Key Points
Patient-specific pathways of resistance to venetoclax can be identified by high-content screening of clinical samples with a KI library.
Sunitinib may overcome resistance to venetoclax for many patients by downregulating the expression of Bcl-xl, Mcl-1, and A1 in CLL cells.
Abstract
Novel agents such as the Bcl-2 inhibitor venetoclax (ABT-199) are changing treatment paradigms for chronic lymphocytic leukemia (CLL) but important problems remain. Although some patients exhibit deep and durable responses to venetoclax as a single agent, other patients harbor subpopulations of resistant leukemia cells that mediate disease recurrence. One hypothesis for the origin of resistance to venetoclax is by kinase-mediated survival signals encountered in proliferation centers that may be unique for individual patients. An in vitro microenvironment model was developed with primary CLL cells that could be incorporated into an automated high-content microscopy-based screen of kinase inhibitors (KIs) to identify agents that may improve venetoclax therapy in a personalized manner. Marked interpatient variability was noted for which KIs were effective; nevertheless, sunitinib was identified as the most common clinically available KI effective in overcoming venetoclax resistance. Examination of the underlying mechanisms indicated that venetoclax resistance may be induced by microenvironmental signals that upregulate antiapoptotic Bcl-xl, Mcl-1, and A1, which can be counteracted more efficiently by sunitinib than by ibrutinib or idelalisib. Although patient-specific drug responses are common, for many patients, combination therapy with sunitinib may significantly improve the therapeutic efficacy of venetoclax.
Introduction
Chronic lymphocytic leukemia (CLL) is compartmentalized in the circulation and in proliferation centers (PCs) in lymphoid organs and bone marrow. CLL cells in PCs are generally much less sensitive to cytotoxic agents than cells in the circulation.1-4 Accordingly, the importance of eradicating tumor cells in PCs to cure CLL requires that novel treatment strategies be evaluated in this compartment.
A promising new strategy to kill cancer cells is to directly target the apoptotic machinery that is tightly controlled by Bcl-2 family proteins and ultimately determines cell survival.5-7 The antiapoptotic protein Bcl-2 is overexpressed in the majority of CLL cases due to deletion of miR-15a and 16-1,6 whereas the antiapoptotic proteins Mcl-1 and Bcl-xl are transcriptionally upregulated by microenvironmental survival signals.7 These proteins inhibit apoptosis by binding proapoptotic BH3 proteins and preventing activation of proapoptotic Bax and Bak. Venetoclax specifically binds and inhibits Bcl-2, releasing BH3 proteins to activate Bax and/or Bak and cause mitochondrial outer membrane permeabilization.8-10 Venetoclax has been recently approved for previously treated CLL patients.9 However, despite an overall response rate of 71% to 79%, the complete remissions rate for venetoclax monotherapy was relatively low (20%).9 These observations suggest the need for new strategies to improve the efficacy of venetoclax in the microenvironments that produce drug resistance.
Genetic heterogeneity and activation of patient-specific bypass pathways likely contribute to therapy resistance.11 Overcoming these barriers and being able to rapidly identify drugs or drug combinations that would be effective in individual patients would be an important advance.11 To meet this need, we have developed an in vitro model of the leukemic microenvironment that is amenable to high-content image-based screening. This model recapitulated the clinical phenomenon of venetoclax resistance in the microenvironment. Given that other BCL-2 family members such as Mcl-1 and Bcl-xl are transcriptionally upregulated by microenvironmental survival signals and could mediate resistance to venetoclax,7 we screened a kinase inhibitor (KI) library of over 300 members and found that venetoclax resistance could be overcome by adding KIs. Although the optimal KI was patient-specific, sunitinib emerged as the most common clinically available drug that significantly augmented cell killing by venetoclax. Biochemical analyses suggest that changes in antiapoptotic Bcl-2 family protein expression in cells contributed to the observed drug responses. Thus, kinase-mediated signaling in response to microenvironmental cues may underlie CLL cell drug resistance in PCs, and sunitinib is a candidate to improve the efficacy of venetoclax in many patients.
Materials and methods
Heparinized blood was obtained from consenting patients with CLL (Table 1). Protocols were approved by the Sunnybrook Ethics Review Board, and informed consent was obtained in compliance with the Declaration of Helsinki. For stimulation of CLL cells, resiquimod and interleukin 2 (IL2) were used at 1 µg/mL and 500 U/mL, respectively, as previously described.12,13 These cells are hereafter referred to as “2S” cells.14 IL4 was used at a final concentration of 20 ng/mL. For image-based screening, 2S-stimulated CLL cells seeded into 384-well plates were treated with 320 KIs at 1 µM, a commonly used dose in primary preclinical drug screens,11,15 with or without 10 nM venetoclax. Cells stained with Annexin V Alexa Fluor 488, tetramethylrhodamine ethyl ester (TMRE), and Draq5 were evaluated for cytotoxicity by automated live-cell high-content confocal fluorescence microscopy (Opera QEHS high-content screening system; PerkinElmer). Acquired images were analyzed using Acapella 2.0 (PerkinElmer). Fluorescence characteristics for cells exposed to dimethyl sulfoxide (DMSO) as negative control or the pan-KI staurosporine (STS) plus venetoclax as positive control were used for single and multiparametric analysis using custom Acapella software (available at www.andrewslab.ca) and exported to MATLAB (MathWorks) for further analysis as described in the supplemental Methods (available on the Blood Web site).
Table 1.
Patient characteristics
| Patient no.* | Sex | Age, y | Time, y† | WBC | Stage‡ | CD38, % | β2M§ | Cytogenetic | Treatments|| | IGHV status¶ | CLL methylation# | ZAP70 methylation call (<0.2)** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 72 | 10 | 175 | 3 | 1 | 3.8 | NA | 0 | MU | HP | High |
| 2 | F | 79 | 4 | 112 | 4 | 1 | 11 | Normal | 1 | NA | NA | NA |
| 3 | M | 62 | 8 | 102 | 4 | 35 | 3.2 | 11q | 0 | U | LP | Low |
| 4 | F | 77 | 10 | 46 | 4 | 12 | 3 | NA | 0 | MU | IP | Low |
| 5 | M | 59 | 12 | 589 | 4 | 15 | 4.9 | Normal | 2 | NA | HP | High |
| 6 | M | 67 | 8 | 161 | 4 | 1 | 2.6 | 13q | 0 | NA | HP | High |
| 7 | M | 71 | 13 | 147 | 4 | 3 | 3.8 | 13q | 3 | NA | HP | Low |
| 8 | M | 67 | 4 | 103 | 4 | 1 | 5.4 | Normal | 1 | NA | NA | NA |
| 9 | M | 75 | 21 | 154 | 4 | 18 | 6.1 | NA | 0 | NA | NA | NA |
| 10 | F | 76 | 3 | 33 | 4 | 1 | 2.9 | NA | 0 | NA | NA | NA |
| 11 | M | 78 | 1 | 46 | 4 | 25 | 4.3 | NA | 0 | MU | NA | NA |
| 12 | F | 67 | 12 | 44 | 3 | 1 | 2.4 | 13q, 17p | 3 | NA | NA | NA |
| 13 | M | 58 | 4 | 36 | 2 | 1 | 2.2 | NA | 0 | NA | HP | High |
| 14 | F | 76 | 11 | 70 | 4 | NA | 4.3 | NA | 2 | NA | NA | NA |
| 15 | M | 62 | 14 | 20 | 2 | 1 | 2.1 | 13q | 5 | MU | HP | High |
| 16 | F | 82 | 11 | 75 | 4 | 1 | 4 | Normal | 0 | NA | HP | High |
| 17 | M | 76 | 12 | 38 | 4 | NA | 6 | t12 | 2 | NA | LP | Low |
| 18 | F | 79 | 3 | 147 | 3 | 1 | 6.4 | 17p | 3 | MU | HP | High |
| 19 | F | 81 | 3 | 94 | 4 | 3 | 4.5 | NA | 1 | NA | LP | Low |
| 20 | F | 66 | 24 | 74 | 4 | 1 | 3.5 | 13q | 3 | NA | LP | Low |
| 21 | M | 75 | 3 | 144 | 4 | NA | 6.1 | NA | 0 | NA | NA | NA |
| 22 | M | 85 | 6 | 237 | 3 | 1 | 2.2 | NA | 0 | MU | IP | Low |
| 23 | M | 55 | 2 | 99 | 2 | 1 | 3 | 13q | 0 | MU | IP | Low |
| 24 | F | 79 | 5 | 313 | 3 | 1 | 7.4 | 13q, 17p | 2 | U | LP | Low |
| 25 | F | 60 | 24 | 72 | 4 | 2 | 2.7 | 13q | 0 | NA | NA | NA |
| 26 | F | 73 | 10 | 146 | 4 | 1 | 5 | 13q | 1 | MU | IP | Low |
| 27 | F | 87 | 18 | 14 | 4 | 8 | 15.6 | t12 | 3 | NA | NA | NA |
| 28 | F | 57 | 1 | 112 | 4 | 0 | 5.1 | 13q, 17p | 2 | U | LP | Low |
| 29 | M | 68 | 12 | 59 | 2 | 1 | 3.6 | 13q | 1 | MU | HP | High |
| 30 | M | 66 | 14 | 85 | 2 | 1 | 2 | Normal | 1 | NA | HP | High |
| 31 | F | 64 | 4 | 36 | 4 | 16 | 4.1 | NA | 0 | NA | NA | NA |
| 32 | F | 56 | 5 | 147 | 2 | NA | 2.4 | NA | 0 | MU | HP | High |
| 33 | F | 76 | 7 | 22 | 2 | 2 | 3.5 | NA | 0 | U | IP | Low |
| 34 | M | 57 | 5 | 251 | 3 | NA | 2.9 | t12 | 1 | NA | HP | High |
| 35 | F | 73 | 9 | 296 | 4 | 1 | 5.6 | 13q | 2 | NA | LP | Low |
| 36 | M | 62 | 2 | 56 | 1 | 2 | 3.2 | NA | 0 | NA | NA | NA |
| 37 | M | 63 | 2 | 344 | 4 | NA | 3.4 | 17p | 1 | NA | NA | NA |
| 38 | M | 61 | 4 | 40 | 1 | 2 | 2.2 | NA | 0 | NA | NA | NA |
| 39 | F | 85 | 10 | 53 | 4 | 4 | 5.9 | 13q | 1 | NA | NA | NA |
| 40 | F | 56 | 6 | 240 | 2 | NA | 2.3 | NA | 0 | NA | NA | NA |
| 41 | F | 86 | 7 | 101 | 1 | 1 | 4 | NA | 0 | MU | NA | NA |
| 42 | M | 68 | 1 | 38 | 2 | 14 | 2.1 | NA | 0 | NA | NA | NA |
| 43 | M | 54 | 2 | 82 | 4 | 23 | 3 | t12 | 0 | NA | NA | NA |
β2M, β2-microglobulin; F, female; HP, highly programmed CLL; IP, intermediate-programmed CLL; LP, low-programmed CLL; M, male; MU, mutated; NA, not available; U, unmutated; WBC, white blood cell count.
Corresponding patient numbers are maintained throughout this manuscript.
Time since diagnosis.
Rai stage: 0, lymphocytosis; I, with adenopathy; II, with hepatosplenomegaly; III, with anemia; IV, with thrombocytopenia.
Normal range is 0.6 to 2.3 µg/mL.
Including alkylator-, fludarabine-, high-dose glucocorticoid-, and/or antibody-based treatments, indicated treatments were stopped at least 3 months prior to time of analysis.
IGVH mutation status.
DNA methylation.
ZAP70 CpG+223 methylation, <0.2 = low, >0.2 = high.
Results
Sensitivity of CLL cells to venetoclax depends on microenvironmental signals
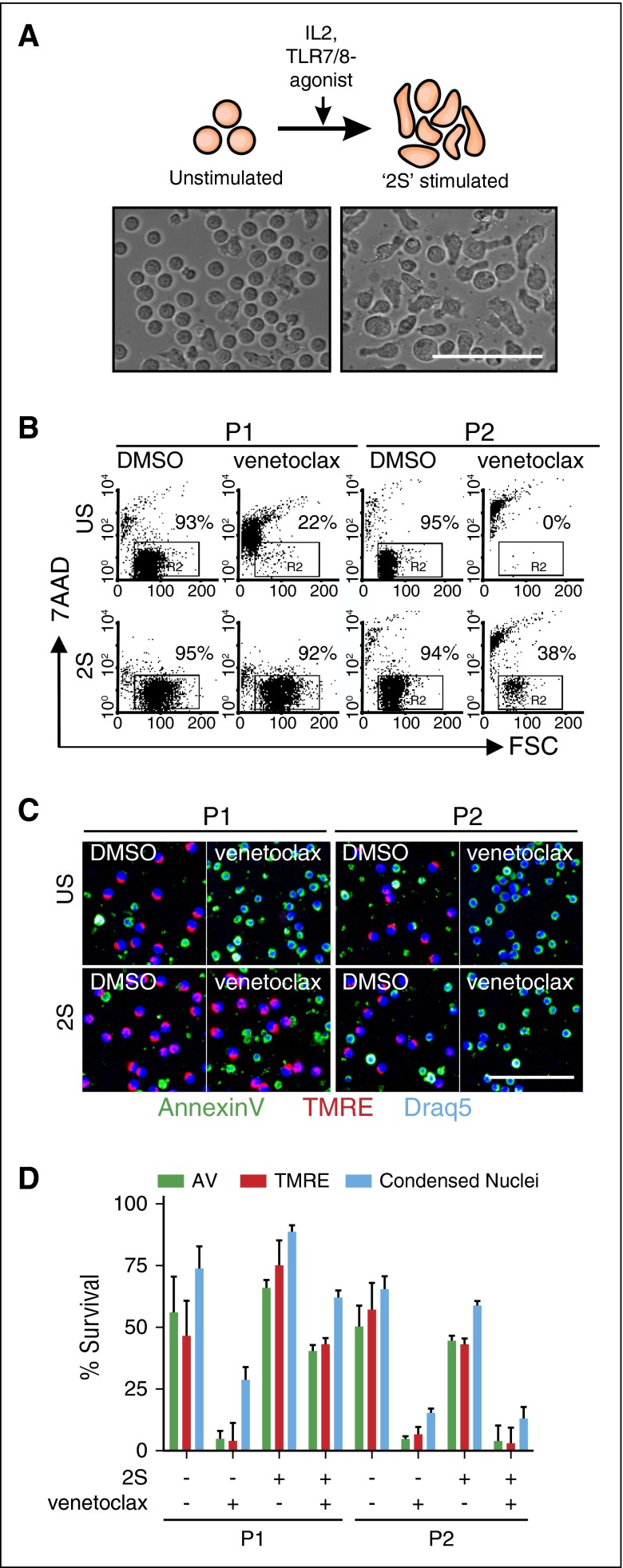
Sensitivity of CLL cells to drug treatment can be influenced strongly by the leukemic microenvironment.16-18 To address the effect of venetoclax on cells in PCs, a previously characterized in vitro model was used where CLL cells from patient-derived blood are stimulated in serum-free conditions with IL2 to represent T-cell signaling and Toll-like receptor 7/8 (TLR-7/8) agonist resiquimod to provide NF-κB–activating signals (Figure 1A).12-14,19 Such stimulated CLL cells (called “2S” cells) increase in size, change their morphology from a round to elongated shape, and proliferate in clusters (Figure 1A).12,13 Venetoclax rapidly killed unstimulated CLL cells from most patients (Figure 1B, top panel), whereas 2S-stimulated cells exhibited varying degrees of resistance to 10 nM venetoclax, a concentration that is considered to be on-target for BH3-mediated killing.20 For example, a moderate effect was obtained in 2S cells from P2, but complete resistance was observed in cells from P1 (Figure 1B, bottom panel).
Figure 1.
Resistance to venetoclax induced by microenvironmental signals. (A) Schematic model of CLL cell activation by the tumor microenvironment and corresponding bright-field images. CLL cells derived from peripheral blood (unstimulated [US]) change morphology and proliferate in vitro in response to IL2 (500 U/mL) and the Toll-like receptor-7/8 agonist Resiquimod (1 µg/mL) (“2S” stimulated). Phase-contrast micrographs of cells incubated and imaged (EVOS FL automicroscope [Life Technologies] with a 40× LPlan FLPH objective [AMG EP4683]) in serum-free AIM-V culture media (scale bar, 50 μm). (B) Flow cytometric analysis of cell 7-aminoactinomycin D (7AAD) staining (viability) for US (top panel) and 2S cells (2S; bottom panel) from 2 representative patients (P1, P2) cultured with or without venetoclax (10 nM) for 48 hours after addition of drug. The percentages of viable cells that exclude 7AAD (7AAD−, enclosed box R2) are shown in the dot plots as numbers. (C) Image-based detection of cell death and survival. US (top panels) and 2S-stimulated (2S; bottom panels) CLL cells from the patients in panel B were cultured with DMSO as a negative control or with venetoclax (10 nM) as indicated. After 72-hour incubation, cells were stained with standard fluorescence dyes for detection of apoptotic cell death (Annexin V conjugated to Alexa Fluor 488, green), mitochondrial membrane potential (TMRE, red), and nuclear size and morphology (Draq5, blue). Automated confocal fluorescence microscopy (Opera QEHS high-content screening system; PerkinElmer) was used to acquire 3 images from each well for 4 replicate wells of a 384-well imaging plate using a 20× 0.45 numerical aperture (NA) air lens at 37°C and 5% CO2. Representative images are shown (scale bar, 50 μm). (D) Cell survival determined by automated analysis of micrographs such as those in (C) for each of unstimulated (–2S) and stimulated cells (+2S) with or without venetoclax (10 nM) as indicated. Viability was defined by either the absence of Annexin V staining intensity (green bars), the presence of TMRE staining intensity (red bars), or the absence of condensed nuclei stained by Draq5 (blue bars). Error bars ± standard deviation (std dev), n = 12 fields of view (FOV). AV, Annexin V; FSC, forward scatter.
To examine patient-specific responses to venetoclax, we developed a high-content fluorescence microscopy method to assess cell stress and death based on automated image analysis of 3 fluorescence channels (Figure 1C-D). Annexin V (green) staining was used to capture the externalization of phosphatidylserine as a result of disturbed lipid asymmetry in the plasma membrane of cells, a well-characterized event in apoptosis. TMRE (red), a lipophilic cationic redistribution dye, reports loss of mitochondrial membrane potential; Draq5 (blue) enabled measurements of both nuclear/chromatin condensation and total cell counts.
Drug responses to 10 nM venetoclax (Figure 1C) were quantified by automatically identifying the cells using the Draq5 micrograph, and applying intensity and area thresholds established from DMSO-treated control samples to the appropriate fluorescence channel (Figure 1D). Consistent with the flow cytometry results, a high sensitivity to venetoclax was detected in unstimulated cells as indicated by loss of TMRE+ cells, increased Annexin V+ cells, and increased condensed nuclei (Figure 1D). The patient-specific effect of venetoclax in 2S-stimulated cells was also confirmed as a response to venetoclax in cells from P2 but a marked resistance in cells from P1 as shown by each of the single parameters (Figure 1C-D). Hence, our data are in agreement with previous studies reporting a microenvironment-induced resistance to venetoclax16 and further suggest that activated signaling pathways exhibit heterogeneity across patients.
High-content imaged-based screening identifies drugs that overcome resistance to venetoclax in individual patients
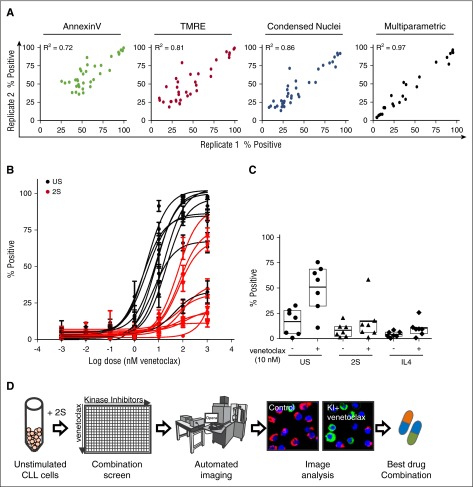
To validate the image-based assays for screening, technical replicates of 2S-stimulated cells obtained from 1 blood sample from 1 patient were treated with a panel of control drugs with and without venetoclax (10 nM) (Figure 2A). Comparison of drug responses in technical-replicate samples revealed a linear correlation for each of the single assays (Annexin V, R2 = 0.72; TMRE, R2 = 0.81; and condensed nuclei, R2 = 0.86), confirming the precision and reproducibility of the system.
Figure 2.
Multiparametric image analysis and high-content screening enables automated analysis of cell viability for CLL patients’ cells exposed to KIs and venetoclax alone and in combination. (A) Correlation of technical replicates for 2S cells from 1 representative patient treated with negative control (DMSO), positive control (STS + venetoclax), and a panel of drugs. Comparison of fluorescence intensity or area threshold analyses to identify positive (dead) cells based on loss of lipid asymmetry (Annexin V, green), loss of mitochondrial membrane potential (TMRE, red), and nuclear condensation (Condensed Nuclei, blue) with a multiparametric classifier (black). Images of cells were acquired as described in Figure 1C. For threshold analysis, cells were classified as in Figure 1D; for multiparametric analysis, cells were classified as nonviable (percentage positive) if they were more similar to the positive control (STS + venetoclax treated) training group than the negative control (DMSO) group based on 8 quantified image features using a random forest classifier. (B) Patient-specific dose response fit for venetoclax (1 pM to 1 µM) in unstimulated (C, black) and 2S CLL cells (2S, red) assessed by multiparametric analysis as in the description of panel A. Data for 9 representative patients were fit using nonlinear least squares with GraphPadPrism 5.03 (GraphPad Software) as mean ± standard deviation (std dev) for cell images from 8 micrographs per condition. (C) Microenvironment-induced resistance to venetoclax is independent of model. Response to 10 nM venetoclax for 72 hours was measured for unstimulated and 2S- or IL4-stimulated CLL cells from 7 patients. Drug response assessed by multiparametric analysis of cells classified as percentage positive as described in panel A. (D) Schematic overview of image-based high-content drug screening in primary CLL cells. Patient-derived CLL cells were stimulated with 2S media, seeded into 384-well plates and treated with 320 KIs at a screening concentration of 1 µM with or without venetoclax (10 nM). Negative (DMSO) and positive controls (STS + venetoclax) used for training of the classifier were included in each plate. Automated fluorescence imaging was performed as described in Figure 1.
Given that cell death is a complex process associated with a wide range of measureable intracellular changes,21,22 combining a variety of measurements from each cell can improve the accuracy of cell death detection.23 Therefore, multiparametric analysis was used to integrate the information obtained from the micrographs into 1 cell death index indicating the percentage of dead cells classified as similar to the dead cell (positive) training control of STS plus venetoclax (Figure 2A, right panel, percentage positive). Multiparametric analysis increased linear correlations between replicate samples (R2 = 0.97), compared with threshold analyses for single assays (Figure 2A, right panel), and therefore was used for subsequent high-throughput experiments.
Dose-response studies in CLL cells from 9 patients confirmed the resistance to venetoclax under PC microenvironmental conditions (Figure 2B). In contrast to the high efficacy of venetoclax in unstimulated cells that induced 25% to 100% cell death at 10 nM and higher, 2S cells of all patients were protected against 10 nM venetoclax. Higher concentrations of 100 and 1000 nM were sufficient to kill 60% to 80% of cells from only 3 of 9 patients (Figure 2B). Accordingly, 10 nM was selected as the optimal screening dose for venetoclax to identify KIs that enhance cell death due to venetoclax by blocking cell survival signals.
To confirm these results, IL4 was used as an alternative method to mimic the leukemic microenvironment.24-27 The venetoclax dose response in 2S-stimulated cells was plotted against the dose response in IL4-stimulated cells and positive correlations were obtained for each of the single assays (Annexin V, R2 = 0.80; TMRE, R2 = 0.72; Draq5, R2 = 0.80; supplemental Figure 1A). Similar to stimulation with 2S and consistent with previous studies in vivo,24,28-30 IL4 stimulation of CLL cells induced complete resistance to 10 nM venetoclax (Figure 2C). Moreover, the response of cells to venetoclax (10 nM) was not significantly different in the presence of 1% pooled patient plasma or 5% fetal bovine serum (supplemental Figure 1B).
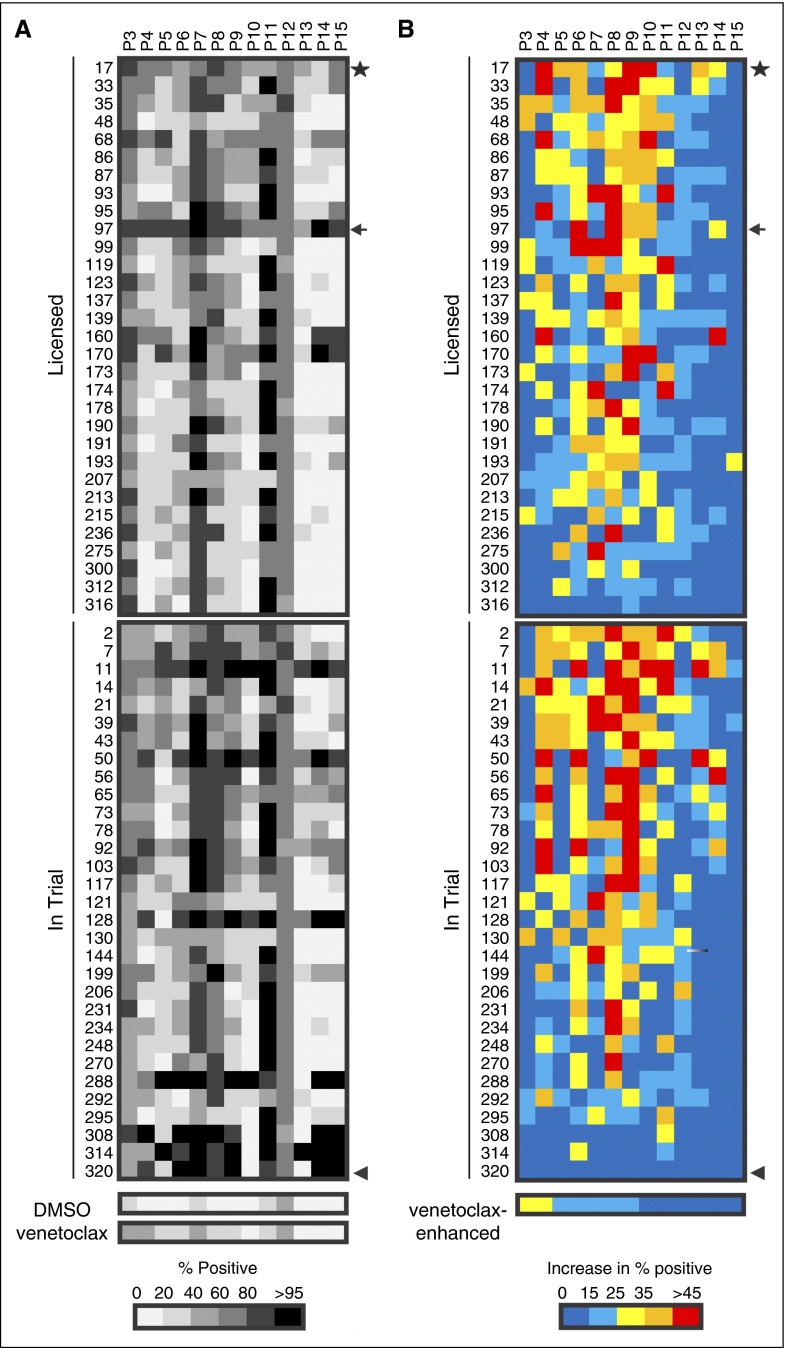
We hypothesized that resistance to venetoclax could be counteracted by KIs that block microenvironmental survival signals. Therefore, 320 KIs (supplemental Table 1) were screened in combination with 10 nM venetoclax in 2S-stimulated cells from 13 patients with varying but low sensitivity (<60% positive) to 10 nM venetoclax (Figure 3; supplemental Figure 2) to identify the most effective combinations of venetoclax and KIs for individual patients (Figure 2D). The efficacy of the combination of venetoclax and all KIs was assessed by determining the percentage of cells classified as positive (ie, dead) (supplemental Figure 2A). To enable detection of drug pairs likely to act synergistically, and to prevent KIs with high single-agent toxicity from dominating the analysis, we determined the extent to which addition of 10 nM venetoclax increased the number of dead cells for the same KIs (supplemental Figure 2B). The increase in dead cells above that with KI treatment alone is called the “venetoclax-enhanced effect.” Based on the value of total kill induced by the drugs in combination, a cutoff to identify hit combinations was set at 25% for venetoclax-enhanced effect which corresponds to a mean total kill of 63% across screen positives.
Figure 3.
Identification of KIs currently licensed or in trial that overcome resistance to venetoclax in 2S-stimulated cells from individual patients. Heatmaps of (A) total cytotoxicity expressed as percentage positive (classified similarity to STS + venetoclax control) and (B) venetoclax-enhanced cytotoxicity expressed as an increase in percentage positive for 2S-stimulated CLL cells from 13 patients incubated with KIs that are licensed or in clinical trials (1 μM) in combination with venetoclax (10 nM), as indicated. Rows indicate individual KIs; columns indicate different patient samples. The grayscale bar shows percentage of cells classified as positive as indicated at the bottom in panel A, and the color bar indicates venetoclax-enhanced percentage positive in panel B. A combination of KI and venetoclax is considered to be effective if venetoclax increases the percentage-positive classification by >25% above the value for KI alone, corresponding to a mean total kill of 63% in screen positives (yellow [B]). A combination of KI and venetoclax was considered to be highly effective if addition of venetoclax increased the amount of cell death by >45% (red [B]), corresponding to a total kill of >80% for the drug combination (A). The controls below the heatmaps show percentage-positive cells for the negative control (DMSO) and venetoclax alone in panel A, and venetoclax-enhanced percentage-positive cells compared with DMSO (ie, the difference between venetoclax and DMSO alone) in panel B, for individual patients. The columns are sorted in decreasing order of venetoclax-enhanced percentage-positive compared with DMSO; rows are ranked according to frequency of effective KI-venetoclax combinations. For combinations with the same frequency, the rows were further sorted based on the average value for venetoclax-enhanced effect across positive screens. Results are shown for 62 of the 320 KIs (results for all KIs are in supplemental Figure 2). Sunitinib (★) was identified for further analysis as the licensed compound with a high frequency (8 of 13 patient samples) and degree of venetoclax-enhanced kill (mean value of 45% enhanced kill in screen positives). The arrow and arrowhead refer to dasatinib and alvocidib, respectively (see “Discussion” for more details). Patient samples were analyzed by fluorescence-based high-content screening for percentage positive as described in Figure 2. The 10 nM dose of venetoclax was selected for screening as it was the highest concentration that exhibited minimal activity against 2S-stimulated CLL cells (Figure 2B-C).
Responses of patient samples in this screen were highly variable, with some exhibiting sensitivity to a broad spectrum of KIs (P7, P11), whereas others had a poor response to the majority of drugs (P13, P15; supplemental Figure 2). However, for most patients (12 of 13) there were KIs for which the venetoclax-enhanced effect resulted in an increase in cell killing of >45% (supplemental Figure 2B red) corresponding to a mean total kill of 78%.
KIs were subdivided into 31 US Food and Drug Administration (FDA) licensed drugs (Figure 3, top panel) and another 31 compounds currently being tested in clinical trials (phase 1-3) (Figure 3, bottom panel). Importantly, although every patient was sensitive to at least 1 clinically used KI in combination with venetoclax, very few KIs were identified as optimal for multiple patients (Figure 3B).
The KI that most often produced a high venetoclax-enhanced effect was sunitinib (Sutent), a licensed multitargeted tyrosine KI (average enhanced effect for 8 of 13 patients of 45%; Figure 3, star).31-35 In comparison, venetoclax alone had a <25% enhanced effect above DMSO for most patients (Figure 3B colored bar at the bottom).
Consistent with its lack of efficacy as monotherapy in CLL,36 sunitinib had only moderate single-agent activity in 2S-stimulated cells with an average kill of 30% (approximately the same as the KI library mean of 31% single-agent kill) and ranked in position 132 of 320 KIs (supplemental Table 1).
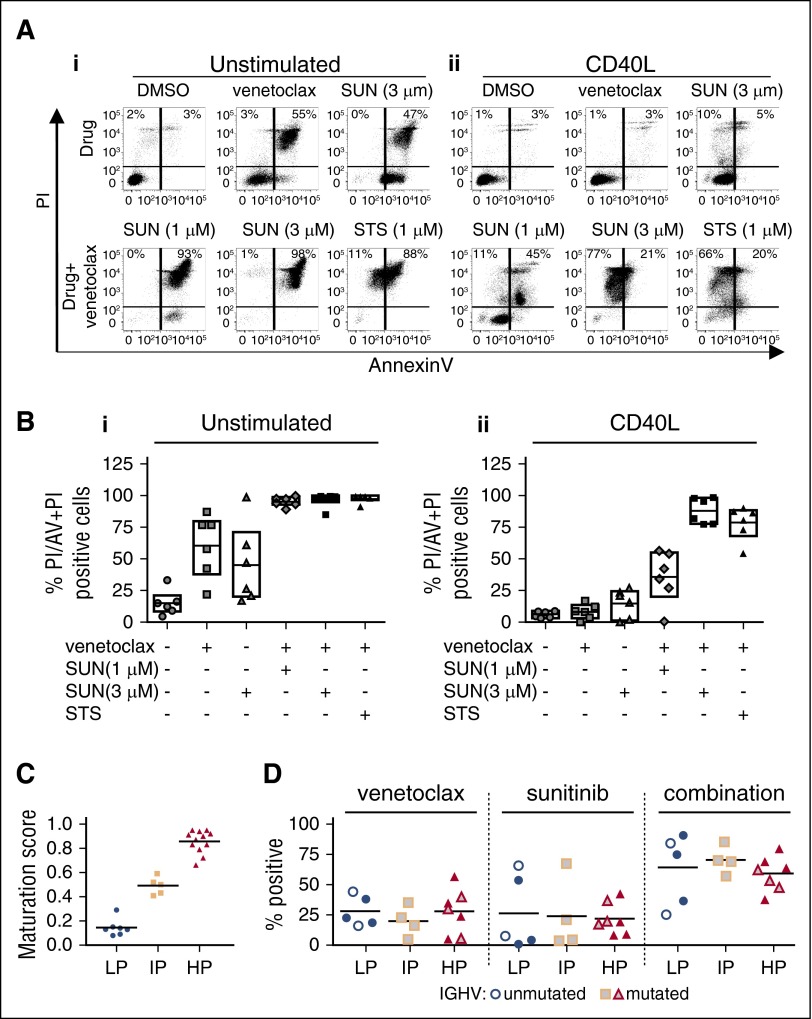
No correlations were observed between the augmentation of venetoclax killing and clinical prognostic markers (Table 1), including the degree of mutation of the immunoglobulin heavy chain variable (IGHV) region gene assessed by sequencing (Table 1; Figure 4D) or by the recently described DNA methylation subgrouping that correlates with IGHV status and ZAP70 methylation (Figure 4C-D).37-41
Figure 4.
Coculture of primary CLL cells promotes resistance to venetoclax that was overcome by sunitinib independent of IGHV mutation status. Primary CLL cells from 6 patients were cultured in suspension (unstimulated) or in coculture with bone marrow stromal cells (OP9) expressing human CD40L (CD40L) and treated with the indicated concentrations of venetoclax, sunitinib (SUN) alone or in combination or as a positive cell death control with STS and venetoclax. Drug response was determined by flow cytometric analysis of Annexin V (AV) and propidium iodide (PI)-stained CLL cells 48 hours after drug treatment. (A) Dot plots are shown for unstimulated suspension CLL cells (i) and CD40L-stimulated CLL cells (ii) for 1 representative patient. Numbers in dot blots indicate percentages of PI+ cells (top left quadrant) and Annexin V + PI++ cells (top right quadrant). (B) Percentage of PI/Annexin V + PI+ cells for unstimulated CLL cells (i) and CD40L-stimulated CLL cells (ii) from 6 patients 48 hours after the indicated drug treatment. Cells double stained with Annexin V and PI (AV + PI, top right in dot blots) or PI+ cells (top left corner in dot blots) were counted as nonviable. Cells positive for only Annexin V (bottom right corner) were excluded due to possible interference from sunitinib fluorescence. On average, venetoclax induced only 8% cell kill (n = 6) in CD40-cocultured cells compared with 60% cell kill in unstimulated cells. Sunitinib (3 µM) increased the total kill to >80%. Similar results were obtained for cells cocultured with OP9 stromal cells (supplemental Figure 3). (C) DNA methylation maturation scores for 24 CLL samples separated into methylation subtypes as assessed by MassARRAY. Consensus clustering of DNA methylation levels of a panel of 6 genes was used to classify 3 clusters of CLL cases based on their degree of DNA methylation maturation (blue: low [LP-CLL]; yellow: intermediate [IP-CLL]; and red: high [HP-CLL]) programmed CLL (LP-CLL, n = 7; IP-CLL, n = 5; HP-CLL, n = 12). (D) Drug response to venetoclax (10 nM), sunitinib (1 µM) alone or in combination in 2S-stimulated CLL cells from 16 patients separated by DNA methylation subgroup as in panel C and IGHV mutation status. Gray filled symbols, mutated IGHV; white filled symbols, unmutated IGHV; solid color symbols, IGHV status data not available. IGHV sequence homology of <98% vs germline was considered as mutated. Percentage positive indicates percentage of dead cells determined by multiparametric image analysis as described in Figure 2. Data for CLL cells cocultured with OP9 control stromal cell lines are shown in supplemental Figure 2.
Coculture of primary CLL cells with stromal cells confirms resistance to venetoclax and effect of sunitinib
As stromal cells have been found to guard CLL cells from drug-induced apoptosis,3,42,43 CLL cells were cocultured with murine bone marrow stromal cells (OP9) or OP9 cells expressing human CD40L to combine stromal elements with T-cell effects.43-47 Venetoclax (10 nM) induced cytotoxicity was significantly decreased in both coculture systems, with an average cell kill of 8% (n = 6) for CD40L-cocultured cells and 30% (n = 4) for OP9-cocultured cells compared with 60% kill in unstimulated (suspension) CLL cells (n = 6) (Figure 4A-B; supplemental Figure 3A-B). Consistent with the cytokine models (Figures 1-2), sunitinib had minor single-agent activity (average of 15% cell kill in CD40L-cocultured CLL cells and 29% in OP9-cocultured CLL cells), but augmented cell killing by venetoclax to a total kill of >80% in cocultured CLL cells of all patients (average 88% with CD40L [n = 6] and 84% with OP9 [n = 4]; Figure 4A-B; supplemental Figure 3A-B).
Drug dose-response validation studies confirm sunitinib-venetoclax combination efficacy in CLL
The potentiating effect of sunitinib for venetoclax was studied in more detail in 12 additional patient samples. Sunitinib alone at concentrations from 0.03 to 30 µM was relatively nontoxic to both 2S-stimulated and unstimulated cells (Figure 5A, top left panel; supplemental Figure 4A, top left panel). At concentrations of 3 µM and higher, sunitinib induced >80% total kill in most samples when combined with 10 nM venetoclax (Figure 5A, bottom left panel and B; supplemental Figure 4A). In unstimulated cells, the high total kill (>95%) measured for the drug combination can be attributed to the single-agent effect of venetoclax (supplemental Figure 4A, bottom panel). Concentrations of venetoclax below 10 nM did not kill in this system, alone or in combination with sunitinib (Figure 5B).
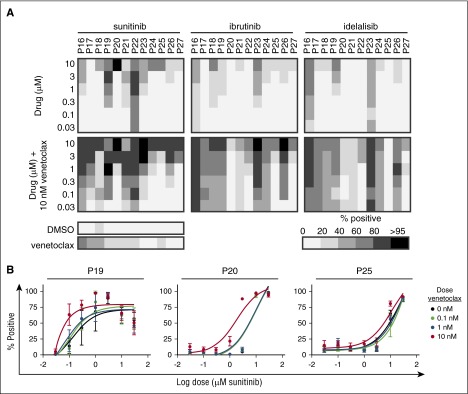
Figure 5.
Sunitinib is more efficient than idelalisib or ibrutinib in killing 2S-stimulated CLL cells in combination with venetoclax. 2S-stimulated CLL cells from 12 patients were treated with sunitinib, ibrutinib, or idelalisib alone or in combination with venetoclax, as indicated. Drug response was measured as described in Figure 2. (A) Dose-response heatmap of total cytotoxicity expressed as percentage-positive cells for cell death induced by sunitinib, ibrutinib, and idelalisib as single agents (top panels) and in combination with venetoclax (10 nM) (bottom panels). The shade indicates the mean for all the cells in 8 micrographs for each condition according to the grayscale bar shown below. Individual patient samples are shown in columns and drug concentrations in rows. Cell death (percentage positive) in negative control (DMSO) and for venetoclax (10 nM) as single agents are shown as bars below the heatmaps for individual patients. Across 12 patients, the average single-agent activity for 1 µM sunitinib, ibrutinib, and idelalisib was 24%, 15%, and 19%, respectively. (B) Dose-response data fit by nonlinear least squares for sunitinib alone (black dots and line) and in combination with the indicated concentrations of venetoclax (colored lines) for cells from 3 patients (numbers above the panels) analyzed as in panel A. Points are mean ± standard deviation (std dev), n = 8 FOV.
We compared sunitinib to ibrutinib and idelalisib, which are licensed for CLL48-50 but not included in our KI library. As single agents, both ibrutinib and idelalisib were relatively nontoxic (Figure 5A, top, middle, and right panels; supplemental Figure 4A, top, middle, and right panels) resulting in >80% cell kill in 2S-stimulated cells from only 5 of 12 and 2 of 12 patients, respectively. For venetoclax in combination with 10 µM ibrutinib and idelalisib, respectively, the average total cell kill for the 12 patient samples was 76% and 57% (Figure 5A, bottom, middle, and right panels). In contrast, under the same conditions, sunitinib and venetoclax resulted in >80% total kill for the majority of patients (9 of 12) with an average of 83% total kill for all 12. When the KI inhibitor concentration was reduced to 3 µM, the average total kill in combination with venetoclax remained high for sunitinib (80%) but was less for ibrutinib (57%) and idelalisib (53%) for 2S-stimulated and IL4-stimulated (supplemental Figure 4B) cells. Addition of human plasma revealed a slightly protective effect for sunitinib, but not ibrutinib and idelalisib alone and with venetoclax (supplemental Figure 5A-B, and C, left panel). Combination with venetoclax was slightly less effective in serum-containing media for all 3 drugs (supplemental Figure 5C-D). Taken together, the results obtained were remarkably robust to variations in culture conditions.
Microenvironmental survival signals induce patient-specific changes in expression levels of Bcl-2 family proteins that can be prevented by sunitinib
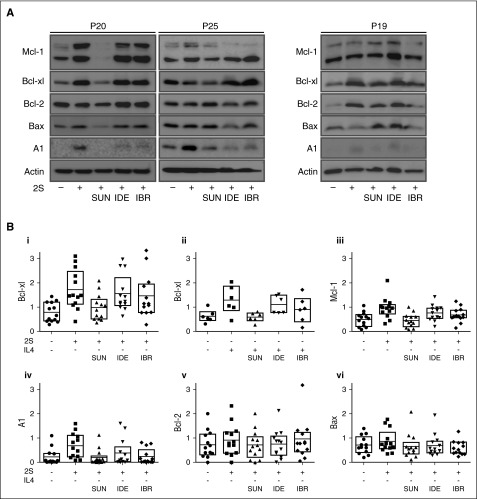
The above results (Figures 1-2) suggest that microenvironmental survival signals confer resistance to venetoclax in CLL cells from most patients that can be overcome by KIs. Previously identified mechanisms for venetoclax resistance include activation of signaling pathways that upregulate antiapoptotic proteins other than Bcl-2 itself or downregulate proapoptotic Bcl-2 family proteins. Therefore, changes in Bcl-2 family protein levels were examined in unstimulated and stimulated (2S or IL4) cells from individual patients in response to sunitinib, idelalisib, or ibrutinib (Figure 6; supplemental Figures 6-7). These analyses revealed substantial variations between patients (Figure 6A), some of which correlated well with the observed drug responses (Figure 5B). For example, for P20, there was a significant upregulation of Bcl-xl, Mcl-1, and A1 in both 2S- and IL4-stimulated cells that correlated with resistance to both venetoclax and sunitinib individually. However, these cells were substantially more sensitive to the drugs in combination, a result that correlates with downregulation of Mcl-1, Bcl-xl, and A1 in response to sunitinib combined with inhibition of Bcl-2 by venetoclax.
Figure 6.
Microenvironment survival signals induce patient-specific changes in expression of Bcl-2 family proteins that was prevented by sunitinib but not ibrutinib or idelalisib. Comparison of protein levels for unstimulated CLL cells (−) and 2S- or IL4-stimulated cells (+) before and after treatment with 1 µM sunitinib (SUN), idelalisib (IDE), and ibrutinib (IBR). Protein lysates for stimulated patient cells (n = 13 for 2S stimulation, n = 6 for IL4 stimulation) collected 18 to 20 hours after drug treatment were analyzed by immunoblotting using antibodies to the antigens indicated. (A) Representative results for patients P20, P25, and P19. (B) Quantification of relative protein levels for Bcl-xl (i-ii), Mcl-1 (iii), A1 (iv), Bcl-2 (v), and Bax (vi) by analysis of chemiluminescence signals recorded using a CCD camera corrected to the intensities obtained with the β-actin antibody using NIH ImageJ 1.48p software. Data are for 13 patients in subpanels i, iii, iv, v, and vi and 6 patients in subpanel ii; boxes indicate 25th to 75th percentile with the mean indicated as a line. For sunitinib treatment in 2S-stimulated cells, 21 patients were analyzed by immunoblotting (data not shown, significance tests in supplemental Table 2).
In other cases, such as P25 where expression of all of the antiapoptotic proteins was readily apparent in unstimulated cells and in which incubation in 2S medium upregulated primarily A1, sunitinib only marginally decreased Mcl-1 and Bcl-xl and the cells were relatively resistant to the drugs used in combination (Figure 5B, right panel). Changes in expression of Bcl-2 family proteins were similar when cells were stimulated with IL4 (supplemental Figure 6A). Consistent with the patient-specific effects reported in Figure 3 and supplemental Figure 2 described in the second section of “Results,” cells from P19 responded to 2S stimulation by upregulating only Bcl-xl but downregulating Bax (Figure 6A, right panel). Although most striking for Bax, both effects were inhibited by sunitinib correlating with the enhanced kill of these cells by the drugs in combination (Figure 5B, left panel).
Notwithstanding these “patient-specific mechanisms,” 2S stimulation induced an increase in Bcl-xl, Mcl-1, and A1 levels on average 2.9-, 2.4-, and 10.0-fold, respectively, compared with unstimulated controls (Figure 6B; supplemental Figure 6B; supplemental Table 2). Addition of sunitinib reduced these increases to 2.0-, 1.1-, 3.2-fold compared with unstimulated controls, respectively. In addition, downregulation of the proapoptotic sensitizer Bim was seen in 5 of 6 patients that was also reversed by sunitinib (supplemental Figure 7A-B). Taken together, these observations suggest sunitinib counteracts resistance to venetoclax by complex regulation of the Bcl-2 family.
The effect of sunitinib on Bcl-2 family proteins was compared with ibrutinib and idelalisib in cells stimulated with 2S (n = 13) and with IL4 (n = 6). In both models, the increase in Bcl-xl, Mcl-1 and A1 was blocked significantly by sunitinib, but not by idelalisib (Figure 6B; supplemental Figure 6; supplemental Table 2). On average, ibrutinib decreased only slightly the levels of A1 and Mcl-1 in the 2S model, but not in the IL4 model (supplemental Table 2). Across patient samples, significant changes were not detected for Bcl-2, whereas changes in Bax were found for only a minor fraction. However, sunitinib consistently decreased levels of Mcl-1, Bcl-xl, and A1 but not Bcl-2. Thus, when venetoclax was used in combination with sunitinib there was a substantial reduction/inhibition in protein levels for all of the known antiapoptotic Bcl-2 family proteins that were associated with increased cell kill.
Resistance to venetoclax is frequently mediated by upregulation of Bcl-xl
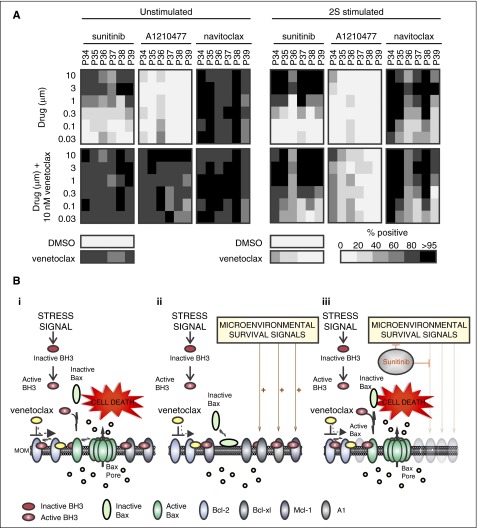
Although microenvironmental stimulation appeared to affect several Bcl-2 family proteins with major effects on Bcl-xl and Mcl-1 in the majority of patients, we identified Bcl-xl as the most important protein accountable for resistance to venetoclax (Figure 7A). Navitoclax, a drug that inhibits both Bcl-xl and Bcl-2, was highly effective in killing CLL cells from 6 patients, where cell death was induced at 30 nM in unstimulated cells and 1 µM in stimulated cells. In 2S-stimulated cells, response to navitoclax was similar to the dose response to sunitinib and venetoclax (compare patterns in the heatmaps for sunitinib and venetoclax with navitoclax in 2S-stimulated cells; Figure 7A). As expected, addition of venetoclax did not increase single-agent effect of navitoclax (Figure 7A, bottom panel). In contrast, the Mcl-1 inhibitor A1210477 failed to induce cell kill in unstimulated and 2S-stimulated cells at all concentrations assayed (30 nM to 10 µM), and when used in combination with venetoclax was inefficient to induce >60% of total kill in 2S-stimulated cells of all patients, except 1 (P34; Figure 7A, right panel). Corresponding immunoblot studies in cells from the same patients confirmed upregulation of Bcl-xl under 2S conditions, reverted by sunitinib to the level in unstimulated control cells (supplemental Figure 7A-B), suggesting that reduced expression of Bcl-xl may be of greatest importance for sunitinib to augment venetoclax effects. However, both navitoclax and sunitinib dose responses achieved <60% cell kill in samples from patient P36 (Figure 7A), for which Mcl-1 and A1 levels were less altered by sunitinib compared with its effect on Bcl-xl (P36; supplemental Figure 7A), suggesting that the overall drug response of some patients is not related to the regulation of a single antiapoptotic protein.
Figure 7.
Resistance in 2S-stimulated cells is mainly mediated by Bcl-xl. (A) Dose-response heatmap of total cytotoxicity expressed as percentage-positive cells for cell death induced by sunitinib, A1210477, and navitoclax as single agents (top panels) and in combination with venetoclax (10 nM) (bottom panels) in unstimulated and 2S-stimulated cells from 6 patients. Drug response was determined by multiparametric analysis as described in Figure 2. The shade indicates the mean for all the cells in 8 micrographs for each condition according to the grayscale bar shown below. Columns are individual patient samples and rows are drug concentrations. Cell death (percentage positive) in negative control (DMSO) and for venetoclax (10 nM) as a single agent are shown as bars below the heatmaps. (B) Model illustrating potential cellular responses to venetoclax and sunitinib. (i) Apoptotic response of unstimulated CLL cells treated with venetoclax. Bcl-2 family proteins control cell death by regulating the permeabilization of the mitochondrial outer membrane (MOM) through a series of competitive binding interactions among themselves. For illustrative purposes, inhibition of BH3 proteins (mode 2) is shown although the process is predicted to be similar for activated Bax bound to and inhibited by antiapoptotic proteins (mode 1). Upon receiving an apoptotic stress signal, inactive proapoptotic BH3 proteins (red) are activated (red and white) and migrate to mitochondria where they are either bound by 1 of the antiapoptotic proteins (Bcl-2, Bcl-xl, Mcl-1, or A1) or trigger apoptosis by binding to inactive Bax (light green) and activating it at the membrane (dark green). Activated Bax oligomerizes and permeabilizes the MOM, enabling release of proapoptotic proteins including cytochrome c, endonuclease G, and Smac (orange circles) from the mitochondrial intermembrane space into the cytoplasm to activate the effector caspases that execute the cell. Venetoclax (yellow) binds Bcl-2 displacing an activator BH3 protein, allowing the downstream activation of Bax, permeabilization of MOM, and cell death. (ii) Apoptotic response of CLL cells stimulated by microenvironment. Microenvironmental survival signals increase expression of the antiapoptotic Bcl-2 family members Bcl-xl, Mcl-1, and A1 that are not targeted by venetoclax and/or the downregulation of the proapoptotic protein Bax. The excess antiapoptotic proteins bind active BH3 proteins displaced from Bcl-2 by venetoclax preventing them from activating Bax thereby inhibiting apoptosis. (iii) Sunitinib turns off microenvironmental prosurvival signals preventing increased expression of the antiapoptotic proteins other than Bcl-2 and thereby restoring sensitivity to venetoclax.
Discussion
Variable levels of Bcl-2 family proteins in different cellular contexts may help explain different responses to venetoclax by unstimulated and stimulated CLL cells. We propose that apoptotic stress signals are an inevitable consequence of leukemic transformation and lead to the activation of proapoptotic BH3 proteins, such that sufficient antiapoptotic Bcl-2 family proteins must then be present to prevent apoptosis, a condition called “primed.”51 Circulating CLL cells appear to depend mainly on BCL-2 itself to survive. Accordingly, venetoclax displaces active BH3 proteins from preloaded Bcl-2 thereby allowing Bax activation, pore formation in the mitochondrial outer membrane, and subsequent cell death (Figure 7Bi).
In contrast, stimulation with 2S, IL4, or stromal cells to mimic microenvironmental survival signals caused resistance to venetoclax in most patient cells (Figures 1, 2, and 4). This resistance may be explained in part by upregulation of the antiapoptotic proteins Mcl-1, Bcl-xl, and A1 and downregulation of proapoptotic Bim (Figure 7Bii). Bcl-xl and A1 cause resistance to ABT-737, an inhibitor of Bcl-2 and Bcl-xl, in a lymph node model of CLL.4 NF-κB–mediated upregulation of antiapoptotic Bcl-2 family members in lymph nodes correlates inversely with patient survival,7,52 and increased levels of Bcl-xl, Mcl-1, and A1 levels are known to mediate venetoclax resistance in CD40-activated cells.16 Consistent with these observations, upregulation of antiapoptotic Bcl-2 proteins and downregulation of proapoptotic Bim were also detected in our CD40L-cocultured CLL cells (data not shown), most likely accounting for the stroma-induced resistance to venetoclax (Figure 4A-B; supplemental Figure 3A-B).
Our study provides insight into patient-specific mechanisms of microenvironmental-induced resistance to venetoclax and suggests that combination with KIs may improve the therapeutic efficacy of venetoclax. Many signal transduction pathways control the transcription, translation, posttranslational modification, or degradation of Bcl-2 family members.53,54 Therefore, the pathway(s) activated by the microenvironment likely differ from patient to patient, as substantiated by our studies revealing patient-specific patterns in protein levels (Figure 6A; supplemental Figure 6A) and lack of correlation with conventional CLL prognostic markers (Figure 4C-D). Our identification that broad-spectrum KIs are effective when used in combination with venetoclax is consistent with preventing the upregulation of antiapoptotic proteins requiring inhibition of multiple pathways similar to the regulation of Mcl-1 and A1 in a model of acute myeloid leukemia.55
Importantly, and despite marked interpatient variability in drug response, this screen identified sunitinib, a broad-specificity KI, as the most effective FDA-licensed KI that was sufficient to counteract venetoclax resistance in the majority of patient cells. Sunitinib had only modest activity as a single agent in CLL at doses that achieved a plasma concentration of up to 0.25 µM.36,56-60 Indeed, our studies confirmed the lack of efficacy for sunitinib as monotherapy in unstimulated and 2S-/IL4-stimulated CLL cells at these concentrations. The sunitinib response was associated with an effect on Bcl-2 family proteins other than Bcl-2 itself, likely explaining its failure to kill CLL cells as single agent (average of 30% kill as single agent; supplemental Table 1). Instead, sunitinib was sufficient to block the microenvironmental upregulation of Mcl-1, Bcl-xl, and A1, and the downregulation of the proapoptotic protein Bim, thereby overcoming resistance to venetoclax (Figure 7Biii). Interestingly, navitoclax revealed high efficacy in killing cells of most patients, likely due to simultaneous inhibition of Bcl-2 and Bcl-xl. Consistent with the data reported in Figures 4 and 5, sunitinib in combination with venetoclax was as effective as navitoclax, likely due to indirect inhibition of Bcl-xl and other antiapoptotic Bcl-2 proteins. As an indirect inhibitor of Bcl-xl, sunitinib may be a better alternative for clinical use without the on-target side effect of thrombocytopenia caused by navitoclax.61-64 Sunitinib targets multiple tyrosine kinases, such as PDFRs, KIT, FLT3, and VEGF, suggesting a role for these pathways in CLL biology that is relatively poorly explored.
In contrast, the currently approved BTK inhibitor ibrutinib, recently suggested for combination therapy with venetoclax,65 as well as the PI3Kδ inhibitor idelalisib, were not as effective as sunitinib in killing stimulated CLL cells in combination with venetoclax. The drug concentrations tested in our validation studies were in the range of clinically achievable concentrations for each of the KIs, with achievable plasma concentrations of 0.25 µM, 0.5 µM, and up to 1.1 µM reported for sunitinib,56-60 ibrutinib (NCT01105247),66 and idelalisib (NCT00710528, NCT01090414),48 respectively.
Other KIs such as dasatinib67-71 and flavoperidol72-74 were identified as having a high total kill with venetoclax (Figure 3A, arrow and arrowhead, respectively). In combination with venetoclax in vitro, dasatinib ranked in position 10 of 31 licensed drugs and its venetoclax-enhanced effect was intermediate and detected in only 5 of 13 patients (Figure 3A-B, arrow). Accordingly, it was not examined in more detail. The KI with the highest single-agent toxicity of the 62 FDA licensed and in-trial drugs was the cyclin-dependent kinase (CDK) inhibitor alvocidib (flavopiridol), showing an average kill of 86% as monotherapy (rank order 1 of 320 KIs; supplemental Table 1). In combination with venetoclax, a high total kill (Figure 3A, arrowhead) and therefore a near-zero venetoclax-enhanced effect (Figure 3B, arrowhead) was detected. Alvocidib had high clinical activity in patients with advanced fludarabine-refractory CLL; however, acute tumor lysis syndrome limited its use clinically.74 Given that tumor lysis syndrome is associated with both alvocidib and venetoclax,9,74 combining the 2 agents might be too toxic.
Our results suggest that a personalized drug selection based on screening may identify clinically relevant drugs that can overcome resistance to venetoclax by altering microenvironmental signaling pathways (Figure 7Biii). High-content screening of large numbers of KIs for individual patients is a feasible and efficient way to identify effective personal combination therapies with venetoclax. Given that our screening results obtained with 2S- and IL4-mediated stimulation of CLL cells were further confirmed in stromal coculture models, our assay system provides a reasonable model that may emphasize the circumstances under which CLL in vivo is most resistant to therapy, enabling the identification of drugs with effects in those compartments. However, the direct relevance of our results to resistance in vivo remains to be established.
For clinical use of an identified drug combination, dose adaption is likely required, considering a high plasma protein binding reported for venetoclax (99.9%)9 as well as for the kinase inhibitors sunitinib (95%),75 ibrutinib (97%),76 and idelalisib (85%).48 However, our studies indicated only a slightly decreased efficacy for sunitinib and venetoclax, but not ibrutinib and idelalisib, when applied to cells in media containing pooled human plasma (supplemental Figures 1 and 5).
Similarities in pharmacokinetics and pharmacodynamics, including metabolism through CYP3A4 for venetoclax and the 3 KIs,48,75-78 would necessitate careful coadministration in vivo. Importantly, in combination with sunitinib, venetoclax was effective at 10 nM, a concentration well below the clinically tolerated dose9 which indeed may allow a dose adaption. A clinical phase 1/2 trial combining venetoclax with sunitinib in CLL is required to test our hypothesis.
Acknowledgments
The authors thank Spencer Gibson and Michelle Queau, Research Institute in Oncology and Hematology, University of Manitoba (Winnipeg, MB, Canada), for IGHV sequencing studies and sequence analysis.
This work was supported by grants from the Canadian Institutes of Health Research (CIHR; MOP130479 [D.E.S.] and FRN 12517 [D.W.A. and B.L.]), a CIHR Foundation grant (D.W.A.), the Leukemia & Lymphoma Society of Canada (D.E.S.), and the Canada Research Chairs program (D.W.A. and J.C.Z.-P.). P.M.B. was supported by a CIHR Postdoctoral Fellowship award.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.O., B.L., D.W.A., and D.E.S. designed the research, interpreted the data, and wrote the manuscript; B.L., D.W.A., and D.E.S. supervised the project; S.O. established and performed primary cell and coculture, biochemical, and flow cytometric experiments and data analysis; S.O. performed and J.Y. contributed to imaged-based experiments and image analysis; Y.S. helped purifying cells; S.H. and J.Y. wrote custom software scripts for image analysis; J.C.Z.-P. and P.M.B. provided OP9 and OP9-CD40L cell lines; P.M.B. sorted CD40L+ cells; and C.C.O. performed and analyzed MassARRAY studies and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David W. Andrews, Biological Sciences Platform, Sunnybrook Research Institute, University of Toronto, Toronto, ON, Canada, M4N 3M5; e-mail: david.andrews@sri.utoronto.ca; and David E. Spaner, Biological Sciences Platform, Sunnybrook Research Institute, University of Toronto, Toronto, ON, Canada, M4N 3M5; e-mail: spanerd@sri.utoronto.ca.
References
- 1.Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14(9):2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- 2.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–2663. [PubMed] [Google Scholar]
- 3.Kurtova AV, Balakrishnan K, Chen R, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114(20):4441–4450. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogler M, Butterworth M, Majid A, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113(18):4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 5.Brahmbhatt H, Oppermann S, Osterlund EJ, Leber B, Andrews DW. Molecular pathways: leveraging the BCL-2 interactome to kill cancer cells--mitochondrial outer membrane permeabilization and beyond. Clin Cancer Res. 2015;21(12):2671–2676. doi: 10.1158/1078-0432.CCR-14-0959. [DOI] [PubMed] [Google Scholar]
- 6.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2 [published correction appears in Proc Natl Acad Sci USA. 2006;103(7):2464]. Proc Natl Acad Sci USA. 2005;102(39):13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smit LA, Hallaert DY, Spijker R, et al. Differential Noxa/Mcl-1 balance in peripheral versus lymph node chronic lymphocytic leukemia cells correlates with survival capacity. Blood. 2007;109(4):1660–1668. doi: 10.1182/blood-2006-05-021683. [DOI] [PubMed] [Google Scholar]
- 8.Anderson MA, Deng J, Seymour JF, et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53 independent mechanism. Blood. 2016;127(25):3215–3224. doi: 10.1182/blood-2016-01-688796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 11.Crystal AS, Shaw AT, Sequist LV, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346(6216):1480–1486. doi: 10.1126/science.1254721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaner DE, Lee E, Shi Y, et al. PPAR-alpha is a therapeutic target for chronic lymphocytic leukemia. Leukemia. 2013;27(5):1090–1099. doi: 10.1038/leu.2012.329. [DOI] [PubMed] [Google Scholar]
- 13.Tomic J, White D, Shi Y, et al. Sensitization of IL-2 signaling through TLR-7 enhances B lymphoma cell immunogenicity. J Immunol. 2006;176(6):3830–3839. doi: 10.4049/jimmunol.176.6.3830. [DOI] [PubMed] [Google Scholar]
- 14.Spaner DE, Shi Y, White D, et al. Immunomodulatory effects of Toll-like receptor-7 activation on chronic lymphocytic leukemia cells. Leukemia. 2006;20(2):286–295. doi: 10.1038/sj.leu.2404061. [DOI] [PubMed] [Google Scholar]
- 15.Shen M, Zhang Y, Saba N, Austin CP, Wiestner A, Auld DS. Identification of therapeutic candidates for chronic lymphocytic leukemia from a library of approved drugs [published correction appears at http://dx.doi.org/10.1371/annotation/e2536fcb-3ab3-44a0-8eab-91aaeb8e49b6]. PLoS One. 2013;8(9):e75252. doi: 10.1371/journal.pone.0075252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thijssen R, Slinger E, Weller K, et al. Resistance to ABT-199 induced by microenvironmental signals in chronic lymphocytic leukemia can be counteracted by CD20 antibodies or kinase inhibitors. Haematologica. 2015;100(8):e302–e306. doi: 10.3324/haematol.2015.124560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crassini K, Stevenson WS, Mulligan SP, Best OG. The MEK1/2 inhibitor, MEKi-1, induces cell death in chronic lymphocytic leukemia cells under conditions that mimic the tumor microenvironment and is synergistic with fludarabine. Leuk Lymphoma. 2015;56(12):3407–3417. doi: 10.3109/10428194.2015.1032963. [DOI] [PubMed] [Google Scholar]
- 18.Crassini K, Mulligan SP, Best OG. Targeting chronic lymphocytic leukemia cells in the tumor microenviroment: a review of the in vitro and clinical trials to date. World J Clin Cases. 2015;3(8):694–704. doi: 10.12998/wjcc.v3.i8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Shi Y, McCaw L, et al. Microenvironmental interleukin-6 suppresses toll-like receptor signaling in human leukemia cells through miR-17/19A. Blood. 2015;126(6):766–778. doi: 10.1182/blood-2014-12-618678. [DOI] [PubMed] [Google Scholar]
- 20.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7(12):989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 21.Galluzzi L, Bravo-San Pedro JM, Vitale I, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22(1):58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G. Cell death assays for drug discovery. Nat Rev Drug Discov. 2011;10(3):221–237. doi: 10.1038/nrd3373. [DOI] [PubMed] [Google Scholar]
- 23.Collins TJ, Ylanko J, Geng F, Andrews DW. A versatile cell death screening assay using dye-stained cells and multivariate image analysis. Assay Drug Dev Technol. 2015;13(9):547–557. doi: 10.1089/adt.2015.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douglas RS, Capocasale RJ, Lamb RJ, Nowell PC, Moore JS. Chronic lymphocytic leukemia B cells are resistant to the apoptotic effects of transforming growth factor-beta. Blood. 1997;89(3):941–947. [PubMed] [Google Scholar]
- 25.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 26.Panayiotidis P, Ganeshaguru K, Jabbar SA, Hoffbrand AV. Interleukin-4 inhibits apoptotic cell death and loss of the bcl-2 protein in B-chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 1993;85(3):439–445. doi: 10.1111/j.1365-2141.1993.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 27.Steele AJ, Prentice AG, Cwynarski K, et al. The JAK3-selective inhibitor PF-956980 reverses the resistance to cytotoxic agents induced by interleukin-4 treatment of chronic lymphocytic leukemia cells: potential for reversal of cytoprotection by the microenvironment. Blood. 2010;116(22):4569–4577. doi: 10.1182/blood-2009-09-245811. [DOI] [PubMed] [Google Scholar]
- 28.de Totero D, Reato G, Mauro F, et al. IL4 production and increased CD30 expression by a unique CD8+ T-cell subset in B-cell chronic lymphocytic leukaemia. Br J Haematol. 1999;104(3):589–599. doi: 10.1046/j.1365-2141.1999.01219.x. [DOI] [PubMed] [Google Scholar]
- 29.Kay NE, Han L, Bone N, Williams G. Interleukin 4 content in chronic lymphocytic leukaemia (CLL) B cells and blood CD8+ T cells from B-CLL patients: impact on clonal B-cell apoptosis. Br J Haematol. 2001;112(3):760–767. doi: 10.1046/j.1365-2141.2001.02605.x. [DOI] [PubMed] [Google Scholar]
- 30.Rossmann ED, Lewin N, Jeddi-Tehrani M, Osterborg A, Mellstedt H. Intracellular T cell cytokines in patients with B cell chronic lymphocytic leukaemia (B-CLL). Eur J Haematol. 2002;68(5):299–306. doi: 10.1034/j.1600-0609.2002.01612.x. [DOI] [PubMed] [Google Scholar]
- 31.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25(7):884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 32.Christensen JG. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann Oncol. 2007;18(suppl 10):x3–x10. doi: 10.1093/annonc/mdm408. [DOI] [PubMed] [Google Scholar]
- 33.Davis MI, Hunt JP, Herrgard S, et al. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29(11):1046–1051. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- 34.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA. 2007;104(43):17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson VC, Demko N, Hakala A, et al. A meta-analysis of threats to valid clinical inference in preclinical research of sunitinib. eLife. 2015;4:e08351. doi: 10.7554/eLife.08351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shanafelt T, Zent C, Byrd J, et al. Phase II trials of single-agent anti-VEGF therapy for patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2010;51(12):2222–2229. doi: 10.3109/10428194.2010.524327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claus R, Wilop S, Hielscher T, et al. A systematic comparison of quantitative high-resolution DNA methylation analysis and methylation-specific PCR. Epigenetics. 2012;7(7):772–780. doi: 10.4161/epi.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kröber A, Seiler T, Benner A, et al. V(H) mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood. 2002;100(4):1410–1416. [PubMed] [Google Scholar]
- 39.Mertens D, Stilgenbauer S. Prognostic and predictive factors in patients with chronic lymphocytic leukemia: relevant in the era of novel treatment approaches? J Clin Oncol. 2014;32(9):869–872. doi: 10.1200/JCO.2013.53.8421. [DOI] [PubMed] [Google Scholar]
- 40.Oakes CC, Seifert M, Assenov Y, et al. DNA methylation dynamics during B cell maturation underlie a continuum of disease phenotypes in chronic lymphocytic leukemia. Nat Genet. 2016;48(3):253–264. doi: 10.1038/ng.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Queirós AC, Villamor N, Clot G, et al. A B-cell epigenetic signature defines three biologic subgroups of chronic lymphocytic leukemia with clinical impact. Leukemia. 2015;29(3):598–605. doi: 10.1038/leu.2014.252. [DOI] [PubMed] [Google Scholar]
- 42.Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111(2):846–855. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 43.Purroy N, Abrisqueta P, Carabia J, et al. Targeting the proliferative and chemoresistant compartment in chronic lymphocytic leukemia by inhibiting survivin protein. Leukemia. 2014;28(10):1993–2004. doi: 10.1038/leu.2014.96. [DOI] [PubMed] [Google Scholar]
- 44.Néron S, Pelletier A, Chevrier MC, Monier G, Lemieux R, Darveau A. Induction of LFA-1 independent human B cell proliferation and differentiation by binding of CD40 with its ligand. Immunol Invest. 1996;25(1-2):79–89. doi: 10.3109/08820139609059292. [DOI] [PubMed] [Google Scholar]
- 45.Soderquist R, Bates DJ, Danilov AV, Eastman A. Gossypol overcomes stroma-mediated resistance to the BCL2 inhibitor ABT-737 in chronic lymphocytic leukemia cells ex vivo. Leukemia. 2013;27(11):2262–2264. doi: 10.1038/leu.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willimott S, Baou M, Naresh K, Wagner SD. CD154 induces a switch in pro-survival Bcl-2 family members in chronic lymphocytic leukaemia. Br J Haematol. 2007;138(6):721–732. doi: 10.1111/j.1365-2141.2007.06717.x. [DOI] [PubMed] [Google Scholar]
- 47.Willimott S, Baou M, Huf S, Deaglio S, Wagner SD. Regulation of CD38 in proliferating chronic lymphocytic leukemia cells stimulated with CD154 and interleukin-4. Haematologica. 2007;92(10):1359–1366. doi: 10.3324/haematol.11340. [DOI] [PubMed] [Google Scholar]
- 48.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woyach JA, Johnson AJ. Targeted therapies in CLL: mechanisms of resistance and strategies for management. Blood. 2015;126(4):471–477. doi: 10.1182/blood-2015-03-585075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117(1):112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tromp JM, Tonino SH, Elias JA, et al. Dichotomy in NF-kappaB signaling and chemoresistance in immunoglobulin variable heavy-chain-mutated versus unmutated CLL cells upon CD40/TLR9 triggering. Oncogene. 2010;29(36):5071–5082. doi: 10.1038/onc.2010.248. [DOI] [PubMed] [Google Scholar]
- 53.Davids MS, Brown JR. Targeting the B cell receptor pathway in chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(12):2362–2370. doi: 10.3109/10428194.2012.695781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herishanu Y, Pérez-Galán P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahmani M, Aust MM, Attkisson E, Williams DC, Jr, Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood. 2012;119(25):6089–6098. doi: 10.1182/blood-2011-09-378141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abrams TJ, Murray LJ, Pesenti E, et al. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with “standard of care” therapeutic agents for the treatment of breast cancer. Mol Cancer Ther. 2003;2(10):1011–1021. [PubMed] [Google Scholar]
- 57.Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2(5):471–478. [PubMed] [Google Scholar]
- 58.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–337. [PubMed] [Google Scholar]
- 59.Murray LJ, Abrams TJ, Long KR, et al. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20(8):757–766. doi: 10.1023/b:clin.0000006873.65590.68. [DOI] [PubMed] [Google Scholar]
- 60.O’Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101(9):3597–3605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 61.Debrincat MA, Pleines I, Lebois M, et al. BCL-2 is dispensable for thrombopoiesis and platelet survival. Cell Death Dis. 2015;6:e1721. doi: 10.1038/cddis.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaefer A, Yang J, Noertersheuser P, et al. Mechanism-based pharmacokinetic/pharmacodynamic meta-analysis of navitoclax (ABT-263) induced thrombocytopenia. Cancer Chemother Pharmacol. 2014;74(3):593–602. doi: 10.1007/s00280-014-2530-9. [DOI] [PubMed] [Google Scholar]
- 63.Leverson JD, Phillips DC, Mitten MJ, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015;7(279):279ra40. doi: 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- 64.Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30(5):488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cervantes-Gomez F, Lamothe B, Woyach JA, et al. Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res. 2015;21(16):3705–3715. doi: 10.1158/1078-0432.CCR-14-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez Marignac VL, Smith S, Toban N, Bazile M, Aloyz R. Resistance to dasatinib in primary chronic lymphocytic leukemia lymphocytes involves AMPK-mediated energetic re-programming. Oncotarget. 2013;4(12):2550–2566. doi: 10.18632/oncotarget.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pitini VV. The role of p53 and autophagy in dasatinib resistance of CLL lymphocytes. Leuk Res. 2011;35(1):32–33. doi: 10.1016/j.leukres.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 69.Amrein PC. The potential for dasatinib in treating chronic lymphocytic leukemia, acute myeloid leukemia, and myeloproliferative neoplasms. Leuk Lymphoma. 2011;52(5):754–763. doi: 10.3109/10428194.2011.555890. [DOI] [PubMed] [Google Scholar]
- 70.Amrein PC, Attar EC, Takvorian T, et al. Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clin Cancer Res. 2011;17(9):2977–2986. doi: 10.1158/1078-0432.CCR-10-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eley T, Luo FR, Agrawal S, et al. Phase I study of the effect of gastric acid pH modulators on the bioavailability of oral dasatinib in healthy subjects. J Clin Pharmacol. 2009;49(6):700–709. doi: 10.1177/0091270009333854. [DOI] [PubMed] [Google Scholar]
- 72.Hussain SR, Lucas DM, Johnson AJ, et al. Flavopiridol causes early mitochondrial damage in chronic lymphocytic leukemia cells with impaired oxygen consumption and mobilization of intracellular calcium. Blood. 2008;111(6):3190–3199. doi: 10.1182/blood-2007-10-115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin TS. Novel agents in chronic lymphocytic leukemia: efficacy and tolerability of new therapies. Clin Lymphoma Myeloma. 2008;8(suppl 4):S137–S143. doi: 10.3816/CLM.2008.s.009. [DOI] [PubMed] [Google Scholar]
- 74.Lanasa MC, Andritsos L, Brown JR, et al. Final results of EFC6663: a multicenter, international, phase 2 study of alvocidib for patients with fludarabine-refractory chronic lymphocytic leukemia. Leuk Res. 2015;39(5):495–500. doi: 10.1016/j.leukres.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goodman VL, Rock EP, Dagher R, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13(5):1367–1373. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 76.Kronabel D. Possible influence of some foods on the metabolism of ibrutinib. Clin Lab. 2015;61(3-4):443–444. doi: 10.7754/clin.lab.2015.150116. [DOI] [PubMed] [Google Scholar]
- 77.Rais R, Zhao M, He P, Xu L, Deeken JF, Rudek MA. Quantitation of unbound sunitinib and its metabolite N-desethyl sunitinib (SU12662) in human plasma by equilibrium dialysis and liquid chromatography-tandem mass spectrometry: application to a pharmacokinetic study. Biomed Chromatogr. 2012;26(11):1315–1324. doi: 10.1002/bmc.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weiss J, Gajek T, Köhler BC, Haefeli WE. Venetoclax (ABT-199) might act as a perpetrator in pharmacokinetic drug-drug interactions. Pharmaceutics. 2016;8(1) doi: 10.3390/pharmaceutics8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]