Abstract
Aim and Objective:
To compare and evaluate the effect of ultraviolet (UV) stabilizers (Chimassorb 81 and Uvinul 5050) on the color change of pigmented elastomer.
Materials and Methods:
Two pigments - Red (P112 Brilliant Red) and Yellow (P106 Yellow) and two UV stabilizers Chimassorb 81 and Uvinul 5050 were studied. A total of six groups of 10 samples each were fabricated using a combination of the above colors and stabilizers: Group A1 - Red control, Group A2 - Red + Chimassorb 81, Group A3 - Red + Uvinul 5050. Group B1 - Yellow control, Group B2 - Yellow + Chimassorb 81, Group B3 - Yellow + Uvinul 5050. All samples were subjected to ageing in an accelerated weathering chamber (Weather-Ometer). Color values L, a, and b were measured at 500 and 1000 h for all samples before and after weathering and change in color (Delta E) was calculated.
Results:
All groups showed a significant color change. At 500 h, Chimassorb 81 showed a statistically significant lesser change in both colors (red - 3.66 and yellow - 2.8) compared to their control groups (red - 5.19 and yellow - 4.9). At 1000 h, both UV stabilizers showed lesser color change (A2 - 5.49, B2 - 4.28, A3 - 7.47 and B3 - 4.09) as compared to their respective control groups (A1 - 9.57 and B1 - 5.91). Overall, the change in the color with Group A was more than Group B.
Conclusion:
Addition of UV stabilizers helped the reduction of color change. Chimassorb 81 showed a greater reduction in color change in both colors consistently at 500 and 1000 h.
Keywords: Color change, maxillofacial prosthesis, pigments, ultraviolet stabilizers
INTRODUCTION
Rehabilitation of extra-oral maxillofacial defects with silicone elastomers is a predictable and common treatment modality. Patients present with such extra-oral defects commonly due to cancer resections, trauma, burns, or even congenitally compromised body parts.[1]
The success of prosthesis lies in it being biocompatible, durable, inert, and easy to manipulate and color. One of the main drawbacks of prostheses made from silicone elastomers is that they degrade physically and discolor over a period of time.[2,3,4,5,6,7] Unfortunately, this requires the prostheses to be remade periodically, generally every 1–2 years, increasing the cost of rehabilitation.
The color change in silico ne elastomer has been attributed to factors such as ultraviolet (UV) radiation, temperature changes, humidity, and the use of adhesives, cosmetics, cleansing agents, and exposure to body fluids.[8,9,10,11,12,13]
Current research strategies have been directed toward improving the mechanical properties and color stability of pigmented elastomers. The addition of UV stabilizers, thermochromic pigments, and opacifiers to improve the color stability of pigmented elastomers have been tried in the past with varied results.[14,15,16,17,18]
UV stabilizers are a broad term including UV absorbers (UVAs) and hindered amine light stabilizers (HALS), both having different modes of action.
UV degradation occurs on constant exposure to UV rays produced by the sun and depends to a large extent on their duration, extent, and intensity. UV stabilizers have been used extensively in the polymer,[19] paint, cosmetic, and plastic industries as well as for wood[20] and cellulose fabrics[21] to prevent color degradation and increase the life of these products.
In order to understand the effect of the UV stabilizers in the presence of pigments, their effect in terms of color change over a period of time must be analyzed. The change in color following ageing, expressed as Delta E is calculated. A Delta E value >1 (Kiat-Amnuay et al.,[14] Lemon et al.,[8] Haug et al.[22]), 2 (Beatty et al.,[23] Polyzois et al.[24]), and 3 (Kantola et al.[15]) has been considered perceptible to the human eye.
Previous studies by Haug et al.[22] and Hatamleh and Watts[25] on pigmented samples concluded that color change in elastomer occurs as a result of weathering, silicones having inherent pigment instability. Gary et al.[26] and Hatamleh and Watts[25] have shown that the change in color has been attributed to the degradation in the elastomer itself.
Tran et al.[16] studied the effect of UV stabilizers Tinuvin 213 and Tinuvin 123 on organic and inorganic pigments. They demonstrated some color change in all samples at both their weathering locations. The amount of color change decreased significantly in certain pigmented groups. No study has evaluated the effect of commercial pigments in additive stabilized elastomers.
The use of weathering machine has been commonly used in research studies as it follows standard parameters taking into account humidity, temperature changes, and UV radiation and gives a better estimate of the overall weathering.[7,8,17]
This research study is an attempt to evaluate the effect of UV stabilizers on the color change of pigmented silicone elastomer using two pigments – red and yellow.
MATERIALS AND METHODS
Two commonly used pigments - Red (P112 Brilliant Red, Technovent Pvt. Ltd., UK) and Yellow (P106 Yellow, Technovent Pvt. Ltd., UK) were tested in this study. The UV stabilizers that were used as additives were Food and Drug Administration (FDA) approved UVA Chimassorb 81 (BASF, India) and HALS Uvinul 5050 (BASF, India).
A commonly used platinum based maxillofacial silicone elastomer Z004 (Technovent Pvt. Ltd., UK) mixed in a 1:1 base:catalyst ratio was used for the study.
A total of six groups of 10 samples each were fabricated using a combination of the above pigments and stabilizers. Groups A and B were the principle groups of the pigments red and yellow, respectively, which are further subdivided according to the stabilizer used.
The resultant groups were as follows [Table 1].
Table 1.
Groups and subgroups studied

Group A (Red): Group A1 - Red, Group A2 - Red + Chimassorb 81, Group A3 - Red + Uvinul 5050.
Group B (Yellow): Group B1 - Yellow, Group B2 - Yellow + Chimassorb 81, Group B3 - Yellow + Uvinul 5050.
The A1 and B1 Groups served as control groups with only pigment and no UV stabilizer.
The fabrication of the mold for the samples involved stainless steel sheets of 4 mm thickness that were wire cut (“Precision Wire EDM” machine, Sodick, Germany) into square plates of 10 cm × 10 cm. These were paired into sets of 3. The middle sheet was further wire cut to obtain square shaped cavities for samples of 2 cm × 2 cm. Holes to retain nuts and bolts were made at the four corners of the sheets to secure the assembly.
One percentage by weight of respective UV stabilizer was added in Groups A2, A3, and B2, B3. 0.2% by weight of the respective pigment was added to the subgroups of A and B. These were as per recommendations by Tran et al.,[16] Beatty et al.[23] and Gary et al.[26] The stabilizer and pigment for Groups A2, A3 and B2, B3 and the pigment alone for Groups A1 and B1 were weighed carefully using a digital weighing scale, added and thoroughly spatulated together with the silicone (base + catalyst) manually for 5 min to obtain a homogenous mix. The silicone was vacuum mixed for 20 min under 30 inch Hg. The molds were carefully loaded and clamped under pressure for 24 h to allow the silicone to polymerize. Care was taken to slowly fill the molds to prevent the possibility of air entrapment. The cured samples were examined carefully and those with surface porosities or impurities were discarded.
The samples obtained were cleaned thoroughly with acetone and cotton. They were tested at 0 h to obtain the baseline color.
The samples were subjected to ageing in a Weather-Ometer (Xenon Arc Ci4000, Atlas Material Testing Technology, USA). Alternating light and dark cycles for a total of 180 min completed one cycle. The light cycle (120 min) included an irradiance of 340 nm of 0.55 W/m2, humidity of 50% and a chamber temperature of 47°C with water spray for 60 min. This was followed by 60 min without water spray. The dark cycle lasted for 60 min with a temperature of 38°C, humidity of 95% and irradiance at 340 nm of 0.55 W/m2. These parameters were selected keeping in mind tropical climatic conditions.
Testing of the samples was carried out at intervals of 0, 100, 500, and 1000 h. The samples for all groups were thoroughly cleaned with cotton and acetone prior to testing. Each sample was measured 3 times, and an average of the readings was considered for its analysis.
The color variables L, a, b according to the Commission Internationale d’Eclairage Lab system were measured using a spectrophotometer (TES-135 Color Meter, Instruments and Machinery Sales Corporation, Mumbai, India) before and after ageing.
The L* parameter corresponds to the degree of lightness and darkness (100 ideal white, 0 ideal black), while a* and b* coordinates correspond to red or green chroma (+a = red, −a = green) and yellow or blue chroma (+b = yellow, −b = blue), respectively. The Delta E (change in color) was calculated for each sample using a software with the formula: Delta E = ([Delta L*]2+ [Delta a*]2+ [Delta b*]2)1/2, where Delta L*, Delta a*, and Delta b* are the difference in L, a, and b values before and after ageing.
RESULTS
Average Delta E values obtained for Groups A and B are seen in [Tables 2 and 3] and represented in Figures 1 and 2.
Table 2.
Average color change (Delta E values) after 500 h of ageing

Table 3.
Average color change (Delta E values) after 1000 h of ageing

Figure 1.

Color change at 500 h
Figure 2.
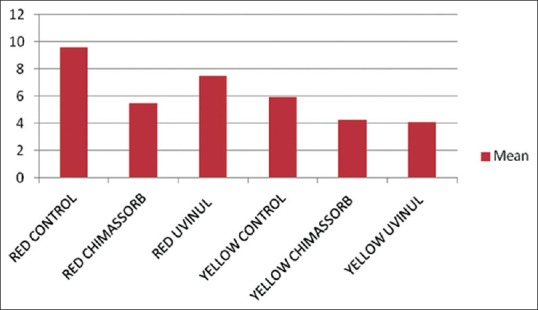
Color change at 1000 h
A one-way ANOVA was applied to compare the mean values of the six groups. A further analysis, the post-hoc Tukey test was carried out to compare between groups. The significance level was considered at 0.05.
At 500 h, for the red pigment, both the UV stabilizers showed a lesser color change than the control (5.19). When the two UV stabilizers were compared, Chimassorb 81 showed the least change (3.66). The color change between Chimassorb 81 and the control group was highly significant (P < 0.01).
At 500 h, for the yellow pigment, both the UV stabilizers showed a lesser color change than the control (4.99). A comparison between the UV stabilizers resulted in Chimassorb 81 showing the least change (2.81). The color change between Chimassorb 81 and the control group was highly significant (P < 0.01).
At 1000 h, for the red pigment, both the UV stabilizers showed a lesser color change than the control (9.57). In comparison between the UV stabilizers, Chimassorb 81 showed the least change (5.49). The color change between Chimassorb 81 and the control group was highly significant (P < 0.01) whereas that between Uvinul 5050 and the control was significant (P < 0.05).
At 1000 h, for the yellow pigment, both the UV stabilizers showed a lesser color change than the control (5.91). When the two UV stabilizers were compared, Uvinul 5050 showed the least change (4.09). However, the color change between Chimassorb 81 and the control group as well as Uvinul 5050 and the control group was significant (P < 0.05).
A comparative analysis among the UV stabilizers revealed that the performance of the Chimassorb 81 was consistent. Samples with Chimassorb 81 (Groups A2, B2) showed a lower Delta E value at 500 and 1000 h except for in the yellow pigment at 1000 h.
Amongst the pigments (Groups A and B), Group A (Red) showed a much greater color change than Group B (Yellow).
DISCUSSION
Silicone elastomers have shown to degrade by undergoing a color change and deterioration of their mechanical properties during the course of their use. This is attributed to a multifactorial etiology involving primarily the exposure to UV rays.[27]
The literature has reported on UV degradation of elastomers and a subsequent change in their mechanical and optical properties and is said to be dependent on the duration, extent, and intensity of the exposure.[7,8,9,10,11,24]
Research strategies for stabilization of elastomers have included the use of UV stabilizers, thermochromic pigments, and opacifiers.[8,14,15,16,17,18] UV stabilizers have been used extensively in the polymer, paint, cosmetic, and plastic industries as well as for wood and cellulose fabrics to prevent color degradation and increase the life of the products.[19,20,21]
A UV stabilizer is a broad term including UVAs and HALS, both of which have different mechanisms of actions. The empirical requirements for optimal action of the stabilizers are high solubility, minimal diffusion, and high distribution homogeneity.[19]
Such attempts at stabilization of elastomers have yielded mixed results. Kantola et al.[15] found that thermochromic pigments were very sensitive to UV radiation and not suitable for prosthetic application whereas Kiat-Amnuay et al.[14] studied various concentrations of opacifiers and found that they prevent color degradation overtime. In this study, Chimassorb 81, a benzophenone UVA and Uvinul 5050, an oligomeric HALS were studied.
In the past, varying concentrations of UV stabilizers have been documented by researchers with mixed results. Chu and Fischer[17] used 1.5% weight UVA and found that it was effective in reducing yellowing of the elastomer, Lemon et al.[8] used 12%, 25% by weight UVA and found that these did not protect the samples and Tran et al.[16] used 75% by weight UVA and HALS and found that this was effective only for certain pigmented groups. In this study, 1% by weight of UVA (Chimassorb 81) and 1% by weight of HALS (Uvinul 5050) were used for Groups A2, B2 and A3, B3, respectively. These additives are FDA approved biocompatible additives manufactured by BASF Chemicals, Mumbai, India Division.
To know the effect of these UV stabilizers on color change, an accelerated weathering chamber is used to simulate weathering conditions and includes parameters such as UV lighting and radiation, water spray, humidity, and temperature. A combined effect of these can probably cause a more pronounced change compared to that produced by one parameter alone. UV irradiation can contribute to enhancing cross linking leading to breaking down of chain bonds and decomposition of the elastomer.[25]
The effect of UV radiation has been known to enhance cross linking, break down of polymer chain bonds, reduce polymerization, and decompose the elastomers, all of which may contribute to color instability.[25,28] It is likely that Chimassorb 81 compound is able to better absorb UV rays from sunlight and dissipate this energy throughout the polymer matrix, thereby preventing degradation.
As reported by Mancuso et al.,[29] color change of the elastomer itself can be attributed to inherent chemical alterations in the silicone and a probability of absorption and adsorption of substances that can take place from the surface of the silicone.
Among the stabilizers studied in this research, Chimassorb 81 (UVA) showed the least color change. Chimassorb 81, chemical name methanone, (2-hydroxy-4-[octyloxy] phenyl) phenyl, and molecular weight of 326.4 g/mol is preferably used for thick films of materials (>100 µm) especially plasticized polyvinylchloride and rubbers. The thickness of the samples or absorption depth in this study was 4 mm contributing to protection of the polymer.[30]
Chimassorb 81 is an UVA and as the name suggests, soaks up harmful UV rays from sunlight converting this energy into heat energy which is then dissipated.[30] It prevents the formation of harmful free radicals. It is probable that Chimassorb 81 absorbs heat energy, preventing photosensitization. Its color is transparent to visible light and does not alter the appearance of the elastomer thereby contributing to its stabilizing effect.[19]
Uvinul 5050 on the other hand is a HALS that protects the basic material structure and helps neutralize the harmful photochemical free radicals. HALS regenerate themselves and hence probably provide protection over a longer period of time.[31] This explains for its better performance over UVA Chimassorb 81 at 1000 h.
Both red and yellow pigments are commonly used in color formulations to obtain skin colors. However, red pigment degraded to a larger extent that yellow. These results are consistent with previous studies by Kiat-Amnuay et al.[14,30] and Beatty et al.[23] These may be attributed to the organic nature of the red pigment being more affected by irradiation.[25]
Future research strategies may involve different types of elastomers, including those different in their compositions and manufacturing protocols. A variety of other UV stabilizers, their combinations and concentrations can be evaluated on physical and mechanical properties. Evaluation over longer periods of time, combining methods of ageing to simulate natural conditions and stabilization of the pigments can be researched on.
CONCLUSION
Within the limitations of the study, it can be concluded that both the UV stabilizers performed well and helped to reduce the color change in pigmented samples as compared to the control samples. UVA Chimassorb 81 consistently showed a lesser color change in both pigments. A comparison between the pigments revealed that those samples fabricated with red pigment showed a greater color change than yellow.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bulbulian AH. Maxillofacial prosthetics: Evolution and practical application in patient rehabilitation. J Prosthet Dent. 1965;15:544–69. [PubMed] [Google Scholar]
- 2.Gunay Y, Kurtoglu C, Atay A, Karayazgan B, Gurbuz CC. Effect of tulle on the mechanical properties of a maxillofacial silicone elastomer. Dent Mater J. 2008;27:775–9. doi: 10.4012/dmj.27.775. [DOI] [PubMed] [Google Scholar]
- 3.Haug SP, Moore BK, Andres CJ. Color stability and colorant effect on maxillofacial elastomers. Part II: Weathering effect on physical properties. J Prosthet Dent. 1999;81:423–30. doi: 10.1016/s0022-3913(99)80009-2. [DOI] [PubMed] [Google Scholar]
- 4.Hulterström AK, Ruyter IE. Changes in appearance of silicone elastomers for maxillofacial prostheses as a result of aging. Int J Prosthodont. 1999;12:498–504. [PubMed] [Google Scholar]
- 5.Mohite UH, Sandrik JL, Land MF, Byrne G. Environmental factors affecting mechanical properties of facial prosthetic elastomers. Int J Prosthodont. 1994;7:479–86. [PubMed] [Google Scholar]
- 6.Beumer J, Curtis TA, Maurinick MT. Maxillofacial Rehabilitation: Prosthodontic and Surgical Considerations. St. Louis: Elsevier Publications; 1996. p. 436. [Google Scholar]
- 7.Kheur MG, Sethi T, Coward T, Jambhekar SS. A comparative evaluation of the change in hardness, of two commonly used maxillofacial prosthetic silicone elastomers, as subjected to simulated weathering in tropical climatic conditions. Eur J Prosthodont Restor Dent. 2012;20:146–50. [PubMed] [Google Scholar]
- 8.Lemon JC, Chambers MS, Jacobsen ML, Powers JM. Color stability of facial prostheses. J Prosthet Dent. 1995;74:613–8. doi: 10.1016/s0022-3913(05)80314-2. [DOI] [PubMed] [Google Scholar]
- 9.Chen MS, Udagama A, Drane JB. Evaluation of facial prostheses for head and neck cancer patients. J Prosthet Dent. 1981;46:538–44. doi: 10.1016/0022-3913(81)90244-4. [DOI] [PubMed] [Google Scholar]
- 10.Fine L, Robinson JE, Barnhart GW, Karl L. New method for coloring facial prostheses. J Prosthet Dent. 1978;39:643–9. doi: 10.1016/s0022-3913(78)80075-4. [DOI] [PubMed] [Google Scholar]
- 11.Jani RM, Schaaf NG. An evaluation of facial prostheses. J Prosthet Dent. 1978;39:546–50. doi: 10.1016/s0022-3913(78)80191-7. [DOI] [PubMed] [Google Scholar]
- 12.Hanson MD, Shipman B, Blomfield JV, Janus CE. Commercial cosmetics and their role in the coloring of facial prostheses. J Prosthet Dent. 1983;50:818–20. doi: 10.1016/0022-3913(83)90098-7. [DOI] [PubMed] [Google Scholar]
- 13.Andres CJ, Haug SP, Munoz CA, Bernal G. Effects of environmental factors on maxillofacial elastomers: Part I – Literature review. J Prosthet Dent. 1992;68:327–30. doi: 10.1016/0022-3913(92)90339-c. [DOI] [PubMed] [Google Scholar]
- 14.Kiat-Amnuay S, Johnston DA, Powers JM, Jacob RF. Color stability of dry earth pigmented maxillofacial silicone A-2186 subjected to microwave energy exposure. J Prosthodont. 2005;14:91–6. doi: 10.1111/j.1532-849X.2005.00017.x. [DOI] [PubMed] [Google Scholar]
- 15.Kantola R, Lassila LV, Tolvanen M, Valittu PK. Color stability of thermochromic pigment in maxillofacial silicone. J Adv Prosthodont. 2013;5:75–83. doi: 10.4047/jap.2013.5.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran NH, Scarbecz M, Gary JJ. In vitro evaluation of color change in maxillofacial elastomer through the use of an ultraviolet light absorber and a hindered amine light stabilizer. J Prosthet Dent. 2004;91:483–90. doi: 10.1016/S002239130400112X. [DOI] [PubMed] [Google Scholar]
- 17.Chu CC, Fischer TE. Evaluation of sunlight stability of polyurethane elastomers for maxillofacial use. I. J Biomed Mater Res. 1978;12:347–59. doi: 10.1002/jbm.820120308. [DOI] [PubMed] [Google Scholar]
- 18.Han Y, Zhao Y, Xie C, Powers JM, Kiat-Amnuay S. Color stability of pigmented maxillofacial silicone elastomer: Effects of nano-oxides as opacifiers. J Dent. 2010;38(Suppl 2):e100–5. doi: 10.1016/j.jdent.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Feldman D. Polymer weathering: Photo-oxidation. J Environ Polym Degrad. 2002;10:163–73. [Google Scholar]
- 20.George B, Suttie E, Merlin A, Deglis X. Photo degradation and photo stabilisation of wood – The state of the art. Polym Degrad Stab. 2005;88:268–74. [Google Scholar]
- 21.Czajkowski W, Paluszkiewicz J, Stolarski R, Kaźmierska M, Grzesiak E. Synthesis of reactive UV absorbers, derivatives of monochlorotriazine, for improvement in protecting properties of cellulose fabrics. Dyes Pigm. 2006;71:224–30. [Google Scholar]
- 22.Haug SP, Andres CJ, Moore BK. Color stability and colorant effect on maxillofacial elastomers. Part II: Weathering effect on color. J Prosthet Dent. 1999;81:431–8. doi: 10.1016/s0022-3913(99)80010-9. [DOI] [PubMed] [Google Scholar]
- 23.Beatty MW, Mahanna GK, Dick K, Jia W. Color changes in dry-pigmented maxillofacial elastomer resulting from ultraviolet light exposure. J Prosthet Dent. 1995;74:493–8. doi: 10.1016/s0022-3913(05)80351-8. [DOI] [PubMed] [Google Scholar]
- 24.Polyzois GL, Tarantili PA, Frangou MJ, Andreopoulos AG. Physical properties of a silicone prosthetic elastomer stored in simulated skin secretions. J Prosthet Dent. 2000;83:572–7. doi: 10.1016/s0022-3913(00)70017-5. [DOI] [PubMed] [Google Scholar]
- 25.Hatamleh MM, Watts DC. Effect of extraoral aging conditions on color stability of maxillofacial silicone elastomer. J Prosthodont. 2010;19:536–43. doi: 10.1111/j.1532-849X.2010.00627.x. [DOI] [PubMed] [Google Scholar]
- 26.Gary JJ, Huget EF, Powell LD. Accelerated color change in a maxillofacial elastomer with and without pigmentation. J Prosthet Dent. 2001;85:614–20. doi: 10.1067/mpr.2001.114683. [DOI] [PubMed] [Google Scholar]
- 27.Polyzois GL. Color stability of facial silicone prosthetic polymers after outdoor weathering. J Prosthet Dent. 1999;82:447–50. doi: 10.1016/s0022-3913(99)70032-6. [DOI] [PubMed] [Google Scholar]
- 28.Goiato CM, Pesqueira AA, Moreno A, Micheline Dos Santos D, Haddad MF, Bannwart LC. Effects of pigment, disinfection, and accelerated aging on the hardness and deterioration of a facial silicone elastomer. Polym Degrad Stab. 2012;97:1577–80. [Google Scholar]
- 29.Mancuso DN, Goiato MC, Dekon SF, Gennari-Filho H. Visual evaluation of color stability after accelerated aging of pigmented and nonpigmented silicones to be used in facial prostheses. Indian J Dent Res. 2009;20:77–80. doi: 10.4103/0970-9290.49073. [DOI] [PubMed] [Google Scholar]
- 30.Kiat-Amnuay S, Mekayarajjananonth T, Powers JM, Chambers MS, Lemon JC. Interactions of pigments and opacifiers on color stability of MDX4-4210/type A maxillofacial elastomers subjected to artificial aging. J Prosthet Dent. 2006;95:249–57. doi: 10.1016/j.prosdent.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Jia H, Wang H, Wenxiu C. The combination effect of hindered amine light stabilizers with UV absorbers on the radiation resistance of polypropylene. Radiat Phys Chem. 2007;76:1179–88. [Google Scholar]


