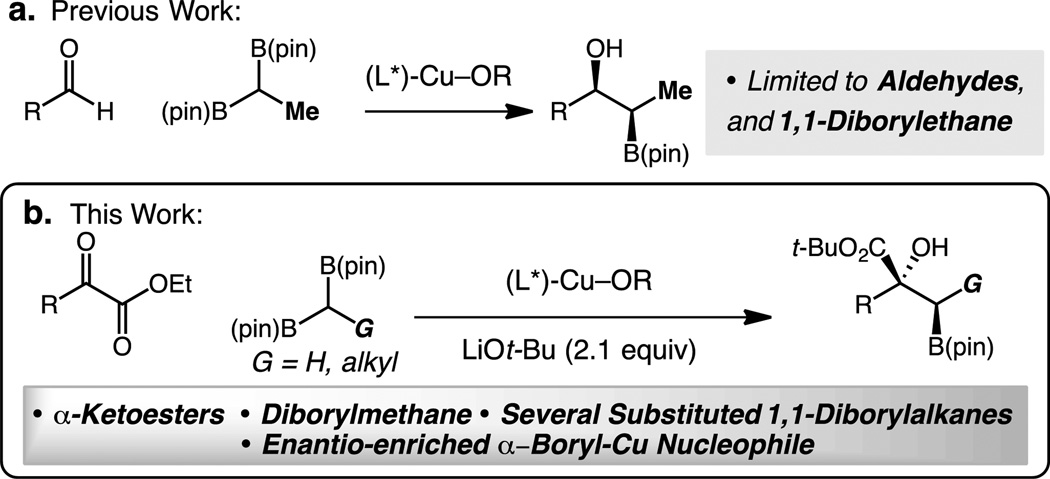
Abstract
Catalytic enantioselective synthesis of boronate-substituted tertiary alcohols through additions of diborylmethane and substituted 1,1-diborylalkanes to α-ketoesters is reported. The reactions are catalyzed by readily available chiral phosphine–copper(I) complexes and produce β-hydroxyboronates containing up to two contiguous stereogenic centers in up to 99:1 er, and >20:1 dr. The utility of the organoboron products is demonstrated through several chemoselective functionalizations. Evidence indicates reactions occur via enantio-enriched α-boryl-Cu-alkyl intermediate.
Keywords: diboron; 1,2-addition; copper; catalysis; enantioselective
Graphical abstract
Catalytic enantioselective synthesis of boronate-substituted tertiary alcohols through additions of diborylmethane and substituted 1,1-diborylalkanes to α-ketoesters is reported. The reactions are catalyzed by readily available chiral phosphine–copper(I) complexes and produce β-hydroxyboronates containing up to two contiguous stereogenic centers in up to 99:1 er, and >20:1 dr. The utility of the organoboron products is demonstrated through several chemoselective functionalizations. Evidence indicates reactions occur via enantio-enriched α-boryl-Cu-alkyl intermediate.
Tertiary alcohols are valuable functional groups found within many biologically active organic molecules. As a result, the development of efficient and selective methods for their preparation is an important challenge in organic chemistry.[1–2] The catalytic addition of sp3 carbon-based nucleophiles to ketones provides one of the most efficient strategies to access enantiomerically enriched tertiary alcohols;[3] nevertheless, despite recent progress in this area, a number of challenges still remain to be addressed. Of particular significance are protocols that deliver non-racemic tertiary alcohols and incorporate chemically versatile functional groups, such as alkyl boronic esters, for further manipulation.[4] Stereoselective synthesis of β-boryl alcohols by the 1,2-addition of α-boryl alkyl nucleophiles provides a direct approach for the generation of such versatile chemical motifs.[5] Catalytic methods of incorporating boronate esters via carbon-carbon bond formation remain limited, as current stoichiometric protocols involve the use of cuprate reagents[6] and alkylation of reactive dimesityl boron-stabilized α–boryl carbanions.[7] More recently, 1,1-organodiboronate esters have been developed as α–boryl carbanions in stereoselective deborylative carbon-carbon bond forming reactions.[8] Furthermore, catalytic enantioselective reactions that employ substituted 1,1-diborylalkanes are scarce.[8d–e, h], [5a]
We have shown that α-boryl-Cu species can be accessed through stereoselective deborylative transmetalation of 1,1-diboron compounds with Cu-based catalysts. We recently demonstrated that such catalytically generated intermediates engage in 1,2-additions for the enantio- and diastereoselective preparation of secondary alcohols bearing a vicinal boron-containing stereogenic center.[5] We proposed that a similar strategy could be employed for the synthesis of tertiary alcohols containing 1,2-hydroxyboronates by adding to α-ketoesters. The difficulty of such a catalytic method arises from the ability to efficiently form a new C–C bond between sterically congested vicinal C-sp3 stereogenic centers.
Herein, we report the catalytic enantio- and diastereoselective synthesis of β-boryl tertiary alcohols through the Cu-catalyzed additions of α-boryl nucleophiles to α-ketoesters. The utility of the products is illustrated through further alkyl boronate ester functionalizations (e.g., homologation, oxidation, and iodoetherification). Moreover, addition to a symmetrical ketone demonstrates the reaction occurs via the formation of a non-racemic α-boryl-Cu-alkyl intermediate.
We initiated our studies by evaluating the Cu-catalyzed enantioselective addition of unsubstituted 1,1-diborylmethane (5) to different α-ketoesters (4a–c) (Table 1). We first examined the ability of the chiral Cu complex derived from commercially available monodentate L1, which had emerged as the optimal ligand for 1,2-additions involving aldehydes.[5] We found that, with 5 mol % Cu(NCMe)4PF6, 10 mol % L1, and 2.05 equiv LiOt-Bu in thf at 22 °C, the reaction proceeds to afford 43% conversion to 6a (91:9 er) in 24 h and 24% eliminated product 7 (entry 1). At lower temperatures formation of boron-Wittig product 7 is suppressed, and the conversion to, and enantioselectivity of, 6a increases; for example, 6a is generated in 92:8 er and 96:4 er at 4 °C and −10 °C, respectively (entries 2 and 3).[9] Entry 4 illustrates that the Cu(I)-catalyzed reaction is less efficient at −25 °C, furnishing 6a in 51% conversion and in 95:5 er.[10] Remarkably, reaction of both the tert-butyl and methyl α-ketoesters 4b and 4c, afford 6a in similar conversions (63% and 70%) and similar enantioselectivities (94:6 and 93:7 er respectively). These data indicate that (i) trans-esterification of 4a and 4c, or the Et and Me ester products, occurs faster than 1,2-addition (entries 3 and 6 vs 5),[9] and (ii) C–C bond formation occurs primarily through the t-Bu ester 4b (entry 3 vs 6).[11–12] This is further supported by the reaction of ethyl ester 4a with one equivalent of LiOt-Bu (entry 7), which proceeds to >98% conversion of 4a to afford β-boryl tertiary alcohol 6a (26%, 91:9 er), and α-ketoester 4b (18%).[13] Use of NaOt-Bu results in a less efficient transformation (entry 8) as 6a is formed in 37% NMR yield and 81:19 er. Further reaction optimization using various mono-and bidentate chiral phosphines did not result in a more efficient or stereoselective process (see Table 1). As there is minimal difference in reaction efficiency between 4a–c, ethyl α-ketoesters were used for the remainder of the study due to their ready availability.
Table 1.
Initial Examination of Chiral Cu-Complexes.[a]
 | |||||
|---|---|---|---|---|---|
| entry | R | base | temp (°C) | 4b:6a:7 (%)[b–C] | er 6a[d] |
| 1 | Et | LiOt-Bu | 22 | <2: 43: 24 | 91:9 |
| 2 | Et | LiOt-Bu | 4 | <2: 60: 12 | 92:8 |
| 3 | Et | LiOt-Bu | −10 | <2: 74: <2 | 96:4 |
| 4 | Et | LiOt-Bu | −25 | 35: 51: <2 | 95:5 |
| 5[e] | t-Bu | LiOt-Bu | −10 | <2: 63: 2 | 94:6 |
| 6 | Me | LiOt-Bu | −10 | <2: 70: <2 | 93:7 |
| 7[e] | Et | LiOt-Bu | −10 | 18: 26: 8 | 91:9 |
| 8 | Et | NaOt-Bu | −10 | 32: 37: <2 | 81:19 |
 | |||||
Reactions performed under N2 atm.
>98% conv. of α-ketoester starting material in all cases.
1H NMR yield values determined by analysis of 400 or 600 MHz 1H NMR spectra of unpurified mixtures with dmf as internal standard.
Determined by NaBO3.4H2O oxidation to diol and HPLC analysis; see the Supporting Information for details.
One equivalent of LiOt-Bu was used.
We began exploring the scope of the catalytic reaction by synthesizing various unsubstituted hydroxy boronates. Due to the varying stability of unsubstituted hydroxy boronates (e.g., 6a), the products were oxidized to the corresponding diol. Isolation and non-oxidative workup of the primary alkylboron products can be achieved through hydroxyl protection (vide infra). Using the optimal conditions in Table 1, the catalytic transformations can be performed with various aryl-substituted α-ketoesters (Table 2), including those that carry para (8a–f), or meta (8g–i) substituents. Transformations with electron-donating and electron-withdrawing groups proceed to similar 1H NMR yields (58–82%) and high enantioselectivities (92:8–97:3 e.r.) in 24 h at −10 °C. Only in the presence of p-MeO-substituted phenyl α-ketoesters is a diminution in enantioselectivity observed; tertiary alcohol 8b (entry 2), is formed in 72% 1H NMR yield and 85:15 er.[14] The transformation is sensitive to sterically congested α-ketoesters; 2-naphthyl proceeds to 70% and 96:4 er (entry 10), while ortho-Me-substituted tertiary alcohol 8k is generated in 43% and 66:34 er (entry 11). Synthesis of pyridyl-substituted product 8l demonstrates that N-heterocyclic α-ketoesters can serve as effective substrates, albeit oxidation to the corresponding diol proceeds with lower efficiency (entry 12).
Table 2.
Cu(I)-Catalyzed 1,2-Addition of 1 to Aryl α-Ketoesters.[a]
 | ||||
|---|---|---|---|---|
| entry | product |
1H NMR yield of 6 (%)[b–C] |
yield of 8 (%)[d] |
er[e] |
| 1 | 8a; Ar = Ph | 74 | 65 | 96:4 |
| 2 | 8b; Ar = p-OMeC6H4 | 72 | 60 | 85:15 |
| 3 | 8c; Ar = p-CIC6H4 | 80 | 64 | 97:3 |
| 4 | 8d; Ar = p-t-BuC6H4 | 77 | 70 | 92:8 |
| 5 | 8e; Ar = p-NO2C6H4 | 62 | 46 | 94:6 |
| 6 | 8f; Ar = p-CF3C6H4 | 58 | 54 | 97:3 |
| 7 | 8g; Ar = m-MeC6H4 | 76 | 63 | 96:4 |
| 8 | 8h; Ar = m-OMeC6H4 | 82 | 62 | 96:4 |
| 9 | 8i; Ar = m-CIC6H4 | 81 | 64 | 97:3 |
| 10 | 8j; Ar = 2-napthyl | 70 | 62 | 96:4 |
| 11 | 8k; Ar = o-MeC6H4 | 43 | 27 | 66:34 |
| 12 | 8l; Ar = 3-pyridyl | 72 | 36 | 97:3 |
See Table 1.
Yields of the corresponding purified diol; yields are an average of two runs.
Determined by HPLC analysis; see the Supporting Information for details.
Access to the β-boryl substituted products 6, can be easily achieved by protection of the tertiary alcohol as the silyl ether product prior to purification. The conversion of 4a into 9 in Eq 1 is representative; treatment of the crude product 6a (70% conv) with (Me)3SiCl and imidazole (dmf, 22 °C, 14 h) affords alkylboronate (9) in 46% overall yield and 96:4 er after silica gel chromatography.
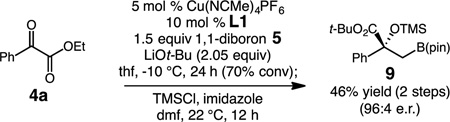 |
(1) |
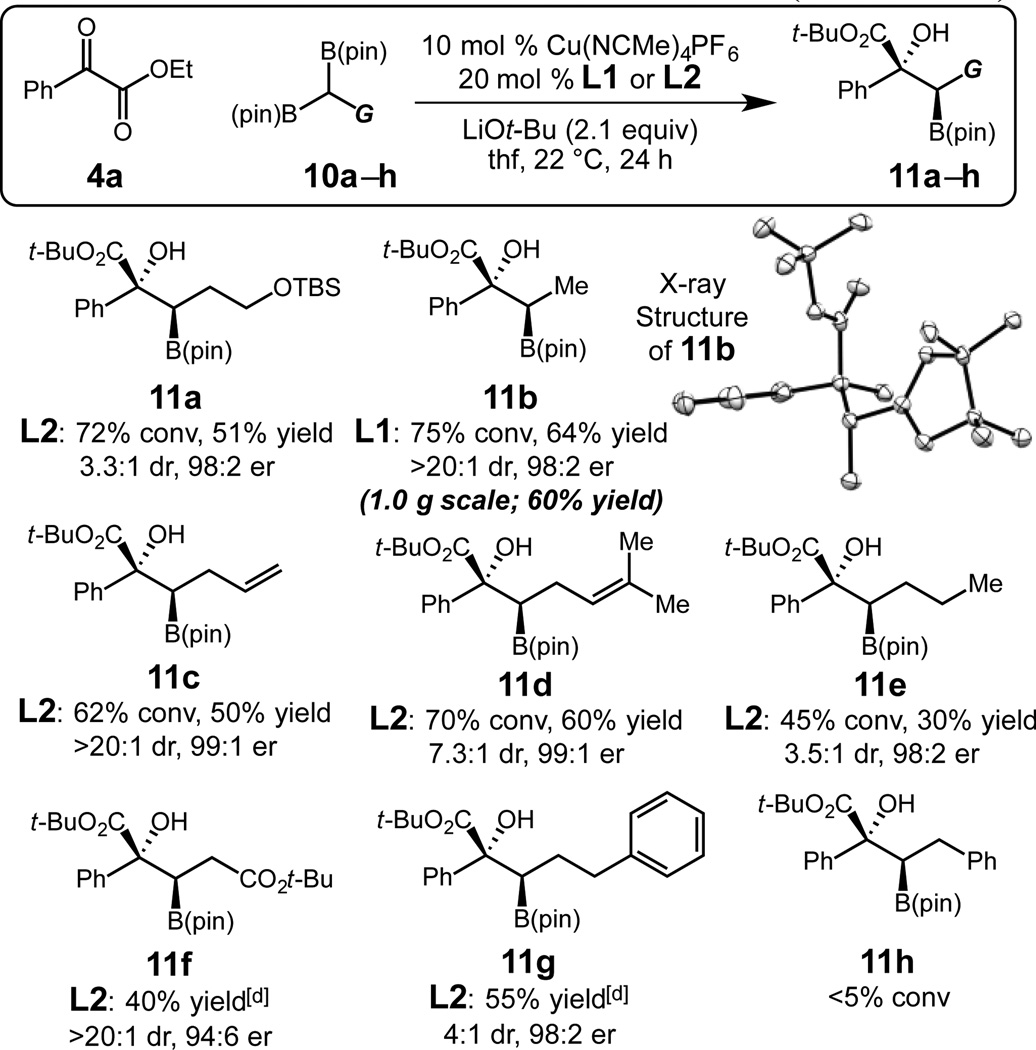
The Cu-catalyzed protocol can be extended to more challenging processes that utilize substituted alkyl 1,1-diboron reagents to afford congested contiguous 3° and 2° stereogenic centers. Reactions require 10 mol % Cu catalyst derived from L1 or L2 at 22 °C to proceed to good conversion and high enantioselectivity. Notably, the secondary alkyl hydroxyboronate products are stable to silica gel column chromatography; for example, β-boryl tertiary alcohols containing silyl ether (11a), alkyl (11b–c), and alkenyl (11d–e) functional groups are isolated in up to 64% yield, >20:1 dr, and 99:1 er. The reaction is easily performed on gram scale; subjection of 1.0 g of 4a to 10b lead to the formation of 11b in 75% conversion 60% yield, >20:1 dr, and 98:2 er. The reaction of a gem-diboron reagent that contains an ester (e.g., 11f), although remarkably diastereo-(>20:1 dr) and enantioselective (94:6 er), results in only 40% 1H NMR yield after 24 h; likely due to the presence of acidic α-protons.[15] Increasing the size of the substituent proximal to the 1,1-diboron group also results in decreased reactivity; under optimal conditions phenethyl containing 11g is generated in 55% yield (4:1 dr, 98:2 er). The lack of formation (<5% conv) of benzyl 11h highlights the sensitivity of the Cu-catalyzed protocol to increased sterics of the substituted nucleophilic components. The absolute and relative stereochemistry of the 1,2-hydroxyboronates synthesized through the Cu-catalyzed protocol was determined by X-ray crystallographic analysis of tertiary alcohol 11b (Scheme 2). The stereochemical assignment of 11b is (R,R) with an anti relationship between the hydroxyl and B(pin) units, corresponding to the addition of an (R)-alkyl copper species to the Si face of the α-ketoester.16
Scheme 2.
Enantio- and Diastereoselective Addition of Substituted 1,1-Diboronates to α-Ketoesters.[a] [a–c] See Table 1. [d] 1H NMR yield. L2 = (R)-(+)-5,5’,6,6’,7,7’,8,8’-octahydro-1,1’-bi-2-naphthol derived MonoPhos.
The substituted organoboron compounds formed by the reported catalytic reaction can be transformed to afford a number of valuable enantio- and disastereoenriched molecules (Scheme 3a–d). Boron oxidation of 11a delivers trans diol 12 in 91% yield, which is equivalent to the dihydroxylation of a stereo-defined tri-substituted alkene. While the secondary alkyl B(pin) functional groups synthesized through the Cu-catalyzed protocol (e.g., 13) proved to be unsuitable reagents for Pd-catalyzed cross-coupling,[17] they readily participate in metal-free homologations. Sterically hindered secondary alkyl B(pin) silyl ether 13 (formed in 60% yield) undergoes stereospecific C–B to C–C conversion, to afford primary alkylboron tertiary silyl ether 14 in 50% yield. The functional group rich organoboron molecules can also be chemoselectively functionalized leaving the C(sp3)–B bond intact. As illustrated in Scheme 3c, allyl substituted hydroxyboronate 11c undergoes efficient cross-metathesis;[18] in the presence of 10 mol % ruthenium catalyst 17 and 2 equivalents of cis-alkene 15, allylic alcohol 16 is formed in 63% yield and 16:1 E/Z. Additionally, the prenyl-substituted β-boryl tertiary alcohols can be readily converted to tetrahydropyrans via iodoetherification[19] without loss of the B(pin) unit; treatment of 11d with I2 and NaHCO3 at −35 °C in MeCN for 2 h furnishes substituted tetrahydropyran 18 in 56% conversion and 3:1 dr, and silica gel purification delivers 18 as a single diastereoisomer in 45% yield.
Scheme 3.
Representative Functionalizations of α–Boryl Tertiary Alcohols.
A proposed catalytic reaction sequence for the Cu-catalyzed process is outlined in Scheme 4. Enantioselective transmetalation between a Cu-alkoxide (A) and 1,1-diboron 1, generates chiral α-boryl-alkyl-Cu B. Stereoselective 1,2-addition to α-ketoester 2 affords Cu-alkoxide C containing two contiguous stereogenic centers. Reaction with LiOt-Bu furnishes the lithium alkoxide product D, which is converted to 3 after acid workup, and regenerates copper catalyst A.
Scheme 4.
Working Catalytic Cycle for Cu-Catalyzed 1,2-Addition.
While the chiral phosphine-copper catalyst controls addition of the α-boryl-Cu intermediate to the Si face of the α-ketoester (B→C) for diborylmethane, and substituted variants, it is unclear if substituted 1,1-diborons react through an enantio-enriched (R)-α-boryl-Cu-alkyl nucleophile (e.g., B R=Me, Scheme 4). To gain insight into the formation of an enantio-enriched α-boryl-Cu-alkyl intermediate we examined the Cu-catalyzed 1,2-addition of 10b to a symmetrical carbonyl electrophile (Equation 2). Treatment of benzophenone (19) with 10b under optimal reaction conditions in Scheme 2 (10 mol % (L1)2CuOt-Bu), affords (R)-20 in 42% 1H NMR yield and 97:3 er.20 This result indicates that the sterocenter of the α-boryl-Cu-alkyl nucleophile is formed in high er, and that the catalyst functions to control both the formation of the Cu-alkyl and the facial selectivity.21
 |
(2) |
In summary, we present the first catalytic protocol for the enantio- and diastereoselective synthesis of β-boryl tertiary alcohols. The approach is applicable to diboroylmethane and other readily accessible substituted 1,1-diborylalkanes, and α-ketoesters. Reactions are promoted by a phosphine-Cu complex and proceed via 1,2-addition of α-boryl nucleophiles to generate up to two contiguous stereogenic centers. The versatility of the β-boryl tertiary alcohol products synthesized is underlined by representative stereospecific and chemoselective functionalizations to provide useful chemical fragments. Further mechanistic studies, application to multistep complex molecule syntheses, and the development of related stereoselective catalytic reactions are in progress.
Supplementary Material
Scheme 1.
Previous work: a. Catalytic enantio- and diastereoselective 1,2-addition of 1,1-diborylethane to aldehydes. This work: b. Catalytic enantio- and diastereoselective 1,2-addition of 1,1-diborylmethane and functionalized 1,1-diborylalkanes to α-ketoesters.
Acknowledgments
Financial support was provided by the United States National Institutes of Health, Institute of General Medical Sciences (GM-116987) and the University of North Carolina at Chapel Hill.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx
References
- 1.Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis; Christophers J, Baro A, editors. Weinheim: Wiley-VCH; 2006.
- 2.For recent reviews on catalytic enantioselective additions to ketones, see: Riant O, Hannedouche J. Organic & Biomolecular Chemistry. 2007;5(6):873. doi: 10.1039/b617746h. Shibasaki M, Kanai M. Chem. Rev. 2008;108:2853–2873. doi: 10.1021/cr078340r.
- 3.For representative examples of catalytic enantioselective additions of sp3 carbon-based nucleophiles to ketones, see: García C, LaRochelle LK, Walsh PJ. J. Am. Chem. Soc. 2002;124:10970–10971. doi: 10.1021/ja026568k. DiMauro EF, Kozlowski MC. J. Am. Chem. Soc. 2002;124:12668–12669. doi: 10.1021/ja026498h. Funabashi K, Jachmann M, Kanai M, Shibasaki M. Angew. Chem. Int. Ed. 2003;42:5489–5492. doi: 10.1002/anie.200351650. Angew. Chem2003, 115, 5647–5650. Betancort JM, García C, Walsh PJ. Synlett. 2004;5:749–760. Siewert J, Sandmann R, von Zezschwitz P. Angew. Chem. Int. Ed. 2007;46:7122–7124. doi: 10.1002/anie.200701087. Angew. Chem. 2007, 119, 7252–7254. Hatano M, Miyamoto T, Ishihara K. Org. Lett. 2007;9:4535–4538. doi: 10.1021/ol702074a. Friel DK, Snapper ML, Hoveyda AH. J. Am. Chem. Soc. 2008;130:9942–9951. doi: 10.1021/ja802935w. Madduri AVR, Harutyunyan SR, Minnaard AJ. Angew. Chem. Int. Ed. 2012;51:3164–3167. doi: 10.1002/anie.201109040. Angew. Chem. 2012, 124, 3218–3221. Rong J, Pellegrini T, Harutyunyan SR. Chem. Eur. J. 2016;22:3558–3570. doi: 10.1002/chem.201503412.
- 4. Winbush SM, Roush WR. Org. Lett. 2010;12:4344–4347. doi: 10.1021/ol101789g. Blaisdell TP, Caya TC, Zhang L, Sanz-Marco A, Morken JP. J. Am. Chem. Soc. 2014;136:9264–9267. doi: 10.1021/ja504228p. Blaisdell TP, Morken JP. J. Am. Chem. Soc. 2015;137:8712–8715. doi: 10.1021/jacs.5b05477. For a recent in direct approach to 1,2-hydroxyborons, see: Chen D, Zhang X, Qi W-Y, Xu B, Xu M-H. J. Am. Chem. Soc. 2015;137:5268–5271. doi: 10.1021/jacs.5b00892.
- 5.Joannou MV, Moyer BS, Meek SJ. J. Am. Chem. Soc. 2015;137:6176–6179. doi: 10.1021/jacs.5b03477. [DOI] [PubMed] [Google Scholar]; (b) Joannou MV, Moyer BS, Goldfogel MJ, Meek SJ. Angew. Chem. Int. Ed. 2015;54:14141–14145. doi: 10.1002/anie.201507171. Angew. Chem. 2015, 127, 14347–14351. [DOI] [PubMed] [Google Scholar]
- 6.(a) Knochel P. J. Am. Chem. Soc. 1990;112:7431–7433. [Google Scholar]; (b) Sakai M, Saito S, Kanai G, Suzuki A, Miyaura N. Tetrahedron. 1996;52:915–924. [Google Scholar]
- 7.Pelter A, Peverall S, Pitchford A. Tetrahedron. 1996;52:1085–1094. [Google Scholar]
- 8. Endo K, Ohkubo T, Hirokami M, Shibata T. J. Am. Chem. Soc. 2010;132:11033–11035. doi: 10.1021/ja105176v. Endo K, Ohkubo T, Shibata T. Org. Lett. 2011;13:3368–3371. doi: 10.1021/ol201115k. Endo K, Ohkubo T, Ishioka T, Shibata T. J. Org. Chem. 2012;77:4826–4831. doi: 10.1021/jo3004293. Sun C, Potter B, Morken JP. J. Am. Chem. Soc. 2014;136:6534–6537. doi: 10.1021/ja500029w. Potter B, Szymaniak AA, Edelstein EK, Morken JP. J. Am. Chem. Soc. 2014;136:17918–17921. doi: 10.1021/ja510266x. Hong K, Liu X, Morken JP. J. Am. Chem. Soc. 2014;136:10581–10584. doi: 10.1021/ja505455z. Coombs JR, Zhang L, Morken JP. J. Am. Chem. Soc. 2014;136:16140–16143. doi: 10.1021/ja510081r. Sun HY, Kubota K, Hall DG. Chem. Eur. J. 2015;21:1–10. doi: 10.1002/chem.201406680. Kim J, Park S, Park J, Cho SH. Angew. Chem. Int. Ed. 2016;55:1498–1501. doi: 10.1002/anie.201509840. Angew. Chem. 2016, 128, 1520–1523. Shi Y, Hoveyda AH. Angew. Chem. Int. Ed. 2016;55:3455–3458. doi: 10.1002/anie.201600309. Angew. Chem. 2016, 128, 3516–3519. Zhang Z-Q, Zhang B, Lu X, Liu J-H, Lu X-Y, Xiao B, Fu Y. Org. Lett. 2016;18:952–955. doi: 10.1021/acs.orglett.5b03692. Park J, Lee Y, Kim J, Cho SH. Org. Lett. 2016;18:1210–1213. doi: 10.1021/acs.orglett.6b00376. Zhan M, Li R-Z, Mou Z-D, Cao C-G, Liu J, Chen Y-W, Niu D. ACS Catal. 2016;6:3381–3386. For recent syntheses of 1,1-alkylbisboronate esters, see: Zhang Z-Q, Yang C-T, Liang L-J, Xiao B, Lu X, Liu J-H, Sun Y-Y, Marder TB, Fu Y. Org. Lett. 2014;16:6342–6345. doi: 10.1021/ol503111h. Batsanov AS, Cabeza JA, Crestani MG, Fructos MR, García-Álvarez P, Gille M, Lin Z, Marder TB. Angew. Chem. Int. Ed. 2016;55:4707–4710. doi: 10.1002/anie.201601121. Angew. Chem. 2016, 128, 4785–4788. Cook AK, Schimler SD, Matzger AJ, Sanford MS. Science. 2016;351:1421–1424. doi: 10.1126/science.aad9289. Smith KT, Berritt S, González-Moreiras M, Ahn S, Smith MR, III, Baik M-H, Mindiola DJ. Science. 2016;351:1424–1427. doi: 10.1126/science.aad9730.
- 9.Absolute stereochemistry of tertiary alcohol 6a formed with (R)-MonoPhos is (R) (see Supporting Information).
- 10.Reactions run for 48 h at −25 °C did not lead to an increase in yield.
- 11.Treatment of 4a with 2 equivalents of LiOt-Bu (no Cu or L1) at −10 °C for 2.5 h results in >98% conversion of 4a and 90% conversion to 6. Treatment of 4a with 2 equivalents of LiOt-Bu, 5 mol % Cu(NCMe)4PF6, and 10 mol % L1) at −10 °C for 2.5 h results in >98% conversion of 4a but only 70% to 6.
- 12.For an example of Cu(I)-catalyzed trans-esterification, see: Munro-Leighton C, Delp SA, Blue ED, Gunnoe TB. Organometallics. 2007;26:1483–1493.
- 13.Decreased er is likely due to reaction with LiOEt.
- 14.The lower enantioselectivity of 9b is not due to partial racemization during purification; re-subjection of 9b to silica column chromatography affords 9b in 85:15 er.
- 15.Increasing the equivalents of LiOt-Bu or decreasing the reaction temperature does not improve conversion to 10e.
- 16.For examples of stereoretentive reactions of organocopper-alkyls, see: Campbell MJ, Johnson JS. Org. Lett. 2007;9:1521–1524. doi: 10.1021/ol0702829. Lee Y, Hoveyda AH. J. Am. Chem. Soc. 2009;131:3160–3161. doi: 10.1021/ja809382c. Zhong C, Kunii S, Kosaka Y, Sawamura M, Ito H. J. Am. Chem. Soc. 2010;132:11440–11442. doi: 10.1021/ja103783p. Matsuda N, Hirano K, Satoh T, Miura M. J. Am. Chem. Soc. 2013;135:4934–4937. doi: 10.1021/ja4007645. Jia T, Cao P, Wang B, Lou Y, Yin X, Wang M, Liao J. J. Am. Chem. Soc. 2015;137:13760–13763. doi: 10.1021/jacs.5b09146. Yang Y, Shi S-L, Niu D, Liu P, Buchwald SL. Science. 2015;349:62–66. doi: 10.1126/science.aab3753. For an example stereoinvertive reactions of organocopper-alkyls, see: (g) ref 16c Logan KM, Smith KB, Brown MK. Angew. Chem. Int. Ed. 2015;54:5228–5231. doi: 10.1002/anie.201500396. Angew. Chem. 2015, 127, 5317–5320.
- 17.Steric hinderance of the 1° and 2° alkyl boronic esters likely inhibits reaction.
- 18.Connon SJ, Blechert S. Angew. Chem. Int. Ed. 2003;42:1900–1923. doi: 10.1002/anie.200200556. Angew. Chem. 2003, 115, 1944–1968. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Kaur P, Mittal A, Singh P. Tetrahedron. 2006;62:4018–4026. [Google Scholar]
- 20.24% unreacted benzophenone; Er determined by conversion to the known corresponding diol (see Supporting Information for details)
- 21.Studies to elucidate the boron enantiotopic group-selectivity during transmetalation are on going.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.