Abstract
Plants, as sessile organisms, can suffer serious growth and developmental consequences under cold stress conditions. Glutathione transferases (GSTs, EC 2.5.1.18) are ubiquitous and multifunctional conjugating proteins, which play a major role in stress responses by preventing oxidative damage by reactive oxygen species (ROS). Currently, understanding of their function(s) during different biochemical and signaling pathways under cold stress condition remain unclear. In this study, using combined computational strategy, we identified 65 Brassica oleracea glutathione transferases (BoGST) and characterized them based on evolutionary analysis into 11 classes. Inter-species and intra-species duplication was evident between BoGSTs and Arabidopsis GSTs. Based on localization analyses, we propose possible pathways in which GST genes are involved during cold stress. Further, expression analysis of the predicted putative functions for GST genes were investigated in two cold contrasting genotypes (cold tolerance and susceptible) under cold condition, most of these genes were highly expressed at 6 h and 1 h in the cold tolerant (CT) and cold susceptible (CS) lines, respectively. Overall, BoGSTU19, BoGSTU24, BoGSTF10 are candidate genes highly expressed in B. oleracea. Further investigation of GST superfamily in B. oleracea will aid in understanding complex mechanism underlying cold tolerance in plants.
Keywords: cold, glutathione transferase (GST), Brassica oleracea, contrasting genotypes, tolerance
1. Introduction
Cold stress is detrimental to plant growth and development, thus affecting agricultural productivity worldwide. During low temperature conditions, cold-tolerant plants activate a tolerance mechanism called cold acclimation, which prevents ice formation within the vegetative cells. However, many agricultural crops lack this cold acclimation mechanism. In general, plants achieve tolerance to cold stress by modifying biochemical and physiological factors at the cellular and molecular level. Cold acclimation involves expression of a set of so-called cold-regulated (COR) genes, induction of which is mediated by effector genes and various transcription factors [1]. In addition to their basic role of protecting cells during cold stress conditions, COR genes regulate signal transduction of defense-related and secondary metabolite genes [2]. Cold stress responses involve cis-acting elements such as ABREs (abscisic acid response element), DREs (c-repeat/dehydration responsive elements), and LTREs (low-temperature-responsive elements), which are regulated by abscisic acid (ABA)-dependent or -independent pathways [3]. ABA and reactive oxygen species (ROS) play vital roles as second messengers via Ca2+ signaling, inducing COR genes such as ERD6 [1], LOS5, FRO1 [4], AP2/ERF [5,6], bZIP [7] and CBFs [8]. Notably, there is also an increase in secondary metabolite accumulation in the plant cell during cold stress. Cold conditions induce increased accumulation of sugar [9], polyamines [10], anthocyanins [11], and glucosinolates [12]. Increased activity of enzymatic (superoxide dismutase (SOD), GST, glutathione reductase (GR), and glutathione peroxidases (GPX)) and non-enzymatic antioxidants (GSH, tripeptide thiols, and vitamins) are also evident in cold-stressed tissues [13]. Additionally, post-translational modifications, such as sumoylation (conjugation of small ubiquitin-like modifier (SUMO) proteins to target proteins) carried out by SIZ1 gene products during stress, play roles in cold tolerance [14]. SIZ1 regulates the expression of CBF/DREB genes by inhibiting MYB genes, thereby enhancing low temperature tolerance [15].
Plant glutathione transferases (GSTs, EC 2.5.1.18) represent a complex superfamily of proteins with multiple roles, involving site-specific (G-site) tripeptide conjugation (glutathione, GSH, and γ-Glu-Cys-Gly) leading to reduction of a wide range of electrophilic and hydrophobic substrates and redox buffering. GSTs play a vital role in glucosinolate biosynthesis and metabolism [16], transport of anthocyanin [17,18], and xenobiotic metabolism [19]. Thus far, evolutionary analysis of GSTs found in eukaryote photosynthetic organisms elucidated 14 major classes in this superfamily [20,21]. Currently, in plants, GSTs are grouped into tau, phi, theta, zeta, lambda, DHAR (dehydroascorbate reductase), EF1G (elongation factor 1 γ), TCHQD (tetrachlorohydroquinone dehalogenase), GHR (glutathionyl hydroquinone reductase), GST2N (glutathione transferases with two thioredoxin), and mPGES2 (microsomal prostaglandin e synthase type 2) classes based on sequence similarity, immunological reactivity, kinetic properties, and structural conformation. Among these classes, tau, phi, DHAR, and lambda GSTs are plant-specific proteins. GST proteins in all phyla typically contain GST N-domain (thioredoxin-like domain, conjugation of GSH moiety, G-site) and GST C-domain (bind to hydrophobic substrates, H-site) except GST2N class, which contains two repeated GST N-domain and lack terminal GST C-domain. A serine residue in the N-terminal region acts as the active site of GST proteins [22], except in those of the lambda, DHAR, GHR, and mPGES2 classes wherein the serine is replaced with a cysteine residue in their active site [21,23]. GSTs were originally considered to serve mainly for xenobiotic detoxification until the discovery of their functions in preventing oxidative damage to cells due to biotic and abiotic stresses such as pathogen infection, ROS, UV radiation, and heavy metal toxicity [24,25,26]. Dixon et al. (2009) reported that tau and phi class GSTs have a wide range of substrate specificity in Arabidopsis, which is correlated with high tolerance against cold, dehydration, UV, oxidative stress, salt, and heavy metals [27]. In Arabidopsis, phi class glutathione transferases (GSTF2) has high affinity to heterocyclic compounds and aids in intracellular transport of defense-related genes [28]. DHAR maintains a reduced ascorbic acid pool within the cell, acting as an antioxidant protein [29], and shows up-regulation during abiotic stresses such as light and drought [21]. Further, lambda GST genes show increased expression under heavy metal stress compared to other GST genes [30]. Among the GST superfamily, few tau, phi and theta GSTs have been known for high glutathione peroxidase (GPOX) activity [22,31], and Euphorbia esula GSTT1 exhibits up-regulation during drought stress and treatment with xenobiotics [32]. Zeta GSTs are known for their roles in tyrosine catabolism [33] and aid during the germination stage under chilling and salt stress in Euphorbia esula [32]. Little information is interpreted related to GHR and mPGES2 classes roles during environmental conditions in plants, however microarray data of Arabidopsis show differential regulation during various abiotic stress [21].
Brassica oleracea includes many important crops in the Brassicaceae family, namely cabbage (B. oleracea var. capitata), broccoli (B. oleracea var. italica), cauliflower (B. oleracea var. botrytis), kale (B. oleracea var. acephala), Brussels sprouts (B. oleracea var. gemmifera), collard greens (B. oleracea var. acephala), kohlrabi (B. oleracea var. gongylodes), and Chinese broccoli (B. oleracea var. alboglabra). Most Brassica species contain high levels of proteins and diversified secondary metabolites [16] that have unique phytochemical characteristics including anti-carcinogenic properties in humans [34]. Cabbage and broccoli are the major agricultural crops of B. oleracea. In this study, a combined computational strategy comprising HMM (hidden Markov model) profile scan coupled with BLAST analysis of the sequenced B. oleracea genome data from Brassica databases was employed to identify GST genes, and further raw data were processed and screened for candidate genes based on coding and protein sequences. We identified and characterized 65 BoGST genes. Moreover, genome-wide expression analysis was performed in two contrasting inbred lines of B. oleracea, Bo106 (cold tolerant (CT)) and Bo107 (cold susceptible (CS)), during cold treatment (4 °C) to reveal possible candidate gene involved during cold tolerance.
2. Results and Discussion
2.1. Identification of GST Genes in B. oleracea
The release of the draft genome of B. oleracea to public databases (Brad, Bolbase and EnsemblPlants) has made it possible to analyze gene families based on annotation from the Arabidopsis genome. Here, we identified a comprehensive set of GST genes from the B. oleracea genome using combined computational analysis comprising HMM profiling and BLAST analyses. A series of systematic analyses was performed to assemble the final set of protein sequence. Firstly, Arabidopsis GST proteins (55 proteins) were collected using locus IDs published in Dixon and Edwards 2010. Based on the 3D structure of GST proteins in Arabidopsis, Armstrong [35] reported that GST N- and C-domains are the basic domain constituents of GST proteins. To spot the functional domains of those sequences, domain analysis was carried out and domain sequences of those proteins were used as input to build a GST-specific HMM profile. Secondly, two peptide datasets, from Bolbase and EnsemblPlants databases, were used as queries against the GST-specific HMM profile. We obtained 107 and 1109 proteins, respectively, from the datasets. From the HMMER results, multiple hits of the same genes were inferred, signifying that the assembled draft genome of B. oleracea contains multiple copies or fractional copies of the same gene. Manually, we identified 89 putative annotated GST genes from Brassica databases. We evaluated all 1305 identified proteins (which includes redundancy) to identify GST genes based on domain occurrence. In total, 65 non-redundant GST proteins were identified based on the HMM results and annotation searches showing they contained only GST-specific domains. Finally, we verified the annotations of these 65 protein sequences using local BLASTP against the Arabidopsis genome and NCBI database using customized E values (1 × 10−3, 1 × 10−10, 1 × 10−30 and 1 × 10−50). The results from each analysis were in agreement, showing paralogous and orthologous genes among B. oleracea (37 proteins), B. napus (21 proteins), B. rapa (6 proteins), and Zea mays (1 proteins) (Table S1). Also, results obtained from GST-specific HMM profile retrieved form Pfam database were similar to that of Arabidopsis GST-specific HMM profile (data not shown). GSTs are studied in a range of plant species such as Arabidopsis [22], barley [36], soybean [37], maize [37], rice [38], tomato [39], and Populus trichocarpa [40], and the total numbers of GST genes in selected crops are summarized in Table 1. Results of BLAST searches of BoGST proteins against the Arabidopsis genome were similar to those for orthologous genes as annotated in the Brad database (data not shown).
Table 1.
Number of GST genes content in Brassica oleracea, Arabidopsis thaliana, Hordeum vulgare, Populus trichocarpa, Solanum lycopersicum, Oryza sativa, Zea mays and Glycine max.
| Plant | Brassica oleracea | Arabidopsis thaliana | Hordeum vulgare | Populus trichocarpa | Solanum lycopersicum | Oryza sativa | Zea mays | Glycine max |
|---|---|---|---|---|---|---|---|---|
| GST Family | Number | Number | Number | Number | Number | Number | Number | Number |
| Tau | 28 | 28 | 50 | 58 | 56 | 40 | 28 | 20 |
| Phi | 14 | 13 | 21 | 9 | 5 | 16 | 12 | 4 |
| Theta | 2 | 2 | 1 | 2 | 4 | 2 | N/A a | N/A a |
| Zeta | 2 | 2 | 5 | 2 | 2 | 3 | 2 | 1 |
| Lambda | 3 | 3 | 2 | 3 | 5 | N/A a | N/A a | N/A a |
| DHAR | 4 | 4 | 2 | 3 | 6 | N/A a | N/A a | N/A a |
| TCHQD | 1 | 1 | 1 | 1 | 1 | N/A a | N/A a | N/A a |
| EF1G | 3 | 2 | 2 | 3 | 1 | N/A a | N/A a | N/A a |
| Others | 8 | N/A a | N/A a | N/A a | 1 | N/A a | N/A a | N/A a |
| Total | 65 | 55 | 84 | 81 | 81 | 61 | 42 | 25 |
| Reference | [44] | [36] | [40] | [39] | [38] | [37] | [37] |
a, not available; GST, glutathione transferase; DHAR, dehydroascorbate reductase; TCHQD, tetrachlorohydroquinone dehalogenase; EF1G, elongation factor 1 γ.
Our domain analysis results confirmed the presence of both GST N- (thioredoxin-like) and GST C-domains (hydrophobic or electrophilic binding) in all GST proteins except two proteins (Bol010024 and Bol015341) which contains two repeats of thioredoxin domain and lack terminal C-domain (Table S2). From our prediction results, we also found other specific and multi domains such as EF1G (elongation factor 1 γ, pfam00647), GSTA (glutathione S-transferase multi-complex domain, COG0625), MaiA (maleylacetoacetate isomerase, TIGR01262) and ECM4 (glutathionyl-hydroquinone reductase, COG0435). Similarly, domain analysis of AtGST and BrGST proteins (uncharacterized) using the SMART and conserved domains database (CDD) database generated similar results (data not shown). The thioredoxin-like domain comprises βαβαββα motifs that make three layers of β-sheet enclosed with two α-helixes on either side (α/β/α) [41], and there is a small peptide sequence between the N-terminus and C-terminus called the linker region (8–15 aa) that connects these two termini [42]. Mohsenzadeh et al. (2011) reported that the C-terminal region of GST confers the substrate selectivity between the GST classes [43]. Secondary structure prediction using the garnier program employing the GOR method in the EMBOSS server showed a higher percentage of α-helix than β-sheets (Table S3). Overall, the 65 BoGST proteins have open reading frames ranging from 570–1248 bp, with predicted protein lengths between 189 and 415 amino acids, and different numbers of exons in their gene structure. The predicted molecular weights, pIs, and stability indexes ranged between 21.37–46.57 (kDa), 4.96–9.5 and 27.33–60.31, respectively. Based on their stability index values, 41 and 24 proteins were characterized as stable and unstable proteins, respectively (Table S3).
2.2. Classification and Sequence Characterization of BoGST Genes
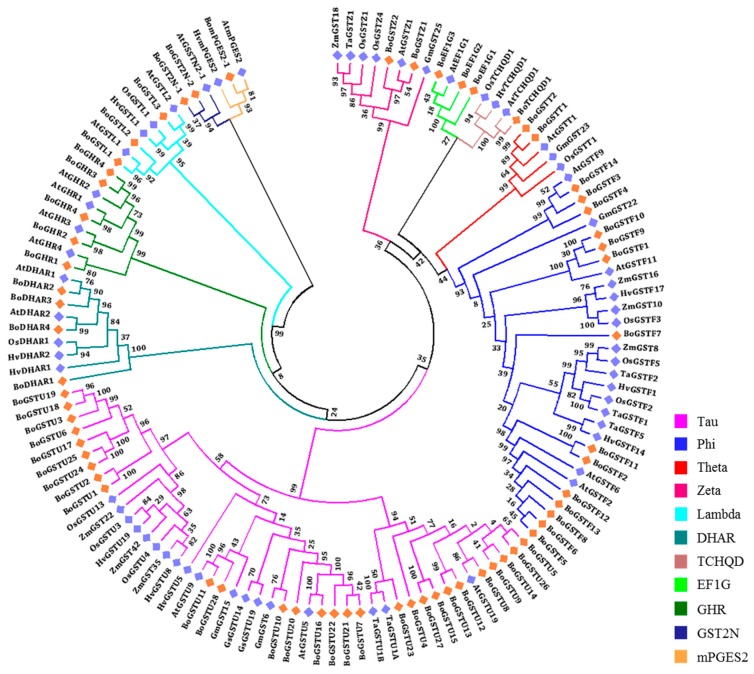
To distinguish between mammalian GSTs and plant GSTs, a classification system was first proposed by Droog (1997) [45], and later modified [46] based on gene association within the genome. Finally, a unified nomenclature system for plant GSTs was adopted [22]. Plant GSTs are classified into eight major classes, namely tau (U), phi (F), theta (T), zeta (Z), lambda (L), DHAR, TCHQD, and EF1G. To investigate the evolutionary relationships of BoGST proteins, known GST proteins from GST superfamilies characterized in monocots (rice, maize, wheat, and barley) and dicots (Arabidopsis, soybean and wild soybean) were collected (Tables S4 and S5). Here, we classified the BoGSTs based on their evolutionary relationships with GSTs from other species. The two largest and most abundant classes, tau and phi, had 28 and 14 closely grouped BoGSTs, respectively, whereas three BoGSTs were separately placed in the lambda and EF1G classes and two BoGSTs in theta and zeta classes. Among the rest of the BoGSTs, four and one were clustered in DHAR, and TCHQD classes, respectively (Figure 1, Figure S1). An additional eight proteins (Bol001864, Bol004474, Bol012366, Bol012372, Bol024359, Bol010024, Bol015341, and Bo035968) were not placed into any of the above mentioned plant classes. Based on BLAST results and domain analysis, five proteins (Bol001864, Bol004474, Bol012366, Bol012372, and Bol024359) were annotated as glutathionyl hydroquinone reductase (GHR) and one protein (Bol035968) as microsomal prostaglandin E synthase type 2 (mPGES-2) with both GST N- and GST C-domain, so we named these classes of proteins as BoGHR and BomPGES2, respectively (data not shown). The remaining two proteins (Bol010024 and Bol015431) consist two repeats of GST N-domain and lack C-terminal, hence these two proteins were classified as GST2N class. A summary of all GST genes in B. oleracea is given in Table 2.
Figure 1.
Phylogenetic trees and classification of glutathione transferase (GST) proteins of B. oleracea using Arabidopsis, rice, barley, wheat, sorghum, maize, and soybean published GST proteins. The unrooted phylogenetic trees was constructed based on multiple sequence alignment using ClustalW followed by maximum-likelihood method using JTT model MEGA6.06 software. Highlighted genes with diamond symbol, orange color represents BoGSTs and blue color represents orthologous GST genes.
Table 2.
Characterization of GST genes in Brassica oleracea.
| Sr. No. | Class | No. of GST Genes | Nucleotide Length Range (bp) | ORF Range (bp) | No. of Exons | Protein | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Length Range (aa) | Mol. Wt. Range (kDa) | pI Range | Average Domain Range | ||||||||
| GST N-Region | GST C-Region | EF1G Region | |||||||||
| 1 | Tau | 28 | 705–4093 | 570–942 | 1–2 | 189–313 | 21.56–35.13 | 4.96–8.85 | 72–75 | 110–146 | - |
| 2 | Phi | 14 | 780–1198 | 603–777 | 3 | 200–258 | 22.59–28.84 | 5.13–8.21 | 60–75 | 114–118 | - |
| 3 | Theta | 2 | 1406–1470 | 726–738 | 7 | 241–245 | 27.38–27.66 | 9.36–9.5 | 75 | 128 | - |
| 4 | Zeta | 2 | 1994–2097 | 591–714 | 9 | 196–237 | 22.26–26.35 | 5.29–6.91 | 44–77 | 118–119 | - |
| 5 | Lambda | 3 | 1430–1776 | 708–906 | 8–9 | 187–301 | 21.37–34.39 | 5.08–8.82 | 77–68 | 88–121 | - |
| 6 | DHAR | 4 | 851–1421 | 633–774 | 3–6 | 210–257 | 23.22–28.63 | 5.76–8.28 | 56–72 | 118–121 | - |
| 7 | TCHQD | 1 | 1071 | 801 | 2 | 266 | 31.46 | 9.26 | 72 | 99 | - |
| 8 | EF1G | 3 | 1902–2326 | 1239–1248 | 6–7 | 412–415 | 46.4–46.57 | 5.56–5.64 | 71–81 | 107–120 | 106–108 |
| 9 | GHR | 5 | 1290–1576 | 954–1212 | 3–5 | 317–403 | 36.43–44.9 | 6.32–8.2 | 88–106 | 111–141 | - |
| 10 | GST2N | 2 | 2196–2401 | 1011–1017 | 11–12 | 336–338 | 36.93–36.95 | 8.81–9.26 | 76–77 | - | - |
| 11 | mPGES2 | 1 | 1464 | 942 | 6 | 313 | 35.13 | 8..85 | 72 | 146 | - |
Sr. No., serial number; bp, base pair; Mol. Wt., molecular weight; aa, amino acid; kDa, Kilodalton; pI, Iso-electric point; GST, Glutathione transferase; N-, N-terminal; C-, C-terminal; EF1G, Elongation factor 1 γ.
In plants, various classes of GST genes respond to cold stress in different species. Specifically, tau GST members in Arabidopsis [47], phi GSTs in Solanum species [48], theta GSTs in Euphorbia [32], and zeta GSTs as well as the TCHQD gene in O. sativa [49,50] show cold-responsive expression. Members of a cold-induced antioxidant enzyme family, DHAR, show activity specifically for rapid H2O2 scavenging in the chloroplast through the water-water cycle to remove H2O2 from the cell [51]. Ahsan et al. (2008) reported that GST omega class genes are closely related to human glutathione S-transferase omega (GSTO) genes and are involved in heavy metal detoxification in rice roots [52]. However, the roles of other GST classes in plants needs to be elucidated during cold stress and other abiotic stresses. Mapping of enzymes or proteins in biochemical pathways can help in understanding their biological function. AtGSTZ1-1 shows maleylacetone isomerase (MAI) activity with a GSH-dependent reaction, involved in tyrosine metabolism in Arabidopsis [33]. Cytochrome P450 (CYP)-mediated detoxification of drugs and xenobiotics requires phase II detoxification enzymes, namely glutathione transferases, for the final degradation process [53]. In addition to these pathways, GSTs are known to play roles in secondary metabolite [54] and auxin metabolism [55]. We mapped the BoGST proteins in the kyoto encyclopedia of genes and genomes (KEGG) database using Blast2Go software [56] to identify their possible roles in the plant. Three major pathways were predicted for BoGST proteins, of which 54 were assigned to glutathione metabolism (map00480), drug metabolism (map00982) and xenobiotics metabolism via cytochrome P450 (map00980). Besides these pathways, BoGST proteins were related to phenylpropanoid biosynthesis (map00940, 18 proteins), ascorbate and aldarate metabolism (map00053, four proteins), arachidonic acid metabolism (map00590, three proteins), tyrosine metabolism (map00350, two proteins), pyruvate metabolism (map00620, four proteins), styrene degradation (map00643, two proteins), and aminoacyl-tRNA biosynthesis (map00970, one protein). Pathway distributions among the different classes of BoGSTs are detailed in Table 3. Overall, the previous pathway-related studies on GST genes in plants have reported similar predicted pathways as those for these BoGST genes in B. oleracea.
Table 3.
Organization of GST super-family in B. oleracea based on pathway analysis using KEGG database.
| Class | Abbreviation | Genes | Predicted Function |
|---|---|---|---|
| Tau | GSTU | 28 | Drug and Xenobiotics metabolism-cytochrome P450, Glutathione metabolism, Pyruvate metabolism, Phenylpropanoid biosynthesis |
| Phi | GSTF | 14 | Drug and Xenobiotics metabolism-cytochrome P450, Glutathione metabolism, Pyruvate metabolism, Phenylpropanoid biosynthesis, Arachidonic acid metabolism |
| Theta | GSTT | 2 | Drug and Xenobiotics metabolism-cytochrome P450, Glutathione metabolism, Phenylpropanoid biosynthesis |
| Zeta | GSTZ | 2 | Drug metabolism-cytochrome P450, Glutathione metabolism, Styrene degradation, Tyrosine and Pyruvate metabolism |
| Lambda | GSTL | 3 | Drug and Xenobiotics metabolism-cytochrome P450, Glutathione metabolism |
| DHAR | DHAR | 4 | Drug and Xenobiotics metabolism-cytochrome P450, Glutathione metabolism, Pyruvate metabolism, Phenylpropanoid biosynthesis, Ascorbate and aldarate metabolism, Aminoacyl-tRNA biosynthesis |
| TCHQD | TCHQD | 1 | Drug and Xenobiotics metabolism-cytochrome P450, Glutathione metabolism |
| EF1G | EF1G | 3 | N/A a |
| GHR | GHR | 5 | Drug and Xenobiotics metabolism-cytochrome P450, Glutathione metabolism |
| GST2N | GST2N | 2 | N/A a |
| mPGES2 | mPGES2 | 1 | N/A a |
a, Not available.
Among various GST classes, intron–exon organization is well categorized. The total numbers of exons within the different classes are included Table 2, and individual exons for the genes are listed in Table S3, Figure S2a,b. Members of the largest class, the tau class, contain two exons in their gene structure except BoGSTU11 (1 exon) (Table S3). The presence of a single exon in this group may possibly be due to selection pressure or genomic duplication. Tau class GSTs were first identified as being encoded by an auxin-inducible gene and are involved in responses to wide range of stresses such as wounding, pathogen infection, herbicides, and extreme temperature. BoGST members of the phi, theta, zeta, TCHQD and mPGES2 classes contained conserved gene orientation with 3, 7, 9, 2, and 6 exons, respectively, in their genomic structure. By contrast, the lambda, DHAR, EF1G, GHR and GST2N gene structures varied within classes. The results of our predicted intron–exon analysis were similar to those for rice GST genes [38]. However, we observed slight deviation when compared to Arabidopsis intron–exon GST results [42], which may be due to the genome size and evolution within the Brassicaceae family. Zeta GST genes from human, carnation, and C. elegans share a common gene structure with three exons, demonstrating that the zeta class gene structure evolved before these lineages diverged, and hence represents the ancestral class [57].
During cold stress, cis-acting factors are involved in activation and overexpression of zeta GST proteins in transgenic rice line [58]. There is no previous report of LTRE (low-temperature-responsive element) regulatory elements in GST superfamily members, although there are other elements such as ABREs (ABA-responsive elements) in wheat GST genes [59], an ERE (ethylene-responsive element) in carnation GST1 [60] and AuxREs (auxin-responsive elements) in tobacco GST [61]. To investigate cis-elements in BoGST genes, the sequences 1000 bp upstream from the 5′-end of the coding region were analyzed for conserved DRE and LTRE motifs. Nine BoGST genes were found to have both putative elements, whereas 10 and 21 genes were predicted to contain either DRE or LTRE cis-acting elements, respectively (Table S6, Figure S3). BoGSTU1, BoGSTU11, BoGSTU16, BoGSTU19, BoGSTL3 and BoGHR3 contained more putative LTRE elements in the sense strand of the promoter region, whereas only BoDHAR1, BoGST2N-1 and BomPGES2-1 had LTRE elements in the antisense region of the promoter. Based on computational analysis revealed several regions of DRE and LTRE elements are present in various promoter regions of BoGST genes, which might be involved during various abiotic stresses.
Moons et al (2003) reported that OsGSTU4 and OsGSTU3 proteins each contain one potential N-glycosylation site similar to that of sorghum [62], and that this site is necessary for the activity of GSTs. Prediction using NetNGlyc (Asn-Xaa-Ser/Thr), showed that 33 BoGSTs have a potential site for N-glycosylation (Table S3). In addition, protein motif searching identified 10 motifs in BoGST proteins, and motif conservation was higher within the classes than between them (Figure S4a,b). Motif 1, present in 57 BoGSTs, contained the serine active site residue at the 18th position (Figure S5). In rice, out of 79 GSTs, 72 were found to contain a serine residue as their active site in their own motif region [50]. Determination of the sub-cellular localization of a protein is an important step toward learning the function of the protein. Little information regarding GST localization is available in the literature, and due to the lack of targeting peptide sequences in their N-terminal regions [27], most GST proteins are thought to be localized in the cytoplasm. Similarly, we found that most BoGST proteins were predicted to be cytoplasmic by Protcomp (61.5%, 40 proteins), tabulated in Table S3. Understanding the sub-cellular localization of GST proteins will aid in finding other possible proteins associated with GST, as well as determining their associated reactions with other proteins and substrates/ligands.
2.3. Chromosomal Distribution, Gene Duplication and Syntenic Regions
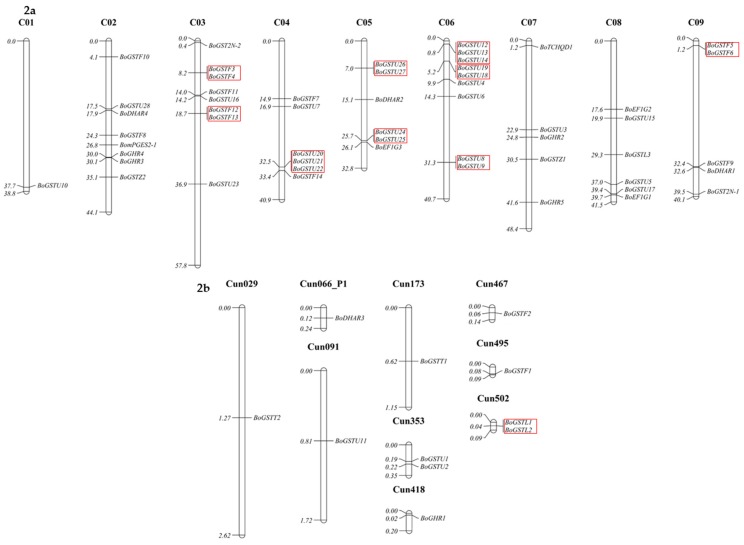
Analysis of gene distribution in the chromosome showed that BoGST genes were spread throughout the genome. The most genes were found in scaffold regions (Cun, 11 genes) and C06 (nine genes), whereas C01 had the fewest GST genes (one gene). Among the tau GSTs, 13 were located in C04 and C06, whereas as a total of eight phi GSTs were in C03 and C09 (Figure 2). Clustering of GST genes was present only in the largest gene classes tau and phi with six clusters (highest) and three clusters, respectively, whereas we observed one cluster (lowest) in the scaffold regions with lambda GSTs. Similar gene distribution and clustering was reported in Arabidopsis [22]. To examine the sequence similarity of BoGST proteins within and between the classes, multiple sequence alignment was performed using DiAlign [63]. As expected, the similarity within the classes was higher than that between classes. GST proteins such as BoGSTU2, BoGSTU6, BoGSTU10, BoGSTU11, BoGSTU23, BoGSTU28, BoGSTF7, BoGSTL3, and BoGHR1 showed less than 60% similarity within their class, compared to not more than 40% between the classes (Tables S7–S10). These results raise the possibility of diversified roles, such as in secondary metabolite metabolism and substrate specificity, within members of a class due to relatively high levels of divergence between the proteins. Thirteen pairs of phi BoGST proteins shared more than 82% similarity, and three pairs of tau BoGSTs had similarity higher than 80%, which indicate high rate of gene duplication within these classes. Such gene duplication might be due to evolutionary pressure imposed on these GST gene family or whole genome triplication event in Brassicaceae family. As mentioned above, zeta GSTs represent the oldest known class and share high similarity among members from different kingdoms, e.g., humans and carnation share 38% identity, whereas humans and C. elegans have 49% similarity. These levels of similarity indicate that significant stretches of sequences have been conserved over a long evolutionary period, thus suggesting common biological function in all organisms [64].
Figure 2.
Chromosomal distribution of GST genes in B. oleracea genome plotted using Mapchart software. The red box indicates genes that are clustered within their GST class.
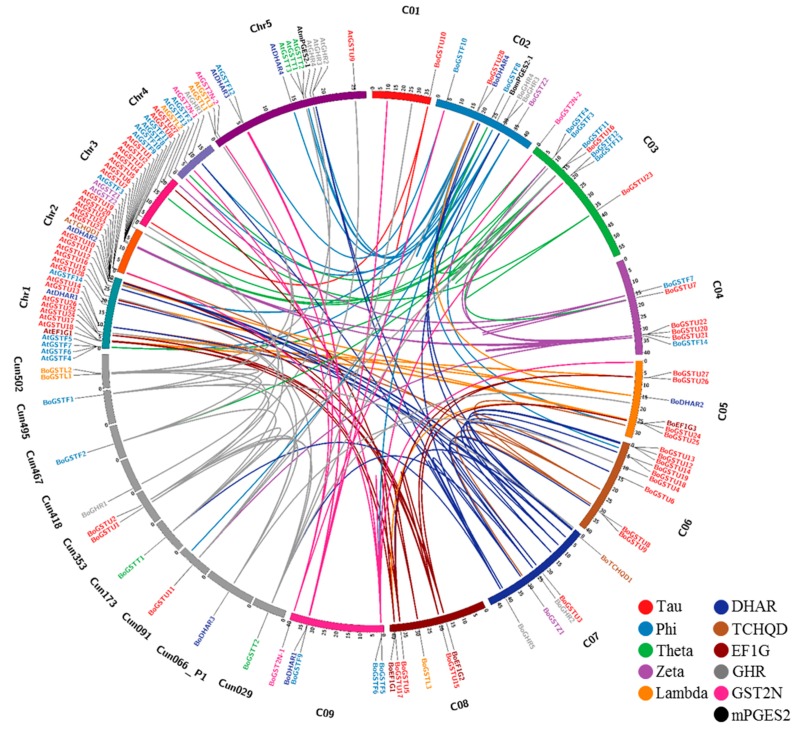
The Brassica and Arabidopsis lineages diverged 20–24 million years ago [65]. To pinpoint the conserved blocks related to GSTs within the Arabidopsis and B. oleracea genomes, syntenic mapping was examined (Figure 3). Within the B. oleracea genome, C06 had more syntenic regions (18.8%) than other chromosomes, whereas C01, with the least (0.1%) GST genes showed fewer syntenic regions (Tables S11 and S12). Syntenic regions of BoGST genes shared 48.3% similarity (highest) in observed regions with Arabidopsis Chr1 (Chromosome 1) and 5.3% (lowest) with Chr4 (chromosome 4). In Arabidopsis, Chr1 contains 26 of 55 AtGSTs, which is the reason for high sequence conservation of BoGST genes with Chr1, is suggestive of genome evolution of duplicated genes from the same chromosome. Surprisingly, in Arabidopsis, Chr5 contains more GST genes than Chr4, but there were fewer syntenic regions than in Chr4 [22]. Genome duplication and gene losses during evolution of Arabidopsis to Brassica are likely responsible for this distribution of synteny between the two species.
Figure 3.
Comparative genome mapping of orthologous and paralogous GST’s genes between B. oleracea and Arabidopsis chromosomes. A high level of conserved syntenic regions between the two species was evident.
2.4. Gene-Specific and Organ-Specific Expression Analysis in Non-Treated Samples
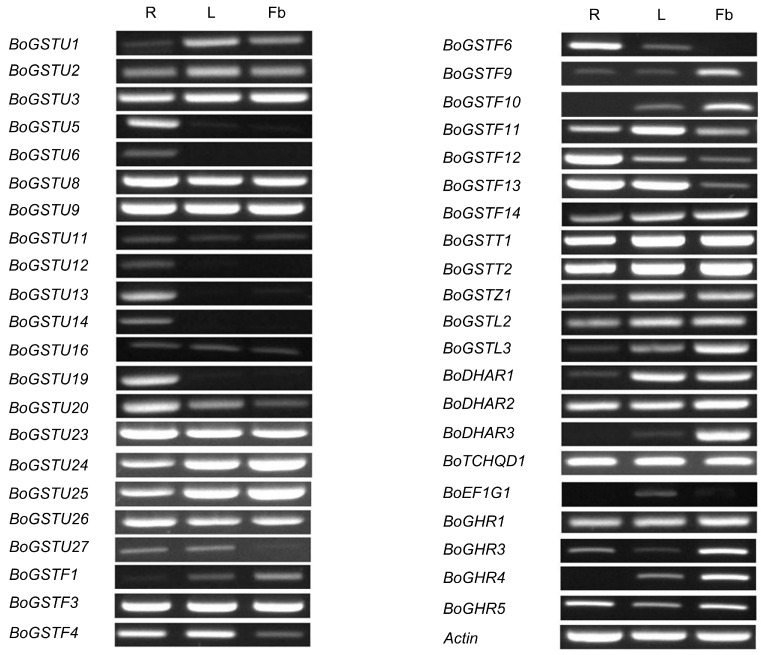
Gene-specific primer pairs for 65 BoGSTs (Table S13) were used for reverse transcription-PCR (RT-PCR) analysis of class-wise expression patterns of BoGSTs in different organs (root, leaf, flower buds) of healthy non-treated B. oleracea (line Bo106). From semi-quantitative RT-PCR analysis among 65 genes, 43 genes were found to express in any one of the investigated tissues (Figure 4). As stated earlier, similarity within the classes was very high, which made it impossible to analyze gene-specific expression of the remaining genes. Of all the expressed genes in organ tissues, 33 genes were expressed in all organs ubiquitously. Notably, BoGSTU6, BoGSTU14 transcripts expressed exclusively in the roots, thus suggesting it is a root-specific gene that could be possibly involved in root associated biological reactions. In rice, tau class OsGSTU3 and OsGSTU4 genes are expressed only in root organs and also show high activity against various abiotic stresses [66]. Among the rest, members of all GST classes were expressed in all organs expect EF1G class, BoGSTF10, BoDHAR3, which were not expressed in root samples. Specifically, BoGSTF1 was expressed in leaf and flower bud, consistent with its reported function in aliphatic GSL biosynthesis in B. oleracea leaves [16]. Overall, the organ-specific expression patterns of BoGSTs were in parallel with those found in organ-specific microarray expression analysis of AtGSTs [22] and OsGSTs [50]. These results shows that GST genes are predominantly expressed in all organs suggest that GSTs may have regulatory functions within developing plant cells.
Figure 4.
BoGST mRNAs showed distinct expression patterns in B. oleracea organs (R—Root, L—Leaf, and Fb—Flower bud). Transcripts specific for each GST genes amplified by reverse transcription-PCR, visualized using 1.5% agarose gel.
2.5. Differential Expression Pattern under Cold Stress
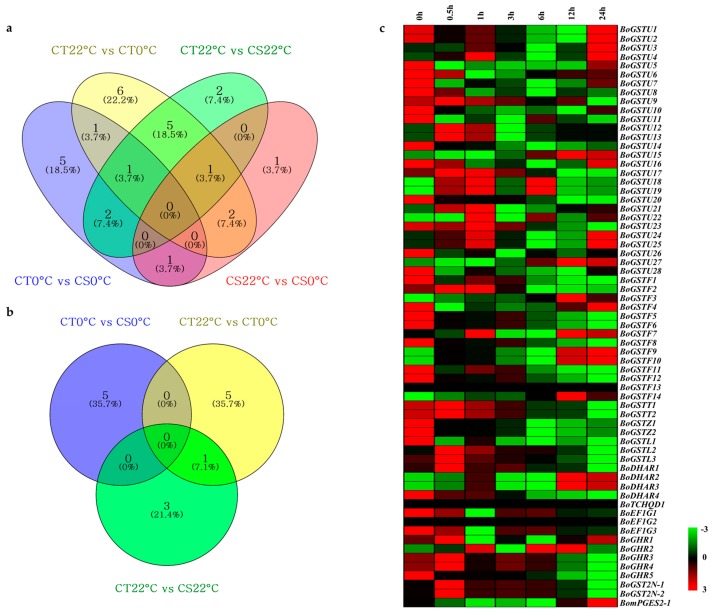
GST genes are among cold-inducible genes in Arabidopsis [47], rice [58], Solanum sp. [48], Euphorbia esula [32], and S. bicolor [67]. Here, cold transcriptome data of the two contrasting lines (cold tolerant (CT) and cold susceptible (CS)) were used for BLAST searches coupled with data mining for our BoGST sequences and revealed a total of 33 unigenes differentially expressed during cold stress (Figure 5a,b; Table S14). For further expression profiling analysis based on Arabidopsis orthologous genes, cold microarray profiles for aerial parts were downloaded using the AtGenExpress visualization tool to investigate cold stress responses of GST genes. A total of 48 orthologous AtGST gene expression profiles were obtained for 65 corresponding BoGST genes. Relative gene expression using cluster analysis revealed that different class of GST genes showed up (red) and down (green) regulation at different time points of cold stress (Figure 5c).
Figure 5.
The Venn diagram shows differentially expressed GST genes from cold transcriptome data of CT and CS lines: (a) up-regulated genes and (b) down-regulated genes. Expression profile cluster analysis of BoGST superfamily using Arabidopsis orthologous microarray data under cold stress: (c) aerial (leaf) sample. Expression cluster with red color indicates up-regulated genes and green color indicates down-regulated genes.
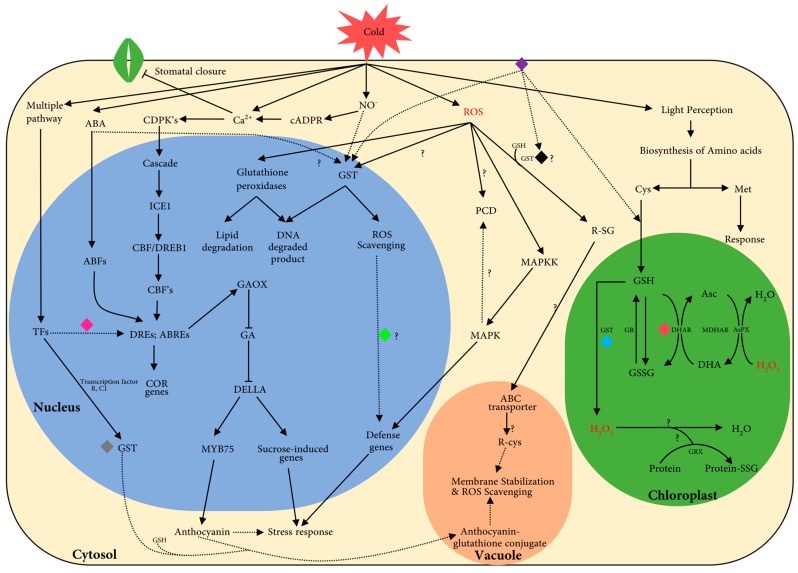
Based on our data and previous reports on GST proteins during stress conditions, a simple schematic model for GST roles in cold stress is proposed in Figure 6; details for a few genes in different pathways during cold stress are tabulated in Table 4. During cold stress, plant cells sense cold via changes in membrane fluidity and protein conformations, which elicit primary signals, such as ABA, Ca2+ (calcium ions), and NO− (nitric oxide) [68], and secondary signals like ROS, stomatal closure, and light perception. The best-known cold response pathway in plants is mediated by transcription factors that bind ABRE and DRE elements in promoters, and further induce COR genes (COR15, COR47, RD22, and RD29A). In addition, AP2/ERF (apetala2/ethylene response factor) elements are also involved in induction of cold-related genes [5,8,69]. During ROS formation (oxidative burst), GST and GPX proteins are known to be highly induced, and in turn detoxify lipid peroxides, DNA degradation products, and ROS. Further, increased cellular levels of ROS, which are second messengers during cold stress conditions, lead to PCD (programmed cell death) [54]. Besides these functions, ROS are also known to induce MAPKK (mitogen-activated protein kinase kinase) proteins, which further induce defense-related genes via MAPKs, and a conjugation process involving GSH by GST enzyme was further localized in vacuole by transporters (ATP-binding cassette (ABC) transporter cascade) for degradation. In the chloroplast, two types of ROS degradation take place: (i) via the ascorbate-glutathione system mediated by DHAR class members [70]; and (ii) conjugation of GSH aided by GST proteins [71]. ROS degradation in chloroplasts is strongly affected by light perception signal transduction by photosystem, Cys, Met and GSH biosynthesis [70]. Targeting of GST proteins to specific organelles within a plant cell during stress conditions reveals likely roles for those proteins. Based on the expression profile and localization of GST proteins, with especially tau and theta class members targeted inside the nucleus and showing differential gene expression (DGE) in cluster analysis (Figure 5c), some members of the tau and theta class are candidates to play roles as transcription factors [22,55]. By contrast, DHAR protein activity is high in thylakoid membranes of the chloroplast [27] and expression analysis during cold stress showed short-term and long-term induction of genes within the class. In general, cold tolerance of plants positively correlates with levels of anthocyanins, which probably protect chlorophyll from over-excitement during cold stress [13]. However in the phi class, GSTF12 is regulated by transcription factors R (bHLH family) and C1 (R2R3-MYB protein family), and is involved in translocation of anthocyanins to vacuoles via ABC transporters, which suggests a possible role in membrane stabilization and ROS scavenging during cold stress [72]. Additionally, TCHQD members are plasma membrane proteins whose function is uncharacterized [27], but interact with DHAR, tau, theta and lambda classes in protein–protein interaction network predicted using STRING database (data not shown), suggesting a possible role in signal perception.
Figure 6.
Simplified overview of GST genes involved in cold pathway, colored diamond shape represents the possible role of different GST classes as mentioned in Table 4. Potential deleterious compound are shown in red color, black solid arrow indicates proven experimental evidence available in literature, black dotted arrow indicates possible predicted function based on localization and protein–protein interaction and the question mark indicates the unknown cellular reaction during cold stress. Role of GSH: photosystem in light perception are proposed pathways for GST genes during cold stress. TFs, transcription factor; Transcription factor R bHLH family; C1, R2R3-MYB protein family; ABA, abscisic acid; ABRE, abscisic acid responsive element; COR, cold responsive element; NO−, nitric oxide; cADPR, cyclic adenosine triphosphate ribose; Ca2+, calcium ion signal; CDPK, calcium dependent protein kinases; ICE1, inducer of CBF expression 1; CBF/DREB1, C-repeat binding factor/dehydration responsive-element binding; DRE, dehydration responsive element; GAOX, gibberellic acid oxidase; GA, gibberellic acid; MYB, myeloblastic transcription factor; ROS, reactive oxygen species; GST, glutathione transferase; PCD, programmed cell death; R-SG, conjugated compound with GSH; MAPK, mitogen-activated protein kinase; MAPKK, MAPK kinase; Cys, cysteine; Met, methionine; GSH, glutathione; GSSG, reduced glutathione; DHAR, dehydroascorbate reductase; DHA, dehydroascorbate; MDHAR- mono dehydroascorbate reductase; Asc, ascorbate; AsPX, ascorbate peroxidase; H2O2, hydrogen peroxide; GR, glutathione reductase; GRX, glutaredoxin.
Table 4.
Predicted BoGST genes involving in various pathways during cold stress in B. oleracea.
| Sr. No. | Image | Possible Role | Localization | GST Family | Possible GST Class Involvement |
|---|---|---|---|---|---|
| 1 |  |
TFs | Nucleus | Tau and Theta | BoGSTU1, BoGSTU2, BoGSTU3, BoGSTU6, BoGSTU10, BoGSTU17, BoGSTU18, BoGSTU19, BoGSTU24, BoGSTU25, BoGSTT1, BoGSTT2 |
| 2 |  |
Gene induction | Nucleus | Unknown | Unknown classes |
| 3 |  |
H2O2 Reduction | Chloroplast | DHAR | BoDHAR1, BoDHAR2, BoDHAR3, BoDHAR4 |
| 4 |  |
H2O2 Reduction | Chloroplast | Lambda | BoGSTL1, BoGSTL2 |
| 5 |  |
GSH conjugation | Cytoplasm | Unknown | Unknown classes |
| 6 |  |
Anthocyanin-transporting gene | Cytoplasm | Phi | BoGSTF9, BoGSTF10 |
| 7 |  |
Signaling protein | Plasma membrane | TCHQD | BoTCHQD1 |
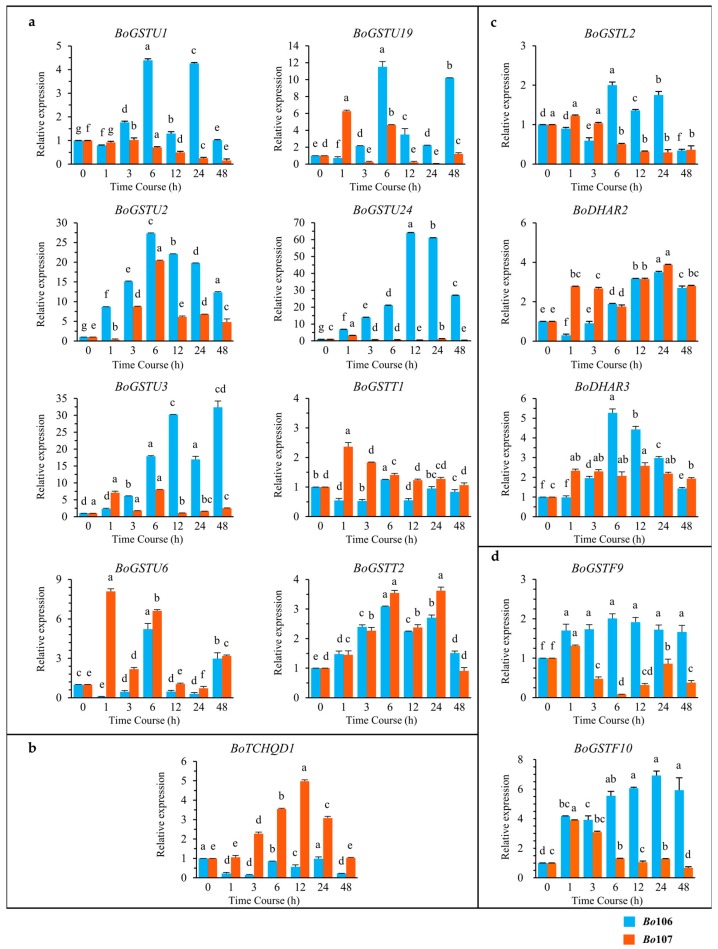
Further to validate the expression of BoGSTs in response to cold, we used leaf samples of two contrasting B. oleracea lines, CS and CT, for RT-PCR and qRT-PCR experiments. RT-PCR results for all GST genes expressed in the organ were analyzed in cold-treated samples, and all genes were successfully amplified except BoGSTU14 in leaves of both lines, and BoGSTU5 in leaf samples of the CS line (Figure S6). Considering previously published results, in silico localization analysis and deduced pathways for GST genes during cold stress, we selected BoGST genes for further analysis that possibly function as transcription factors (eight genes), H2O2 reduction in chloroplast (three genes), and signal perception in the plasma membrane (one gene), as well as co-regulated genes in anthocyanin sequestration to the vacuole (two genes). For these genes, qRT-PCR analysis revealed that transcript levels differed in leaf samples along the time course of cold stress (Figure 7). Of the eight genes analyzed based on possible roles at TFs in the GST superfamily (Figure 7a), BoGSTU2 and BoGSTT2 showed up-regulation in both inbred lines, showing the highest gene expression at 6 h of cold treatment, followed by down-regulation. Their similar expression patterns in both lines suggest that these two genes are not affected by any varying internal factors. In the CT inbred line, BoGSTU1, BoGSTU3, BoGSTU6, BoGSTU19 and BoGSTU24 were positively differentially regulated in response to cold stress; however, high transcript levels of these genes occurred at different times, followed by gradual decreases over the time course. Notably, BoGSTU3 showed continuous upregulation except at 24 h during cold stress. Moreover, BoGSTT1 showed no significant expression during stress time. In the CS inbred line, BoGSTU1, BoGSTU19, BolGSTU24, and BoGSTT1 showed down-regulation along the time period, whereas no significant expression change was observed in BoGSTU3, BoGSTU6 and BoGSTU19 genes during cold treatment.
Figure 7.
Relative quantitative (RQ) expression analysis of 14 BoGST genes which are involved in: (a) transcription activation; (b) signal perception in plasma membrane; (c) H2O2 reduction in chloroplast; and (d) co-regulated genes in anthocyanin sequestration to the vacuoles after cold stress treatment in Brassica oleracea. X-axis represents Time Course (0, 1, 3, 6, 12, 24, and 48 h) and Y-axis represents relative mRNA expression. Graph with orange line is CT line (Bo106), blue line is CS line (Bo107). For each gene, data are represented as relative expression levels to the levels measured at 0 h (2−ΔΔCt). Graph shows the mean of three biological replicates ± standard deviation. (a–e) lowercase letters represent significant differences between different time courses (one-way ANOVA, Tukey’s Test, p < 0.05).
For theta class GST proteins, a myb-like transcription factor regulates gene expression during oxidative stress [22]. Similarly, in drought and cold, GSTT1 in Euphorbia esula showed higher expression than under control conditions [32]. By contrast, parA (tau class) in tobacco possibly functions in transcription regulation in addition to its GST activity [27,55]. In Arabidopsis, AtGSTU7 showed changes in expression within 3 h of different stresses [47]; additionally, AtGSTU17 acts as negative regulator in drought- and salt-mediated signal transduction [73]. Overall, among putative TFs in the GST superfamily, genes positively expressed were induced early in cold stress, and thus are likely to be positively involved in regulation of cold-related genes, secondary metabolite biosynthesis and metabolism and ROS reduction. Investigation of TCHQD revealed contrasting mRNA transcript levels in CT and CS lines, showing down-regulation and up-regulation respectively, consistent with this gene acting as a negative regulator during cold stress (Figure 7b). However, in rice, OsTCHQD1 accumulated after 3 h of cold treatment, significantly lower when compared to drought and salt stress [50], and it is also involved in reduction of pesticides [27]. In the GST superfamily, few genes encode antioxidant enzymes that reduce ROS produced due to cold stress in chloroplasts. Evaluating transcripts of antioxidant enzymes in B. oleracea revealed that BoDHAR2 was up-regulated in both inbred lines, showing high accumulation at 24 h of cold stress. This activation of DHAR genes occurs after high accumulation of ROS levels inside chloroplasts and these genes are also induced after prolonged cold stress (Figure 7c). In the CT line, BoDHAR3 showed up-regulation with a peak at 6 h, whereas BoGSTL2 showed no significant expression during the stress period. Different expression patterns were observed for BoGSTL2 (down-regulation from 3 h) and BoDHAR3 (no response) in the CS line. In wheat, DHAR shows up-regulation in contrasting seasonal varieties during prolonged cold [51], and the level of transcripts was also elevated in transgenic Arabidopsis lines compared to wild type under salt stress [74]. In addition, lambda GST activity is increased in response to heavy metals in Arabidopsis [30], although there are no reports on other abiotic stresses. Anthocyanin biosynthesis-related genes are induced during cold stress, conferring tolerance to the plants. Anthocyanin biosynthesis-related genes are highly induced during cold stress in kale, a B. oleracea member [75], and GST genes are also highly induced regulated during high light (HL) stress along with anthocyanin biosynthesis genes in Arabidopsis [76]. In Arabidopsis, especially GSTF12 promotes the transport of anthocyanins to vacuoles for increased tolerance during cold stress. Examination of two orthologous genes of AtGSTF12 in B. oleracea revealed that in the CT line, BoGSTF9 showed static expression and BoGSTF10 was significantly up-regulated over time during cold stress, whereas the BoGSTF10 failed to accumulate in the CS line, which might lead to the cold-susceptibility of the plant (Figure 7d). Two-way ANOVA statistical analysis support that expression levels of genes were significant (i.e., significant at 0.1% level of significance) at different time point within and between genotypes (Table S15).
In sum, BoGSTU24 exhibited strongly contrasting expression patterns in CT and CS line, showing up-regulation and down-regulation, respectively, during cold stress, whereas BoGSTU19 showed opposite pattern. In CT lines, high accumulation of GST transcripts was observed at or after 6 h of cold stress, although in the CS line, the levels were highest at or after 1 h of cold stress. These findings suggest that there are differences in GST gene induction between the two inbred lines, which may be due to induced formation of ROS during cold stress. However, the phenotype and genotype of the two inbred line also affect the transcript levels of GST genes as well as ROS levels. In silico analysis of BoGST superfamily members supported the transcript expression study of BoGSTs in B. oleracea. Overall, these findings indicate that BoGSTU19, BolGSTU24, and BoGSTF10 are potential genes up-regulated during cold conditions. Further investigation on their functional behavior might help in understanding the cold tolerance mechanism conferred by GST superfamily genes. There are also reports on GST genes induced through the ABA pathway [29], and the GST superfamily may thus also be involved in conferring tolerance to other abiotic stress such as salt, drought, and wounding.
3. Materials and Methods
3.1. GST Sequence Retrieval
A search was conducted based on annotation for glutathione transferase (GST) genes, and corresponding Bo (B. oleracea) coding (CDS) and protein sequences were retrieved from BRAD [77,78], Bolbase database [79,80] and EnsemblPlants database [81,82]. Furthermore, a complementary method that exploits advanced probabilistic methods, called HMM-profiling, was implemented to increase the accuracy in identifying candidate genes within a genome. For this method, we defined Arabidopsis GST amino acid sequences as a primary source of GST-specific domains (GST N- and GST C-domains) in the HMM analysis. Sequences of 55 proteins from Arabidopsis with GST-specific domains were aligned using Clustal Omega [83,84]. We used those aligned sequences (Stockholm alignment format) as an input for HMMBUILD program in HMMER 3.1b2 software [85] to construct our GST-specific HMM profile. This user-defined GST-HMM profile was used as model to search against the B. oleracea genome acquired from the Bolbase and Ensemble databases using the HMMSEARCH program. Further, the results were subjected to domain analysis using the SMART database [86,87] and CDD (Conserved Domain Database) [88,89] to remove sequences with false domains or partial domain architecture of classic GST proteins. Screening and post-processing of the results were done on the basis of default cutoff values and on the presence of GST-specific domains in their protein structure. The retrieved proteins from the B. oleracea genome were revalidated using local BLASTP searches against the NCBI database for confirmation of putative GST functions. The results obtained from GST-specific HMM profile using Arabidopsis GSTs were confirmed using another GST-specific profile retrieved from Pfam database (GST_N-PF02798 and GST_C-PF00043).
3.2. In Silico Approach for Identification and Characterization of GST Genes
To understand the evolutionary relationship among BoGST proteins, processed GST proteins were aligned using CLUSTALW [90,91] with other known GST sequences from Arabidopsis, rice, maize, barley, soybean, wild soybean, and wheat (Table S5) with BLOSUM matrix employing default parameters and the alignment was condensed manually. A molecular phylogenetic tree was constructed using the ML (Maximum likelihood) procedure with the JTT (Jones, Taylor, Thornton) matrix-based amino acid substitution method in MEGA6.06 [92] and 1000 bootstrap replications to access tree topology and reliability. Primary analysis of the predicted molecular weights, pIs, and stability indexes was done using ProtParam [93,94]. Further, N-glycosylation sites were predicted using NetNGlyc 1.0 server [95]. Subcellular localization prediction of predicted BoGST proteins was performed using Protcomp 9.0 from Softberry [96]. Secondary structures of GST proteins were predicted using the garnier script tool from EMBOSS-6.6.0 [97]. Motif analysis of proteins was performed using MEME (Multiple Em for Motif Elicitation v4.10.1) [98] with the following parameters: (1) number of motifs = 10; (2) Motif width ≥6 and ≤50. Gene Structure Display Server (GSDS) web tool [99,100] was used to determine the number of introns and exons, using GFF3 (General file format) and aligning CDS and genomic region of the GST genes. Prediction of putative cis-acting regulatory elements in BoGST genes, using the regions about 1000-bp upstream from the translation initiation site (ATG), was carried out using PlantCARE [101] and PLACE [102], and manually validated as reported by Ibraheem et al. [103].
3.3. Chromosomal Location and Syntenic Regions of BoGSTs
BoGST gene information, chromosome, gene position, strand, and syntenic regions between B. oleracea and Arabidopsis were retrieved using the gene locus search option from Bolbase database [80]. Chromosomal positions of GST genes were drawn using MapChart 2.3 [104] software program. GST genes of B. oleracea were aligned against the B. oleracea and Arabidopsis genome using SyMap v3.4 [105] to obtain syntenic regions within the genome. Subsequently, the derived syntenic regions within the genome and syntenic regions between B. oleracea GST genes and the Arabidopsis genome from the database were used as input for Circos [106] software for visualization of syntenic regions of GST genes.
3.4. Sampling and Preparation of Plant Material
To study the expression patterns of BoGST genes, two contrasting lines, Bo106 (cold tolerant (CT)) and Bo107 (cold susceptible (CS)) [8,107] previously referred to as BN106 (cold tolerant (CT)) and BN107 (cold susceptible (CS)) in the reference [5], were grown at the Department of Horticulture, Sunchon National University, Korea. Seeds of the two lines were aseptically inoculated in half-strength murashige and skoog (MS) medium in a growth chamber. The growth chamber was maintained at 25 ± 1 °C for 16 h light/8 h dark conditions. For organ-specific analysis, samples from fresh roots, leaves, and flower buds were removed from three weeks old healthy plants, frozen immediately in liquid nitrogen, and stored at −80 °C until RNA extraction. Three-week-old seedlings were subjected to cold treatment (4 °C) with three replications. Samples of cold-treated leaves were excised at different time points (0, 0.5, 1, 3, 6, 12, 24, and 48 h), frozen immediately in liquid nitrogen, and stored at −80 °C until RNA extraction. Frozen organ samples and cold-treated samples were subjected to total RNA extraction using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA), subsequently RNA cleanup by DNase I treatment, (Takara Bio, Inc., Shiga, Japan). Isolated RNA was quantified using an ND-1000 Spectrophotometer and NanoDrop v3.7 software (NanoDrop Technologies, Wilmington, DE, USA). Synthesis of cDNA from RNA extracts was performed with Superscript III® First-strand Synthesis Supermix kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions.
3.5. Qualitative and Quantitative PCR Expression Analysis
Initial analysis of expression patterns was carried out using microarray data of Arabidopsis with orthologous loci downloaded from the AtGenExpress visualization tool (AVT) [108] during cold stress (leaf samples). Expression cluster analysis of GST genes were performed with the Cluster program [109] and results were visualized using GenePattern software [56,110]. Additionally, cold transcriptome data of B. oleracea for Bo106 (CT) and Bo107 (CS) lines were downloaded from the NCBI database [111] using TSA (Transcriptome Shotgun assembly; GAQY00000000) and SRA (Sequence Read Archive; SRS490050). Further, qualitative expression analysis using RT-PCR was conducted using one-step EmeraldAmp GT PCR Master Mix (Takara, Bio, Inc., Shiga, Japan). Gene-specific primer pairs for BoGSTs were used for RT-PCR and the actin gene from B. oleracea (JQ435879) was used as a housekeeping control gene (Table S13). RT-PCR was performed using 50 ng cDNA (1 µL) from organ and cold-treated samples as template in a master mix consisting of 2 µL primer pairs (10 pmol each of forward and reverse primer), 8 µL sterile water, and 9 µL Emerald master mix, in a total volume of 20 µL. PCR conditions were set as follows: initial denaturation 94 °C, succeeded by 35 cycles (30 cycles for organ samples) of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 45 s, with a final extension of 5 min at 72 °C for cold-treated samples. PCR products were visualized using 1.5% agarose gels (Duchefa Biochemie, Haarlem, The Netherlands).
Real-Time quantitative PCR (qRT-PCR) was executed using 1 µL cDNA in a 20-µL reaction volume with iTaqTM SYBR® Green Super-mix with ROX (Foster City, CA, USA). Class-wise gene-specific primers for qRT-PCR were employed in this experiment (Table S13). Thermal-cycler conditions were set as follows: 5 min at 95 °C, followed by 40 cycles at 95 °C for 10 s, 60 °C for 10 s, 72 °C for 20 s, and then melting at 72 °C for 60 s and 97 °C for 1 s. The fluorescence was assessed following the last step of each cycle. Product amplification, detection, and data inspection were carried out using LightCycler96 (Roche, Basel, Switzerland). Relative gene expression levels were calculated using the ∆∆Ct method. Actin was used as housekeeping gene.
3.6. Data Statistics
Statistical data analysis was performed for the relative gene expression levels from three biological replicates under each treatment (time-point) × genotype (inbred line) combinations. The log-transformed values were analyzed by two-way analysis of variance (ANOVA) following a generalized linear model using the MINITAB 16 (Minitab Inc., State College, PA, USA) statistical software. To separate the means under each treatment, a Tukey’s pairwise comparison test was performed.
4. Conclusions
This is the first report on genome-wide characterization of GSTs in B. oleracea. In short, using a combined computational strategy, we identified 65 BoGSTs in the B. oleracea genome and characterized them based on domain, gene, and protein structures, sequence similarities, and expression patterns in response to cold stress conditions. Using two contrasting lines, BoGST genes were found to possess potential functions against cold stress in B. oleracea. Overall, the roles of GST along with GSH conjugation in various pathways and degradation process are important to consider for engineering of these candidates gene in recombinant DNA technology for the development of suitable and elite transgenic cultivars that can withstand various abiotic stresses.
Acknowledgments
This research was supported by Golden Seed Project (Center for Horticultural Seed Development, No. 213003-04-4-SB110), Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA) and Korea Forest Service (KFS).
Abbreviations
| ABA | Abscisic acid |
| BoGST | Brassica oleracea Glutathione transferases |
| CS | Cold susceptible |
| CT | Cold tolerant |
| DHAR | Dehydro ascorbate reductase |
| GHR | Glutathionyl Hydroquinone reductase |
| GPX | Glutathione peroxidases |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GST | Glutathione transferases |
| GST2N | GST protein with two repeats of thioredoxin domain |
| mPGES2 | Microsomal Prostaglandin E Synthase type 2 |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/8/1211/s1.
Author Contributions
Ill-Sup Nou, Mi-Young Chung, and Jong-In Park conceived of the study. Harshavardhanan Vijayakumar carried out the experimental work. Harshavardhanan Vijayakumar and Senthil Kumar Thamilarasan drafted the manuscript. Senthil Kumar Thamilarasan, Ashokraj Shanmugam and Sathishkumar Natarajan performed in-silico analysis. Hee-Jeong Jung provided support for data analysis. HyeRan Kim and Senthil Kumar Thamilarasan contributed in manuscript preparation. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Thomashow M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi-Shinozaki K., Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi-Shinozaki K., Shinozaki K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005;10:88–94. doi: 10.1016/j.tplants.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Lee B., Lee H., Xiong L., Zhu J.-K. A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell. 2002;14:1235–1251. doi: 10.1105/tpc.010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed N.U., Jung H.-J., Park J.-I., Cho Y.-G., Hur Y., Nou I.-S. Identification and expression analysis of cold and freezing stress responsive genes of Brassica oleracea. Gene. 2015;554:215–223. doi: 10.1016/j.gene.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 6.Du C., Hu K., Xian S., Liu C., Fan J., Tu J., Fu T. Dynamic transcriptome analysis reveals AP2/ERF transcription factors responsible for cold stress in rapeseed (Brassica napus L.) factors responsible for cold stress in rapeseed (Brassica napus L.) Mol. Genet. Genom. 2016;291:1053–1067. doi: 10.1007/s00438-015-1161-0. [DOI] [PubMed] [Google Scholar]
- 7.Hwang I., Jung H.-J., Park J.-I., Yang T.-J., Nou I.-S. Transcriptome analysis of newly classified bZIP transcription factors of Brassica rapa in cold stress response. Genomics. 2014;104:194–202. doi: 10.1016/j.ygeno.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Thamilarasan S.K., Park J.-I., Jung H.-J., Nou I.-S. Genome-wide analysis of the distribution of AP2/ERF transcription factors reveals duplication and CBFs genes elucidate their potential function in Brassica oleracea. BMC Genom. 2014;15:1211. doi: 10.1186/1471-2164-15-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livingston D.P., Premakumar R., Tallury S.P. Carbohydrate partitioning between upper and lower regions of the crown in oat and rye during cold acclimation and freezing. Cryobiology. 2006;52:200–208. doi: 10.1016/j.cryobiol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Kovács Z., Simon-Sarkadi L., Szucs A., Kocsy G. Differential effects of cold, osmotic stress and abscisic acid on polyamine accumulation in wheat. Amino Acids. 2010;38:623–631. doi: 10.1007/s00726-009-0423-8. [DOI] [PubMed] [Google Scholar]
- 11.Christie P.J., Alfenito M.R., Walbot V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta. 1994;194:541–549. doi: 10.1007/BF00714468. [DOI] [Google Scholar]
- 12.Baskar V., Gururani M.A., Yu J.W., Park S.W. Engineering glucosinolates in plants: Current knowledge and potential uses. Appl. Biochem. Biotechnol. 2012;168:1694–1717. doi: 10.1007/s12010-012-9890-6. [DOI] [PubMed] [Google Scholar]
- 13.Janska A., Maršík P., Zelenková S., Ovesná J. Cold stress and acclimation-what is important for metabolic adjustment? Plant Biol. 2010;12:395–405. doi: 10.1111/j.1438-8677.2009.00299.x. [DOI] [PubMed] [Google Scholar]
- 14.Chinnusamy V., Zhu J., Zhu J.-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007;12:444–451. doi: 10.1016/j.tplants.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Miura K., Jin J.B., Lee J., Yoo C.Y., Stirm V., Miura T., Ashworth E.N., Bressan R.A., Yun D.-J., Hasegawa P.M. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell. 2007;19:1403–1414. doi: 10.1105/tpc.106.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S., Liu Y., Yang X., Tong C., Edwards D., Parkin I.A.P., Zhao M., Ma J., Yu J., Huang S., et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014;5:3930. doi: 10.1038/ncomms4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura S., Shikazono N., Tanaka A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004;37:104–114. doi: 10.1046/j.1365-313X.2003.01943.x. [DOI] [PubMed] [Google Scholar]
- 18.Alfenito M.R., Souer E., Goodman C.D., Buell R., Mol J., Koes R., Walbot V. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell. 1998;10:1135–1149. doi: 10.1105/tpc.10.7.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon D.P., Cummins I., Cole D.J., Edwards R. Glutathione-mediated detoxification systems in plants. Curr. Opin. Plant Biol. 1998;1:258–266. doi: 10.1016/S1369-5266(98)80114-3. [DOI] [PubMed] [Google Scholar]
- 20.Oakley A. Glutathione transferases: A structural perspective. Drug Metab. Rev. 2011;43:138–151. doi: 10.3109/03602532.2011.558093. [DOI] [PubMed] [Google Scholar]
- 21.Lallement P.-A., Brouwer B., Keech O., Hecker A., Rouhier N. The still mysterious roles of cysteine-containing glutathione transferases in plants. Front. Pharmacol. 2014;5:192. doi: 10.3389/fphar.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon D.P., Edwards R. Glutathione transferases. Arabidopsis Book. 2010;8:e0131. doi: 10.1199/tab.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards R., Dixon D.P. Plant glutathione transferases. Methods Enzymol. 2005;401:169–186. doi: 10.1016/S0076-6879(05)01011-6. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal G.K., Jwa N.-S., Rakwal R. A pathogen-induced novel rice (Oryza sativa L.) gene encodes a putative protein homologous to type II glutathione S-transferases. Plant Sci. 2002;163:1153–1160. doi: 10.1016/S0168-9452(02)00331-X. [DOI] [Google Scholar]
- 25.Mueller L.A., Goodman C.D., Silady R.A., Walbot V. AN9, a petunia glutathione S-transferase required for anthocyanin sequestration, is a flavonoid-binding protein. Plant Physiol. 2000;123:1561–1570. doi: 10.1104/pp.123.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kampranis S.C., Damianova R., Atallah M., Toby G., Kondi G., Tsichlis P.N., Makris A.M. A novel plant glutathione S-transferase/peroxidase suppresses Bax lethality in yeast. J. Biol. Chem. 2000;275:29207–29216. doi: 10.1074/jbc.M002359200. [DOI] [PubMed] [Google Scholar]
- 27.Dixon D.P., Hawkins T., Hussey P.J., Edwards R. Enzyme activities and subcellular localization of members of the arabidopsis glutathione transferase superfamily. J. Exp. Bot. 2009;60:1207–1218. doi: 10.1093/jxb/ern365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dixon D.P., Sellars J.D., Edwards R. The Arabidopsis phi class glutathione transferase AtGSTF2: Binding and regulation by biologically active heterocyclic ligands. Biochem. J. 2011;438:63–70. doi: 10.1042/BJ20101884. [DOI] [PubMed] [Google Scholar]
- 29.Moons A. Regulatory and functional interactions of plant growth regulators and plant glutathione S-transferases (GSTs) Vitam. Horm. 2005;72:155–202. doi: 10.1016/S0083-6729(05)72005-7. [DOI] [PubMed] [Google Scholar]
- 30.Dixon D.P., Davis B.G., Edwards R. Functional divergence in the glutathione transferase superfamily in plants: Identification of two classes with putative functions in redox homeostasis in Arabidopsis thaliana. J. Biol. Chem. 2002;277:30859–30869. doi: 10.1074/jbc.M202919200. [DOI] [PubMed] [Google Scholar]
- 31.Dixon D.P., Lapthorn A., Edwards R. Plant glutathione transferases. Genome Biol. 2002;3:3004.1–3004.10. doi: 10.1186/gb-2002-3-3-reviews3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson J.V., Davis D.G. Abiotic stress alters transcript profiles and activity of glutathione S-transferase, glutathione peroxidase, and glutathione reductase in Euphorbia esula. Physiol. Plant. 2004;120:421–433. doi: 10.1111/j.0031-9317.2004.00249.x. [DOI] [PubMed] [Google Scholar]
- 33.Dixon D.P., Cole D.J., Edwards R. Characterisation of a zeta class glutathione transferase from Arabidopsis thaliana with a putative role in tyrosine catabolism. Arch. Biochem. Biophys. 2000;384:407–412. doi: 10.1006/abbi.2000.2125. [DOI] [PubMed] [Google Scholar]
- 34.Keck A.-S., Finley J.W. Cruciferous vegetables: Cancer protective mechanisms of glucosinolate hydrolysis products and selenium. Integr. Cancer Ther. 2004;3:5–12. doi: 10.1177/1534735403261831. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong R.N. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem. Res. Toxicol. 1997;10:2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- 36.Rezaei M.K., Shobbar Z.-S., Shahbazi M., Abedini R., Zare S. Glutathione S-transferase (GST) family in barley: Identification of members, enzyme activity, and gene expression pattern. J. Plant Physiol. 2013;170:1277–1284. doi: 10.1016/j.jplph.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 37.McGonigle B., Keeler S.J., Lau S.C., Koeppe M.K., Keefe D.P.O. A Genomics Approach to the comprehensive analysis of the glutathione S-transferase gene family in soybean and maize. Plant Physiol. 2000;124:1105–1120. doi: 10.1104/pp.124.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soranzo N., Sari Gorla M., Mizzi L., de Toma G., Frova C. Organisation and structural evolution of the rice glutathione S-transferase gene family. Mol. Genet. Genom. 2004;271:511–521. doi: 10.1007/s00438-004-1006-8. [DOI] [PubMed] [Google Scholar]
- 39.Csiszar J., Horvath E., Vary Z., Galle A., Bela K., Brunner S., Tari I. Glutathione transferase supergene family in tomato: Salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol. Biochem. 2014;78:15–26. doi: 10.1016/j.plaphy.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Lan T., Yang Z.-L., Yang X., Liu Y.-J., Wang X.-R., Zeng Q.-Y. Extensive functional diversification of the populus glutathione S-transferase supergene family. Plant Cell. 2009;21:3749–3766. doi: 10.1105/tpc.109.070219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheehan D., Meade G., Foley V.M., Dowd C.A. Structure, function and evolution of glutathione transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. J. Biochem. 2001;16:1–16. doi: 10.1042/bj3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frova C. The plant glutathione transferase gene family: Genomic structure, functions, expression and evolution. Physiol. Plant. 2003;119:469–479. doi: 10.1046/j.1399-3054.2003.00183.x. [DOI] [Google Scholar]
- 43.Mohsenzadeh S., Esmaeili M., Moosavi F., Shahrtash M. Plant glutathione S-transferase classification, structure and evolution. Afr. J. Biotechnol. 2011;10:8160–8165. [Google Scholar]
- 44.Wagner U., Edwards R., Dixon D.P., Mauch F. Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol. Biol. 2002;49:515–532. doi: 10.1023/A:1015557300450. [DOI] [PubMed] [Google Scholar]
- 45.Droog F. Plant glutathione S-transferases, a tale of theta and tau. J. Plant Growth Regul. 1997:95–107. doi: 10.1007/PL00006984. [DOI] [Google Scholar]
- 46.Edwards R., Dixon D.P., Walbot V. Plant glutathione S-transferases: Enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000;5:193–198. doi: 10.1016/S1360-1385(00)01601-0. [DOI] [PubMed] [Google Scholar]
- 47.Sappl P.G., Carroll A.J., Clifton R., Lister R., Whelan J., Harvey Millar A., Singh K.B. The Arabidopsis glutathione transferase gene family displays complex stress regulation and co-silencing multiple genes results in altered metabolic sensitivity to oxidative stress. Plant J. 2009;58:53–68. doi: 10.1111/j.1365-313X.2008.03761.x. [DOI] [PubMed] [Google Scholar]
- 48.Seppänen M.M., Cardi T., Borg Hyökki M., Pehu E. Characterization and expression of cold-induced glutathione S-transferase in freezing tolerant Solanum commersonii, sensitive S. tuberosum and their interspecific somatic hybrids. Plant Sci. 2000;153:125–133. doi: 10.1016/S0168-9452(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 49.Tsuchiya T., Takesawa T., Kanzaki H., Nakamura I. Genomic structure and differential expression of two tandem-arranged GSTZ genes in rice. Gene. 2004;335:141–149. doi: 10.1016/j.gene.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 50.Jain M., Ghanashyam C., Bhattacharjee A. Comprehensive expression analysis suggests overlapping and specific roles of rice glutathione S-transferase genes during development and stress responses. BMC Genom. 2010;11:1211. doi: 10.1186/1471-2164-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baek K.-H., Skinner D.Z. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci. 2003;165:1221–1227. doi: 10.1016/S0168-9452(03)00329-7. [DOI] [Google Scholar]
- 52.Ahsan N., Lee D.G., Alam I., Kim P.J., Lee J.J., Ahn Y.O., Kwak S.S., Lee I.J., Bahk J.D., Kang K.Y., et al. Comparative proteomic study of arsenic-induced differentially expressed proteins in rice roots reveals glutathione plays a central role during As stress. Proteomics. 2008;8:3561–3576. doi: 10.1002/pmic.200701189. [DOI] [PubMed] [Google Scholar]
- 53.Kumar S., Jin M., Weemhoff J. Cytochrome P450-mediated phytoremediation using transgenic plants: A need for engineered cytochrome P450 enzymes. J. Pet. Environ. Eng. 2012;29:997–1003. doi: 10.4172/2157-7463.1000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marrs K.A. The functions and regulation of glutathione S-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi Y., Hasezawa S., Kusaba M., Nagata T. Expression of the auxin-regulated parA gene in transgenic tobacco and nuclear localization of its gene products. Planta. 1995;196:111–117. doi: 10.1007/BF00193224. [DOI] [PubMed] [Google Scholar]
- 56.Blast2GO. [(accessed on 11 January 2016)]. Available online: https://www.blast2go.com/
- 57.Blackburn A.C., Woollatt E., Sutherland G.R., Board P.G. Characterization and chromosome location of the gene GSTZ1 encoding the human Zeta class glutathione transferase and maleylacetoacetate isomerase. Cytogenet. Cell Genet. 1998;83:109–114. doi: 10.1159/000015145. [DOI] [PubMed] [Google Scholar]
- 58.Takesawa T., Ito M., Kanzaki H., Kameya N., Nakamura I. Over-expression of ζ glutathione S-transferase in transgenic rice enhances germination and growth at low temperature. Mol. Breed. 2002;9:93–101. doi: 10.1023/A:1026718308155. [DOI] [Google Scholar]
- 59.Pandey B., Sharma P., Pandey D.M., Varshney J., Sheoran S., Singh M., Singh R., Sharma I., Chatrath R. Comprehensive computational analysis of different classes of glutathione S-transferases in Triticum aestivum L. Plant Omics J. 2012;5:518–531. [Google Scholar]
- 60.Itzhaki H., Maxson J.M., Woodson W.R. An ethylene-responsive enhancer element is involved in the senescence-related expression of the carnation glutathione-S-transferase (GST1) gene. Proc. Natl. Acad. Sci. USA. 1994;91:8925–8929. doi: 10.1073/pnas.91.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van der Zaal B.J., Droog F.N., Pieterse F.J., Hooykaas P.J. Auxin-sensitive elements from promoters of tobacco GST genes and a consensus as-1-like element differ only in relative strength. Plant Physiol. 1996;110:79–88. doi: 10.1104/pp.110.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gronwald J.W., Plaisance K.L. Isolation and characterization of glutathione S-transferase isozymes from sorghum. Plant Physiol. 1998;117:877–892. doi: 10.1104/pp.117.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Genomatix Software Suit. [(accessed on 23 January 2016)]. Available online: http://www.genomatix.de/cgi-bin/dialign/dialign.pl.
- 64.Board P.G., Baker R.T., Chelvanayagam G., Jermiin L.S. Zeta, a novel class of glutathione transferases in a range of species from plants to humans. Biochem. J. 1997;328:929–935. doi: 10.1042/bj3280929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziolkowski P.A., Kaczmarek M., Babula D., Sadowski J. Genome evolution in Arabidopsis/Brassica: Conservation and divergence of ancient rearranged segments and their breakpoints. Plant J. 2006;47:63–74. doi: 10.1111/j.1365-313X.2006.02762.x. [DOI] [PubMed] [Google Scholar]
- 66.Moons A. Osgstu3 and osgtu4, encoding tau class glutathione S-transferases, are heavy metal- and hypoxic stress-induced and differentially salt stress-responsive in rice roots. FEBS Lett. 2003;553:427–432. doi: 10.1016/S0014-5793(03)01077-9. [DOI] [PubMed] [Google Scholar]
- 67.Chi Y., Cheng Y., Vanitha J., Kumar N., Ramamoorthy R., Ramachandran S., Jiang S.-Y. Expansion mechanisms and functional divergence of the glutathione S-transferase family in sorghum and other higher plants. DNA Res. 2011;18:1–16. doi: 10.1093/dnares/dsq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chinnusamy V., Zhu J., Zhu J.K. Gene regulation during cold acclimation in plants. Physiol. Plant. 2006;126:52–61. doi: 10.1111/j.1399-3054.2006.00596.x. [DOI] [Google Scholar]
- 69.Jung H.-J., Dong X., Park J.-I., Thamilarasan S.K., Lee S.S., Kim Y.-K., Lim Y.-P., Nou I.-S., Hur Y. Genome-wide transcriptome analysis of two contrasting brassica rapa doubled haploid lines under cold-stresses using Br135K oligomeric chip. PLoS ONE. 2014;9:1211. doi: 10.1371/journal.pone.0106069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tausz M., Šircelj H., Grill D. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot. 2004;55:1955–1962. doi: 10.1093/jxb/erh194. [DOI] [PubMed] [Google Scholar]
- 71.Zagorchev L., Seal C.E., Kranner I., Odjakova M. A central role for thiols in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2013;14:7405–7432. doi: 10.3390/ijms14047405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petrussa E., Braidot E., Zancani M., Peresson C., Bertolini A., Patui S., Vianello A. Plant flavonoids-biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013;14:14950–14973. doi: 10.3390/ijms140714950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J.-H., Jiang H.-W., Hsieh E.-J., Chen H.-Y., Chien C.-T., Hsieh H.-L., Lin T.-P. drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol. 2012;158:340–351. doi: 10.1104/pp.111.181875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ushimaru T., Nakagawa T., Fujioka Y., Daicho K., Naito M., Yamauchi Y., Nonaka H., Amako K., Yamawaki K., Murata N. Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J. Plant Physiol. 2006;163:1179–1184. doi: 10.1016/j.jplph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Zhang B., Hu Z., Zhang Y., Li Y., Zhou S., Chen G. A putative functional MYB transcription factor induced by low temperature regulates anthocyanin biosynthesis in purple kale (Brassica Oleracea var. acephala f. tricolor) Plant Cell Rep. 2012;31:281–289. doi: 10.1007/s00299-011-1162-3. [DOI] [PubMed] [Google Scholar]
- 76.Vanderauwera S., Zimmermann P., Van Breusegem F., Langebartels C., Gruissem W., Inze D., Breusegem F. Van Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005;139:806–821. doi: 10.1104/pp.105.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng F., Liu S., Wu J., Fang L., Sun S., Liu B., Li P., Hua W., Wang X. BRAD, the genetics and genomics database for Brassica plants. BMC Plant Biol. 2011;11:1211. doi: 10.1186/1471-2229-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brassica Database. [(accessed on 20 November 2015)]. Available online: http://brassicadb.org/brad/
- 79.Yu J., Zhao M., Wang X., Tong C., Huang S., Tehrim S., Liu Y., Hua W., Liu S. Bolbase: A comprehensive genomics database for Brassica oleracea. BMC Genom. 2013;14:1211. doi: 10.1186/1471-2164-14-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bolbase Database. [(accessed on 20 November 2015)]. Available online: http://www.ocri-genomics.org/bolbase/
- 81.Kersey P.J., Allen J.E., Christensen M., Davis P., Falin L.J., Grabmueller C., Hughes D.S.T., Humphrey J., Kerhornou A., Khobova J., et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.EnsemblPlants Database. [(accessed on 20 November 2015)]. Available online: http://plants.ensembl.org/Brassica_oleracea/Info/Index.
- 83.Eddy S.R. A new generation of homology search tools based on probabilistic inference. Genome Inform. 2009;23:205–211. [PubMed] [Google Scholar]
- 84.Clustal Omega. [(accessed on 9 December 2015)]. Available online: http://www.ebi.ac.uk/Tools/msa/clustalo/
- 85.Schultz J., Milpetz F., Bork P., Ponting C.P. SMART, a simple modular architecture research tool: Identification of signaling domains. Proc. Natl. Acad. Sci. USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marchler-Bauer A., Derbyshire M.K., Gonzales N.R., Lu S., Chitsaz F., Geer L.Y., Geer R.C., He J., Gwadz M., Hurwitz D.I., et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;43:D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.SMART Database. [(accessed on 26 November 2015)]. Available online: http://smart.embl-heidelberg.de/
- 88.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Conserved Domain Database. [(accessed on 26 November 2015)]; Available online: http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml.
- 90.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.CLUSTALW. [(accessed on 9 December 2015)]. Available online: http://www.genome.jp/tools/clustalw/
- 92.Gasteiger E., Hoogland C., Gattiker A., Duvaud S., Wilkins M.R., Appel R.D., Bairoch A. The Proteomics Protocols Handbook. Humana Press; New York, NY, USA: 2005. Protein identification and analysis tools on the ExPASy server; pp. 571–607. [Google Scholar]
- 93.Gupta R., Jung E., Brunak S. Prediction of N-glycosylation sites in human proteins. 2004 in press. [Google Scholar]
- 94.ProtParam. [(accessed on 14 February 2016)]. Available online: http://web.expasy.org/protparam/
- 95.Bailey T.L., Williams N., Misleh C., Li W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006;34:369–373. doi: 10.1093/nar/gkl198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Softberry. [(accessed on 14 February 2016)]. Available online: http://linux1.softberry.com/berry.phtml.
- 97.EMBOSS-6.6.0. [(accessed on 4 March 2016)]. Available online: http://emboss.sourceforge.net/apps/
- 98.Hu B., Jin J., Guo A.-Y., Zhang H., Luo J., Gao G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics. 2014;31:1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rombauts S., Déhais P., Van Montagu M., Rouzé P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999;27:295–296. doi: 10.1093/nar/27.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gene Structure Display Server (GSDS) Web Tool. [(accessed on 5 March 2016)]. Available online: http://gsds.cbi.pku.edu.cn/index.php.
- 101.Higo K., Ugawa Y., Iwamoto M., Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ibraheem O., Botha C.E.J., Bradley G. In silico analysis of cis-acting regulatory elements in 5′ regulatory regions of sucrose transporter gene families in rice (Oryza sativa Japonica) and Arabidopsis thaliana. Comput. Biol. Chem. 2010;34:268–283. doi: 10.1016/j.compbiolchem.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 103.Voorrips R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002;93:77–78. doi: 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]
- 104.Soderlund C., Bomhoff M., Nelson W.M. SyMAP v3.4: A turnkey synteny system with application to plant genomes. Nucleic Acids Res. 2011;39:1–9. doi: 10.1093/nar/gkr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., Jones S.J., Marra M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kayum M.A., Park J.I., Ahmed N.U., Jung H.J., Saha G., Kang J.G., Nou I.S. Characterization and stress-induced expression analysis of Alfin-like transcription factors in Brassica rapa. Mol. Genet. Genom. 2015;290:1299–1311. doi: 10.1007/s00438-015-0993-y. [DOI] [PubMed] [Google Scholar]
- 107.Reich M., Liefeld T., Gould J., Lerner J., Tamayo P., Mesirov J.P. GenePattern 2.0. Nat. Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 108.AtGenExpress Visualization Tool (AVT) [(accessed on 18 March 2016)]. Available online: http://jsp.weigelworld.org/expviz/expviz.jsp.
- 109.Cluster Program. [(accessed on 21 March 2016)]. Available online: http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm.
- 110.GenePattern Software. [(accessed on 21 March 2016)]. Available online: http://genepattern.broadinstitute.org/gp/pages/index.jsf.
- 111.NCBI Database. [(accessed on 24 November 2015)]; Available online: http://www.ncbi.nlm.nih.gov/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.