Abstract
Loss of light perception (LP) after open globe injury (OGI) does not necessarily mean the patient will have permanent complete visual loss. Findings that seem to be associated reliably with permanent profound vision loss after OGI include optic nerve avulsion, optic nerve transection, and profound loss of intraocular contents, which can be identified with CT/MRI imaging albeit with varying degrees of confidence. Eyes with NLP after OGI that undergo successful primary repair with intact optic nerves may be considered for additional surgery, particularly if there is: (1) recovery of LP on the first day after primary repair; (2) treatable pathology underlying NLP status (e.g., extensive choroidal hemorrhage, dense vitreous and subretinal hemorrhage); (3) NLP in the fellow eye. We counsel patients that the chance of recovering ambulatory vision under these circumstances is very low (~5%).
Key words: Choroidal Hemorrhage, Eye Trauma, No Light Perception, Optic Nerve Avulsion, Optic Nerve Transection, Retinal Detachment, Ruptured Globe, Vitrectomy
INTRODUCTION
Severe ocular trauma is a major cause of profound visual loss among young persons, who may be at higher risk of subsequent injury to their fellow eye. Traditional practice has been to observe or enucleate eyes with no light perception (NLP) after open globe injury (OGI) to reduce the risk of sympathetic ophthalmia or for cosmetic reasons.[1,2] Loss of light perception (LP) after open globe injury, however, does not necessarily mean that the patient will have permanent visual loss.[3,4,5,6,7,8,9,10,11,12] Reported visual recovery rates from NLP to LP or better after globe rupture range from 4%-33%.[3,4,5,6,7,8,9,10,11,12] In one study of 73 NLP eyes (73 patients) with OGI that underwent primary repair, final visual acuity was LP or better in 17 (23%) eyes.[12] In this series, final vision was 20/100 in 1 eye (1%), counting fingers in 2 eyes (3%), hand motions in 9 eyes (12%), LP in 5 eyes (7%), and NLP in 56 eyes (77%).[12]
PATIENT SELECTION
There is no single finding, except optic nerve transection or avulsion, that allows clinicians to be certain that NLP status is permanent after OGI.[3,4,5,6,7,8,9,10,11,12] Unfortunately, optic nerve avulsion can be difficult to confirm in the setting of severe globe trauma because the dural sheath often remains attached to the globe even if retrolaminal neural tissue is disrupted.[13,14,15] In contrast to a complete optic nerve transection, an optic nerve avulsion may be partial, and partial return of visual function has been documented in some cases.[15]
Data from our institution indicate that neither patient gender, the type of injury (e.g. rupture vs. laceration), the presence of retinal detachment/vitreous hemorrhage/choroidal hemorrhage, occurrence of intraocular tissue prolapse, nor ocular trauma score is significantly predictive of visual prognosis [Table 1].[12] Although there was a suggestion that eyes with zone 2 injury might be more likely to recover from NLP, this result was of borderline statistical significance and is probably not clinically important.
Table 1.
Prognostic factors in no light perception eyes after open globe injury[12]
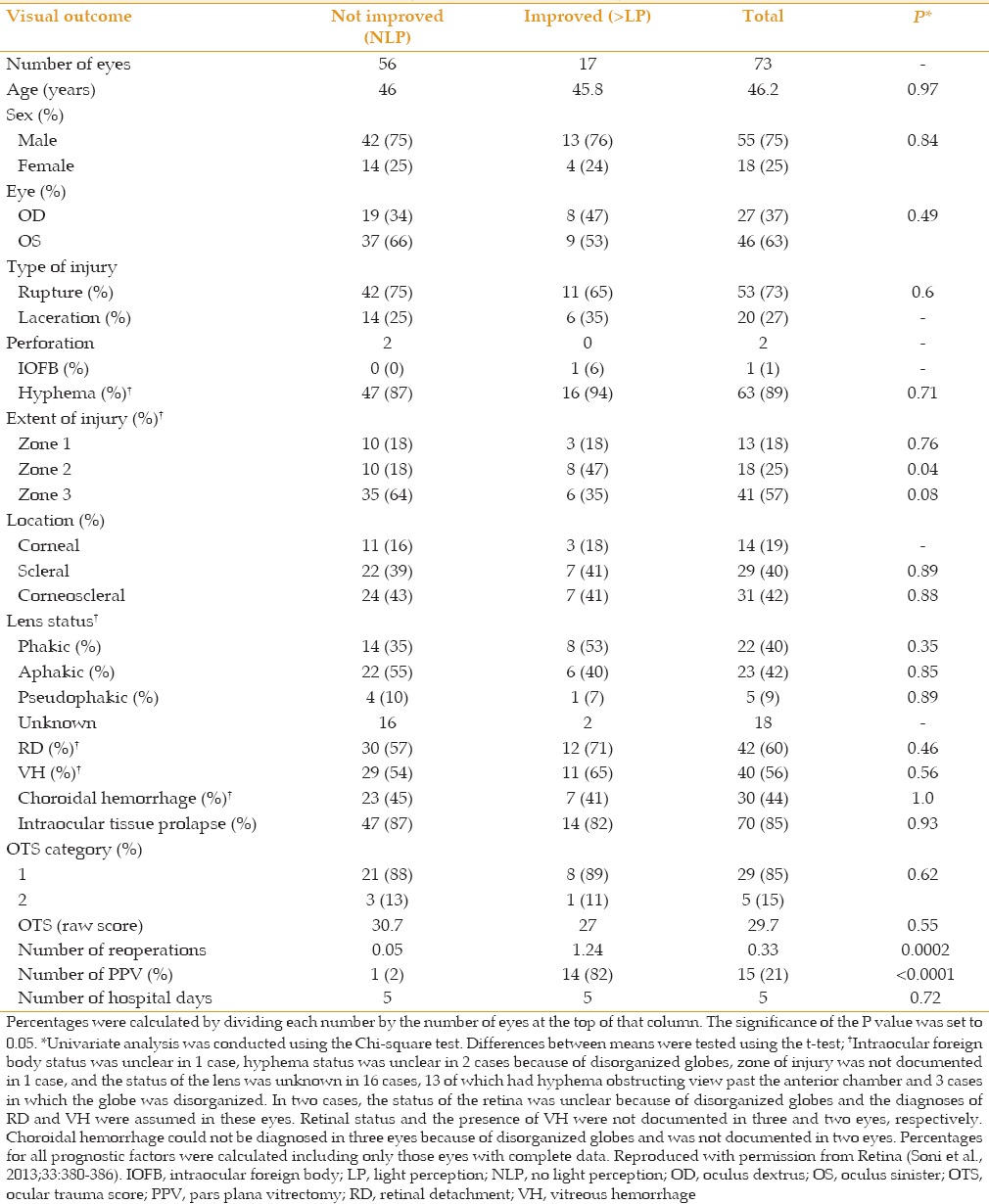
Unfortunately, the information gained from clinical examination is often of limited value. Assessment of LP with the indirect ophthalmoscope, for example, is not always reliable.[16] Cognitive status (e.g., patient age, unconscious, intoxicated, incompetent) influences the result as can anatomic changes that once reversed allow recovery of LP (e.g., choroidal or subretinal hemorrhage ± dense vitreous hemorrhage, particularly in the presence of retinal detachment). Even a severe hyphema can be associated with a relative afferent pupillary defect.[17]
For this reason, it may be more useful to focus on anatomic findings rather than psychophysical findings when assessing visual prognosis. First, one attempts to identify findings that are amenable to surgical repair the correction of which may reverse NLP status, e.g., retinal detachment with subretinal hemorrhage or choroidal hemorrhage [Figure 1]. These findings are often well demonstrated with B-scan echography. Second, one attempts to identify findings that are consistent with irreversible visual loss, e.g., optic nerve avulsion, optic nerve transection, or profound loss of intraocular contents. These findings can be demonstrated with CT or MRI imaging, but optic nerve avulsion may be difficult to confirm with neuro-imaging in the setting of acute severe globe trauma [Figure 2].[13,15,18] Even in the setting of complete optic nerve transection, neuroimaging may reveal what appears to be a surprisingly intact optic nerve complex [Figure 3]. Nonetheless, in some cases, the only way the status of the globe and optic nerve can be explored reliably is with CT/MRI imaging [Figure 4].
Figure 1.
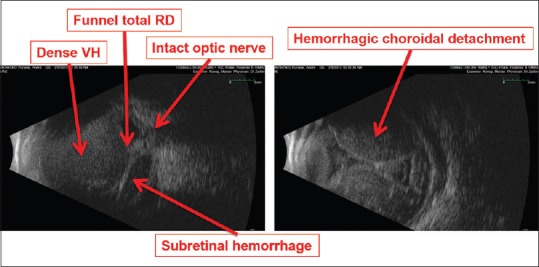
Anatomic findings consistent with reversible NLP status. A 46 year-old man with history of congenital glaucoma, high myopia, S/P enucleation OD, S/P hemorrhagic choroidal detachment during PK for corneal ulcer in the left eye 5 days ago now with NLP vision. NLP, no light perception; PK, penetrating keratoplasty; RD, retinal detachment; S/P, status post; VH, vitreous hemorrhage
Figure 2.
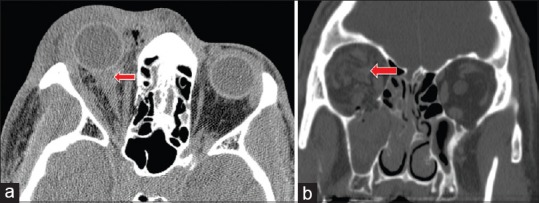
Peribulbar and intra-nerve sheath hemorrhage. (a) Peribulbar and perineural hemorrhage (arrow) misidentified as intraneural hemorrhage on noncontrast axial CT scan. (b) Intra-nerve sheath hemorrhage identified on coronal CT (arrow) in a patients with blunt trauma, right orbital floor fracture, and retrobulbar intraconal hemorrhage. Courtesy of Roger Turbin, MD. CT, computed tomography
Figure 3.
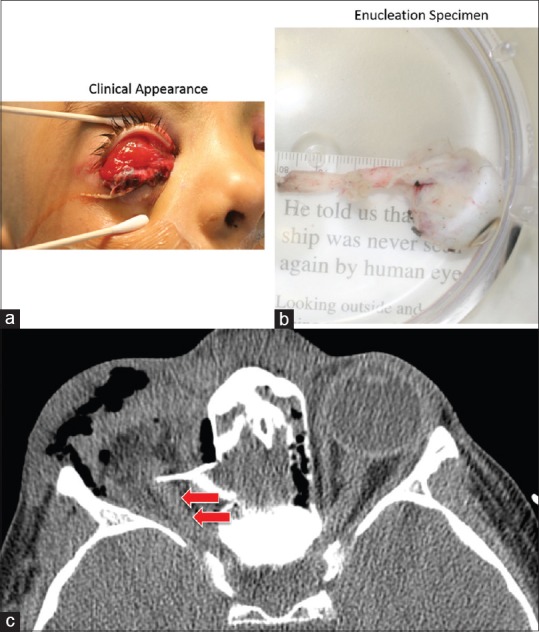
Traumatic intracranial optic nerve and globe avulsion. (a and b) A 5-year-old boy was struck in the forehead by a falling emergency escape ladder that broke free. He completely avulsed the globe and a 3.5 cm segment of optic nerve, the posterior edge of which extended to the intracranial portion of the nerve. Axial CT: Absent right globe, orbital emphysema, bone fragment, and appearance of “normal nerve sheath complex” (arrows). However, the entire orbital optic nerve (3.5 cm) was avulsed from the intracranial segment of the nerve, and the “intact” segment actually represents an artifact of blood and edema. Courtesy of Roger Turbin, MD. CT, computed tomography
Figure 4.
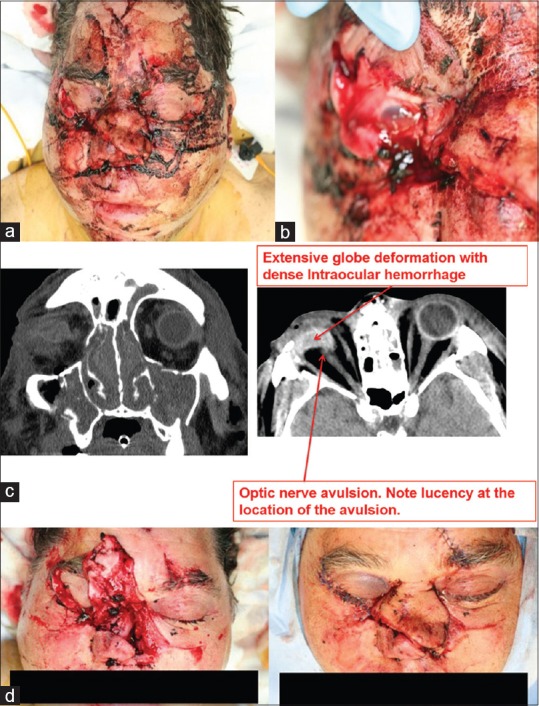
Anatomic findings consistent with irreversible NLP status. (a) Appearance at presentation of a 61-year-old man who sustained multiple craniofacial injuries after a propane tank explosion. (b) Close up view of ruptured globe. (c) CT findings are illustrated. (d) Appearance after surgical repair. Courtesy of Roger Turbin, MD. CT, computed tomography; NLP, no light perception
SURGICAL APPROACH: STAGED SURGERY
The first procedure typically involves repair of the globe rupture/laceration (if possible), removal of intraocular foreign bodies, excision or removal of damaged tissue (e.g., extruded/contaminated tissue, cataractous lens), and drainage of choroidal hemorrhage (usually not possible). If the globe cannot be closed, it is reasonable to consider primary enucleation [Figure 5].
Figure 5.
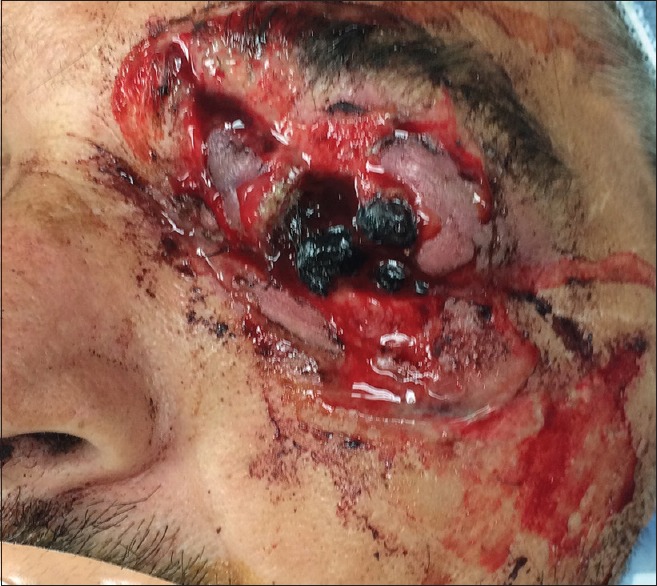
Case for possible primary enucleation. A 51-year-old man was grinding masonry without eye protection. The grinding belt exploded sending pieces of masonry into his face. Deep penetrating orbital wounds and extensive globe laceration are illustrated. Courtesy of Roger Turbin, MD. Foreign bodies were removed from deep soft tissue and the skull base, penetrating the sphenoid wing, orbital roof, and frontal sinus
Ideally, the second procedure is undertaken when the suprachoroidal hemorrhage has liquefied (determined by serial echography), typically within 2 weeks of the injury [Figure 6]. Surgical timing may be very important for increasing the chance of anatomic and functional success. Techniques involved in the second procedure depend on the nature of the injury. If the optical axis of the cornea is damaged, an intraocular keratoprosthesis is used with penetrating keratoplasty completed at the end of the case. If choroidal hemorrhage is present, use of a 6 mm infusion cannula may facilitate infusion into the vitreous cavity (vs. inadvertent infusion into the suprachoroidal space). Alternatively, one may infuse balanced salt solution into the vitreous cavity via a limbal infusion system. In many cases, residual lens and/or foreign material may be present and should be removed. Typically, this scenario occurs in patients with multiple glass intraocular foreign bodies (e.g., after motor vehicle accident), where small slivers of glass may remain hidden in clots of blood. Experimental studies demonstrated detection rates of 57% and 11% for helical CT and MRI, respectively, depending on the size of the glass foreign body.[19] In addition to excision of vitreous hemorrhage, subretinal hemorrhage should be removed if there is an open retinal break or if there is substantial subfoveal blood. Subretinal fibrosis can develop surprisingly rapidly in the setting of post-traumatic rhegmatogenous retinal detachment and may prevent retinal reattachment. Typically, a large retinotomy is made to access the subretinal space if there is posterior peripapillary (“napkin ring”) subretinal fibrosis, and bimanual dissection with chandelier illumination is used to remove the tissue. Often, perfluorodecalin is used to reattach the retina and express subretinal hemorrhage, particularly if a 360-degree retinotomy has been created.[20] In such cases, the residual peripheral retina is excised completely to forestall the development of anterior proliferative vitreoretinopathy with cyclitic membrane formation and hypotony. If retina is incarcerated in the wound or if there is an extensive retinal break, silicone oil tamponade is used typically. Cyclitic membrane formation can begin to develop relatively soon after OGI. Routinely, one should examine the epiciliary space using scleral indentation and the co-axial illumination of the operating microscope to identify and excise such tissue.
Figure 6.
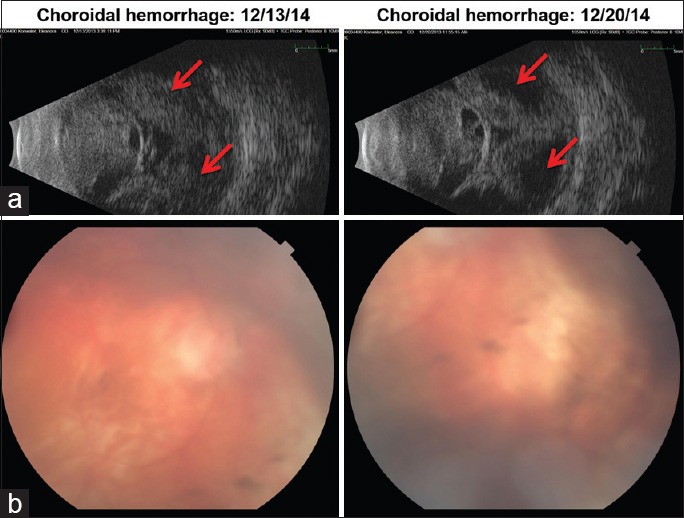
Timing of surgery. A 75-year-old woman with severe Sjogren disease and corneal ulcer in the right eye and amblyopia in the left eye presented with a large perforated corneal ulcer on the right and developed expulsive choroidal hemorrhage during tectonic penetrating keratoplasty (PK). (a) Preoperative ultrasound. Arrows point to suprachoroidal hemorrhage at different times. (b) Postoperative status 3 months after PK, drainage of suprachoroidal hemorrhage, pars plana vitrectomy, retinal reattachment, and silicone oil infusion. Visual acuity was 1/200 on the right and 20/200 in the left eye.
BENEFIT OF PARS PLANA VITRECTOMY
In our series, 15 (21%) of 73 eyes that presented with NLP after OGI, were LP at postoperative day-1 after open globe repair, and underwent pars plana vitrectomy to manage retinal detachment, vitreous hemorrhage, proliferative vitreoretinopathy, and/or choroidal hemorrhage.[12] Of these, 14 (93%) had final vision of LP or better. Of the 17 eyes with final vision of LP or better, 14 (82%) underwent pars plana vitrectomy. Eyes that underwent pars plana vitrectomy were significantly more likely to achieve LP or better final vision than those that did not (odds ratio: 257, 95% confidence interval: 25-2659).
PREDICTING VISUAL PROGNOSIS AFTER RUPTURED GLOBE REPAIR
Among the 21 eyes that presented with NLP but that recovered LP or better after ruptured globe repair on postoperative day-1, 13 (62%) recovered LP or better vision at last follow-up [Figure 7]. Among the 52 NLP eyes (71%) that did not recover LP on postoperative day-1 after ruptured globe repair, only 4 (8%) recovered LP or better vision at last follow-up.
Figure 7.
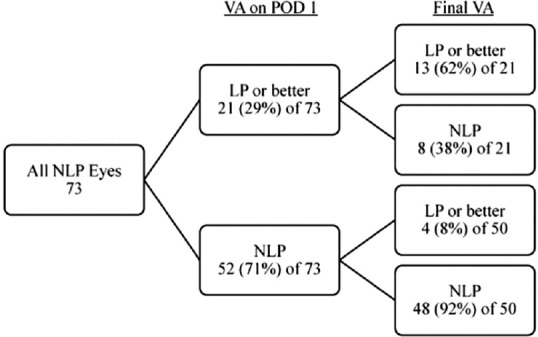
Predicting prognosis based on sensory status after ruptured globe repair.[12] LP, light perception; NLP, no light perception; POD, postoperative day; VA, visual acuity. Reproduced with permission from Retina (Soni et al, 2013;33:380-386).
FUTURE CONSIDERATIONS
We consider enucleation for eyes with OGI and NLP and major loss of retinal tissue or confirmed optic nerve avulsion/transection. Eyes with NLP after OGI that undergo successful primary closure with intact optic nerves may be considered for additional surgery, particularly if there is: (1) Recovery of LP on postoperative day-1 after primary repair; (2) treatable pathology underlying the NLP status (e.g. extensive choroidal hemorrhage, extremely dense vitreous hemorrhage; and/or subretinal hemorrhage; (3) NLP in the fellow eye. Patients should be counseled that the chance of recovering ambulatory vision is very low (~5%) under these circumstances.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial Support and Sponsorship
Supported in part by the New Jersey Lions Eye Research Foundation and the Gene C Coppa Memorial Fund.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Grant WF, Jr, Swan KG. Gunshot wounds of the orbit. J Trauma. 1980;20:809–811. doi: 10.1097/00005373-198009000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Moshfeghi DM, Moshfeghi AA, Finger PT. Enucleation. Surv Ophthalmol. 2000;44:277–301. doi: 10.1016/s0039-6257(99)00112-5. [DOI] [PubMed] [Google Scholar]
- 3.Hui Y, Wang L, Shan W. Exploratory vitrectomy for traumatized eyes with no light perception and dense vitreous hemorrhage. Zhonghua Yan Ke Za Zhi. 1996;32:450–452. [PubMed] [Google Scholar]
- 4.Matthews GP, Das A, Brown S. Visual outcome and ocular survival in patients with retinal detachments secondary to open- or closed-globe injuries. Ophthalmic Surg Lasers. 1998;29:48–54. [PubMed] [Google Scholar]
- 5.Rofail M, Lee GA, O'Rourke P. Prognostic indicators for open globe injury. Clin Experiment Ophthalmol. 2006;34:783–786. doi: 10.1111/j.1442-9071.2006.01309.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt GW, Broman AT, Hindman HB, Grant MP. Vision survival after open globe injury predicted by classification and regression tree analysis. Ophthalmology. 2008;115:202–209. doi: 10.1016/j.ophtha.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Weichel ED, Colyer MH, Ludlow SE, Bower KS, Eiseman AS. Combat ocular trauma visual outcomes during operations iraqi and enduring freedom. Ophthalmology. 2008;115:2235–2245. doi: 10.1016/j.ophtha.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Salehi-Had H, Andreoli CM, Andreoli MT, Kloek CE, Mukai S. Visual outcomes of vitreoretinal surgery in eyes with severe open-globe injury presenting with no-light-perception vision. Graefes Arch Clin Exp Ophthalmol. 2009;247:477–483. doi: 10.1007/s00417-009-1035-4. [DOI] [PubMed] [Google Scholar]
- 9.Heidari E, Taheri N. Surgical treatment of severely traumatized eyes with no light perception. Retina. 2010;30:294–299. doi: 10.1097/IAE.0b013e3181babd75. [DOI] [PubMed] [Google Scholar]
- 10.Feng K, Shen L, Pang X, Jiang Y, Nie H, Wang Z, et al. Case-control study of risk factors for no light perception after open-globe injury: Eye injury vitrectomy study. Retina. 2011;31:1988–1996. doi: 10.1097/IAE.0b013e318213d8c7. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal R, Wei HS, Teoh S. Predictive factors for final outcome of severely traumatized eyes with no light perception. BMC Ophthalmol. 2012;12:16. doi: 10.1186/1471-2415-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soni NG, Bauza AM, Son JH, Langer PD, Zarbin MA, Bhagat N. Open globe ocular trauma: Functional outcome of eyes with no light perception at initial presentation. Retina. 2013;33:380–386. doi: 10.1097/IAE.0b013e318263cefb. [DOI] [PubMed] [Google Scholar]
- 13.Galor A, Perry JD, Ratliff N, Kaiser PK, Bakri SJ, Lee MS. Failure of imaging to detect optic nerve avulsion: An explanation based on histopathology. Eye (Lond) 2006;20:965–967. doi: 10.1038/sj.eye.6702077. [DOI] [PubMed] [Google Scholar]
- 14.Kline LB, McCluskey MM, Skalka HW. Imaging techniques in optic nerve evulsion. J Clin Neuroophthalmol. 1988;8:281–282. [PubMed] [Google Scholar]
- 15.Foster BS, March GA, Lucarelli MJ, Samiy N, Lessell S. Optic nerve avulsion. Arch Ophthalmol. 1997;115:623–630. doi: 10.1001/archopht.1997.01100150625008. [DOI] [PubMed] [Google Scholar]
- 16.Abrams GW, Knighton RW. Falsely extinguished bright-flash electroretinogram. Its association with dense vitreous hemorrhage. Arch Ophthalmol. 1982;100:1427–1429. doi: 10.1001/archopht.1982.01030040405005. [DOI] [PubMed] [Google Scholar]
- 17.Striph GG, Halperin LS, Stevens JL, Chu FC. Afferent pupillary defect caused by hyphema. Am J Ophthalmol. 1988;106:352–353. doi: 10.1016/0002-9394(88)90374-1. [DOI] [PubMed] [Google Scholar]
- 18.Sawhney R, Kochhar S, Gupta R, Jain R, Sood S. Traumatic optic nerve avulsion: Role of ultrasonography. Eye (Lond) 2003;17:667–670. doi: 10.1038/sj.eye.6700411. [DOI] [PubMed] [Google Scholar]
- 19.Gor DM, Kirsch CF, Leen J, Turbin R, Von Hagen S. Radiologic differentiation of intraocular glass: Evaluation of imaging techniques, glass types, size, and effect of intraocular hemorrhage. AJR Am J Roentgenol. 2001;177:1199–1203. doi: 10.2214/ajr.177.5.1771199. [DOI] [PubMed] [Google Scholar]
- 20.Kolomeyer AM, Grigorian RA, Mostafavi D, Bhagat N, Zarbin MA. 360° retinectomy for the treatment of complex retinal detachment. Retina. 2011;31:266–274. doi: 10.1097/IAE.0b013e3181eef2c7. [DOI] [PubMed] [Google Scholar]


