Abstract
Polar auxin transport is dependent on the family of PIN-formed proteins (PINs), which are membrane transporters of anionic indole-3-acetic acid (IAA−). It is assumed that polar auxin transport may be essential in the development and meristematic activity maintenance of Medicago truncatula (M. truncatula) root nodules. However, little is known about the involvement of specific PIN proteins in M. truncatula nodulation. Using real-time quantitative PCR, we analyzed the expression patterns of all previously identified MtPIN genes and compared them between root nodules and root tips of M. truncatula. Our results demonstrated significant differences in the expression level of all 11 genes (MtPIN1–MtPIN11) between examined organs. Interestingly, MtPIN9 was the only PIN gene with higher expression level in root nodules compared to root tips. This result is the first indication of PIN9 transporter potential involvement in M. truncatula nodulation. Moreover, relatively high expression level in root nodules was attributed to MtPINs encoding orthologs of Arabidopsis thaliana PIN5 subclade. PIN proteins from this subclade have been found to localize in the endoplasmic reticulum, which may indicate that the development and meristematic activity maintenance of M. truncatula root nodules is associated with intracellular homeostasis of auxins level and their metabolism in the endoplasmic reticulum.
Keywords: Medicago truncatula, root nodule, auxin, PIN
1. Introduction
Nitrogen is the primary and most important nutrient for plants, since it is an element for amino acids and nucleobases biosynthesis. Its availability in soil is frequently a major limiting factor for plant growth and, consequently, crop yield. The Earth’s atmosphere is a rich source of dinitrogen, but, unfortunately, plants cannot directly assimilate it in this form. However, plant species from the Fabaceae family have evolved an ability of establishing symbiosis with nitrogen-fixing bacteria, collectively called rhizobia, which allows them to exploit atmospheric nitrogen sources. This type of symbiosis is an effective evolutionary adaptation, which enables fabaceans to accumulate high levels of nitrogen in their tissues [1].
Medicago truncatula is a model fabacean species in the studies of plant interactions with rhizobia. The symbiosis initiates with a molecular dialogue in the rhizosphere between microorganisms and plant’s roots. Such molecular communication enables rhizobia to find their compatible host plant. Flavonoids, which are secreted by fabacean roots, induce bacterial Nod factors synthesis in rhizobia [2]. The recognition of specific Nod factors triggers root hair deformation relying on their curling around microsymbionts’ cells [3]. Subsequently, rhizobia induce local plant cell wall rebuilding, which gives rise to the infection thread, allowing bacteria the colonization of host tissues. At the same time, the nodule primordium starts to develop. When the infection thread reaches the nodule primordium, rhizobia are endocytosed by host cells. Each bacterial cell is surrounded by a peribacteroid membrane originating from the infection thread’s plasma membrane. This structure is called the symbiosome, and the bacteria located inside—bacteroid [4]. In bacteroids, metabolism shifts from the processes specific for free-living bacteria to increased activity of proteins associated with dinitrogen fixation and ATP synthesis, which is crucial for dinitrogen reduction [5]. Host plants provide to bacteroids the energy source and metabolites essential for dinitrogen fixation. The main carbon sources supplied are C4-dicarboxylic acids such as malate, which are intermediates in the citric acid cycle [6]. Furthermore, the host plant needs to ensure a microaerobic environment in the infected cells. This is indispensable for the proper activity of nitrogenase, an oxygen-labile enzyme directly responsible for the dinitrogen fixation. Such an environment is created by the presence of symbiotic leghemoglobins, hemoproteins present in root nodules. Like human hemoglobin, they have strong affinity towards oxygen, and thus effectively decrease their concentration in nodule tissues [7]. In symbiosomes, dinitrogen is converted into ammonium and subsequently secreted for the uptake by plant tissues. Since ammonium is toxic to plant cells, it is immediately assimilated by presumably three interdependent metabolic pathways engaging asparagine synthetase, glutamine synthetase or glutamate dehydrogenase [8].
A mature Medicago truncatula (M. truncatula) root nodule has several distinct zones. From the apical part of the nodule, the following developmental zones can be distinguished: (I) the rhizobial-free meristematic zone, providing indeterminate nodule growth; (II) the infection zone, in which bacterial cells are released from the infection threads and cell differentiation begins; (II/III) the interzone, usually consisting of one to three layers of cells that accumulate starch rapidly; (III) the dinitrogen-fixing zone with mature bacteroids; (IV) the senescence zone, which contains degenerated symbiosomes [9]; and (V) the saprophytic zone with rod-shaped rhizobia colonizing degraded host’s cells [10]. The nodule meristem—together with the bacteroid-containing tissue are surrounded with lateral nodule tissues—an inner cortex with vascular bundles, a nodule endodermis and an outer cortex [9].
An essential role of auxins (principally indole-3-acetic acid—IAA) in nodulation has been assumed for a long time. These plant hormones are involved in numerous, sometimes very divergent physiological processes, such as embryogenesis, organogenesis, plant tropisms, maintenance of meristematic activity, differentiation of vascular tissues, root elongation, fruit development, apical dominance and responses to many environmental stimuli. This phenomenon can be explained by the ability of auxins to be directionally transported and accumulated in particular cells and tissues. Auxins are synthesized in young apical tissues and can be transported by two different pathways: by phloem parenchyma cells (non-polar transport) or by cell-to-cell transport as a result of polar auxin transport (PAT). The second pathway is crucial for the local accumulation of these hormones and is dependent on polar auxin transporters such as auxin efflux carriers—PIN-formed proteins (PINs), PGP proteins that work both as influx and efflux carriers, as well as auxin influx carriers—AUX1/LAX [11]. The protonated form of IAA (IAAH) has the ability to penetrate cell membranes in accordance with the concentration gradient. In cytosolic environments (pH = 7), IAAH dissociates into IAA– and H+. Deprotonated IAA− is trapped inside the cell and cannot passively diffuse through the plasma membrane. Thus, auxin efflux carriers (PINs) are needed to transport IAA– from the cell [11,12,13].
PIN proteins are polar carriers, which means that their location in the plasma membrane of transport-competent cells is asymmetric. They are predominantly positioned on only one side of the cell, which empowers required direction of auxin transport [13]. However, in Arabidopsis thaliana (A. thaliana) cells, PIN proteins have also been found to localize in endoplasmic reticulum (ER) membranes. AtPIN5, AtPIN6 and AtPIN8, belonging to PIN5 subclade, mediate auxins transport from cytosol to ER lumen [14,15,16,17].
Eleven genes encoding PIN proteins have been characterized in M. truncatula: MtPIN1–MtPIN11 [18,19]. Insightful phylogenetic analyses of genomic and protein sequences of MtPINs revealed conserved N- and C-terminal regions of transmembrane domains and variable middle region of cytoplasmic domain. Evolutionary relations and possible origins of MtPINs have also been explained, which enabled identification of A. thaliana and M. truncatula probable orthologs [18,19].
The subcellular localization of PIN proteins, their mode of action, and involvement in specific molecular pathways are well-known in A. thaliana. However, our knowledge concerning PIN proteins in M. truncatula, especially their function in the nodulation process, is still very limited. Although there are some data indicating the role of auxins in the nodulation [20,21,22,23,24,25], and there is a huge gap regarding detailed analysis of PIN involvement in nodule formation and maintenance of their meristematic activity. In this study, we performed a comprehensive analysis of PIN expression, by examining their transcription levels in M. truncatula root tips and nodules. Our results indicate the importance of polar auxins transporters in the nodulation. Based on collected data, we identified these auxin transporters, which may play a crucial role in the nodulation. Our study is the first such detailed one concerning expression of PIN-encoding genes in the model species for rhizobium-fabacean symbiosis—M. truncatula.
2. Results
To perform a detailed expression analysis of PIN genes in M. truncatula root tips and nodules, we employed the real-time quantitative PCR (qPCR) technique. Primers were designed for 11 MtPINs identified previously [18,19]. In order to unambiguously identify the phylogenetic relationship between M. truncatula and A. thaliana orthologs, we performed a BLAST alignment of each MtPIN’s coding DNA sequence (CDS) and full protein sequence with the A. thaliana nucleotide or protein database, respectively. On the basis of this comparison, we identified A. thaliana orthologs that are most closely related to the corresponding MtPINs (Table 1). Our results proved some minor differences between previously identified A. thaliana and M. truncatula orthologs [18,19] and our data. For instance, based on protein alignment, MtPIN7 appeared to be most closely related to AtPIN7, not AtPIN2, as previously described [18,19].
Table 1.
The relationship of M. truncatula PINs and their A. thaliana orthologs according to Schnabel and Frugoli and Peng et al. [18,19] or identified using BLAST search.
| M. truncatula Gene/Protein | A. thaliana Orthologous Gene according to Schnabel and Frugoli and Peng et al. | A. thaliana Orthologous Coding DNA Sequences (CDS) Identified Using BLAST Search | A. thaliana Orthologous Protein Sequences Identified Using BLAST Search |
|---|---|---|---|
| MtPIN1 | AtPIN3 | AtPIN4 | AtPIN4 |
| AtPIN4 | |||
| AtPIN7 | |||
| MtPIN2 | AtPIN2 | AtPIN2 | AtPIN2 |
| MtPIN3 | AtPIN3 | AtPIN3 | AtPIN3 |
| AtPIN4 | |||
| AtPIN7 | |||
| MtPIN4 | AtPIN1 | AtPIN1 | AtPIN1 |
| MtPIN5 | AtPIN1 | AtPIN1 | AtPIN1 |
| MtPIN6 | AtPIN6 | AtPIN6 | AtPIN6 |
| MtPIN7 | AtPIN2 | AtPIN2 | AtPIN7 |
| AtPIN7 | |||
| MtPIN8 | AtPIN8 | AtPIN8 | AtPIN8 |
| MtPIN9 | AtPIN5 | AtPIN5 | AtPIN5 |
| MtPIN10 | AtPIN1 | AtPIN1 | AtPIN1 |
| MtPIN11 | AtPIN8 | AtPIN8 | AtPIN8 |
Absolute, normalized level of MtPINs expression in root tips is presented in Figure 1. Results revealed that all 11 MtPINs were expressed in root tips. However, their expression level differed remarkably. MtPIN1 to MtPIN4 had significantly higher expression level than the other seven MtPINs. The highest expression was found for MtPIN2, whereas the lowest was for MtPIN7 and MtPIN8.
Figure 1.
Absolute, normalized level of PIN expression in M. truncatula root tips. Mean values (±SE) are derived from two biological replicates, for which three individual qPCR reactions were performed (n = 6). Expression level for each PIN was normalized to the endogenous control.
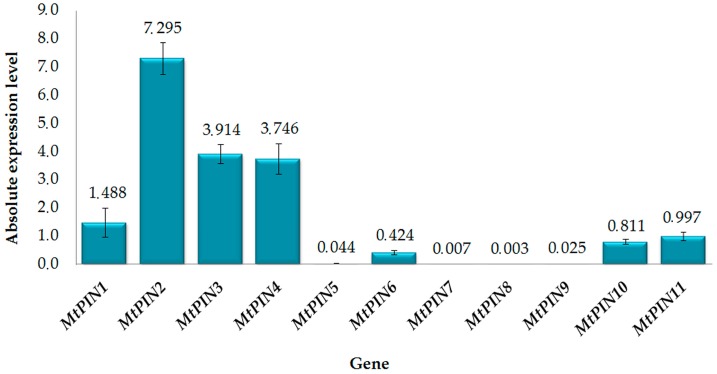
The absolute, normalized level of MtPINs expression in root nodules is presented in Figure 2. Similarly to the results obtained for root tips, the abundance of various PIN transcripts in root nodules differed significantly. The highest expression level, compared to the other MtPINs, was attributed to MtPIN9. Moreover, MtPIN11, MtPIN6 and MtPIN1 had also relatively high expression in root nodules. Expression of the other PIN genes was marginal, besides MtPIN8, which was the only MtPIN for which transcription was not detected at all.
Figure 2.
Absolute, normalized level of PINs expression in M. truncatula root nodules. Mean values (±SE) are derived from two biological replicates for which three individual qPCR reactions were performed (n = 6). Expression levels for each PIN was normalized to the endogenous control.
To compare transcription patterns of MtPINs between root nodules and root tips, relative expression quantification was performed. Statistically significant differences were found for all tested MtPIN genes. Ten out of eleven MtPINs were downregulated in nodules, compared to root tips. The only PIN gene that was up-regulated was MtPIN9 (Table 2).
Table 2.
Comparison of MtPINs expression pattern in root nodules relative to root tips. Statistical analysis was conducted in LinRegPCR [26] and REST2009 [27]. MtRPS7b, encoding ribosomal protein S7, was used as the endogenous reference gene. “DOWN” or “UP” means that particular MtPIN’s expression in nodules in comparison to root tips is significantly lower or higher, respectively.
| Gene | Type | Reaction Efficiency | Expression | Standard Error | 95% Confidence Interval | p-Value | Result |
|---|---|---|---|---|---|---|---|
| MtRPS7b | Reference gene | 0.8951 | 1.000 | ||||
| MtPIN1 | Target gene | 0.7752 | 0.107 | 0.090–0.123 | 0.085–0.137 | 0.001 | DOWN |
| MtPIN2 | Target gene | 0.9265 | 0.001 | 0.001–0.002 | 0.001–0.002 | 0.000 | DOWN |
| MtPIN3 | Target gene | 0.9236 | 0.007 | 0.006–0.011 | 0.004–0.012 | 0.000 | DOWN |
| MtPIN4 | Target gene | 0.9509 | 0.010 | 0.007–0.017 | 0.004–0.023 | 0.000 | DOWN |
| MtPIN5 | Target gene | 0.9748 | 0.288 | 0.194–0.445 | 0.117–0.576 | 0.000 | DOWN |
| MtPIN6 | Target gene | 0.9849 | 0.235 | 0.151–0.351 | 0.105–0.440 | 0.000 | DOWN |
| MtPIN7 | Target gene | 0.8672 | 0.146 | 0.098–0.215 | 0.079–0.300 | 0.001 | DOWN |
| MtPIN8 | Target gene | 0.7412 | 0.012 | 0.009–0.016 | 0.007–0.019 | 0.002 | DOWN |
| MtPIN9 | Target gene | 1.0000 | 29.32 | 19.258–40.578 | 15.769–55.179 | 0.000 | UP |
| MtPIN10 | Target gene | 0.9001 | 0.044 | 0.033–0.055 | 0.023–0.090 | 0.002 | DOWN |
| MtPIN11 | Target gene | 0.9686 | 0.445 | 0.263–0.658 | 0.179–1.515 | 0.007 | DOWN |
Although expressed at lower levels in nodules in comparison to roots (Table 2), MtPIN11, MtPIN6 and MtPIN1 transcript abundance was relatively high in comparison to the remaining PINs (Figure 2). These results imply the highest significance of MtPIN1, MtPIN6, MtPIN9 and MtPIN11, which are the orthologs of Arabidopsis AtPIN4, AtPIN6, AtPIN5 and AtPIN8, respectively, in the nodulation process in M. truncatula.
3. Discussion
It has been shown that PIN-formed proteins play the essential role in the root apical meristem (RAM) activity regulation. By restricting the expression of auxin-inducible PLETHORA genes (main determinants for root stem cells differentiation) in the basal part of embryo, PINs lead to the initiation of root primordium formation [28].
Our data showed that MtPIN2 is the most highly expressed MtPIN gene in root tips. It has been previously found that MtPIN2 expression is upregulated in M. truncatula RAM [29], which is consistent with our results. In A. thaliana, PIN2 plays a pivotal role in the induction of optimal root tip gravitropic response by directional auxins transport from the tip to the root elongation zone [30,31]. Considering such high abundance of MtPIN2 in M. truncatula root tips, it can be concluded that its role is parallel to its A. thaliana ortholog. AtPIN3 has been also demonstrated to be expressed in gravity-sensing tissues [32], thus the high expression of its M. truncatula ortholog, MtPIN3, in root tips seems to be reasonable. Moreover, A. thaliana PIN4 is highly expressed and involved in the establishment and maintenance of auxins gradient within root tip [33]. Previous phylogenetic studies and our comparison of A. thaliana and M. truncatula protein sequences allowed to define MtPIN1 to be most closely related to AtPIN4. In our study, MtPIN1 expression was also relatively high, compared to other MtPINs in root tips. High transcription level was also attributed to MtPIN4—the ortholog of AtPIN1, which is abundantly expressed in vascular tissues [33,34]. Thus, a relatively high expression level of MtPIN4 in root tips was most likely caused by its ubiquity. Furthermore, MtPIN9 has been found to be preferentially expressed in the non-meristematic parts of the root [29], which could explain its almost indiscernible expression in root tips. Nevertheless, based on our results proving high expression of MtPIN9 in indeterminate root nodules, it cannot be excluded that MtPIN9 may play an important role in the nodule meristem functioning.
Additionally, insightful analysis of real-time qPCR results, presented in this study, revealed some interesting facts, especially compared to previous reports. Firstly, our results indicated that, although on a relatively low level, MtPIN5 transcript was detectable in root tips and nodules, in both examined biological repetitions. A previous study by Schnabel and Frugoli from 2004 suggested that MtPIN5 is not expressed in plant tissues, since it originated as a result of MtPIN4 duplication and thus MtPIN5 expression could be silenced, as the one possible fate of duplicated genes [18].
Moreover, the same study reported that expression of MtPIN2 was detected in nodulating roots [18]. However, our analysis showed that although MtPIN2 is highly expressed in root tips, its transcript abundance is very low in mature nodules. Therefore, its contribution in nodulation and meristematic activity maintenance of nodules is uncertain. On the other hand, it should be noted that during our experiment we tested fully differentiated nodules, in which the expression of MtPIN2 could be already suppressed. There are some data demonstrating MtPIN2 expression only in the center and outer cortex of nodules 120 hours after inoculation and in the basal part of nodules 12 days after inoculation, but never in the mature root nodules of M. truncatula. Additionally, silencing MtPIN2, MtPIN3 or MtPIN4 by RNAi resulted in lower number of nodules in M. truncatula roots compared to control plants [21]. Therefore, it has been suggested that these PINs are involved specifically in the regulation of root nodules development. Therefore, this could be the reason why MtPIN2, MtPIN3 and MtPIN4 were no longer highly expressed in mature root nodules tissue in our study. Moreover, a recent report has shown that most MtPIN and MtLAX genes are upregulated in M. truncatula roots after Sinorhizobium meliloti infection, while downregulated in the shoots. The same genes were downregulated in both shoots and roots in dmi3 mutant, which is an infection-resistant mutant of M. truncatula, suggesting the important role of PINs in nodulation. However, that study examined nodulating roots, not root nodules themselves, thus it is hard to draw conclusions from comparing it to our data [23].
Besides MtPIN1, relatively high expression level in root nodules was attributed to MtPIN6, MtPIN9 and MtPIN11, which are the orthologs of A. thaliana AtPIN6, AtPIN5 and AtPIN8, respectively, and encode proteins belonging to the so-called PIN5 subclade. In A. thaliana, PIN proteins from this subclade have been found to localize in the ER [14,15,16,17]. Our results indicated that especially MtPIN9, which is the ortholog of AtPIN5, might be involved in the nodulation in M. truncatula. From all MtPIN genes, MtPIN9 was the only one with statistically significant higher expression level in root nodules in comparison to root tips. Proteins belonging to the PIN5 subclade are responsible for auxins transport from the cytosol to ER lumen, where the enzymes of IAA metabolism pathways are located [35]. Auxins inflow to ER lumen is believed to cause a self-regulation of their metabolism. This particular, intracellular transport results in a decrease of auxins cytosolic concentration, and, consequently, in a decrease of their intercellular flow [14]. Since MtPIN6, MtPIN9 and MtPIN11 are homologous to the transporters from A. thaliana PIN5 subclade, it can be assumed that their subcellular location is the same. This may indicate the essential role of intracellular homeostasis and auxins’ metabolism in ER compartments in the development and meristematic activity maintenance of M. truncatula root nodules.
Furthermore, relatively high expression of MtPIN11 in nodules might have influenced low transcript abundance of MtPIN8. Both of these genes are considered as orthologs of AtPIN8 and probably encode proteins with the same or similar functions, which may result in decreased expression of one of them.
There is some strong evidence indicating the role of auxins and their transporters in the development of indeterminate-type root nodules. Research conducted in 2005 on M. truncatula demonstrated that, in the apical parts of root nodules, de novo IAA synthesis occurs [36]. Moreover, Medicago sativa plants treated with polar auxin transport inhibitors: 1-N-naphthylphthalamic acid (NPA) or 2,3,5-triiodobenzoic acid (TIBA) were shown to produce pseudonodules—structures lacking rhizobia and ineffective in dinitrogen fixation [20]. Similar experiments conducted on M. truncatula plants gave the same results [37].
Interestingly, auxin accumulation in developing Trifolium repens nodules was shown to be preceded by inhibition of PAT [38]. Initial inhibition of auxin flow in the earliest stage of nodulation was demonstrated also in the ethylene-insensitive sickle mutant of M. truncatula. However, 24 h after inoculation, the expression of MtPIN1 and MtPIN2 in M. truncatula sickle mutant roots was increased, which led to boosted auxins accumulation just above the infection site and resulted in a significantly higher number of effective nodules, compared to the wild type [39].
It is also possible that flavonoids, acting as PAT regulators, are essential for nodules formation in M. truncatula. Flavonoid-deficient roots, with reduced abundance of chalcone synthase (CHS), the key enzyme in flavonoids biosynthesis pathway, demonstrated increased auxin transport. These M. truncatula plants were unable to trigger initial reduction of auxin transport and to form root nodules [22].
In 2004, Schnabel and Frugoli identified five M. truncatula genes encoding auxin transporter-like proteins (LAX), which are the auxins influx carriers [18]. Previous research revealed that MtLAX1 is expressed in young, elongating nodule primordia, specifically in their cortical tissues, where the vasculature is formed [40]. Auxins contribution to nodulation of M. truncatula was also indicated by the changes in expression of ARFs (Auxin Response Factors) during the response to S. meliloti infection [23,24,25]. ARFs are transcription factors in auxins signaling cascades, and binding to auxin response elements (AuxREs) in genes promoter regions. There are 24 characterized MtARFs, while AuxREs were found in the promoters of many genes involved in the initiation of nodulation—rhizobial infection response genes [23].
There is also a set of evidence that during symbiosis initiation some phytohormones can be produced by prokaryotic organisms [41]. In a recent study, genetically modified S. meliloti bacteria producing a high amount of IAA were tested in M. sativa inoculation. The results demonstrated that prokaryotic auxins were still detectable after bacteria endocytosis from infection threads, also in IV zone of root nodules. Moreover, such plants showed a higher number of nodules than M. sativa inoculated by wild-type strain, and their transgenic bacteroids had higher expressions of nifH gene, encoding subunits of nitrogenase. These plants also showed up to a 73% increase in the shoot fresh weight and more-branched root system [42].
4. Experimental Section
4.1. Plant Material, Inoculation with Rhizobia and Growth Conditions
Medicago truncatula cv. Jemalong A17 seeds were scarified with 96% sulfuric acid (H2SO4) for 15 min and subsequently washed for five times in sterile water. Seeds were placed in Petri dishes containing 0.8% water agar and stratified for 12 h in the darkness at 4 °C. After stratification, plates were moved to the growth chambers and grown in the following conditions: 16 h photoperiod, photosynthetic photon flux density (PPFD) of 80–100 µE·m−2·s−1 and temperature 25 °C. Root tips were harvested from two-day-old seedlings. To obtain root nodules, four-day-old seedlings were inoculated with Sinorhizobium meliloti strain 1021 culture, which was grown on Tryptone-Yeast (TY) medium until the optical density at 600 nm (OD600) of culture was between 0.6 and 0.8. After inoculation, seedlings were placed in pots filled with perlite and watered with nitrogen-free Fahraeus medium [43] containing 60-times diluted inoculum. Pots were covered with transparent foil for 10 days to ensure seedlings high humidity. Plants were grown for 6 weeks in a 16 h photoperiod, PPFD of 110–170 µE·m−2·s−1 and at 22 °C until fully developed nodules (>1 mm in diameter) were formed. Plants were watered three times per week: twice with distilled water and once with nitrogen-free Fahraeus medium.
4.2. RNA Isolation and cDNA Synthesis
RNA was obtained from M. truncatula root nodules harvested six weeks after inoculation and, separately, from root tips of two-day-old seedlings with primary root active meristems. The reason for using plant material from different developmental stages was that in six-week-old M. truncatula plants, with expanded lateral roots system, the primary root could be delayed in growth and thus difficult to distinguish. Since lateral roots have distinctive root apical meristem (RAM) and geotropism, their PIN distribution can differ from the primary root tip. In order to compare PIN expression level in nodules to a reliable reference point, we used primary roots from young seedlings as well-described control. Total RNA isolation was performed using the GeneMATRIX Universal RNA Purification Kit (EURX, Gdańsk, Poland) with the additional step of on-column DNAse I treatment. RNA concentration, purity and integrity was tested by the spectrophotometric method with Nanodrop 2000 (Thermo Scientific, Waltham, MA, USA) and electrophoretic separation in 1% agarose gel. After equalization of RNA concentration, samples were used for cDNA synthesis using High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA).
4.3. Real-Time Quantitative PCR
Primers used in this study were designed with the help of Primer3 software (Primer3Plus, Free Software Foundation, Inc., Boston, MA, USA) [44] for 11 MtPINs described previously [18,19]. In order to unambiguously identify the phylogenetic relationship between M. truncatula and A. thaliana orthologs, we used the Basic Local Alignment Search Tool (BLAST) [45] within the NCBI database. BLAST alignment was performed for each MtPIN’s coding DNA sequence (CDS) and full protein sequence with the A. thaliana nucleotide or protein database, respectively. For CDSs alignment, we used discontiguous megablast, while, for protein alignment, blastp algorithm. Orthologs were selected according to the highest query cover score. On the basis of this comparison, we identified A. thaliana orthologs that are most closely related to the corresponding MtPINs (Table 1). Each primer pair was designed to amplify 60 to 100 nucleotides long fragments of the first exon of particular MtPIN sequence. Real-time qPCR was conducted in the 7500 Fast Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) using Power SYBR Green Master Mix (Thermo Fisher Scientific), according to the manufacturer’s instruction. Reaction conditions and primers sequences are presented in Table 3 and Table 4, respectively. Each PIN expression was tested for two biological replicates and three technical repetitions. Gene encoding subunit of M. truncatula ribosomal protein S7 (RPS7b) was used as the endogenous reference. The specificity of amplified PCR products was verified by melting curve analysis. Statistical analysis of the results was performed using LinRegPCR [26] (calculation of reaction efficiency) and REST2009 [27] (calculation of relative gene expression level and statistical significance of their differences).
Table 3.
Real-time qPCR conditions.
| Temperature | Time |
|---|---|
| PCR | |
| 50 °C | 20 s |
| 95 °C | 10 min |
| 40 Cycles: | |
| 95 °C | 15 s |
| 60 °C | 1 min |
| Melting curve | |
| 95 °C | 15 s |
| 60 °C | 1 min |
| 95 °C | 30 s |
| 60 °C | 15 s |
Table 4.
Accessions of genes and primers sequences used for real-time qPCR. The exact sequence of each MtPIN first exon fragment that was used for the primer design is also shown (primers binding sites are marked by shading). Underlines represent start codon of each PIN gene and dots (…) represent the discontinuity within the sequence. bp: base pairs.
| Gene/ID | Forward (F) and Reverse (R) Primer Sequence | Sequence of Each MtPIN First Exon Fragment | Product Length |
|---|---|---|---|
| 5′–3′ | (bp) | ||
| MtPIN1 (MTR_4g084870) | F: TCCACTTTACGTAGCCATGATCT | ATGATAACCTGGCACGATCTATACACAGTTTTAACCGCAGTAGTTAGCCTATGGCTCCGTACGGTGGTGGAAAATCCGGCATAAACCGTTTCGTCGC | 74 |
| R: AACATTGGTCCGGTGAGAAT | |||
| MtPIN2 (MTR_4g127100) | F: CGAAGATGAGACATTGAGGATG | ATGATTACCGGTAAGGATATATACGATGTTTTCGCA...CATAAGAAAAGGGGAGGGAGGAGTATGAGGTTCTTACCCTCCTCCAAATCCTATGC | 74 |
| R: CACCATTATTGAACAACTCACCA | |||
| MtPIN3 (MTR_1g030890) | F: CTGGCCTCAACGTGTTCC | ATGATAACACTAAAAGATCTTTACACTGTCTTAACAGCAGTGGTTCCA...GAAATTCGGAACAATCGGAAGAGGGTGCTAGCTGATGAACATAATCAAAA | 68 |
| R: CACCACCATCCTGATCTCCT | |||
| MtPIN4 (MTR_6g069510) | F: TGGTGCCACTTTATGTAGCTATG | ATGATCACTTTAACAGATTTCTACCATGTCATGACAGCAAATCTTAGCTTATGGATCAGTAAAATGGTGGAAAATATTTTCACCTGATTTTGTTGCA | 92 |
| R: ACGGTTGATTCCTGAACATTG | |||
| MtPIN5 (MTR_8g107360) | F: CGTGGCTATGATATTAGCTTATGG | ATGATAACGTTAACAGATTTCTACCATGTGATGACATCAATGGTGCCACTTTATTCAGTGAAATGGTGGAAGATACGGCATCAATCGCTTCGTTGCT | 66 |
| R: GAGCATTGATCGGGAGAGAA | |||
| MtPIN6 (MTR_1g029190) | F: TAAACCGATTCGTCGCAGTT | ATGGTGACAAGAGAAGATTTATACAACGTGATGTGTGCAATGGTACCTC...TTTGCTGTTCCAGTTCTATCTTTTTATCAAATGGACACAAAATTTAT | 67 |
| R: GGATTGTTGAGAGAAATGAAGTGA | |||
| MtPIN7 (MTR_4g127090) | F: TTGTGCCACTATATGTCGCTATG | ATGATTACCGGCAAGGACATATACAATGTTTTAGCGGCGAATATTAGCATATGGTTCGGTCCGATGGTGGAAAATCTTCACACCAGATTGTCTC | 94 |
| R: AAACGGTTTATTCCAGAACATTG | |||
| MtPIN8 (MTR_7g009370) | F: TTTCCTTAGCCAATGTTTATCATGT | ATGAAATAACAACAACTGTCCCATTATATGTAACAATGATACTAGCCTTCACACAAGAACAATGTTCAGGAATAAACAAATTTGTTGC | 95 |
| R: GATCTTAAACCATTTCACTGAGACATA | |||
| MtPIN9 (MTR_7g079720) | F: AGCAGTGGTGCCACTCTATTTT | ATGATTGGGTGGGAAGACGTGTACAAAGTTATTGTGCACTAATATTAGGCTATGGTTCTGTAAGGTGGTGGAAAATTTTCACAAACTAGTTT | 96 |
| R: TTGTTTATTGCATCACATTGTTCTC | |||
| MtPIN10 (MTR_7g089360) | F: TGGTGTTGCTAAAGCTAATGGA | ATGATAAGTGCTTTAGACTTATACCATGTCCTCACAGCAGTAGTACC...AATGGTGGAAATGGCTACCCATTTTTTCACCTGTGGCTAATAAGAAAAA | 61 |
| R: CCCTGCACTATGAGGAGCA | |||
| MtPIN11 (MTR_6g011400) | F: ACAGCCACTGTCCCATTATATGT | ATGATTTCCTTAATTGATGTCTATCATGTAGTAAACTATGTTACTAGCATACATTTGTGTTAAATGGTGGAAACTTTGGCATAAACAAATTTGTAGC | 87 |
| R: TGCACATTGATCTGGTGTGA | |||
| MtRPS7b (MTR_8g087480) | F: GAAACAACACTGCAATTTACAGGA | ATGACCTTACCATACCCATACCATCACCATTGTTGT...AACTATCAGGCAAAGATGTTGTCTTTAGTTCTGTTTCTCAATTTTGATTTTGTTTCATG | 74 |
| R: CCTAAGCCTCAGTAACGGGATA |
5. Conclusions
To conclude, our research revealed possible involvement of MtPIN9 transporter in M. truncatula nodulation, which was not shown ever before. Furthermore, based on the relatively high expression in root nodules of MtPINs, which are the A. thaliana orthologs of genes encoding PIN proteins located in the ER, it can be assumed that development and meristematic activity maintenance of M. truncatula root nodules may be associated with intracellular homeostasis of auxins level and their metabolism in the ER. Our work further supports the hypothesis that M. truncatula nodulation depends on the auxin level. However, further studies are needed to determine auxins role in different developmental stages and particular zones of the nodules. Elucidation of specific auxin transporters functions in nodules formation and maintenance of their meristematic activity should be of great interest in order to fully understand the auxins mode of action during nodulation process.
Acknowledgments
This work was supported by the 0512/IP1/2015/73 project operating within the Iuventus Plus Initiative in years 2015–2017, financed by the Polish Ministry of Science and Higher Education.
Abbreviations
| ATP | adenosine triphosphate |
| AUX1 | AUXIN1 |
| BLAST | basic local alignment search tool |
| bp | base pair |
| CDS | coding DNA sequence |
| cv. | cultivar |
| ER | endoplasmic reticulum |
| IAA− | anionic indole-3-acetic acid |
| IAAH | protonated form of IAA |
| LAX | LIKE AUX1 |
| NCBI | national center for biotechnology information |
| PAT | polar auxin transport |
| PCR | polymerase chain reaction |
| PGP | P-glycoprotein |
| qPCR | quantitative polymerase chain reaction |
| RAM | root apical meristem |
| SE | standard error |
Author Contributions
Weronika Czarnocka conceived and designed the experiments; Izabela Sańko-Sawczenko prepared plant material, harvested nodules and root tips, isolated RNA, and performed cDNA synthesis and real-time qPCRs with the help of Weronika Czarnocka; Izabela Sańko-Sawczenko and Weronika Czarnocka analyzed the data with the help of Barbara Łotocka; Izabela Sańko-Sawczenko wrote the manuscript with the help of Barbara Łotocka and Weronika Czarnocka.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Sprent J.I., Sprent P. Nitrogen Fixing Organisms: Pure and Applied Aspects. Chapman and Hall; London, UK: 1990. [Google Scholar]
- 2.Perret X., Staehelin C., Broughton W.J. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 2000;64:180–201. doi: 10.1128/MMBR.64.1.180-201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esseling J.J., Lhuissier F.G.P., Emons A.M.C. Nod factor-induced root hair curling: Continuous polar growth towards the point of nod factor application. Plant Physiol. 2003;132:1982–1988. doi: 10.1104/pp.103.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewin N.J. Plant cell wall remodelling in the rhizobium-legume symbiosis. Crit. Rev. Plant Sci. 2004;23:293–316. doi: 10.1080/07352680490480734. [DOI] [Google Scholar]
- 5.Barnett M.J., Fisher R.F. Global gene expression in the rhizobial-legume symbiosis. Symbiosis. 2006;42:1–24. [Google Scholar]
- 6.Poole P., Allaway D. Carbon and nitrogen metabolism in Rhizobium. Adv. Microb. Physiol. 2000;43:117–163. doi: 10.1016/s0065-2911(00)43004-3. [DOI] [PubMed] [Google Scholar]
- 7.Ott T., van Dongen J.T., Guenther C., Krusell L., Desbrosses G., Vigeolas H., Bock V., Czechowski T., Geigenberger P., Udvardi M.K. Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Curr. Biol. 2005;15:531–535. doi: 10.1016/j.cub.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Barsch A., Carvalho H.G., Cullimore J.V., Niehaus K. GC-MS based metabolite profiling implies three interdependent ways of ammonium assimilation in Medicago truncatula root nodules. J. Biotechnol. 2006;127:79–83. doi: 10.1016/j.jbiotec.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Vasse J., de Billy F., Camut S., Truchet G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J. Bacteriol. 1990;172:4295–4306. doi: 10.1128/jb.172.8.4295-4306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmers A.C.J., Soupéne E., Auriac M.C., de Billy F., Vasse J., Boistard P., Truchet G. Saprophytic intracellular rhizobia in alfalfa nodules. Mol. Plant Microbe Interact. 2000;13:1204–1213. doi: 10.1094/MPMI.2000.13.11.1204. [DOI] [PubMed] [Google Scholar]
- 11.Vieten A., Sauer M., Brewer P.B., Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Yang H., Peer W.A., Richter G., Blakeslee J.J., Bhandyopadhyay A., Titapiwatanakun B., Undurraga S., Khodakovskaya M., Richards E.L., et al. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science. 2005;310:121–125. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- 13.Michniewicz M., Brewer P.B., Friml J. Polar auxin transport and asymmetric auxin distribution. In: Last R., editor. The Arabidopsis Book. American Society of Plant Biologists; Rockville, MD, USA: 2007. pp. 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mravec J., Skůpa P., Bailly A., Hoyerová K., Křeček P., Bielach A., Petrasek J., Zhang J., Gaykova V., Stierhof Y.-D., et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature. 2009;459:1136–1140. doi: 10.1038/nature08066. [DOI] [PubMed] [Google Scholar]
- 15.Ding Z., Wang B., Moreno I., Dupláková N., Simon S., Carraro N., Reemmer J., Pěnčik A., Chen X., Tejos R., et al. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat. Commun. 2012;3:941. doi: 10.1038/ncomms1941. [DOI] [PubMed] [Google Scholar]
- 16.Sawchuk M.G., Edgar A., Scarpella E. Patterning of leaf vein networks by convergent auxin transport pathways. PLoS Genet. 2013;9:1197. doi: 10.1371/journal.pgen.1003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friml J., Jones A.R. Endoplasmic reticulum: The rising compartment in auxin biology. Plant Physiol. 2010;154:458–462. doi: 10.1104/pp.110.161380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnabel E.L., Frugoli J. The PIN and LAX families of auxin transport genes in Medicago truncatula. Mol. Genet. Genom. 2004;272:420–432. doi: 10.1007/s00438-004-1057-x. [DOI] [PubMed] [Google Scholar]
- 19.Peng J., Ge L., Wang Y., Chen R. Signaling and transport of auxin and plant development. In: Chen R., Baluška F., editors. Polar Auxin Transport. Springer; Berlin & Heidelberg, Germany: 2013. pp. 239–258. [Google Scholar]
- 20.Hirsch A.M., Bhuvaneswari T.V., Torrey J.G., Bisseling T. Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc. Natl. Acad. Sci. USA. 1989;86:1244–1248. doi: 10.1073/pnas.86.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huo X., Schnabel E., Hughes K., Frugoli J. RNAi phenotypes and the localization of a protein: GUS fusion imply a role for Medicago truncatula PIN genes in nodulation. J. Plant Growth Regul. 2006;25:156–165. doi: 10.1007/s00344-005-0106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasson A.P., Pellerone F.I., Mathesius U. Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell. 2006;18:1617–1629. doi: 10.1105/tpc.105.038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen C., Yue R., Bai Y., Feng R., Sun T., Wang X., Yang Y., Tie S., Wang H. Identification and analysis of Medicago truncatula auxin transporter gene families uncover their roles in responses to Sinorhizobium meliloti infection. Plant Cell Physiol. 2015;56:1930–1943. doi: 10.1093/pcp/pcv113. [DOI] [PubMed] [Google Scholar]
- 24.Bustos-Sanmamed P., Mao G., Deng Y., Elouet M., Khan G.A., Bazin J., Turner M., Subramanian S., Yu O., Crespi M., et al. Overexpression of miR160 affects root growth and nitrogen-fixing nodule number in Medicago truncatula. Funct. Plant Biol. 2013;40:1208–1220. doi: 10.1071/FP13123. [DOI] [PubMed] [Google Scholar]
- 25.Breakspear A., Liu C., Roy S., Stacey N., Rogers C., Trick M., Morieri G., Mysore K.S., Wen J., Oldroyd G.E.D., et al. The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for Auxin signaling in rhizobial infection. Plant Cell. 2014;26:4680–4701. doi: 10.1105/tpc.114.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramakers C., Ruijter J.M., Deprez R.H., Moorman A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003;339:62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 27.Pfaffl M.W., Horgan G.W., Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heidstra R., Aida M., Palme K., Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 29.Holmes P., Goffard N., Weiller G.F., Rolfe B.G., Imin N. Transcriptional profiling of Medicago truncatula meristematic root cells. BMC Plant Biol. 2008;8:1197. doi: 10.1186/1471-2229-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller A., Guan C., Gälweiler L., Tänzler P., Huijser P., Marchant A., Parry G., Bennett M., Wisman E., Palme K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman A., Takahashi M., Shibasaki K., Wu S., Inaba T., Tsurumi S., Baskin T.I. Gravitropism of Arabidopsis thaliana roots requires the polarization of PIN2 toward the root tip in meristematic cortical cells. Plant Cell. 2010;22:1762–1776. doi: 10.1105/tpc.110.075317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 33.Friml J., Benková E., Blilou I., Wisniewska J., Hamann T., Ljung K., Woody S., Sandberg G., Scheres B., Jürgens G., et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/S0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- 34.Gälweiler L., Guan C., Müller A., Wisman E., Mendgen K., Yephremov A., Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 35.Woodward A.W., Bartel B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fedorova E.E., Redondo F.J., Koshiba T., Pueyo J.J., de Felipe M.R., Lucas M.M. Aldehyde oxidase (AO) in the root nodules of Lupinus albus and Medicago truncatula: Identification of AO in meristematic and infection zones. Mol. Plant Microbe Interact. 2005;18:405–413. doi: 10.1094/MPMI-18-0405. [DOI] [PubMed] [Google Scholar]
- 37.Rightmyer A.P., Long S.R. Pseudonodule formation by wild-type and asymbiotic mutant Medicago truncatula in response to auxin transport inhibitors. Mol. Plant Microbe Interact. 2011;24:1372–1384. doi: 10.1094/MPMI-04-11-0103. [DOI] [PubMed] [Google Scholar]
- 38.Mathesius U., Bayliss C., Weinman J., Schlaman H.R.M., Spaink H.P., Rolfe B.G., McCully M.E., Djordjevic M.A. Flavonoids synthesized in cortical cells during nodule initiation are early developmental markers in white clover. Mol. Plant Microbe Interact. 1998;11:1223–1232. doi: 10.1094/MPMI.1998.11.12.1223. [DOI] [Google Scholar]
- 39.Prayitno J., Rolfe B.G., Mathesius U. The ethylene-insensitive sickle mutant of Medicago truncatula shows altered auxin transport regulation during nodulation. Plant Physiol. 2006;142:168–180. doi: 10.1104/pp.106.080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Billy F., Grosjean C., May S., Bennett M., Cullimore J.V. Expression studies on AUX1-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Mol. Plant Microbe Interact. 2001;14:267–277. doi: 10.1094/MPMI.2001.14.3.267. [DOI] [PubMed] [Google Scholar]
- 41.Spaepen S., Vanderleyden J., Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007;31:425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 42.Bianco C., Senatore B., Arbucci S., Pieraccini G., Defez R. Modulation of endogenous indole-3-acetic acid biosynthesis in bacteroids within Medicago sativa nodules. Appl. Environ. Microbiol. 2014;80:4286–4293. doi: 10.1128/AEM.00597-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent J.M. A Manual for the Practical Study of Root Nodule Bacteria. Blackwell Scientific Publications Ltd.; Oxford, UK: 1970. [Google Scholar]
- 44.Untergasser A., Nijveen H., Rao X., Bisseling T., Geurts R., Leunissen J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007;35:W71–W74. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]