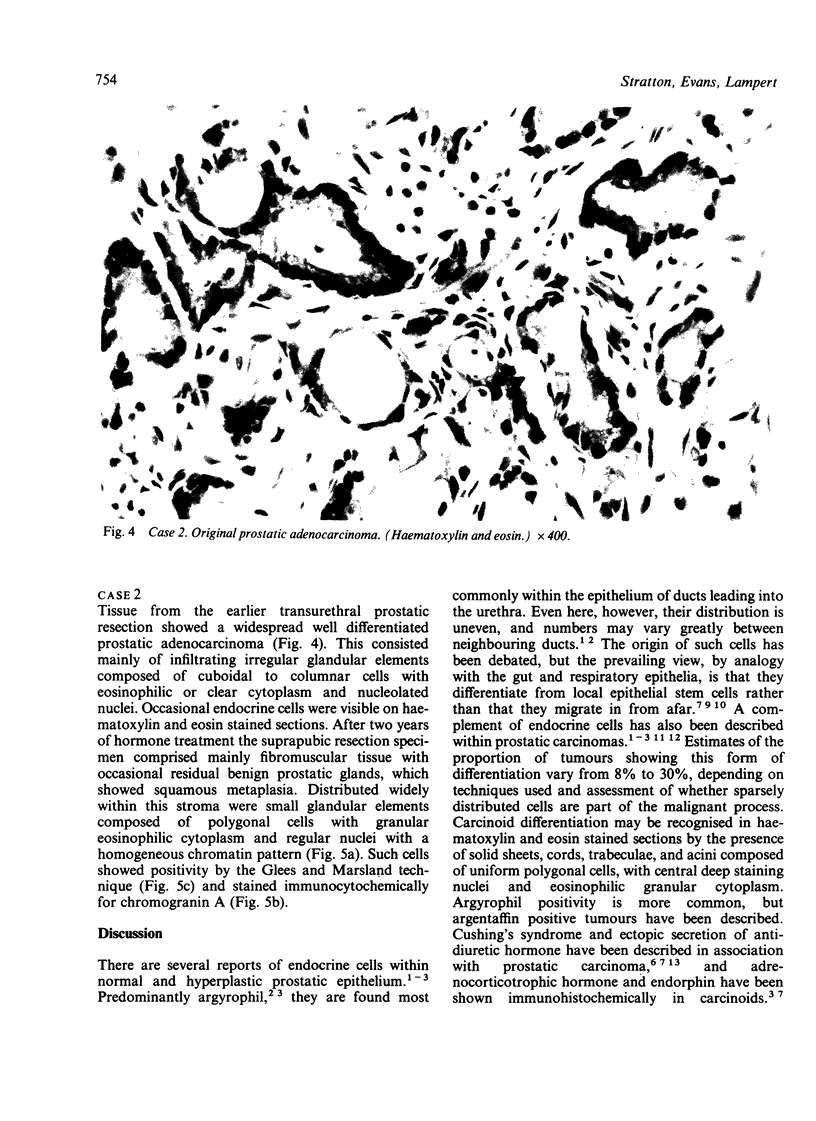
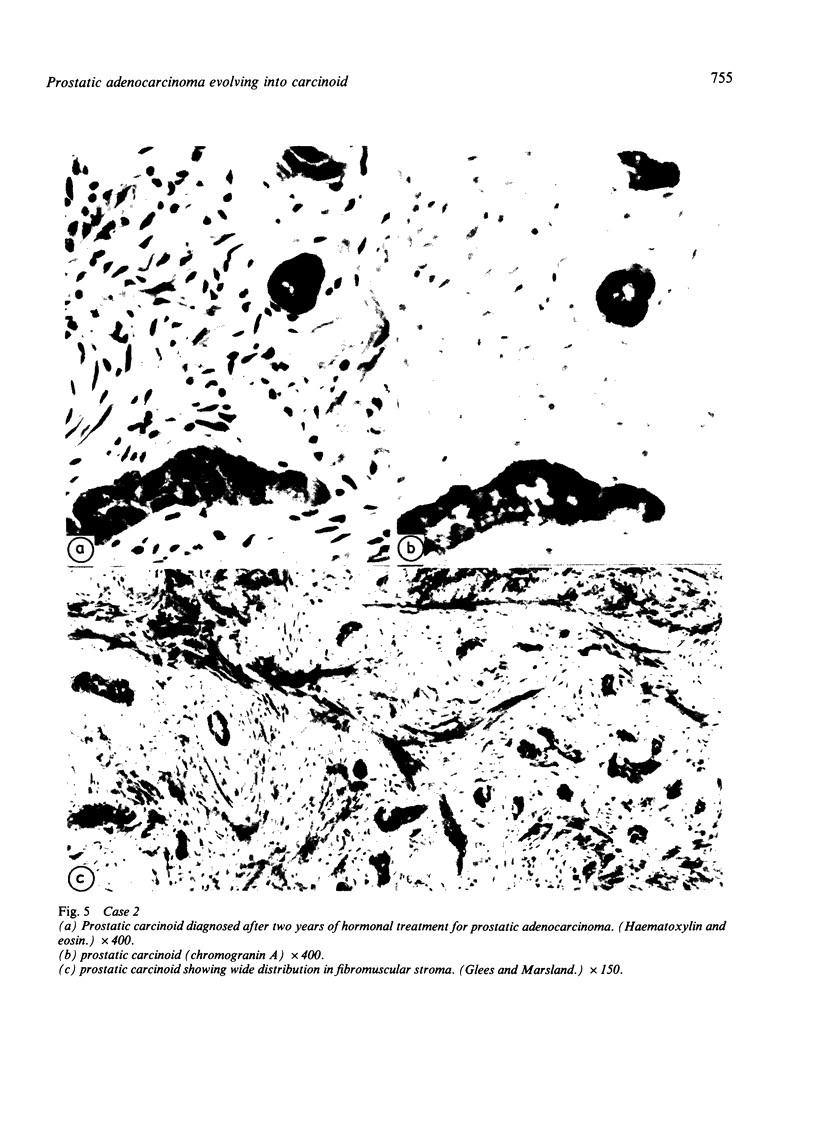
Two patients, aged 72 and 65 years, each underwent two prostatic resections spaced four and two years apart, respectively. In both cases the earlier procedure showed widespread adenocarcinoma with only occasional endocrine cells, while tissue from the later operations showed prostatic carcinoids. It is suggested that the conventional adenocarcinomas were sensitive to hormonal manipulations used in treatment, but that the originally sparse carcinoid components were resistant to this form of treatment and hence became the predominant tumours. These findings imply that endocrine differentiation in prostatic carcinoma leads to lack of sex steroid sensitivity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almagro U. A. Argyrophilic prostatic carcinoma. Case report with literature review on prostatic carcinoid and "carcinoid-like" prostatic carcinoma. Cancer. 1985 Feb 1;55(3):608–614. doi: 10.1002/1097-0142(19850201)55:3<608::aid-cncr2820550322>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Azumi N., Shibuya H., Ishikura M. Primary prostatic carcinoid tumor with intracytoplasmic prostatic acid phosphatase and prostate-specific antigen. Am J Surg Pathol. 1984 Jul;8(7):545–550. doi: 10.1097/00000478-198407000-00007. [DOI] [PubMed] [Google Scholar]
- Azzopardi J. G., Evans D. J. Argentaffin cells in prostatic carcinoma: differentiation from lipofuscin and melanin in prostatic epithelium. J Pathol. 1971 Aug;104(4):247–251. doi: 10.1002/path.1711040406. [DOI] [PubMed] [Google Scholar]
- BAINBOROUGH A. R. Squamous metaplasia of prostate following estrogen therapy. J Urol. 1952 Jul;68(1):329–336. doi: 10.1016/S0022-5347(17)68202-8. [DOI] [PubMed] [Google Scholar]
- Capella C., Usellini L., Buffa R., Frigerio B., Solcia E. The endocrine component of prostatic carcinomas, mixed adenocarcinoma-carcinoid tumours and non-tumour prostate. Histochemical and ultrastructural identification of the endocrine cells. Histopathology. 1981 Mar;5(2):175–192. doi: 10.1111/j.1365-2559.1981.tb01776.x. [DOI] [PubMed] [Google Scholar]
- Ghali V. S., Garcia R. L. Prostatic adenocarcinoma with carcinoidal features producing adrenocorticotropic syndrome. Immunohistochemical study and review of the literature. Cancer. 1984 Sep 15;54(6):1043–1048. doi: 10.1002/1097-0142(19840915)54:6<1043::aid-cncr2820540619>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Kazzaz B. A. Argentaffin and argyrophil cells in the prostate. J Pathol. 1974 Mar;112(3):189–193. doi: 10.1002/path.1711120310. [DOI] [PubMed] [Google Scholar]
- Montasser A. Y., Ong M. G., Mehta V. T. Carcinoid tumor of the prostate associated with adenocarcinoma. Cancer. 1979 Jul;44(1):307–310. doi: 10.1002/1097-0142(197907)44:1<307::aid-cncr2820440152>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Sellwood R. A., Spencer J., Azzopardi J. G., Wapnick S., Welbourn R. B., Kulatilake A. E. Inappropriate secretion of antidiuretic hormone by carcinoma of the prostate. Br J Surg. 1969 Dec;56(12):933–935. doi: 10.1002/bjs.1800561217. [DOI] [PubMed] [Google Scholar]
- Sidhu G. S. The endodermal origin of digestive and respiratory tract APUD cells. Histopathologic evidence and a review of the literature. Am J Pathol. 1979 Jul;96(1):5–20. [PMC free article] [PubMed] [Google Scholar]
- Wasserstein P. W., Goldman R. L. Diffuse carcinoid of prostate. Urology. 1981 Oct;18(4):407–409. doi: 10.1016/0090-4295(81)90405-2. [DOI] [PubMed] [Google Scholar]
- Wasserstein P. W., Goldman R. L. Primary carcinoid of prostate. Urology. 1979 Mar;13(3):318–320. doi: 10.1016/0090-4295(79)90435-7. [DOI] [PubMed] [Google Scholar]
- Wenk R. E., Bhagavan B. S., Levy R., Miller D., Weisburger W. Ectopic ACTH, prostatic oat cell carcinoma, and marked hypernatremia. Cancer. 1977 Aug;40(2):773–778. doi: 10.1002/1097-0142(197708)40:2<773::aid-cncr2820400226>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]