Abstract
This review article aimed to evaluate ocular biometric changes after trabeculectomy. The PubMed database was searched using the keywords “axial length” (AL), “anterior chamber depth” (ACD), “corneal astigmatism,” “corneal topography” and “trabeculectomy.” The extracted studies were categorized based on the evaluated parameters and the biometry method (contact and non-contact). Comparable studies with respect to their sample size were combined for statistical analysis. Twenty-five studies including 690 individuals which met the inclusion criteria were selected. After trabeculectomy, a significant and persistent AL reduction, with a range of 0.1-0.19 and 0.1-0.9 mm measured with contact and non-contact methods, respectively, was observed. With respect to topographic changes, 0.38-1.4 diopters (D) with-the-rule (WTR) astigmatism was induced postoperatively. All studies revealed ACD reduction immediately after surgery, which gradually deepened and approximated its preoperative levels on day 14. ACD reduction was not significant after that period in the majority of cases. In conclusion, changes in ACD is of small amount and of short period, thus it can be ignored; however, reported changes in AL and keratometry are of sufficient magnitude and can affect the refractive prediction of combined cataract surgery and trabeculectomy.
Key words: Anterior Chamber Depth, Axial Length, Keratometry, Trabeculectomy
INTRODUCTION
Trabeculectomy, the most common surgical procedure for long-term reduction of intraocular pressure (IOP) in glaucoma, bypasses the conventional outflow pathway by creating a corneoscleral fistula that leads to subconjunctival bleb formation.[1,2] Maintaining the integrity of the bleb is the key factor for successful outcomes. Intraoperative application of antimetabolites helps to meet this goal and has provided major progresses in achieving better surgical outcomes, especially in those who are at a higher risk of surgical failure.[3] Significant IOP reduction post trabeculectomy could reduce the axial length (AL) of the eye corresponding to the amount of IOP reduction.[4] Anterior chamber depth (ACD) and volume, as well as keratometry could also change after the surgery. These unpredictable changes may affect the accuracy of intraocular lens (IOL) power calculation in cases who need cataract surgery and IOL implantation combined with glaucoma surgery; this is of particular concerns, considering the increased frequency of glaucoma and cataract in elderly patients. In fact, it is well established that even small changes in AL could lead to clinically significant error in IOL power calculation and refractive prediction of cataract surgery.[5] The purpose of the current study is to review articles which studied the effect of trabeculectomy on biometric indices.
METHODS
Search Strategy
The literature search was performed on PubMed database from its inception to April 2015 using the keywords “axial length” (AL), “anterior chamber depth” (ACD), “corneal astigmatism,” “corneal topography” and “trabeculectomy.” The research question was the relationship between ocular biometry and trabeculectomy. The biometry method (contact and non-contact) and evaluated parameters were applied to categorize the extracted articles. The similar articles in terms of sample size were combined to conduct the statistical analysis and finally 25 out of 91 studies were extracted, including 690 individuals were extracted.
The inclusion criteria were: (1) The study had been conducted on adult human subjects (2) the initial surgical intervention was trabeculectomy. The exclusion criteria included (1) studies on congenital or childhood glaucoma (2) studies on patients with history of cataract surgery or combined cataract surgery and trabeculectomy (3) studies on acute glaucoma.
Data Extraction
Information on study location, design, number of patients and their demographic data (age, sex and type of glaucoma), type of surgical intervention, type of applied antimetabolites, the duration of follow-up, type of instruments used to obtain measurements, main outcomes measured and mean changes of the main outcomes in each follow-up were collected.
Statistical Analysis
All analyses were performed using SPSS (Version 22.0, IBM Co., Chicago, IL, USA). The mean and standard deviation with respect to sample size of each study at each examination point was considered and an overall mean was calculated. To evaluate the estimation accuracy, the 95% confidence interval was used.
RESULTS
A total of 25 studies were included in the present review. Tables 1–3 display the characteristics of each study. All articles were published between 1989 and 2015.
Table 1.
Studies on axial length changes after trabeculectomy

Table 3.
Studies on keratometry changes after trabeculectomy

Table 2.
Studies on anterior chamber depth changes after trabeculectomy

Axial Length Changes
Eight studies evaluated the effect of trabeculecotmy on AL and all these studies reported significant AL reduction postoperatively, which became stable nearly 3 months after the surgery. These studies were different in terms of the follow-up period, application of intraoperative antimetabolites and method of AL measurement [Table 4]. At all follow-up periods, the amount of reduction in AL measured by optical devices was less than that measured by ultrasonic tools [Figure 1].
Table 4.
Axial length changes after trabeculectomy in different studies
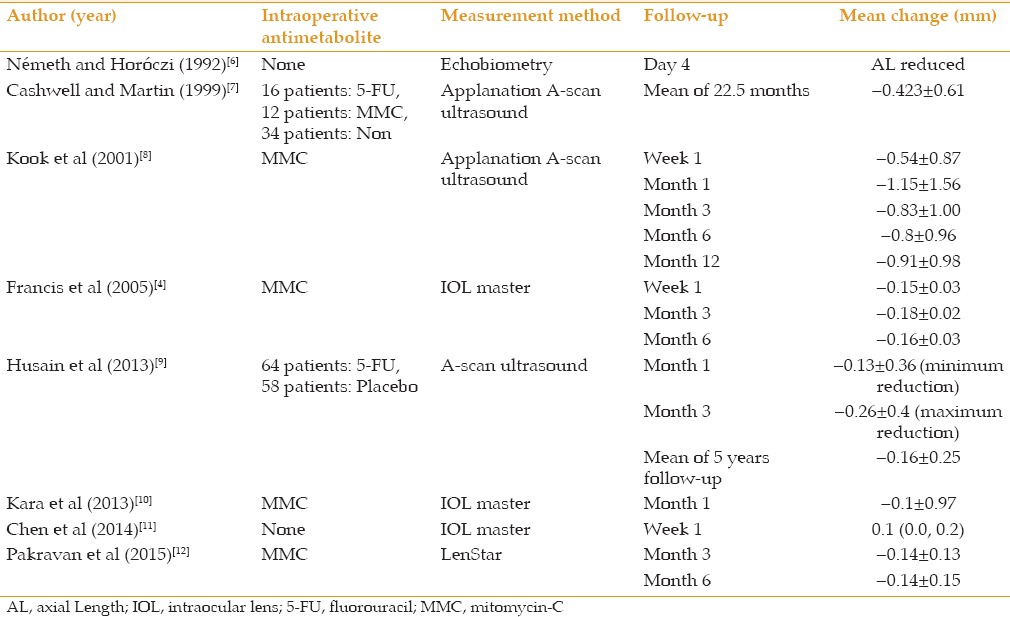
Figure 1.

Axial length changes (mm) after trabeculectomy measured by contact and non-contact biometry during 12-month follow-up.
Anterior Chamber Depth Changes
ACD changes following trabeculectomy were investigated in 9 studies [Table 5]. Measurement methods varied in different studies including: Haag-Streit pachymetry (Haag-Streit AG, Koeniz, Switzerland),[13,16] A-scan (Cinescan; Quantel Medical, Clermont-Ferrand, France),[9,11] ultrasound biomicroscopy (UBM Vumax II, Sonomed Inc., NY, USA),[17,19] and IOL master (Carl Zeiss Meditec AG, Jena, Germany).[18] The studies which evaluated patients in immediate postoperative periods showed that the ACD was the shallowest on days 2-4, and then deepened gradually to reach 87-91% of its preoperative values on day 14. Most of these reports showed that ACD remained less than preoperative values until the last visit.
Table 5.
Anterior chamber depth changes after trabeculectomy in different studies

Keratometry Changes
A total of 12 studies have addressed the effect of trabeculectomy on corneal curvature; they differed in follow-up duration, using intraoperative antimetabolites and method of measurement [Tables 6 and 7]. Four out of these 12 studies compared the keratometric data obtained by keratometry and topography. In all studies, there was an induced with-the-rule (WTR) astigmatism early postoperatively which decreased gradually and shifted toward preoperative values over time [Figure 2].
Table 6.
Keratometry changes after trabeculectomy in different studies

Table 7.
Induced astigmatism (diopter) after trabeculectomy in different studies

Figure 2.
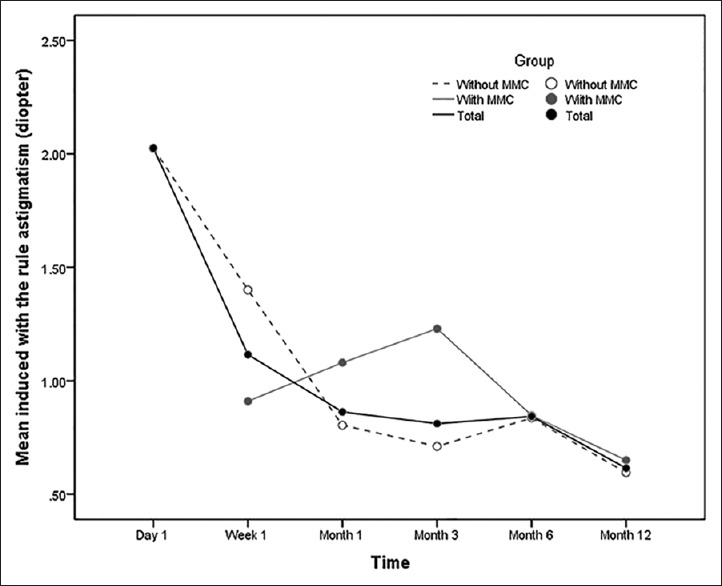
Induced with-the-rule astigmatism after trabeculectomy during one year follow-up. MMC, mitomycin-C.
The AL, ACD and keratometry changes during one year follow-up after trabeculectomy are summarized in Table 8.
Table 8.
Biometric changes after trabeculectomy

DISCUSSION
Trabeculectomy can cause changes in AL, ACD, and keratometry, which may last more than one year.[30] These changes can be significant enough to affect visual acuity, the accuracy of IOL power calculation, and refractive outcomes after combined or future cataract surgery.
The AL is less than preoperative values at all follow-up points after trabeculectomy.[4,6,7,8,9,10,11,12] A reduction of 0.1-0.9 mm has been reported in several studies which would stabilize almost 3 months after the surgery.[4,6,7,8,9,10,11,12] Nemeth and Horoczi [6] noticed the AL reduction 4 days after trabeculectomy for the first time. Cashwell and Martin [7] examined the records of 62 patients who underwent trabeculectomy and compared AL before and after the surgery. Using an applanation ultrasound biometry, they reported a mean AL reduction of 0.423 ± 0.61 mm 22.5 months after trabeculectomy.
Studies on this subject differ mainly in the method of measurement and application of antimetabolites. A subset of the patients who received antimetabolite at the time of surgery had lower IOP and were more likely to experience greater amounts of biometric changes postoperatively.[4,7,8,9,10,12] Kook et al[8] reported a significant reduction in AL after trabeculectomy with mitomycin-C (MMC) which was lower than that reported by other studies. Using an applanation ultrasound biometry, they reported that AL was reduced by 0.83 ± 1.00 mm at the follow-up month 3. In more recent studies in which investigators used non-contact methods for AL measurement, the reported amount of AL reduction were significantly lower than the applanation method. A study by Francis et al[4] showed a reduction of 0.16 ± 0.03 mm in AL post-operatively measured by IOL Master. In a group of their patients who developed hypotony, AL reduction was almost 3 times greater than that in patients without hypotony (0.39 ± 011 vs. 0.14 ± 0.15 mm), which could lead to an error of 1.5 diopters (D) in IOL power calculation.
The reported reduction in AL could last for up to 5 years post-operatively.[9] In a study by Husain et al,[9] mean AL reduction was 0.16 ± 0.25 mm during 5 years of follow-up, with the maximum changes at month 3 after the surgery (0.26 ± 0.4 mm). In a recent prospective study, we used a non-contact optical biometry device (LenStar LS 900) in 34 eyes that underwent trabeculectomy with MMC and found a mean AL reduction of 0.14 ± 0.13 and 0.14 ± 0.15 mm at the postoperative months 3 and 6, respectively.[12] Our results showed that there was no significant difference in the amount of AL reduction between postoperative months 3 and 6, indicating the stabilization of AL changes at least 3 months after the surgery.
Different methods of AL measurement explain disparity between the results of different studies. Ultrasonic methods used in some studies led to more pronounced AL reduction in comparison to non-contact techniques.[7,8,9] Non-contact methods avoid globe indentation and deformation which is in contrast to contact ultrasonic methods tending to deform and underestimate AL in post-trabeculectomy eyes. Therefore, AL may be underestimated by contact methods.
Some studies investigated the causes of AL changes after trabeculectomy and showed that high preoperative IOP, low postoperative IOP, young age, application of antimetabolites, myopic refractive errors and surgical complications such as choroidal detachment and hypotony maculopathy were significantly associated with the AL reduction after the surgery.[4,7,8] Hypotony (IOP ≤6 mmHg) is a common complication after trabeculectomy with a prevalence rate of 10-37%.[31] The amount of AL reduction reported in eyes with hypotony after trabeculectomy is significantly (2.5-3 times) higher than that in non-hypotonic eyes.[4,7] The proposed mechanism of AL reduction after trabeculectomy includes an increase in choroidal and ocular wall thickness associated with IOP reduction after the surgery.[6,8,32] The direct relationship between the amount of IOP reduction, and choroidal thickness increase and AL reduction has been demonstrated in several studies.[8,10] In a study by Husain et al,[9] each one mmHg decrease in IOP led to a 0.01-mm reduction in AL in patients with primary open-angle glaucoma. It has also been shown that patients with open-angle glaucoma are more sensitive to AL changes and experience more AL fluctuations during the first 3 months after trabeculectomy compared to patients with angle-closure glaucoma.[9]
The ACD largely returns to its preoperative values within 14 days following the procedure. After this point, mean ACD is 0.11 ± 0.22 mm smaller compared to preoperative values when obtained by contact ultrasound biometry, and comparable to preoperative values when obtained by non-contact biometry.[13,14,16]
In most studies, it has been shown that ACD becomes shallow immediately after the surgery and reaches its shallowest value on the s2nd and 3rd day post-operatively; thereafter ACD begins to increase gradually to attain its preoperative values on the day 14.[13,14,16] In contrast, Cunliffe et al[15] showed that although ACD returned to its preoperative values in the 3rd week, it was less than preoperative values up to the last visit in the majority of cases (56%). They found a positive relationship between ACD and refractive error changes in their study, such as myopic shift in the first week which returned to preoperative values 3 weeks after trabeculectomy. Martínez-Belló et al[17] examined the anterior chamber angle and depth after trabeculectomy using ultrasound biomicroscopy and showed that despite an increase in the anterior chamber angle, ACD does not change significantly after trabeculectomy.
In contrast to AL measured by non-contact method, ACD seems not to be affected by the procedure, at least 3 weeks post-trabeculectomy; a fact that has been confirmed in a study that used non-contact method to measure ACD.[18] Based on the current literature, it seems that ACD reduction has a minimum contribution to decreased AL observed postoperatively.
Most studies that evaluated keratometric changes after trabeculectomy have revealed induced WTR astigmatism with a mean of 0.81 ± 1.08 D at the postoperative month 3 which tends to resolve within one year.[8,21,24,27,28] This topographic change was first demonstrated by Hugkulstone who observed a reduction in the vertical corneal radius of curvature during 7 weeks after 5 × 5-mm scleral flap trabeculectomy.[20] Similarly, Rosen et al[21] found a mean WTR astigmatism of 1.24 D 3 months after trabeculectomy. This amount of induced astigmatism was confirmed in several other studies.[8,24,27,28]
To further analyze the probable causes of keratometeric changes, some investigators evaluated the amount of induced WTR astigmatism after mini-trabeculectomy (2 mm × 2 mm scleral flap) and reported less amounts of induced astigmatism which lasted for a shorter duration.[27] In contrast, using antimetabolites at the time of surgery is a risk factor for the longer duration of induced WTR astigmatism postoperatively.[25,26] Other suggested mechanisms include tight scleral flap suturing and posteriorly placed wound gape from the internal sclerostomy.[20] Cunliffe et al[15] proposed that internal sclerostomy allows corneal edge of trabeculectomy to retract and subsequently reduced vertical corneal radius. Rosen et al[21] considered cauterization at the time of surgery as one of the main factors for corneal curvature changes. Other explanations put forward include superior corneal steepening secondary to contraction of surrounding tissues at trabeculectomy area, overhanging bleb, and postoperative ptosis.[22]
The mentioned biometric changes may affect IOL power calculation in patients who need cataract surgery and IOL implantation combined with or after glaucoma surgery. For instance, every 0.1-mm change in AL leads to an approximately 0.25-D change in IOL power in an average eye. This error declines to 0.18 D in a very long eye (30 mm eye) and increases to 0.38 D in a very short eye (20 mm eye). Additionally, 1.00-D change in corneal power leads to a 1.00-D change in IOL power.[5] Accurate ACD measurement is important for IOL power calculation using the Haigis, Holladay II or Olsen formulas. Using the Haigis formula in an eye with an AL of 23.5 mm and a mean keratometry of 44.00 D, a difference in ACD of 0.12 mm would result in a change of 0.06D in the target refractive error for a posterior chamber IOL.
The most important implication of biometric changes after trabeculectomy is its effect on refractive outcome of previous, combined, and future cataract surgery. Changes in ACD are of small amount and short lived thus it is not likely to affect the IOL power calculation; however, reported changes in AL and keratometry are of sufficient magnitude to affect refractive prediction of cataract surgery. To achieve the best refractive outcome in these patients, it is better to delay cataract surgery and lens implantation if possible until AL and keratometry changes stabilize. In addition, it is preferable to measure biometric parameters using non-contact optical biometry method instead of contact ultrasound biometry for IOL power calculation in such cases.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Judith EG. Glaucoma Filtering Surgery: Glaucoma Handbook. USA: Butterworth Heinemann; 2001. [Google Scholar]
- 2.Yanoff M, Duker JS. Ophthalmology. 2nd ed. St. Louis: Mosby; 2004. [Google Scholar]
- 3.Morgan WH, Yu DY. Surgical management of glaucoma: A review. Clin Experiment Ophthalmol. 2012;40:388–399. doi: 10.1111/j.1442-9071.2012.02769.x. [DOI] [PubMed] [Google Scholar]
- 4.Francis BA, Wang M, Lei H, Du LT, Minckler DS, Green RL, et al. Changes in axial length following trabeculectomy and glaucoma drainage device surgery. Br J Ophthalmol. 2005;89:17–20. doi: 10.1136/bjo.2004.043950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffer KJ. Intraocular lens power calculation and biometry. In: Vajpayee RB, Sharma N, Pandey SK, Titiyal JS, editors. Phacoemulsification Surgery. New Dehli: Jaypee Brothers Medical Publishers Ltd; 2005. pp. 23–38. [Google Scholar]
- 6.Németh J, Horóczi Z. Changes in the ocular dimensions after trabeculectomy. Int Ophthalmol. 1992;16:355–357. doi: 10.1007/BF00917990. [DOI] [PubMed] [Google Scholar]
- 7.Cashwell LF, Martin CA. Axial length decrease accompanying successful glaucoma filtration surgery. Ophthalmology. 1999;106:2307–2311. doi: 10.1016/S0161-6420(99)90531-6. [DOI] [PubMed] [Google Scholar]
- 8.Kook MS, Kim HB, Lee SU. Short-term effect of mitomycin-C augmented trabeculectomy on axial length and corneal astigmatism. J Cataract Refract Surg. 2001;27:518–523. doi: 10.1016/s0886-3350(00)00646-5. [DOI] [PubMed] [Google Scholar]
- 9.Husain R, Li W, Gazzard G, Foster PJ, Chew PT, Oen FT, et al. Longitudinal changes in anterior chamber depth and axial length in Asian subjects after trabeculectomy surgery. Br J Ophthalmol. 2013;97:852–856. doi: 10.1136/bjophthalmol-2012-302442. [DOI] [PubMed] [Google Scholar]
- 10.Kara N, Baz O, Altan C, Satana B, Kurt T, Demirok A. Changes in choroidal thickness, axial length, and ocular perfusion pressure accompanying successful glaucoma filtration surgery. Eye (Lond) 2013;27:940–945. doi: 10.1038/eye.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Wang W, Gao X, Li Z, Huang W, Li X, et al. Changes in choroidal thickness after trabeculectomy in primary angle closure glaucoma. Invest Ophthalmol Vis Sci. 2014;55:2608–2613. doi: 10.1167/iovs.13-13595. [DOI] [PubMed] [Google Scholar]
- 12.Pakravan M, Alvani A, Yazdani S, Esfandiari H, Yaseri M. Intraocular lens power changes after mitomycin trabeculectomy. Eur J Ophthalmol. 2015;25:478–482. doi: 10.5301/ejo.5000604. [DOI] [PubMed] [Google Scholar]
- 13.Kao SF, Lichter PR, Musch DC. Anterior chamber depth following filtration surgery. Ophthalmic Surg. 1989;20:332–336. [PubMed] [Google Scholar]
- 14.Goins K, Smith T, Kinker R, Lewis J. Axial anterior chamber depth after trabeculectomy. Ophthalmologica. 1990;200:177–180. doi: 10.1159/000310102. [DOI] [PubMed] [Google Scholar]
- 15.Cunliffe IA, Dapling RB, West J, Longstaff S. A prospective study examining the changes in factors that affect visual acuity following trabeculectomy. Eye (Lond) 1992;6(Pt 6):618–622. doi: 10.1038/eye.1992.133. [DOI] [PubMed] [Google Scholar]
- 16.Peng SX, Zhou WB. The anterior chamber depth after trabeculectomy. Zhonghua Yan Ke Za Zhi. 1992;28:214–216. [PubMed] [Google Scholar]
- 17.Martínez-Belló C, Rodriguez-Ares T, Pazos B, Capeáns C, Sánchez-Salorio M. Changes in anterior chamber depth and angle width after filtration surgery: A quantitative study using ultrasound biomicroscopy. J Glaucoma. 2000;9:51–55. doi: 10.1097/00061198-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Karasheva G, Goebel W, Klink T, Haigis W, Grehn F. Changes in macular thickness and depth of anterior chamber in patients after filtration surgery. Graefes Arch Clin Exp Ophthalmol. 2003;241:170–175. doi: 10.1007/s00417-003-0628-6. [DOI] [PubMed] [Google Scholar]
- 19.Man X, Chan NC, Baig N, Kwong YY, Leung DY, Li FC, et al. Anatomical effects of clear lens extraction by phacoemulsification versus trabeculectomy on anterior chamber drainage angle in primary angle-closure glaucoma (PACG) patients. Graefes Arch Clin Exp Ophthalmol. 2015;253:773–778. doi: 10.1007/s00417-015-2936-z. [DOI] [PubMed] [Google Scholar]
- 20.Hugkulstone CE. Changes in keratometry following trabeculectomy. Br J Ophthalmol. 1991;75:217–218. doi: 10.1136/bjo.75.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen WJ, Mannis MJ, Brandt JD. The effect of trabeculectomy on corneal topography. Ophthalmic Surg. 1992;23:395–398. [PubMed] [Google Scholar]
- 22.Claridge KG, Galbraith JK, Karmel V, Bates AK. The effect of trabeculectomy on refraction, keratometry and corneal topography. Eye (Lond) 1995;9(Pt 3):292–298. doi: 10.1038/eye.1995.57. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Peng D, Chen J. Corneal astigmatism following trabeculectomy. Zhonghua Yan Ke Za Zhi. 1996;32:355–358. [PubMed] [Google Scholar]
- 24.Dietze PJ, Oram O, Kohnen T, Feldman RM, Koch DD, Gross RL. Visual function following trabeculectomy: Effect on corneal topography and contrast sensitivity. J Glaucoma. 1997;6:99–103. [PubMed] [Google Scholar]
- 25.Zarnowski T, Haszcz D, Rakowska E, Zagórski Z. Corneal astigmatism after trabeculectomy. Klin Oczna. 1997;99:313–315. [PubMed] [Google Scholar]
- 26.Hong YJ, Choe CM, Lee YG, Chung HS, Kim HK. The effect of mitomycin-C on postoperative corneal astigmatism in trabeculectomy and a triple procedure. Ophthalmic Surg Lasers. 1998;29:484–489. [PubMed] [Google Scholar]
- 27.Vernon SA, Zambarakji HJ, Potgieter F, Evans J, Chell PB. Topographic and keratometric astigmatism up to 1 year following small flap trabeculectomy (microtrabeculectomy) Br J Ophthalmol. 1999;83:779–782. doi: 10.1136/bjo.83.7.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egrilmez S, Ates H, Nalcaci S, Andac K, Yagci A. Surgically induced corneal refractive change following glaucoma surgery: Nonpenetrating trabecular surgeries versus trabeculectomy. J Cataract Refract Surg. 2004;30:1232–1239. doi: 10.1016/j.jcrs.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 29.El-Saied HM, Foad PH, Eldaly MA, Abdelhakim MA. Surgically induced astigmatism following glaucoma surgery in Egyptian patients. J Glaucoma. 2014;23:190–193. doi: 10.1097/IJG.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 30.Law SK, Riddle J. Management of cataracts in patients with glaucoma. Int Ophthalmol Clin. 2011;51:1–18. doi: 10.1097/IIO.0b013e31821e58aa. [DOI] [PubMed] [Google Scholar]
- 31.Shingleton B, Tetz M, Korber N. Circumferential viscodilation and tensioning of Schlemm canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open-angle glaucoma and visually significant cataract: One-year results. J Cataract Refract Surg. 2008;34:433–440. doi: 10.1016/j.jcrs.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 32.Suñer IJ, Greenfield DS, Miller MP, Nicolela MT, Palmberg PF. Hypotony maculopathy after filtering surgery with mitomycin C. Incidence and treatment. Ophthalmology. 1997;104:207–214. doi: 10.1016/s0161-6420(97)30332-7. [DOI] [PubMed] [Google Scholar]


