Abstract
Attention is a component of the working memory system, and as such, is responsible for protecting task-relevant information from interference. Cognitive performance (particularly outside of the laboratory) is often plagued by interference, and the source of this interference, either external or internal, might influence the expression of individual differences in attentional ability. By definition, external attention (also described as “selective attention”) protects working memory against sensorial distractors of all kinds, while internal attention (also called “inhibition”) protects working memory against emotional impulses, irrelevant information from memory, and automatically-generated responses. At present, it is unclear if these two types of attention are expressed independently in non-human animals, and how they might differentially impact performance on other cognitive processes, such as learning. By using a diverse battery of four attention tests (with varying levels of internal and external sources of interference), here we aimed both to explore this issue, and to obtain a robust and general (less task-specific) measure of attention in mice. Exploratory factor analyses revealed two factors (external and internal attention) that in total, accounted for 73% of the variance in attentional performance. Confirmatory factor analyses found an excellent fit with the data of the model of attention that assumed an external and internal distinction (with a resulting correlation of 0.43). In contrast, a model of attention that assumed one source of variance (i.e., “general attention”) exhibited a poor fit with the data. Regarding the relationship between attention and learning, higher resistance against external sources of interference promoted better new learning, but tended to impair performance when cognitive flexibility was required, such as during the reversal of a previously instantiated response. The present results suggest that there can be (at least) two types of attention that contribute to the common variance in attentional performance in mice, and that external and internal attentions might have opposing influences on the rate at which animals learn.
Keywords: Attention, External attention, Internal attention, Working Memory, Learning, Individual Differences, Mice
1. Introduction
Top-down attention is a component of the working memory system that protects task-relevant information from interference (Engle, 2002). Because goal-directed activities are often surrounded by interference, working memory is critically dependent on attention for efficient processing/execution (with “attention” being sometimes referred to “executive control”; Jarrold & Towse, 2006). Variations in attention in humans are a defining feature of disorders like ADHD, schizophrenia, and autism (Allen & Courchesne, 2001; Barch, 2005; Tsal, Shalev, & Mevorach, 2005), and the efficacy of attention is believed to have an influence on human intelligence (Conway, Kane, & Engle, 2003).
Humans that perform well in one particular cognitive task (such as reading comprehension) tend to perform well in tasks that explicitly reflect other intellectual domains (like quantitative reasoning). This positive manifold (i.e., the pattern of positive intercorrelations between domains) is believed to exist due to different intellectual domains either emerging together into (van der Maas et al., 2006) or being under the hierarchical influence of (Mackintosh, 2011) a single “general” factor. This factor, originally referred to as “g” (Spearman, 1904), accounts for as much as 50% of an individual's performance across sets of diverse cognitive tasks, (Carroll, 1993; Jensen, 1998). The attentional component of working memory is widely asserted to impact performance across all (or most) cognitive domains, and thus may be critical to variations in “g” (Dempster & Corkill, 1999; Jarosz & Wiley, 2012).
Previously, we have assessed the relationship between “intelligence” and attention using genetically heterogeneous mice as subjects. Using non-human animals to study cognition provide many methodological advantages, and might allow future lines of genetic and neurophysiological investigation that are impractical with humans (e.g., Kolata et al., 2010; Wass et al., 2013). Mice's performance across diverse learning tasks is positively correlated, and a single factor accounts for 25–48% of the variance in performance across as many as eight tasks (reviewed in Kolata, Light, & Matzel, 2008). This factor correlates with reasoning skills – suggesting that the performance in our learning tests captures other cognitive abilities, too (Wass et al., 2012). Interested in studying attention, we developed a version of the Stroop Test for mice (see review in Matzel & Kolata, 2010), since the original Stroop Test has been the gold-standard of attentional measures in humans (for a review, see MacLeod, 1991). We found that a mouse's performance in the Mouse Stroop Test strongly and consistently predicted its general learning ability (Kolata et al., 2007). However, as a single test, performance on the mouse Stroop task might reflect non-attentional variables, such as foraging capacity, sensory acuity, and motivation, all of which could confound the effect of attention per se. Even more importantly, attention can be divided into several subtypes, and these might be differently related to intelligence (Conway et al., 2005; Mirsky et al., 1991; Schweizer et al., 2005).
The distinction between internal and external attention is of critical functional significance (Mysore & Knudsen, 2013; Nee & Jonides, 2008; Nee et al., 2012). “External attention” protects working memory against external sources of interference (sensorial distractors of all kinds), and is commonly alluded to in the context of “selective attention” and “perceptual attention” (examples in Kane & Engle, 2002; Lavie & Fox, 2000; Tiitinen et al., 1993). “Internal attention”, on the other hand, protects working memory against internal sources of interference (e.g., emotional impulses, irrelevant information from memory, and automatically-generated responses), and is commonly alluded to the literature as representing “inhibition”, “self-control”, and “reflective attention” (examples in Johnson et al., 2002; Kuntsi, Oosterlaan, & Stevenson, 2001; Lister et al., 2013; Tangney, Baumeister, & Boone, 2004). Due to those properties, internal and external attention (or the attention against external and internal sources of interference) are believed to be at the core of the interface between perception, working memory, and long term memory (Chun & Johnson, 2011). At present, it is unclear if or how these two subtypes of attention differentially impact an animal's performance on non-attentional tests, such as those that assess learning.
Here, we aimed to obtain a robust (and less task-specific) measure of attention in mice by using diverse attention tests. In addition, we used our attention battery to explore the attentional property of source of interference, as well as its relationship to general learning performance of mice. The four tests in our “mouse attention battery” involve unique combinations of many non-attentional properties, such as learning, navigation, retrieval, and emotional reaction. Because of this, the common variance obtained from the battery should represent that which the tests share in common: attention. In addition, the interference imposed on the animals during the execution of the tasks originates from internal and external sources (in varying degrees), and thus differentially tax external and internal attentional systems.
For the current study, we focused on the correlational approach in Psychology, using procedures dealing with differences between individuals, not groups. While the experimental approach can easily reveal the causes underlying a simple trait, the correlational approach is better suited to identify causes of variation behind complex traits – determining how much a set of variables contribute to observed differences between individuals (for the importance of using the correlation approach in Psychology, see Cronbach, 1957; Kline, 2011. For its importance in studies on learning and behavior, see Sauce & Matzel, 2013). First, we conducted exploratory factor analyses to examine the patterns of the mice's performances in the four distinct tests of attention. Then, we used confirmatory factor analysis to test if the theoretical model with a single factor (“attention”) as the unique systematic source of variance was able to explain intercorrelations between animals’ performance on the four attentional tests. Then, we assessed a model with two factors of source of interference (where two tests with mostly internal sources of interference were compared to two tests with mostly external sources of interference). Finally, we explored the relationship of attention with learning abilities (using learning data derived from the preparatory steps for the attention tests).
2. Materials and Methods
2.1. Animals and Environment
We used 26 outbred CD-1 male mice (Harlan Sprague Dawley Inc., Indianapolis, IN) that weighed 25-30 g and were approximately 70 days of age upon arrival in our laboratory (Animals were group housed among siblings prior to weaning at 21 days of age, and singly housed thereafter.) They were individually housed in clear shoebox cages (28.5 × 17.5 × 12 cm) inside a temperature-controlled colony room with a 12:12h light-dark cycle. To reduce the effect of individual differences in stress due to handling and removal from the home cage, we removed each animal from its cage and handled it daily for 60 seconds during the two weeks before the start of behavioral testing. (Handling consisted of holding a mouse on the palm of an experimenter's hand, and systematically walking it around the laboratory.) The mice were young adults (approximately 90 days of age) at the start of testing.
2.2. Attention Battery
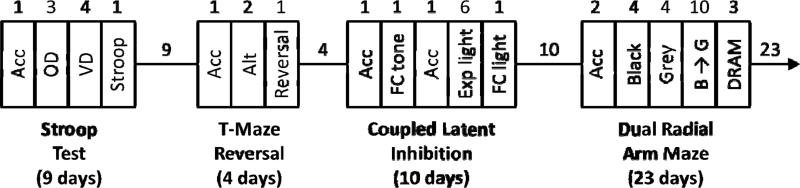
The attention battery consisted of four different tests: Mouse Stroop Test, T-Maze Reversal, Coupled Latent Inhibition, and Dual Radial Arm Maze (administered in this order). In order to reduce confounding effects (such as differential stress responses) on the variation in attentional performance, we administered the tests in the same order for all animals (a standard practice when assessing individual differences). This insures that we are sensitive to innate differences between individuals, rather that differences that might arise were animals to be exposed to tests in different orders. We performed two interleaved runs of the attention battery following the scheme in Figure 1, with 13 mice assigned to each run. For the Mouse Stroop Test, T-Maze Reversal, and Dual Radial Arm Maze, we food deprived the mice by giving them only 90 min of access to food daily, beginning on the day prior to training. This protocol leads to an average loss of 5% of the ad libitum body weight.
Figure 1.
The timeline of procedures for the four attention tests for a single run of animals: Mouse Stroop Test, T-Maze Reversal, Coupled Latent Inhibition, and Dual Radial Arm Maze. The procedures inside the boxes are: Acclimation (Acc), Odor Discrimination (OD), Visual Discrimination (VD), Stroop Test (Stroop), Reinforced Alternation (Alt), Reversal of Alternation (Reversal), Fear Conditioning with Tone (FC Tone), Exposure to Light (Exp Light), Fear Conditioning with Light (FC Light), Radial Arm Maze with the Black Maze (Black), Radial Arm Maze with the Grey Maze (Grey), Radial Arm Maze with the Black Maze in the morning and Grey Maze in the afternoon (Black −> Grey), Dual Radial Arm Maze (DRAM). Numbers indicate duration in days. Space between rectangles indicates the resting time for one run of animals, during which time the other run of animals was tested. Animals were 90 days old (young adult) when the procedures started.
2.2.1. Mouse Stroop Test
When administered to humans, the Stroop Test presents subjects with color-words printed in inks of various colors, and requires them to report the ink color of the word. Subjects show slowed responses and more errors when there is interference from a word naming a color, such as the word ‘red’ printed in blue color. In an analog of this test adapted for mice, mice were first required to associate meaning to odor and visual cues, analogous to the way the human Stroop Test requires subjects to know how to identify words and colors. For this, we trained the mice in two tasks: odor discrimination and visual discrimination.
In the odor discrimination task, mice had to use a specific odor cue to find food. The task was administered in a square box of black Plexiglas, called here “odor box” (45.5 cm ×45.5 cm surface and 20 cm walls), under dim light (10 Lux). Three of the box's four corners always contained cups (that could hold food), and the fourth corner served as a start location. The cups were held in place by vertical structures with small holes creating patterns (see below), and each of these structures could be illuminated from the rear by light bulbs (10 W). These illuminated patterns of holes function as visual cues for the visual discrimination task (see below), but their lights were off during the odor discrimination task. Each cup had a metal mesh separating the bottom from the top, and the bottom contained cotton with 20 μL of flavor extract; one with mint, one with lemon, and one with almond (McCormick flavor extracts). All of the cups had three food pellets (chocolate-flavored crisp rice, 30 mg), but only one cup (marked with the target cue: mint) had one accessible piece of food (above the mesh), while the other two cups had three inaccessible pieces (below the mesh). This controlled for differential degrees of food odor at each cup. On each trial, the corner with a particular odor (and its associated food cup) was systematically re-arranged to avoid any association of the target odor (mint) with specific spatial cues. Thus in this task, the animal's objective was to locate the accessible food using the odor of mint as the discriminative stimulus.
In the visual discrimination task, mice had to use a specific visual cue to find the food. The cotton in the cups did not have flavor extracts, but in this task the lights bulbs illuminated the patterns of holes (a 14 × 14 cm grid comprised of 0.5 cm holes). One pattern of holes formed an X (the target cue), one a circle, and one a rectangle. The visual discrimination was performed in a different box (called here “visual box”) than the odor discrimination. The visual box had the same dimensions, but with vertical white stripes (2 cm wide, with 2 cm between each stripe) on the walls of the box. This difference was important during the critical phase of the Mouse Stroop Test to allow mice to distinguish between the odor box (“mint = food”) and the visual box (“X = food”). All other features of the visual task were as described for the odor task.
The day before starting the first task (odor discrimination), each mouse was acclimated to an empty odor box for 20 min. We then administered three days of odor discrimination training and four days of visual discrimination training, with four trials per day and an 8 min intertrial interval in both tasks. These training parameters have previously been shown to support asymptotic (near errorless) performance in each task (Kolata et al., 2007).
After training separately in the odor and visual discrimination tasks, we then conducted the Mouse Stroop Test. It consisted of a complex discrimination task that requires mice to ignore interference from one of the learned target cues and maintain attention on the context-appropriate cue. The context (the two different training boxes) determined if the relevant cue (the one that marks the available food) is either visual (X) or odor (MINT). For this purpose, we conducted the tests in the visual box with both the previously trained visual cues (with X as the target), and odor cues (with MINT as the task-relevant distractor) present. (During these tests, MINT and X were never presented at the same location.) We conducted similar tests in the odor box, with, in this instance, MINT being the target cue, and the visual X serving as the task-relevant distractor. Mice were judged to have committed an error whenever it put its nose inside/above a non-target cup (i.e., one marked by a non-target cue). Since they naturally rely more on odor cues than visual cues for foraging, the odor distractors in the visual box typically induce more errors (i.e., are more difficult to ignore), and, hence, those results are better suited to detect individual differences in attention. For this reason, we used the average number of errors in the visual box of the Mouse Stroop Test as a measure of attention (better attention = fewer errors).
2.2.1.1. Mouse Stroop Test: Design in relation to the source of interference
Regarding the two different types of interference, it is possible that our mouse version of the Stroop Test, similarly to the human version, taxes both internal and external sources. As described above, the critical test trials in the human Stroop Test (also called “incongruent trials”) have color and word providing conflicting information. This is a clear case of external interference. However, if test trials are preceded by congruent trials (in which color and word match), there is an internal source of interference originating from the repetition of the tendency to read the word (Cohen, Dunbar, & McClelland, 1990; Kane & Engle, 2003). Similarly, in mice, trials in the odor box (odor as the target) during the Mouse Stroop Test might create an error-prone expectation in mice that food will always be on the naturally salient odor cue instead of the visual cue. Hence, in addition to external interference, in the Mouse Stroop Test the animal also needs to ignore internal interference elicited by the native tendency for odors to guide searches for food. However, there were only six test trials in total and they were intercalated, so the error-prone expectation from trials with odor as target never accumulated (unlike in the case of the many congruent trials in a row during the human Stroop Test). For this reason, although the Mouse Stroop Test probably taxed both external and internal attentions, we believe the predominant source of interference originated externally.
2.2.2. T-Maze Reversal
For the T-Maze Reversal task, we first trained the mice in a reinforced alternation task where they must alternate their foraging (for a food reward) between two arms. Then, reversal training began, wherein food was always located in the same arm. This reversal training required animals to ignore the previously learned response and maintain attention to the new task requirements. In other words, the animal must resist a source of interference that originates internally (i.e., the prior learning).
The apparatus consisted of a start arm (9.5 cm wide × 31 cm long) that intersected at its extremity with two choice arms (39.5 cm long × 9.5 cm wide, each), forming a “T” shape that has 10 cm black acrylic glass walls. The initial part of the start arm, the start area (13.5 cm long), had a vertical door that was remotely operated. At the entry of each choice arm, there was another remotely operated, vertical door. To help animals distinguish between arms, one of the arms’ walls had vertical white stripes, and the other had horizontal white stripes.
On the first day of the Reinforced Alternation task, animals were acclimated to the apparatus by giving them four forced choices. A mouse was held in the start area for 30 seconds, and then allowed to pass through, with only the door leading to the right arm opened. After the food was eaten, we returned the mouse to the start area for a 20-second intertrial interval. We then repeated this procedure with the right door closed and the left door opened instead. After the food was eaten, this sequence restarted for a total of four forced-choice trials. On the day following acclimation, we administered training trials in which mice chose between either open arms. On the first trial, food was available in both arms, and the animal was able to make one free choice. On the second trial, food was only available in the arm not chosen on the first trial. If an incorrect choice was made, we allowed the animal to correct its mistake and find the food in the other arm. After the correct choice was made, we placed the animal back in the start area and waited 20 seconds for the following trial, now with food only in the opposite choice arm. Accordingly, the mice had to alternate their choices to most efficiently obtain food. We administered two days of training with 12 trials per day. From our previous experience, this level of training is sufficient to support a high level of efficacy.
One day after the completion of training, the T-Maze Reversal test of attention began. The first four trials were forced alternations (where the animals can only alternate), administered in the same manner as the first four trials of the acclimation day. This was to better instantiate the animals’ tendency to alternate. After this, 12 reversal trials were administered. For this testing, the food was in the same choice arm every trial, which could be either always left or always right depending on a free choice that each animal made at the start of Trial 5. (In this manner, animals could self-select a side for their first choice. Hence, “side biases” should not have differentially impacted the group.) To perform well in this test, a mouse has to learn the new contingency, and thus performance during this phase of testing is a function of both new learning and their capacity to attend exclusively to the new contingency (and ignore the previous alternation contingency). To specifically assess animals’ ability to reverse (i.e., ignore a previously learned response rule, or resist this source of internal interference), we divided the results from the reversal by the average number of correct choices during the same period (trials 5 to 12, out of 24) of the reinforced alternation task. Hence, this measure reflected an animal's reversal performance relative to its respective normal performance (and thus should be relatively independent of learning ability per se). If we had only considered animals’ performance during reversal training (irrespective of their performance during alternation training), an animal that was slow to learn could be evaluated as performing “worse” than an animal that learns more quickly, even though the slow learning animal might have higher attentional abilities. (Such an analysis regimen is crucial, because although learning and attentional abilities tend to co-vary, they are not a single process.) We used this as a measure of mice's attention (better attention = higher values).
2.2.2.1. T-Maze Reversal: Design in relation to the source of interference
The type of interference imposed on the animal during T-Maze Reversal training arises entirely from the mice's learned tendency to alternate arms, so we considered it as taxing internal attention.
2.2.3. Coupled Latent Inhibition
In latent inhibition, an animal is repetitively exposed to a stimulus (that will later serve as a CS) that has no explicit meaning (i.e., it is presented alone). Subsequently, it is difficult for that animal to learn to associate that stimulus with a second stimulus (i.e., associative learning is impaired). Latent inhibition is often regarded as an instance where the animal comes to ignore irrelevant stimuli, and thus is considered to be dependent on variations in attention (Kaplan & Lubow, 2011; Lubow, 1973). Here, we used a Coupled Latent Inhibition procedure that, in principle, could assess variation in attention independently of variation in learning. First, we conducted a fear conditioning task (tone-shock pairings) to determine each mouse's learning rate in the absence of interference (i.e., no prior exposure to the CS). Then, we conducted a fear conditioning task to determine their rate of learning of a light-shock association after extensive latent inhibition trials with non-reinforced exposure to the light (i.e., interference from prior experience). In this later case, the animals had to overcome the habit of ignoring the light, and maintain attention to the new relevance of this stimulus (i.e., its relationship to the shock). Here, the measure of the effects of latent inhibition (interference) was reflected as the difference between the rate of learning of the tone-shock and light-shock associations. (It should be noted that owing to our interest in individual differences, here it would not have been prudent to counterbalance the CSs used in this test. To overcome this possible complication, we chose parameters of the tone and light conditioning regimens based on prior research in which we determined values that supported similar rates of acquisition to each of these stimuli.)
During the initial fear conditioning (tone-shock) task, we placed the animals in a box (26 × 26 × 21 cm; length x width x height) contained within a sound and light-attenuating chamber. The box was brightly lit (100 Lux) with clear Plexiglas walls, and had a stainless steel grid floor (spacing of 5 mm) that could deliver a short (500 ms) 1 mA scrambled foot shock. A camera recorded animals inside the box for later assessment of freezing during the tone that predicted the onset of shock (see below). Animals were acclimated to the box for 30 minutes one day before the start of presentation of any explicit stimuli. During a trial, a mouse was presented with a tone (CS1, emitting at 60 dBs above background) for 20 seconds (cycles of 0.7 second ON and 0.3 second OFF), which terminates with foot shock (US). The mice received eight trials (four per day) with a 4-minute intertrial interval. With successive pairings, animals usually exhibit increased freezing during the CS, a response indicative of fear and defined here as head and body immobilization with the four paws on the floor. We calculated the time of CS1 freezing by measuring the time spent freezing during the 20 seconds of tone, and subtracting the time spent freezing during 20 seconds before the tone (the latter a measure of context freezing). Negative numbers were assigned a value of “0” since they represent an absence of learning.
During the latent inhibition phase of this test, we used the same box and chamber, but now with a different smell (thyme powder added to the inside of the chamber), with illumination coming only from outside the chamber (5 Lux inside the chamber), and with walls covered by vertical black stripes (3 cm wide, with 3 cm between stripes). We did this to minimize any fear associated with the previous training context. Animals were acclimated to the box for 30 minutes one day before the first presentation of any explicit stimuli. Then, they were exposed to 20 seconds (cycles of 0.7 second ON and 0.3 second OFF) of flashes of unfiltered light (CS2, emitting 100 Lux) without any shocks for 48 trials (eight per day for six days), with a 4-min intertrial interval. These presentations of CS2 without shock presumably led animals to ignore (habituate to) the CS2. (Prior work had determined that this pattern of presentation of CS results in subsequent retardation of a light-shock association, i.e., induced latent inhibition.) After all mice had been pre-exposed to CS2, we began to pair CS2 with foot shock (i.e., fear conditioning). This training proceeded in the same manner as for the tone-shock pairings. We calculated time of CS2 (light) freezing in the same way described for CS1 (tone).
Having determined freezing during CS1 and CS2, we then subtracted, for each animal, the average value during acquisition of fear to CS1(in our case, trials 2 to 4) from the average value during the same period(trials 2 to 4) of acquisition of fear to CS2 (which had undergone nominal latent inhibition training). The result was the value for Coupled Latent Inhibition, which is a measure of attention. Since both of the fear conditioning tasks were dependent on animals’ basic learning ability, this subtraction procedure provided a measure of the impact of latent inhibition that was independent of an animal's characteristic learning ability (e.g. a slow animal during fear conditioning was compared to itself during latent inhibition). Relative to the performance in fear conditioning before latent inhibition, a lower performance in fear conditioning after latent inhibition leads to negative values in the Coupled Latent Inhibition measurement. In other words, a good performance in attention requires values after latent inhibition to be close to (or higher than) the performance in fear conditioning before latent inhibition (better attention = higher absolute values).
2.2.3.1. Coupled Latent Inhibition: Design in relation to the source of interference
Interference in Coupled Latent Inhibition arose from predominantly internal sources because mice had to actively inhibit the learned tendency to ignore CS2, and this was the only consistent distractor. It is important to note that in order for the distractor's strength be the same for all animals, the level of habituation (c.f., latent inhibition) to CS2 at the start of CS2-US pairing must be comparable across all animals. Without a comparable level of latent inhibition across all animals, our data would be difficult to interpret. For instance, Sörqvist, Nöstl, & Halin (2012) found that greater working memory in humans is correlated with greater habituation rate, and Light, Grossman, Kolata, Wass, & Matzel (2011) found that higher general learning abilities in CD-1 mice are correlated with higher habituation rates. Consequently, a mouse with higher attention would have habituated more to the CS2, and, consequently, would be slower to learn the CS2-US association (due to a stronger tendency to ignore CS2). Such a pattern would result in our underestimation of the attentional ability of animals of high general intelligence. Here, we minimized this complication by exposing animals to a very high number of latent inhibition trials, which in prior work in our laboratory was sufficient to asymptotically habituate all mice.
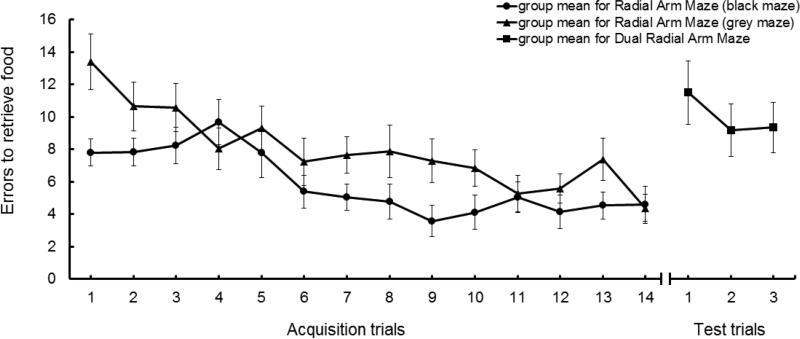
2.2.4. Dual Radial Arm Maze
In the Dual Radial Arm Maze, we assessed animals’ ability to operate simultaneously on two related sets of guidance cues. We first trained the mice in two different (visually distinct) eight-arm radial mazes located in a single testing room (which thus shared extra-maze visual cues). In order to efficiently find food, each maze requires the animals to use spatial cues (distributed around the maze) to guide its search and/or to maintain a memory of arms that have been visited within a trial. After reaching asymptotic (near errorless) performance in each maze, the attentional phase of testing began. During this phase, animals had to alternate choices between the two mazes. The two mazes were located in a single room, so the spatial cues were common to both mazes. Since animals must maintain a memory of the cues segregated according to the appropriate reference maze, the test required animals to maintain attention to the spatial cues relevant to the maze in which it was currently operating, and ignore interference from the cues that were appropriate for the other maze.
Of the two mazes, one was made of black and the other of grey Plexiglas. They both consisted of a central area (15cm of diameter) with eight arms evenly radiating out (40 cm long and 4.5 cm wide). The end of each arm had a depression containing a piece of food (14 mg of Dustless Precision pellets, Bio-Serv). The black maze had walls of clear acrylic glass enclosing the central area with a remotely operated door for each arm. Also, the black maze had walls of clear acrylic glass covering one third of one side of each arm. The grey maze, in contrast, did not have any walls. The two mazes were located next to each other in a room with a variety of visual cues (including architectural details, light strings, and geometric shapes affixed to the walls).
Before training, mice were acclimated to the black maze. During the first day of acclimation, all doors were closed. We placed the animals in one arm at a time; changing arms after a mouse ate the food piece and spent at least 90 seconds in the arm. During the second day of acclimation, we placed the mice in the center of the maze with one door opened at a time; switching the opened door after a mouse ate the food at the end of that arm. Due to its similarity with the black maze, mice were not acclimated to the grey maze.
For training in the black maze, we placed an animal in the central area with all doors closed and all arms baited. Then, all doors were simultaneously opened until an animal made a choice, counted as hind paws passing1/4 of an arm's length. Upon choosing, all of the remaining doors were closed until the subject ate the food at the end of that arm. The process then restarted with the animal re-entering the central area through the arm's door. A trial continued until the animal ate all eight pieces of food. For the grey maze, a mouse, after being placed in the central area, was allowed to move freely until it retrieved the last of the eight pieces of food. Again, choices were counted as hind paws passing 1/4 of an arm's length. If a mouse chose an arm with food but did not eat it, we treated it as neither a correct choice nor an error. We administered four trials (one per day) for the black maze and, subsequently, four trials (one per day) for the grey maze. With this phase of training over, we started training the mice in both mazes during a single day: black maze in the morning and grey maze in the afternoon (four hours after the black maze). This second phase continued for 10 days.
After all animals were performing well at both mazes (a total of 14 trials in each maze, which, from our past experience, supports near errorless performance in each maze), we started the Dual Radial Arm Maze attention test. We placed an animal in the central area of the black maze, and the usual procedure was followed. However, after three correct choices, we removed the mouse from the black maze and placed it in the central area of the grey maze. The animal was then allowed to navigate through the grey maze until it made a total of three correct choices. Following this, we removed the mouse from the grey maze and placed it in the central area of the black maze. These transfers happened again after three more correct choices in the black, three more in the grey, the two final correct choices in the black, and concluded with the two final corrects in the grey maze. This “dual maze” testing continued for three trials (once per day).
We recorded the number of errors that an animal made, and used the average of the three trials (each a sum of errors in the black and grey mazes) during the Dual Radial Arm Maze test as a measure of their attention (better attention = fewer errors).
2.2.4.1. Dual Radial Arm Maze: Design in relation to the source of interference
In the Dual Radial Arm Maze, there was only one test per day, so any internal interference from memory/habit was minimized (see W. A. Roberts, 1984 for review). On the other hand, because many of the visual cues guiding the animals overlapped between mazes, the performance in this task was heavily influenced by external sources of interference.
2.3. Blood Glucose levels
Since three of the dependent measures in our attention battery were dependent on food as a reinforcer, we were concerned that differences in animals’ glucose responses to food deprivation could be a major factor in determining the variation in their performance (independent of attention per se). (Of course, because animals had a typical loss of 5% of their ad libitum body weight before each test, they should all be motivated. However, this could only affect variation in performance if animals are differentially motivated.) To examine this potential interaction, we measured blood glucose levels of each animal twice after the attentional tests were over. We extracted the blood using tail incision and used a blood glucose meter (OneTouch Ultra). For the first extraction, mice were food deprived for one day, while, for the second extraction, they had normal access to food. For the analyses, the relative value (in %) of blood glucoses levels during deprivation were compared to the value during ad libitum. Blood glucose levels are believed to be inversely related to appetite: hypoglycemia can inhibit the release of the hormone leptin, leading to hunger (Mueller et al., 1998). Hence, this measurement allowed us to indirectly infer individual differences in the mice's motivation to obtain food during the attentional tests.
2.4. Statistical analyses
For the separate evaluation of each test of the attention battery, we performed two-tailed t-tests and ANOVAs on SPSS 21. We then categorized each mouse's attention ability based on its performance across the battery. For this, we performed an unrotated exploratory factor analysis (“principal axis factoring”) on SPSS 21 of the scores from the four different tests. The analysis identifies the relationship of variables, and suggests underlying factors that explain (linearly fits) the variation of all observed variables. This results in new (latent) variables with high repeatability, since they aggregate similar measures with minimal error. Since these resulting factors from an exploratory factor analysis are nonhypothesized (have no assumptions about their structure), they are useful to show each measured variable's contribution (or “loading”) before testing hypothetical models in confirmatory factor analysis (Gerbing & Hamilton, 1996). Here, we considered only factors with eigenvalues greater than 1 (a widely accepted standard). To further explore the meaning of each test, we applied a Direct Oblimin rotation on the factor analysis. This technique makes each factor have either large or small loadings on any particular variable by maximizing their variance. Therefore, the Direct Oblimin rotation simplifies the interpretation of different causes for a set of variables because each original variable tends to be associated with one of the factors. In both unrotated and rotated exploratory factor analyses, we considered a factor loading above 0.3 as an indication that a test is meaningfully loading onto the respective factor (Tabachnick & Fidell, 2001).
After the exploratory factory analyses, we then tested the two attention models: 1) The one factor model based on the assumption that a single factor explained the variance across all attentional tests; 2) The two factor model for source of interference assuming that T-Maze Reversal and Coupled Latent Inhibition related to one factor interpreted as internal attention, and the Mouse Stroop Test and Dual Radial Arm Maze related to another factor interpreted as external attention. For this, we used confirmatory factor analyses based on maximum likelihood estimation in AMOS 21. This particular estimation for a confirmatory factor analysis has advantages in cases with relatively small samples (like ours), with fitness indexes working better with maximum likelihood than with other statistical estimation procedures (Chou & Bentler, 1995). We assessed model fit by using three absolute indices (Model Chi-Square, RMSEA, and SRMR) that describe how well the model represents the observed data, where lower values mean better fit (hence, they are also referred to as tests of “badness-of-fit”) (Kline, 2011). For the Model Chi-Square (Χ2M), the null hypothesis is the model itself, so failing to reject it (i.e., a small Model Chi-Square) indicates a good fit (with alpha here set at 0.05) (Kline, 2011). Following a similar reasoning, RMSEA values of 0.06 and below are considered good (while values greater than .10 are considered a poor fit), as well as SRMR values of 0.09 and below (Hu & Bentler, 1999). In addition to these three absolute indices, we also assessed model fit with two incremental indices (CFI and TLI) that describe how well the model fits in comparison a to a baseline model where all variables are uncorrelated and without latent variables, and where higher values mean better fit (“goodness-of-fit” in the literal sense) (Kline, 2011). CFI and TLI indicate an adequate model fit at values of 0.95 or above (Hu & Bentler, 1999). We chose these tests due to their statistical relevance and frequent use (Hooper, Coughlan, & Mullen, 2008; Kline, 2011). For hierarchical comparisons between the one factor (general) model and the two factor (source of interference) model, we performed the Chi-Square Difference Test (Χ2D), where the null hypothesis represents no differences between the models.
In the process of training animals for their ultimate tests of attention, animals underwent acquisition of five basic learning tasks (odor discrimination, visual discrimination, reinforced alternation, fear conditioning, and radial arm maze). Consequently, the rate of acquisition on each of these tasks can be used to construct an aggregate measure of animals’ general learning performance (see Kolata et al., 2008, for details of this procedure). For this purpose, acquisition scores were entered into an unrotated exploratory factor analysis, and a factor score (based on the primary, or “general learning” factor) was computed for each animal. A factor score is analogous to an average z score based on an animal's performance on each individual test, weighted by the degree to which that test loads on the primary factor. Thus factor scores serve to rank each animal on the variable captured by the primary factor (in this case, general learning ability).
3. Results
First, we describe the training phases and summarized results from each test of the attention battery for all 26 animals. We then describe the results of our factor analysis, which revealed the two different, and meaningful, latent variables. Then, we describe the results of the confirmatory factor analyses which estimated the goodness-of-fit for a model with a single latent factor (“attention”) explaining the performance across tests of attention, as well as for a model of external and internal attention as distinct (although correlated) latent factors. Lastly, we examined the relationship of variation in attention to individual animal's general learning performance.
3.1. Individual tests of the attention battery
3.1.1. Mouse Stroop Test
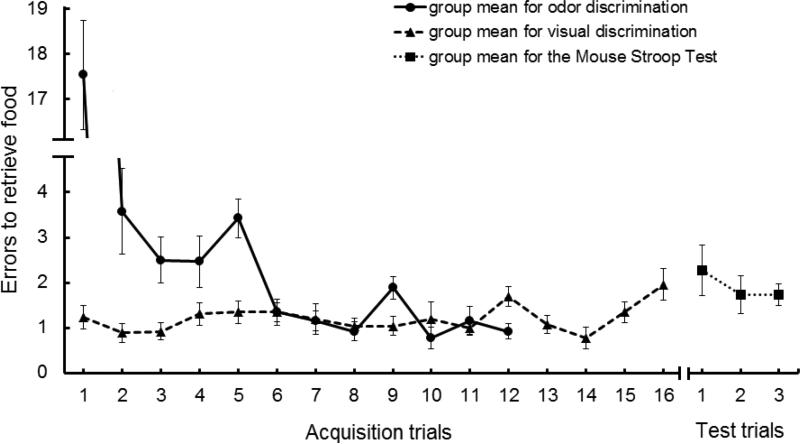
The mean performance of animals during the training for odor discrimination and visual discrimination is presented in Figure 2. At the end of both tasks, mice were performing better than the two errors expected by random chance (although errors could easily exceed two if choices were repeatedly directed toward a non-target food cup). Animals started odor discrimination training making on average 17.54 (± 1.20) errors to find food, and by the 12th trial errors had fallen to 0.92 (± 0.23), a significant decrease across trials (t25 = 13.45, p< 0.0001) with a large effect on performance (Cohen's d = 3.13). For visual discrimination, however, mice began training with a low number of errors, and thus did not improve from trials 1 to 16, (t25 = 1.72, p> 0.05). This likely reflected the fact that animals were previously trained on a task (odor discrimination) which had very similar demands, and thus in this task, animals could focus exclusively on learning the relatively simple visual discrimination.
Figure 2.
Average number of errors for the 26 mice to retrieve food across 12 trials of odor discrimination training, across 16 trials of visual discrimination training, and across three trials in the Mouse Stroop Test (visual discrimination box in the presence of odor distracters). Brackets indicate standard error of the mean.
The right portion in Figure 2 illustrates the group mean performance in the visual box of the Mouse Stroop Test (i.e., in the presence of odor distractors). The group mean number of errors during the first of these tests was significantly higher than the average performance on the last day (Trials 13 to 16) of visual discrimination training (t25 = 2.43, p< 0.05), revealing the negative impact of the addition of the odor distractors.
3.1.2. T-Maze Reversal
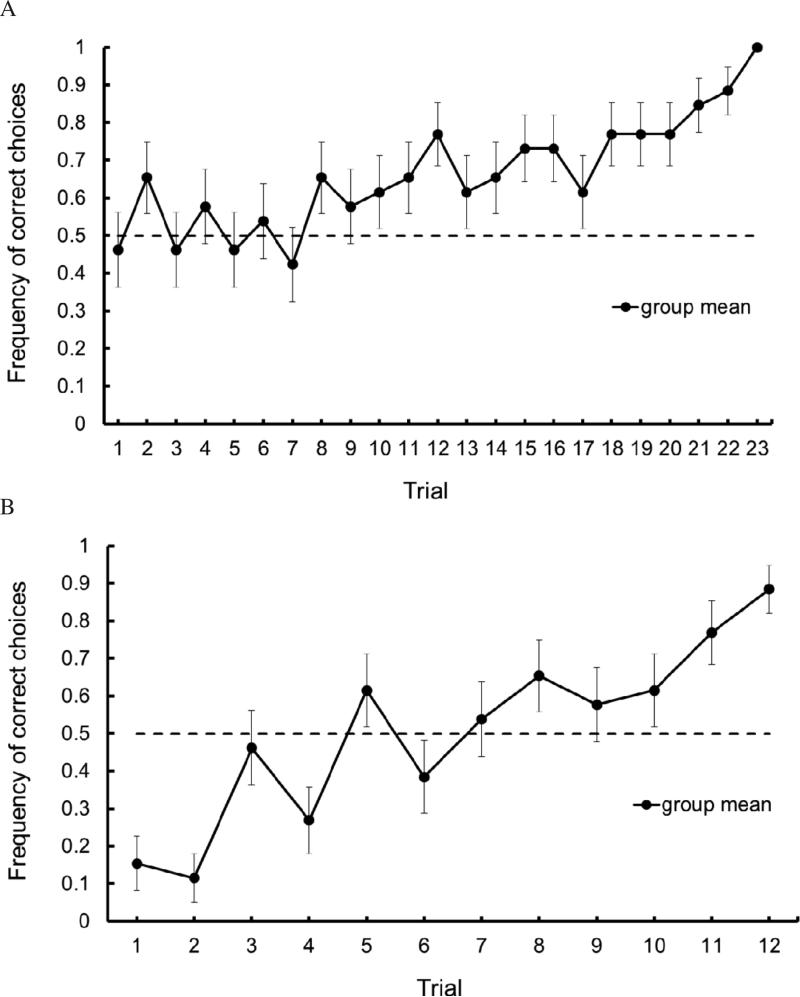
Figure 3A illustrates the relative frequency of correct responses for the group of animals as a function of trials. Animals started the task correctly choosing approximately half of the time, as expected by chance. On the 23rd trial, all animals made a correct choice, a significant difference from the first trial (W = −105, p< 0.001). This indicates that animals had reached a high level of performance prior to the reversal task. The results for the T-Maze Reversal are seen in Figure 3B. During this reversal phase (in which reinforcement was always located on the same side), animals performance fell well below chance levels, indicating a propensity to continue to alternate choices. However, by the 12th trial, animals’ performance had improved to the pre-reversal level.
Figure 3.
A. Average frequency of correct choices for 26 mice across 12 trials of reinforced alternation training. B. Average frequency of correct choices for 26 mice across 12 trials of T-Maze Reversal. Dashed lines indicate a level of random responding (i.e., 50% correct choices.) Brackets indicate standard error of the mean.
3.1.3. Coupled Latent Inhibition
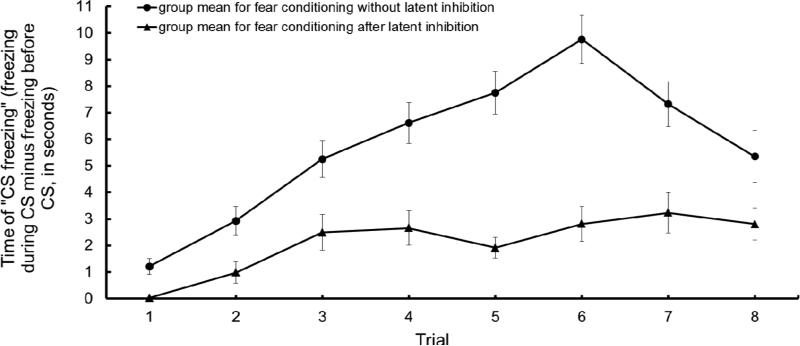
We observed rapid learning of the tone-shock association absent prior exposure to the tone CS (i.e., without prior latent inhibition training; Figure 4A), with the mean time of freezing for CS1 (tone predicting shock, and calculated by the time freezing during the CS1 minus the time freezing before the CS1) increasing more than seven times across trials (i.e., at the start of Trial 6). Even with a drop in performance during the final two pairings (Trials 7 & 8), mice's performance on the last trial (Trial 8) was still significantly different than on the first trial (t22 = 3.82, p< 0.001), indicative of the formation of a tone-shock association (Cohen's d = 0.83).
Figure 4.
A. Average time of “CS freezing” (time spent freezing during the 20 seconds of tone minus the time spent freezing during 20 seconds before the tone) for 26 mice across 8 trials of fear conditioning (upper curve) and fear conditioning to the light after pre-exposure (i.e., latent inhibition) to the light (bottom curve). Brackets indicate standard error of the mean.
The bottom curve in Figure 4A illustrates the acquisition of fear to a light (that was initially presented unpaired) when paired with shock. Mice were slow to acquire a fear response during light-shock pairings following extensive exposure to the light in the absence of shock (i.e., latent inhibition). Acquisition of fear to the light was slower than the acquisition of fear to the tone across all trials (repeated measure ANOVA task × trial, F1, 401 = 93.00, p < 0.0001). However, even after latent inhibition training, animals ultimately acquired the light-shock association, with the mean time of freezing for CS2 (light predicting shock, and calculated by the time freezing during the CS2 minus the time freezing before the CS2) rising 3 seconds at its maximum value, during Trial 7. There was a significant difference from the first and last trials (t25 = 4.62, p< 0.0001), indicative of successful (although weak) acquisition (Cohen's d = 1.21). (It is worth re-iterating that the slow acquisition of fear in response to the light relative to the tone is not likely the result of innate differences in the ability of these stimuli to support learning. The parameters for these stimuli were specifically chosen based on prior work which determined that these stimuli would support similar rates of learning when paired with shock. Instead, the slow learning during light-shock pairings is presumed to reflect the extensive prior experience that the animals had with the unreinforced light.)
The performance of mice for Coupled Latent Inhibition is a measure of the performance on fear conditioning after latent inhibition minus the performance on fear conditioning with no prior latent inhibition. A very high attentional capacity should lead to higher values (close to zero). A value of zero here means that the animal learned about CS2 (after latent inhibition) at the same rate as CS1.
3.1.4. Dual Radial Arm Maze
During training in both the black and grey radial arm mazes, mice gradually improved their performance (Figure 5), making significantly fewer errors during the last trial (Trial 14) compared to the first (t25 = 2.81, p< 0.01 in the black maze, and t25 = 5.20, p< 0.0001 in the grey maze), corresponding to moderate (Cohen's d = 0.57) and large (Cohen's d = 0.87) effect sizes, respectively. In addition, Figure 5 illustrates the performance of all animals in the Dual Radial Arm Maze phase of testing. As illustrated, errors increased in this more complex phase of radial arm maze testing (where animals were simultaneously performing in two mazes that shared an overlapping set of visual guidance cues). For subsequent analyses, fewer errors in this phase indicated better attention.
Figure 5.
Average number of errors for 26 mice across 14 trials of black and grey maze training, and across 3 trials of Dual Radial Arm Maze. Brackets indicate standard error of the mean.
3.2. Exploratory factor analyses of the attention tests
Table 1 shows the descriptive statistics of all 26 animals on each of the four attention tests that contributed to the factor analysis (as well as on the learning tasks described further below). The values entered for each test are as follows: 1) Mouse Stroop Test = average number of errors in the visual box in the presence of task-relevant odor distractors (Trials, 1 to 3), 2) T-Maze Reversal = number of correct choices during the reversal phase divided by the number of correct choices during the alternation learning phase (Trials 5 to 12), 3) Coupled Latent Inhibition = CS2 (light) time of freezing during light-shock pairings (following latent inhibition training) minus CS1 (tone) time of freezing during tone-shock pairing (hence, the existence of negative numbers; Trials 2 to 4), and 4) Dual Radial Arm Maze = average number of errors for the sum of black and grey mazes during trials in which mice alternated choices between the two mazes (Trials, 1 to 3).
Table 1.
Mean, standard deviation, range, and measures of skewness and kurtosis of the performance of the 26 mice in the attention tests and the learning tests.
| Test | Mean | Standard Deviation | Range | Skewness* | Kurtosis* | |
|---|---|---|---|---|---|---|
| Attention† | Mouse Stroop Test | 1.91 | 1.17 | −4.7 to −0.3 | −1.13 | 0.87 |
| T-Maze Reversal | 1.14 | 0.46 | 0.5 to 2.5 | 1.19 | 1.59 | |
| Coupled Latent Inhibition | −2.89 | 3.48 | −10.4 to 2.7 | −0.35 | −0.47 | |
| Dual Radial Arm Maze | 5.00 | 3.21 | −10.8 to −0.3 | −0.36 | −0.90 | |
| Learning‡ | Odor Discrimination | 2.66 | 1.59 | −7.6 to −0.4 | −1.48 | 2.97 |
| Reinforced Alternation | 0.59 | 0.15 | 0.3 to 0.9 | 0.04 | 0.11 | |
| Fear Conditioning | 4.93 | 2.6 | 0.5 to 11.1 | 0.57 | −0.1 | |
| Radial Arm Maze | 8.83 | 2.2 | −13.2 to −4.4 | −0.33 | −0.08 | |
Four tests of attention. Mouse Stroop Test (number of errors), T-Maze Reversal (correct choices in reversal per correct choices in normal learning), Coupled Latent Inhibition (seconds of CS freezing during latent inhibition minus seconds of CS freezing during fear conditioning), and Dual Radial Arm Maze (number of errors).
Four tests of learning. Odor Discrimination (number of errors), Reinforced Alternation (correct choices), Fear Conditioning (seconds of CS freezing), and Radial Arm Maze (number of errors). Each of the four learning tests was part of an attention test.
For skewness, values between −2 and 2 are considered to be inside the acceptable range for normality. For kurtosis, values between −3 and 3 are considered to be inside the acceptable range for normality.
Table 1 also contains skewness and kurtosis measures of the data distribution. For skewness, values between −2 and 2 are considered to be inside the acceptable range for a normal distribution (Gardner, 2001). Note that SPSS uses a formula that estimates the skewness in the population (Zg1), and, hence, its acceptable range differs from the more common formula (g1) of −1 to 1 (Gardner, 2001). For kurtosis, values between −3 and 3 are considered to be inside the acceptable range for a normal distribution (Sheskin, 2003). Hence, dependent measures on all attention tests were well within the range of normalcy and had an adequate distribution for the exploratory factor analyses and the confirmatory factor analyses that followed.
Table 2 presents the results of the unrotated exploratory factor analysis of animals’ performance on the four attentional tests. Regarding the first factor, the Mouse Stroop Test and the Dual Radial Arm Maze (each impacted by external (E) sources of interference) had a considerable loading (>0.5), while the T-Maze Reversal had a modest loading, and the Coupled Latent Inhibition did not load at all. A common influence on attentional performance accounted for approximately 44% of the total variation in test scores between mice. For the second factor, which accounted for 29% of total variation, Coupled Latent Inhibition had a considerable loading (>0.5), T-Maze Reversal had a modest loading (where both tasks are impacted primarily by internal (I) sources of interference), and neither the Mouse Stroop Test nor the Dual Radial Arm Maze loaded.
Table 2.
Factor loadings and variance explained by the first two factors extracted from the four attention tests. (E) and (I) indicate tasks influenced by primarily (E)xternal and (I)nternal sources of interference.
| Attention Test | Factor 1 | Factor 2 |
|---|---|---|
| Mouse Stroop Test (E)* | .64 | −.03 |
| T-Maze Reversal (I)* | .41 | .42 |
| Coupled Latent Inhibition (I)* | −.02 | .58 |
| Dual Radial Arm Maze (E)* | .87 | −.16 |
| Eigenvalue | 1.76 | 1.18 |
| Proportion of total variance | 43.9% | 29.4% |
The Direct Oblimin rotation revealed the same overall pattern found in the unrotated factor analysis, and more clearly separated two distinct factors underlying overall performance across the four tests (Table 3). The Mouse Stroop Test and the Dual Radial Arm Maze loaded strongly on the first factor, while T-Maze Reversal and Coupled Latent Inhibition loaded on the second factor (although the T-Maze Reversal also had a slight loading on the first factor, with a value above the threshold of 0.3). Like the unrotated exploratory factor analysis (Table 2), these results suggest two different influences in our attention battery that match with our expectation of a differential influence of sources of interference (external and internal). Through the confirmatory factor analysis, we were able to further verify this claim.
Table 3.
Factor loadings and variance explained by the first two rotated factors (Direct Oblimin rotation) from the four attention tests. (E) and (I) indicate tasks influenced by primarily (E)xternal and (I)nternal sources of interference.
| Attention Test | Factor 1 | Factor 2 |
|---|---|---|
| Mouse Stroop Test (E) | .65 | .09 |
| T-Maze Reversal (I) | .38 | .50 |
| Coupled Latent Inhibition (I) | −.06 | .56 |
| Dual Radial Arm Maze (E) | .87 | .01 |
3.3. Confirmatory factor analyses of proposed models
The confirmatory factor analyses are described separately for each model: “single attention” and “external/internal attention”.
The model with only a single factor of attention (all tests together under a single potential cause) had a good fit based on three indices (Χ2M 2) = 3.04, p = 0.219, failing to reject the null hypothesis of model and observed data being the same; CFI = 0.97, where values above .95 represent a good fit; SRMR = 0.08, where values below 0.09 represent a good fit), and a relatively poor fit in the other two (TLI = 0.90, where values above .95 represent a good fit; RMSEA = 0.07, where values below 0.06 represent a good fit). This suggests that one factor is not sufficient to capture the common variance among the four attentional tests.
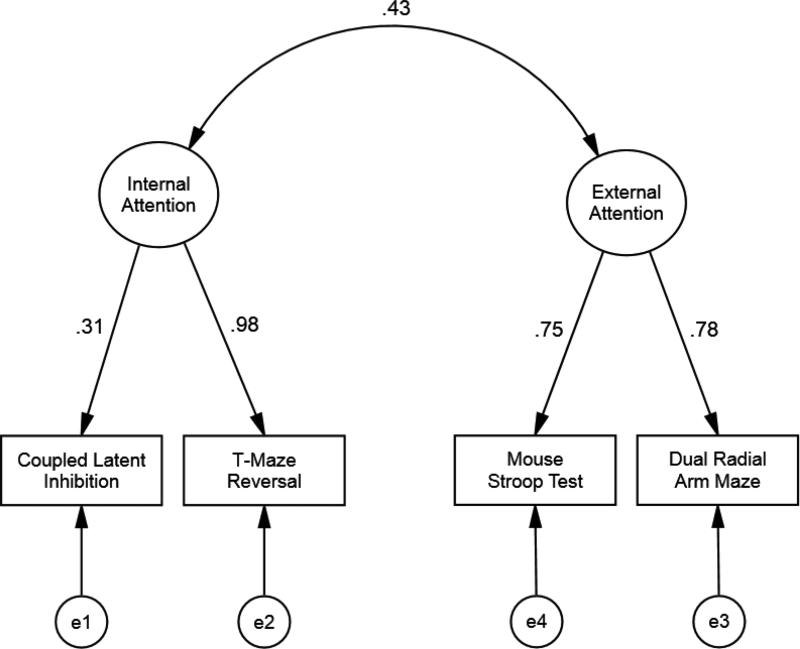
The two factor model of external/internal attention (T-Maze Reversal and Coupled Latent Inhibition with the factor interpreted as internal attention, and Mouse Stroop Test and Dual Radial Arm Maze with the factor interpreted as external attention) had a very good fit to the data (as suggested from the unrotated and rotated exploratory factor analyses). The Model Chi-Square failed to reject the null hypothesis of model and observed data being the same, Χ2M (1) = 0.41, p = 0.522. The RMSEA and the SRMR values were <0.001 and 0.08, respectively, suggesting a very good fit, and, the CFI and the TLI values were 1 and 1.27, respectively, further suggesting that this model explains well the perfomance of the mice in the attention battery. The parameters estimated for this model (Figure 6) show similarly high loading of Mouse Stroop Test and Dual Radial Arm Maze on External Attention, and a very high loading of T-Maze Reversal and a moderate loading of Coupled Latent Inhibition on the hypothesized internal attention factor. The correlation between external and internal attention was 0.43.
Figure 6.
Confirmatory factor analysis of the two factor model with internal and external attentions. Χ2m (1) = 0.41, p = 0.522, RMSEA < 0.001, SRMR = 0.08, CFI = 1, TLI = 1.27. Parameters are standardized.
Even though having a very good fit, the “external/internal attention” model, though, was not significantly different than the “single attention” model, Χ2D (1) = 2.63, p = 0.105. Since the “single attention” model did not fit well in two of the four indices, it might be the case that the external/internal attention model is indeed superior, but we may have failed to detect a significant difference to the single facor model due to our low sample size.
3.4. The influence of blood glucose
We observed a significant reduction in the mice's blood glucose levels under food deprivation (t25 = 11.47, p< 0.001). The percentage of blood glucose after food deprivation relative to ad libitum levels was, on average, 65.39, with SD = 15.22, and did correlate with the ad libitum levels (r24 = −0.23, p > 0.05). This variation in the loss of blood glucose did not load with either of the two resulting factors of an exploratory factor analysis containing the four attention tests (Table 4). Also, the variation in the loss of blood glucose did not correlate with either the first factor (r24 = 0.04, p > 0.05) or the second factor (r24 = −0.21, p > 0.05) extracted from the attention battery.
Table 4.
Factor loadings and variance explained by the tests of attention and the loss in blood glucose levels (% from ad libitum levels).
| Variable | Factor 1 | Factor 2 |
|---|---|---|
| Mouse Stroop Test | .71 | .11 |
| T-Maze Reversal | .32 | .32 |
| Coupled Latent Inhibition | −.22 | .96 |
| Dual Radial Arm Maze | .78 | .09 |
| Loss of Blood Glucose | .14 | −.25 |
| Eigenvalue | 1.77 | 1.37 |
| Proportion of total variance | 35.3% | 27.4% |
3.5. Relationship of attention and learning
Rate of acquisition could be determined for four of the five tests of learning that were administered here. (Animals exhibited near-asymptotic levels of performance on the first trial of visual discrimination training, and thus acquisition rates were not considered.) A factor analysis was performed on the acquisition rates of individual animals on these four learning tasks (descriptive statistics is described in Table 1): Odor Discrimination (Trials 2 to 6), Reinforced Alternation (Trials 5 to 12), Fear Conditioning (Trials 2 to 4), and Radial Arm Maze (Trials 2 to 6). A single factor accounted for 37% of the variance across animals, indicative of a general influence on learning across all tasks. (This level of explanatory value is comparable to most of our previous work with larger batteries of learning tasks and larger sample sizes, e.g., Kolata, Light, & Matzel, 2008; Matzel et al., 2011; Matzel, Grossman, Light, Townsend, & Kolata, 2008.) We then derived factor scores for each animal from this analysis. Scores ranged from −2.99 to 1.10, where higher values indicate better aggregate performance across all tasks, and had a skewness of −1.6 and a kurtosis of 2.9; thus these values are inside the acceptable range of normality. We entered these learning scores into a factor analysis with the performance of the animals on each of the four tests of attention. The results of this analysis are presented in Table 5. On the first factor, all tests but the Coupled Latent Inhibition had a moderate to strong positive loading together with the learning factor scores (the latter of which is indicative of general learning ability). For the second factor, the Mouse Stroop Test and Dual Radial arm maze did not load, while performance on the T-Maze Reversal and Coupled Latent inhibition had positive loadings contrasted with the learning factor's negative loading, suggesting that they are inversely related. The implications of these results are addressed more fully below.
Table 5.
Factor loadings and variance explained by the tests of attention and general learning performance (Learning Factor). (E) and (I) indicate tasks influenced by primarily (E)xternal and (I)nternal sources of interference.
| Variable | Factor 1 | Factor 2 |
|---|---|---|
| Mouse Stroop Test (E) | .72 | −.04 |
| T-Maze Reversal (I) | .40 | .60 |
| Coupled Latent Inhibition (I) | −.06 | .44 |
| Dual Radial Arm Maze (E) | .78 | −.05 |
| Learning Factor | .38 | −.36 |
| Eigenvalue | 1.91 | 1.33 |
| Proportion of total variance | 38.3% | 26.5% |
4. Discussion
Using outbred CD-1 mice, we observed considerable differences in individual performance across four distinct tests of attention. Contrary to our expectation, results here suggest that there is weak (if any) evidence that the described “attention battery” can be used as a robust measure of “general” attention. On the other hand, our results here suggest that the (often ignored!) distinction between internal and external sources of interference could have an important role in how an individual utilizes attention. Furthermore, we found these internal and external attentional systems can potentially explain some of the normal variation in learning abilities. The following examination of results from the exploratory and confirmatory factor analyses makes the implications of our findings more clear.
Considering the design of all tests of the attention battery together (as described in Section 2.2), half the tests had primarily external sources of interference (odor cues of Mouse Stroop Test and overlapping spatial cues of Dual Radial Arm Maze), while the remaining two tests had primarily internal sources of interference (prior learned alternation during T-Maze Reversal, and the habituated CS during latent inhibition training). Regarding our expectations that the source of interference (external and internal) might influence variation in attention, the results of the unrotated factor analysis and the Direct Oblimin rotation fitted well with each test's reliance on these different attentional directions. Performances in the Mouse Stroop Test and the Dual Radial Arm Maze were the strongest predictors of the first rotated factor, and both had external distractors as their main source of interference. In comparison, the T-Maze Reversal and the Coupled Latent Inhibition loaded strongly with the second factor of the Direct Oblimin rotation, and both required attention exclusively (or nearly so) against internal sources of interference (the previously learned response/stimulus). The results from the confirmatory factor analysis further suggest a distinction between internal and external influences on attention, with four independent indices exhibiting a very good fit with a model that assumed such a distinction. Internal attention and external attention are indeed believed to be distinct processes that help balance the levels between abstractions (e.g., recalls from memory and the projection of cognitive maps) and of sensorial input (Johnson, 2006), with different tasks and environmental demands possibly requiring different levels of resistance to internal and external sources of interference. Furthermore, those two systems engage some distinct brain regions in humans, with lateral regions of the PFC being preferentially activated when attention is directed to internal representations (and preferentially connected to regions in the prefrontal and parietal cortex), whereas the medial PFC is activated when attention is directed to sensory events (and connected to the basal ganglia, thalamus, and sensory association cortices) (Henseler, Krüger, Dechent, & Gruber, 2011). Despite the differences, however, internal and external attention do (as one would expect) have very similar cognitive properties (for a review, see Chun & Johnson, 2011) and many of their underlying brain regions overlap in humans (Nobre et al., 2004). Our obtained correlation of 0.43 between internal and external attention might reflect some of these overlapping properties.
Given that three of the four tests in the attention battery used dependent measures that were reliant on food deprivation (Mouse Stroop Test, T-Maze Reversal, and Dual Radial Arm Maze), one might suppose that the common variance between them (indicated by the loading in the first factor of both unrotated and rotated analyses) arises from the animals’ motivation to obtain food. However, this conclusion is unlikely given our results. Blood glucose levels (see Table 4) did not load either onto the first factor (with the three apetitive tests), or onto the second factor (with the apetitive test T-Maze Reversal, and the aversive test Coupled Latent Inhibition). Furthermore, as described above (Section 2.2), the food deprivation procedure consisted of 90 min of free access to food, meaning that individual differences in motivation to obtain food were already limited by this long window of opportunity to gather food. Hence, our results provide evidence that the deprivation (and subsequent decrease in blood glucose levels, and hunger) during three of the four tests in the battery was not a major source of the covariation in performance across tests. It is important to remark that there could still be other explanations behind the variation in the attention battery here that are not related to attention. Task persistence, general level of motivation, or even personality (as discussed below) could be traits representing the factors we obtained. We certainly do not believe that attention is the only trait explaining the common variance in the attention battery. However, from the explicit design/rationale of the tests, we believe it is likely and more parsimonious to infer that attention is playing a major role in establishing the observed factor structure.
The learning factor (general learning ability) had a moderate, positive correlation with the external attention factor (the first attention factor) and a moderate, negative correlation with the internal attention factor (the second attention factor). We can then ask: why is high attention to internal sources of interference related to poor learning? All of the learning data here were derived from tasks that were new to the mice. However, the two internal attention tests (T-Maze Reversal and Coupled Latent Inhibition) had changes in contingencies that were previously well known to the animal; while the two external attention tests (Mouse Stroop Test and Dual Radial Arm Maze) did not. This point suggests that tests of attention that are highly dependent on internal sources of interference (thus reflecting internal attention) may correlate with poor performance in standard learning tasks, while tests of external attention may correlate with good performance in standard learning tasks, but correlate with poor performance in learning tasks with subtle changes in conditions (like in reversals).
We hope that results here can assist in creating better measurements of attention in laboratory animals, and more importantly, a more complete appreciation of the distinct attentional systems and their differential relationship to other aspects of cognitive performance in non-human animals. In sum, the present work adds some insight to our understanding of variations in cognitive performance, and provides a more complete foundation for studies of the neurobiological basis of attention in non-human animals.
Highlights.
The efficacy of attention is critically related to learning and intelligence
Attention can protect against internal or external sources of interference
Individual mice exhibited a conserved ability across four distinct tests of attention
Variations in “external attention” determined attention's influence on learning
Acknowledgements
This work was supported by grants from the National Institute of Aging (R01AG029289), the Busch Foundation, and the Office of Naval Research (N000141210873).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen G, Courchesne E. Attention function and dysfunction in autism. Frontiers in Bioscience : A Journal and Virtual Library. 2001;6(7):D105–19. doi: 10.2741/allen. doi:10.1093/intimm/dxs032. [DOI] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annual Review of Clinical Psychology. 2005;1:321–53. doi: 10.1146/annurev.clinpsy.1.102803.143959. doi:10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Carroll JB. Human cognitive abilities: a survey of factor analytic studies. Cambridge University Press; Cambridge: 1993. [Google Scholar]
- Chou C-P, Bentler PM. Estimates and test in structural equation modeling. In: Hoyle RH, editor. Structural equation modeling: Concepts, issues, and applications. Sage; 1995. [Google Scholar]
- Chun MM, Johnson MK. Memory: enduring traces of perceptual and reflective attention. Neuron. 2011;72(4):520–35. doi: 10.1016/j.neuron.2011.10.026. doi:10.1016/j.neuron.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychological Review. 1990;97(3):332–361. doi: 10.1037/0033-295x.97.3.332. doi:10.1037/0033-295X.97.3.332. [DOI] [PubMed] [Google Scholar]
- Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user's guide. Psychonomic Bulletin & Review. 2005;12(5):769–786. doi: 10.3758/bf03196772. doi:10.3758/BF03196772. [DOI] [PubMed] [Google Scholar]
- Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences. 2003;7(12):547–552. doi: 10.1016/j.tics.2003.10.005. doi:10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ. The two disciplines of scientific psychology. American Psychologist. 1957;12(11):671–684. doi:10.1037/h0043943. [Google Scholar]
- De Waal FBM. Darwin's last laugh. Nature. 2009;460(7252):175. doi: 10.1038/460175a. doi:10.1038/460175a. [DOI] [PubMed] [Google Scholar]
- Dempster FN, Corkill AJ. Individual differences in susceptibility to interference and general cognitive ability. Acta Psychologica. 1999;101(2-3):395–416. doi:10.1016/S0001-6918(99)00013-X. [Google Scholar]
- Engle RW. Working Memory Capacity as Executive Attention. Current Directions in Psychological Science. 2002;11(1):19–23. doi:10.1111/1467-8721.00160. [Google Scholar]
- Gardner R. Psychological statistics using SPSS for Windows. Prentice Hall; Upper Saddle River, New Jersey: 2001. [Google Scholar]
- Gerbing DW, Hamilton JG. Viability of exploratory factor analysis as a precursor to confirmatory factor analysis. Structural Equation Modeling: A Multidisciplinary Journal. 1996;3(1):62–72. doi:10.1080/10705519609540030. [Google Scholar]
- Henseler I, Krüger S, Dechent P, Gruber O. A gateway system in rostral PFC? Evidence from biasing attention to perceptual information and internal representations. NeuroImage. 2011;56(3):1666–76. doi: 10.1016/j.neuroimage.2011.02.056. doi:10.1016/j.neuroimage.2011.02.056. [DOI] [PubMed] [Google Scholar]
- Hooper D, Coughlan J, Mullen M. Structural Equation Modelling : Guidelines for Determining Model Fit. Electronic Journal of Business Research Methods. 2008;6(1):53–60. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. doi:10.1080/10705519909540118. [Google Scholar]
- Jarosz AF, Wiley J. Why does working memory capacity predict RAPM performance? A possible role of distraction. Intelligence. 2012 doi:10.1016/j.intell.2012.06.001. [Google Scholar]
- Jarrold C, Towse JN. Individual differences in working memory. Neuroscience. 2006;139(1):39–50. doi: 10.1016/j.neuroscience.2005.07.002. doi:10.1016/j.neuroscience.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Jensen AR. The g factor: The science of mental ability (human evolution, behavior, and intelligence) Praeger; New York: 1998. [Google Scholar]
- Johnson MK. Memory and reality. The American Psychologist. 2006;61(8):760–71. doi: 10.1037/0003-066X.61.8.760. doi:10.1037/0003-066X.61.8.760. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Reeder J. a, Raye CL, Mitchell KJ. Second Thoughts versus Second Looks: An Age-Related Deficit in Reflectively Refreshing Just-Activated Information. Psychological Science. 2002;13(1):64–67. doi: 10.1111/1467-9280.00411. doi:10.1111/1467-9280.00411. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9(4):637–671. doi: 10.3758/bf03196323. doi:10.3758/BF03196323. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132(1):47–70. doi: 10.1037/0096-3445.132.1.47. doi:10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kaplan O, Lubow RE. Ignoring irrelevant stimuli in latent inhibition and Stroop paradigms: the effects of schizotypy and gender. Psychiatry Research. 2011;186(1):40–5. doi: 10.1016/j.psychres.2010.07.025. doi:10.1016/j.psychres.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. Guilford press; 2011. [Google Scholar]
- Kolata S, Light K, Grossman HC, Hale G, Matzel LD. Selective attention is a primary determinant of the relationship between working memory and general learning ability in outbred mice. Learning & Memory (Cold Spring Harbor, N.Y.) 2007;14(1):22–8. doi: 10.1101/lm.408507. doi:10.1101/lm.408507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolata S, Light K, Matzel LD. Domain-Specific and Domain-General Learning Factors are Expressed in Genetically Heterogeneous CD-1 mice. Intelligence. 2008;36(6):619–629. doi: 10.1016/j.intell.2007.12.001. doi:10.1016/j.intell.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolata S, Light K, Wass CD, Colas-Zelin D, Roy D, Matzel LD. A dopaminergic gene cluster in the prefrontal cortex predicts performance indicative of general intelligence in genetically heterogeneous mice. PloS One. 2010;5(11):e14036. doi: 10.1371/journal.pone.0014036. doi:10.1371/journal.pone.0014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological Mechanisms in Hyperactivity: I Response Inhibition Deficit, Working Memory Impairment, Delay Aversion, or Something Else? Journal of Child Psychology and Psychiatry. 2001;42(2):199–210. doi:10.1111/1469-7610.00711. [PubMed] [Google Scholar]
- Lavie N, Fox E. The role of perceptual load in negative priming. Journal of Experimental Psychology: Human Perception and Performance. 2000;26(3):1038–1052. doi: 10.1037//0096-1523.26.3.1038. doi:10.1037/0096-1523.26.3.1038. [DOI] [PubMed] [Google Scholar]
- Light K, Grossman H, Kolata S, Wass C, Matzel LD. General learning ability regulates exploration through its influence on rate of habituation. Behavioural Brain Research. 2011;223(2):297–309. doi: 10.1016/j.bbr.2011.04.050. doi:10.1016/j.bbr.2011.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Mukamel E. a, Nery JR, Urich M, Puddifoot C. a, Johnson ND, Ecker JR. Global Epigenomic Reconfiguration During Mammalian Brain Development. Science (New York, N.Y.) 2013 doi: 10.1126/science.1237905. doi:10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE. Latent inhibition. Psychological Bulletin. 1973;79(6):398–407. doi: 10.1037/h0034425. doi:10.1037/h0034425. [DOI] [PubMed] [Google Scholar]
- Mackintosh N. IQ and Human Intelligence. Oxford University Press; Oxford: 2011. p. 456. [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109(2):163–203. doi: 10.1037/0033-2909.109.2.163. doi:10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Grossman H, Light K, Townsend D, Kolata S. Age-related declines in general cognitive abilities of Balb/C mice are associated with disparities in working memory, body weight, and general activity. Learning & Memory (Cold Spring Harbor, N.Y.) 2008;15(10):733–46. doi: 10.1101/lm.954808. doi:10.1101/lm.954808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Kolata S. Selective attention, working memory, and animal intelligence. Neuroscience and Biobehavioral Reviews. 2010;34(1):23–30. doi: 10.1016/j.neubiorev.2009.07.002. doi:10.1016/j.neubiorev.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Light K, Wass C, Colas-Zelin D, Denman-Brice A, Waddel AC, Kolata S. Longitudinal attentional engagement rescues mice from age-related cognitive declines and cognitive inflexibility. Learning & Memory (Cold Spring Harbor, N.Y.) 2011;18(5):345–56. doi: 10.1101/lm.2034711. doi:10.1101/lm.2034711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky a F., Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. Analysis of the elements of attention: a neuropsychological approach. Neuropsychology Review. 1991;2(2):109–45. doi: 10.1007/BF01109051. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1844706. [DOI] [PubMed] [Google Scholar]
- Mueller WM, Gregoire FM, Stanhope KL, Mobbs CV, Mizuno TM, Warden CH, Havel PJ. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology. 1998;139(2):551–8. doi: 10.1210/endo.139.2.5716. doi:10.1210/endo.139.2.5716. [DOI] [PubMed] [Google Scholar]
- Mysore SP, Knudsen EI. A shared inhibitory circuit for both exogenous and endogenous control of stimulus selection. Nature Neuroscience. 2013;16(4):473–478. doi: 10.1038/nn.3352. doi:10.1038/nn.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, Jonides J. A Meta-analysis of Executive Components of Working Memory. Cerebral Cortex (New York, N.Y. : 1991) 2012:1–19. doi: 10.1093/cercor/bhs007. Barch 2005 doi:10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Jonides J. Dissociable interference-control processes in perception and memory. Psychological Science. 2008;19(5):490–500. doi: 10.1111/j.1467-9280.2008.02114.x. doi:10.1111/j.1467-9280.2008.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre a C., Coull JT, Maquet P, Frith CD, Vandenberghe R, Mesulam MM. Orienting attention to locations in perceptual versus mental representations. Journal of Cognitive Neuroscience. 2004;16(3):363–73. doi: 10.1162/089892904322926700. doi:10.1162/089892904322926700. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Hager LD, Heron C. Prefrontal cognitive processes: Working memory and inhibition in the antisaccade task. Journal of Experimental Psychology: General. 1994;123(4):374–393. doi:10.1037/0096-3445.123.4.374. [Google Scholar]
- Roberts WA. Some issues in animal spatial memory. In: Roitblat HSTHL, Bever TG, editors. Animal Cognition. Psychology Press; New York, NY: 1984. pp. 425–441. [Google Scholar]
- Sauce B, Matzel LD. The causes of variation in learning and behavior: why individual differences matter. Frontiers in Psychology. 2013;4(July):395. doi: 10.3389/fpsyg.2013.00395. doi:10.3389/fpsyg.2013.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer K, Moosbrugger H, Goldhammer F. The structure of the relationship between attention and intelligence. Intelligence. 2005;33(6):589–611. doi:10.1016/j.intell.2005.07.001. [Google Scholar]
- Sheskin DJ. Handbook of parametric and nonparametric statistical procedures. 3rd ed. CRC Press; 2003. [Google Scholar]
- Sörqvist P, Nöstl A, Halin N. Working memory capacity modulates habituation rate: evidence from a cross-modal auditory distraction paradigm. Psychonomic Bulletin & Review. 2012;19(2):245–50. doi: 10.3758/s13423-011-0203-9. doi:10.3758/s13423-011-0203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. “General Intelligence,” Objectively Determined and Measured. The American Journal of Psychology. 1904;15(2):201–292. [Google Scholar]
- Tabachnick BG, Fidell LS. Principal components and factor analysis. In: Tabachnick BG, Fidell LS, editors. Using multivariate statistics. 4th ed. Allyn and Bacon; Needham: 2001. pp. 582–652. [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High Self-Control Predicts Good Adjustment, Less Pathology, Better Grades, and Interpersonal Success. Journal of Personality. 2004;72(2):271–324. doi: 10.1111/j.0022-3506.2004.00263.x. doi:10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Näätänen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364(6432):59–60. doi: 10.1038/364059a0. doi:10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Tsal Y, Shalev L, Mevorach C. The Diversity of Attention Deficits in ADHD: The Prevalence of Four Cognitive Factors in ADHD Versus Controls. Journal of Learning Disabilities. 2005;38(2):142–157. doi: 10.1177/00222194050380020401. doi:10.1177/00222194050380020401. [DOI] [PubMed] [Google Scholar]
- Van der Maas HLJ, Dolan CV, Grasman RPPP, Wicherts JM, Huizenga HM, Raijmakers MEJ. A Dynamical Model of General Intelligence : The Positive Manifold of Intelligence by Mutualism. 2006;113(4):842–861. doi: 10.1037/0033-295X.113.4.842. doi:10.1037/0033-295X.113.4.842. [DOI] [PubMed] [Google Scholar]
- Wass C, Denman-Brice A, Rios C, Light KR, Kolata S, Smith AM, Matzel LD. Covariation of learning and “reasoning” abilities in mice: evolutionary conservation of the operations of intelligence. Journal of Experimental Psychology. Animal Behavior Processes. 2012;38(2):109–24. doi: 10.1037/a0027355. doi:10.1037/a0027355. [DOI] [PubMed] [Google Scholar]
- Wass C, Pizzo A, Sauce B, Kawasumi Y, Sturzoiu T, Ree F, Matzel LD. Dopamine D1 sensitivity in the prefrontal cortex predicts general cognitive abilities and is modulated by working memory training. Learning & Memory (Cold Spring Harbor, N.Y.) 2013;20(11):617–627. doi: 10.1101/lm.031971.113. doi:10.1101/lm.031971.113. [DOI] [PMC free article] [PubMed] [Google Scholar]