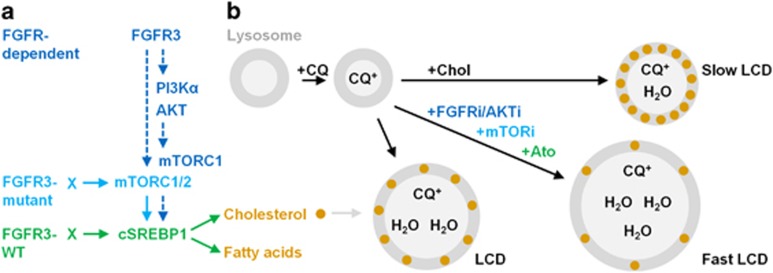
Figure 7.
FGFR signalling and model for the role of cholesterol biosynthesis in the maintenance of lysosomal membrane integrity under CQ- and BafA1-induced stress. (a) FGFR-dependent (blue text) and FGFR3-mutant (light blue text only) bladder cancer cell lines maintain cSREBP1 expression in an mTORC1/2-dependent manner (left). In contrast, FGFR3-WT cells (green text) maintain cSREBP1 independently of mTORC1/2 signalling. (b) CQ is a weak base that diffuses into lysosomes (grey circles), neutralises the pH and becomes trapped by protonation, inducing osmotic stress, lysosomal swelling, cathepsin release and lysosomal cell death (LCD). Inhibition of FGFR, AKT or mTOR signalling in FGFR-dependent cell lines or HMGCR (Ato) in all cell lines, depletes cellular cholesterol and accelerates LCD (Fast LCD). In contrast, saturation of cellular membranes with water-soluble cholesterol (+Chol) prevents CQ-induced lysosomal swelling, cathepsin loss and cell death (Slow LCD). Lysosomal membrane cholesterol is known to regulate permeability to water and ions; however, this is the first study to demonstrate that inhibition of cholesterol biosynthesis underlies the potentiation of CQ-induced LCD in cancer cells. Furthermore, we propose that water-soluble cholesterol can be used to determine the relative contribution of LCD versus autophagy inhibition in future studies examining the potentiation of therapeutic-induced cell death with lysosomal inhibitors.